Abstract
The olfactory epithelium (OE) is one of the few tissues to undergo constitutive neurogenesis throughout the mammalian lifespan. It is composed of multiple cell types including olfactory sensory neurons (OSNs) that are readily replaced by two populations of basal stem cells, frequently dividing globose basal cells and quiescent horizontal basal cells (HBCs). However, the precise mechanisms by which these cells mediate OE regeneration are unclear. Here, we show for the first time that the HBC subpopulation of basal stem cells uniquely possesses primary cilia that are aligned in an apical orientation in direct apposition to sustentacular cell end feet. The positioning of these cilia suggests that they function in the detection of growth signals and/or differentiation cues. To test this idea, we generated an inducible, cell type-specific Ift88 knock-out mouse line (K5rtTA;tetOCre;Ift88fl/fl) to disrupt cilia formation and maintenance specifically in HBCs. Surprisingly, the loss of HBC cilia did not affect the maintenance of the adult OE but dramatically impaired the regeneration of OSNs following lesion. Furthermore, the loss of cilia during development resulted in a region-specific decrease in neurogenesis, implicating HBCs in the establishment of the OE. Together, these results suggest a novel role for primary cilia in HBC activation, proliferation, and differentiation.
SIGNIFICANCE STATEMENT We show for the first time the presence of primary cilia on a quiescent population of basal stem cells, the horizontal basal cells (HBCs), in the olfactory epithelium (OE). Importantly, our data demonstrate that cilia on HBCs are necessary for regeneration of the OE following injury. Moreover, the disruption of HBC cilia alters neurogenesis during the development of the OE, providing evidence that HBCs participate in the establishment of this tissue. These data suggest that the mechanisms of penetrance for ciliopathies in the OE extend beyond that of defects in olfactory sensory neurons and may include alterations in OE maintenance and regeneration.
Keywords: cilia, neurogenesis, olfaction, olfactory sensory neuron, stem cells
Introduction
Neurogenesis occurs from the self-renewal and differentiation of neural stem cells (NSCs; Gage, 2000). While prevalent during development, NSCs are also found in the adult nervous system, in the olfactory epithelium (OE), subventricular zone (SVZ) of the lateral ventricle, and the subgranular zone of the hippocampus (Gage, 2000; Alvarez-Buylla and Garcia-Verdugo, 2002; Lie et al., 2004). Factors and mechanisms that regulate cell proliferation, migration, differentiation, and survival during development can be active in the adult nervous system, allowing for tissue repair and neuroplasticity (for review, see Lie et al., 2004). Unlike most sensory systems, the OE is able to reconstitute both neuronal and non-neuronal populations following injury and neuronal death via the action of resident populations of olfactory stem cells (Graziadei and Graziadei, 1979; Calof and Chikaraishi, 1989; Edge and Chen, 2008). Olfactory sensory neurons (OSNs) are unique in that they directly contact both the external environment and the brain. While this direct contact allows OSNs to detect odors, it also exposes the OE to insults from toxins, bacteria, and viruses leading to cell death. Therefore, the capacity for neurogenesis and the replacement of OSNs is critical for maintaining this important sensory system.
The OE is composed of OSNs, supporting sustentacular (SUS) cells, Bowman's gland duct cells, and two groups of basally located stems cells, globose basal cells (GBCs) and horizontal basal cells (HBCs). GBCs and HBCs are considered to be progenitor or stem cells of the OE, and are capable of promoting regeneration and neurogenesis both for tissue homeostasis and in response to injury (Barber, 1982; Costanzo, 1991; Leung et al., 2007). GBCs are a heterogeneous population of actively cycling cells that are composed of immediate neuronal progenitors and transit-amplifying precursors committed to producing neurons as well as multipotent precursors that can produce non-neuronal cells (Jang et al., 2014). HBCs are a homogeneous population of multipotent, quiescent cells (Holbrook et al., 1995; Carter et al., 2004; Iwai et al., 2008). Following the selective death of olfactory neurons, as occurs in response to olfactory bulbectomy, primarily GBCs replace OSNs through differentiation, while HBCs remain quiescent (Caggiano et al., 1994; Huard et al., 1998; Leung et al., 2007). In lesion models, such as exposure to methyl bromide or ablation with methimazole (MMI), in which SUS cells and GBCs are affected, HBCs have been shown to contribute to the regeneration of the OE (Leung et al., 2007; Iwai et al., 2008). While the cell types involved are known, the precise mechanisms controlling regeneration and homeostasis are unclear. Importantly, the molecular regulation of olfactory basal stem cells, including their activation and proliferation in response to injury and cell fate determination during differentiation, remains largely unknown.
Primary cilia have important roles in cell proliferation, differentiation, and regulation of the cell cycle (for review, see Irigoín and Badano, 2011). There is growing evidence that primary cilia regulate neurogenesis and/or proper differentiation of adult stem cells into amplifying progenitor cells or glial cells in the SVZ and hippocampus (Amador-Arjona et al., 2011; Kumamoto et al., 2012; Tong et al., 2014). Consequently, when cilia are disrupted in these systems, cilia-mediated signaling pathways, such as sonic hedgehog, and cilia-modulated signaling pathways, such as Wnt, are also disrupted (Kumamoto et al., 2012; Tong et al., 2014). These findings demonstrate the important role that cilia play in the signaling pathways that are essential for proper cell differentiation during development and adult homeostasis.
Here, we show that HBCs possess primary cilia, suggesting a potential mechanism for the molecular regulation of olfactory basal stem cells in the OE. In mice in which HBCs lacked cilia, the OE showed limited regeneration following the lesion, with a significant reduction of mature OSNs. Additionally, when HBC cilia were removed early in development, specific regions of the OE were found to have a significant reduction in OSNs. These data identify HBC cilia as a previously unrecognized signaling structure in the OE, provide mechanistic insight into the regulation of HBCs during olfactory regeneration, and indicate a potential role for HBCs in OE development.
Materials and Methods
Mouse strains and genotyping.
All mice were maintained on a mixed genetic background. Transgenic Arl13b-EGFPtg mice were provided by David Clapham (Harvard University, Cambridge, MA). EGFP-CETN2 mice were obtained from The Jackson Laboratory (stock #008234; Higginbotham et al., 2004). Conditional deletion of Ift88 from olfactory horizontal basal cells was achieved by the use of a doxycycline (dox)-inducible Cre recombinase (Cre) mouse model. This model used mice carrying the following three alleles: (1) a Keratin5 (K5) promoter driving expression of the reverse tetracycline transactivator (rtTA; K5rtTA; Diamond et al., 2000); (2) a tetracycline operator (TetO) to permit expression of Cre (TetOCre; Mucenski et al., 2003); and (3) a floxed Ift88fl/Δ to ablate cilia (the Δ allele has exons 4–6 deleted from Ift88; Haycraft et al., 2007). K5rtTA and TetOCre mice provided by Andrzej Dlugosz (University of Michigan, Ann Arbor, MI). Ift88fl/Δ mice were provided by Bradley Yoder (University of Alabama at Birmingham, Birmingham, AL). Removal of Arl13b from olfactory horizontal basal cells was achieved using a similar strategy with a floxed Arl13b (exon 2; Su et al., 2012) mouse provided by Tamara Caspary (Emory University, Atlanta, GA). All mice of either sex were housed and maintained according to the University of Michigan and University of Florida institutional guidelines. All protocols for mouse experimentation were approved by the University of Michigan and the University of Florida Committees on the Use and Care of Animals. Genotyping was performed using primers and PCR parameters from previously published studies, which are referenced above.
Doxycycline transgene induction and olfactory epithelium lesion.
Mice were fed doxycycline chow (200 mg/kg doxycycline, Bio-Serv) and water (200 μg/ml doxycycline, 5% sucrose, Thermo Fisher) starting at either embryonic day 16 (E16) or postnatal day 28 (P28) and remained on a doxycycline-containing diet until they were killed. Based on an approximate daily food intake of 4 g/mouse and water intake of 6 ml/mouse (Bachmanov et al., 2002), mice consumed ∼2 mg of doxycycline/d (0.8 mg in chow and 1.2 mg in water). P28 doxycycline-treated K5rtTA;TetOcre;Ift88fl/fl mice or K5rtTA;TetOcre;Arl13bfl/fl mice and respective control littermates received an intraperitoneal injection of methimazole (2-mercapto-1-methylimidazole, 75 mg/kg in sterilized 1× PBS; Sigma-Aldrich) 4 weeks after the start of the doxycycline-containing diet. These mice were maintained on a doxycycline-containing diet until they were killed 8 weeks after methimazole treatment.
Tissue collection and preparation.
Mice were anesthetized with 30% Fluriso (isoflurane, VetOne), transcardially perfused with 4% paraformaldehyde (PFA), and decapitated, and their heads were fixed in 4% PFA for 12–16 h at 4°C. Tissue was then decalcified in 0.5 m EDTA (Thermo Fisher)/1× PBS overnight at 4°C; cryoprotected in 10% (1 h), 20% (1 h), and 30% sucrose/1× PBS overnight at 4°C; and frozen in OCT compound (Tissue Tek). Sections of the olfactory epithelium and olfactory bulb (OB) that were 10–12 μm in size were collected on a Leica CM1860 cryostat.
Immunohistochemistry.
For all immunofluorescence, antigen retrieval was used. For antigen retrieval, tissue sections were rinsed in 1× PBS to remove OCT then incubated in citrate buffer, pH 6.0, for 30 min at 90°C, cooled for 20 min at room temperature, then washed with distilled water for 5 min. Sections were blocked with 2% donkey or goat serum and 1% BSA in 1× PBS, and were incubated overnight in primary antibody. Antibodies were used at the following dilutions: mIgG2a anti-p63 (1:200; BioCare Medical); mIgG2a anti-ARL13B (1:500; Neuromab); rabbit anti-ARL13B (1:500; Proteintech); mIgG1 anti-γ-tubulin (1:500; Sigma-Aldrich); rabbit anti-K5 (1:2500; Covance); chicken anti-green fluorescent protein (GFP; 1:500; Abcam); rabbit anti-K18 (1:500; Abcam); mIgG1 anti-MASH1 (1:100; BD PharMingen); mIgG2b anti-SEC8 (1:500; BD Transduction Laboratory); rabbit anti-lysine-specific demethylase 1 (LSD1; 1:500; Abcam); goat anti-olfactory marker protein (OMP; 1:1000; Wako Chemicals); mouse anti-Cre (1:500; Millipore); mouse anti-α acetylated tubulin (1:1000; Sigma); rabbit anti-AC3 (1:2000; EnCor Biotechnology); and rabbit anti-tyrosine hydroxylase (TH; 1:500; Millipore). Sections were washed in 1× PBS three times for 5 min each at room temperature and then incubated with Alexa Fluor-conjugated secondary antibodies (1:1000) for 1 h at room temperature. Tissue sections were then incubated with DAPI (5 mg/ml; Invitrogen) for 5 min, washed two times with 1× PBS, and then sealed with coverslips mounted with ProLong Gold (Invitrogen).
For the detection of Cre, tissue sections were rinsed in 1× PBS to remove OCT, puddled with citrate buffer, and steamed for 10 min in a glass jar in a hot water bath. Sections were blocked with 2% donkey or goat serum/5% dry non-fat milk/4% BSA/1% TTX100 in 1× PBS and incubated overnight in primary antibody. For detection of GFP, tissue sections were rinsed in 1× PBS to remove OCT, puddled with citrate buffer, and steamed for 10 min in a glass jar in a hot water bath. Sections were blocked with 2% donkey or goat serum/0.3% TTX100 in 1× PBS and incubated overnight in primary antibody. For triple staining with MASH1, SEC8, and LSD1 or OMP and ATub, mouse antibodies or OMP were incubated together overnight, and LSD1 or ATub, respectively, were incubated for 1 h the following day.
Image processing and quantification.
Images were captured using a Nikon A1R confocal microscope. ImageJ software was used to measure the length of the OE (in micrometers) in each image, to count specific cell types with the cell-counter plugin and to measure the TH intensity. To quantify cell types, 10–15 images were taken from the dorsal-medial, dorsal-lateral, ventral-medial, and ventral-lateral regions, across three to four sections of the OE. Cell counts were averaged and converted to the number of cells per millimeter of OE. Quantification was performed for N = 3–6 mice in all control, iHBC-IFT88, and iHBC-ARL13B groups, unless otherwise stated. Reported N values represent the number of mice examined. Measurements of TH intensity were quantified for all glomeruli in each of three to four sections of the OB in four iHBC-IFT88 and four control mice using ImageJ software. A blind experimental paradigm was used to eliminate bias during image processing and quantification.
Statistics.
Statistical significance was determined using an unpaired t test with GraphPad Prism software. Data are presented as the mean ± SEM with two-tailed p values <0.05 considered to be significant.
Results
Olfactory stem cells possess primary cilia
ARL13B is a small Ras GTPase that regulates ciliogenesis and cilia function (Cantagrel et al., 2008; Higginbotham et al., 2013; Kasahara et al., 2014; Bangs et al., 2015). While immunostaining the OE of wild-type mice for endogenous ARL13B, we made a fortuitous discovery of what appeared to be cilia projecting from cells located just above the lamina propria (Fig. 1). To determine whether these primary cilia were on a specific olfactory cell type, we immunolabeled the OE for the basal body marker γ-tubulin, and the nuclear marker p63, which labels HBCs (Packard et al., 2011). The staining revealed ARL13B+ cilia projecting from basal bodies located within HBCs (Fig. 1A,B). To confirm the presence of cilia using a transgenic marker, we analyzed the OE of mice expressing an ARL13B–GFP fusion protein (Arl13b-EGFPtg; Delling et al., 2013) labeled with a second HBC marker, K5 (Holbrook et al., 1995). Similar to the endogenous staining, ARL13B-GFP also labeled cilia structures projecting from HBCs (Fig. 1C). To further characterize HBC cilia, the OE was immunostained for additional ciliary markers. Adenylate cyclase III (AC3) is a canonical marker of cilia projecting from OSNs but also labels cilia present on other cell types including primary neurons and astrocytes (Bishop et al., 2007; Guadiana et al., 2013; Higginbotham et al., 2013). AC3 colocalized with endogenous ARL13B (Fig. 1D) in wild-type mice, as well as ARL13B-GFP (Fig. 1E) in transgenic mice. The AC3+ cilia were also associated with p63-labeled HBCs similar to ARL13B (Fig. 1F). Finally, we used a second transgenic mouse, EGFP-CETN2, as an additional label for basal bodies (Higginbotham et al., 2004; Bangs et al., 2015). Immunostaining OE sections from this strain revealed the AC3+ projections associated with EGFP-CETN2-labeled basal bodies (Fig. 1G). Together, these data show for the first time that the population of HBCs possesses primary cilia and indicate that OSNs are not the only ciliated cell type in the OE.
Figure 1.
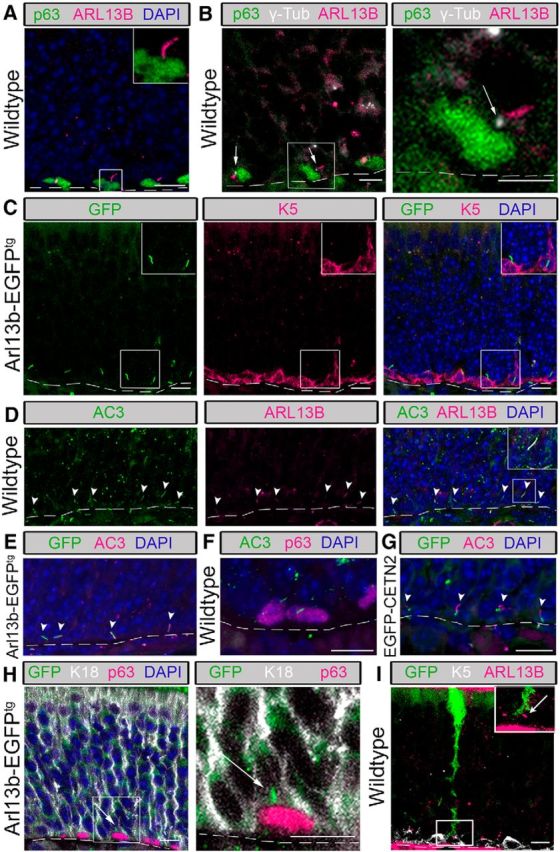
HBCs possess primary cilia. Immunofluorescence staining was performed on tissue from the olfactory epithelium of 3- to 6-week-old wild-type, Arl13b-EGFPtg, and EGFP-CETN2 mice. A, p63-labeled HBCs possess ARL13B-labeled cilia (inset, magnified image). Scale bars, 10 μm. B, Cilia extend from γ-tubulin-labeled basal bodies (arrows). Low-magnification view (left) and high-magnification view (right) of ciliated HBC. Scale bars, 5 μm. C, In Arl13b-EGFPtg mice, K5-labeled HBCs possess GFP+ cilia (inset, magnified image). Scale bars, 10 μm. D, HBC cilia labeled with canonical cilia markers AC3 and ARL13B (arrowheads). E, In Arl13b-EGFPtg mice, AC3 labels GFP+ cilia (arrowheads). F, p63-labled HBCs possess AC3-labeled cilia. G, In EGFP-CETN2 mice, GFP-expressing basal bodies possess AC3+ cilia (arrowheads). H, In Arl13b-EGFPtg mice, GFP+ cilia (arrows) project from p63-labeled HBCs into the interstitial space between HBCs and K18-labeled SUS cell end feet (inset, magnified image). Scale bars, 5 μm. I, In wild-type mice intranasally infected with adenovirus containing GFP, K5-labeled HBCs possess ARL13B-labeled cilia (see arrow) that project into the interstitial space between an HBC and GFP+ end foot of a SUS cell (see inset for higher magnified image). Scale bar, 10 μm. N = 10 total mice for all immunostaining. Dashed lines, Basement membrane.
In the OE, apically located SUS cells possess projections, known as end feet, that juxtapose olfactory basal cells en route to contact with the basal lamina (Doyle et al., 2001). The purpose of these connections has yet to be elucidated; however, it has been proposed that this is a site for communication between basal cells and SUS cells (Jia and Hegg, 2010). In our analysis, we observed that the majority of HBC cilia project from the top of the HBCs toward the SUS cell end feet. To determine whether HBC cilia were associated with SUS cell end feet, analysis of p63+ HBCs and SUS cells immunolabeled with K18 (Holbrook et al., 2011) was performed in Arl13b-EGFPtg mice. As seen in the representative image (Fig. 1H), the cilia projecting from HBCs are in apposition to the end feet. To improve the resolution of a single SUS cell end foot, we analyzed the OE of mice intranasally injected with an adenovirus expressing GFP, in which a small subset of SUS cells are transduced (for methods, see McIntyre et al., 2012). Using this approach, it is clear that the ARL13B+ cilium projects into the interstitial space between the HBC and SUS cell (Fig. 1I). These data suggest the potential for cilia to act as an antenna for communication between basal cells and SUS cells, perhaps in a manner analogous to the immunological synapse (Bromley et al., 2001; Finetti et al., 2011).
Cilia are present on distinct subpopulations of olfactory stem cells
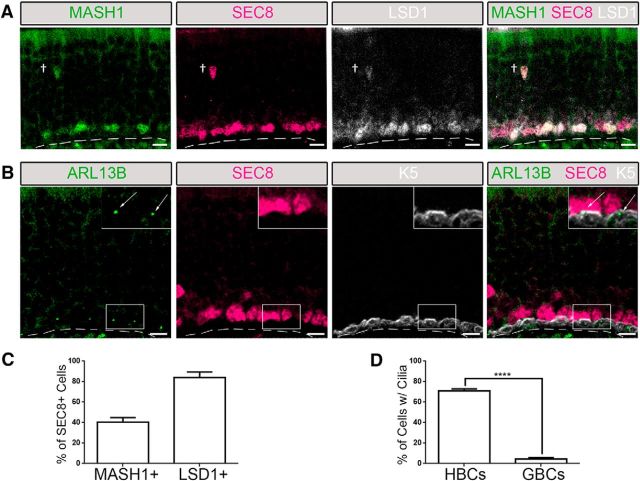
Given the close proximity of GBCs and HBCs at the base of the OE, we assessed whether GBCs also possess primary cilia. We identified a novel pan-GBC marker to distinguish between HBC and GBC subpopulations. SEC8 is one of eight subunits in the exocyst complex that was initially discovered in the secretory pathway of yeast Saccharomyces cerevisae, and was proposed to contribute to various mechanisms, including protein synthesis and vesicle trafficking (Wang et al., 2004). Antibody staining for SEC8 in the OE specifically labeled a large population of GBCs overlapping with previous identified makers of GBC subpopulations (Fig. 2A). Immunostaining with antibodies to either MASH1 (also known as ASCL1; Manglapus et al., 2004), a basic helix-loop-helix transcription factor that is required in the early stages of olfactory neuron lineage (Cau et al., 1997), or LSD1, a chromatin-modifying complex protein (Krolewski et al., 2013), in conjunction with SEC8 confirmed that SEC8 is indeed a pan-GBC marker. All MASH1+ or LSD1+ cells coexpressed SEC8 (Fig. 2A). Approximately 41% of SEC8+ cells were MASH1+, while ∼90% of SEC8+ cells were also LSD1+ (Fig. 2A,C; N = 4). Labeling of the OE with ARL13B and SEC8 antibodies revealed that very few (<5%) SEC8+ GBCs possess cilia, while >70% of K5+ HBCs possess cilia (Fig. 2B,D; N = 6). These data show that HBCs are the predominant ciliated olfactory basal stem cells and suggest a specific role for cilia in this subpopulation of neural progenitors.
Figure 2.
HBCs are the predominant ciliated olfactory basal stem cell. Immunofluorescence staining was performed in the olfactory epithelium of wild-type mice. A, The canonical GBC marker MASH1 colocalizes with a subset of SEC8+ GBCs, while, LSD1 colocalizes with a larger subset of SEC8+ GBCs. B, Few SEC8-labeled GBCs possess ARL13B-labeled cilia (see arrows) compared with K5-labeled HBCs (inset, magnified image). Scale bars, 10 μm. Dashed line, Basement membrane. †Occasional migrating GBC. C, Quantified data of SEC8+ cells that are either MASH1+ (N = 4) or LSD1+ (N = 4). D, The percentage of HBCs (N = 6) and GBCs (N = 6) that possess cilia. ****p < 0.0001 by Student's t test. Data are shown as the mean ± SEM.
Conditional deletion of intraflagellar transport machinery results in the specific loss of cilia on HBCs
To study the cell autonomous function of cilia in HBCs, we used a floxed allele for a gene essential for intraflagellar transport (IFT; Ift88) and an inducible Cre driver specific to HBCs in the OE (see Materials and Methods). IFT88 is a critical component of the bidirectional IFT system (Singla and Reiter, 2006; Pedersen and Rosenbaum, 2008), and conditional knockout of Ift88 ablates cilia from specific cell types (Croyle et al., 2011; Sharma et al., 2013). Mice in which HBC cilia were ablated (K5rtTA;TetOCre;Ift88fl/fl and K5rtTA;TetOCre;Ift88fl/Δ) are hereafter referred to as iHBC-IFT88 mice, while wild-type mice (K5rtTA;TetOCre;Ift88fl/wt) are hereafter referred to as control mice.
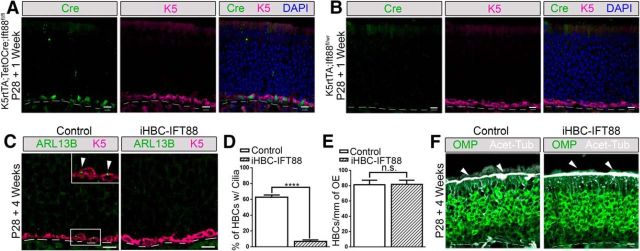
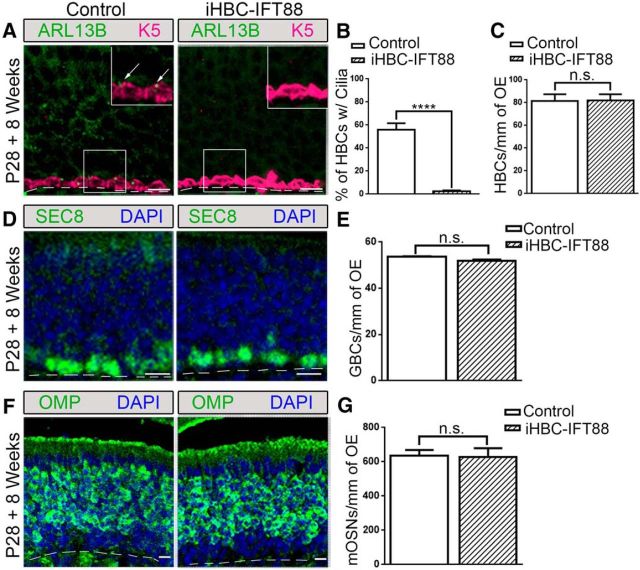
Control and iHBC-IFT88 mice were administered a dox-containing diet to activate Cre expression (Gunther et al., 2002; Grachtchouk et al., 2011) at P28, when the OE is mature (Murdoch and Roskams, 2007). One week after dox administration, ∼90% of HBCs in mice with both TetOCre and K5rtTA alleles showed Cre expression (Fig. 3A). Cre expression was not detected in HBCs of mice that lacked the TetOCre allele (Fig. 3B). More importantly, iHBC-IFT88 mice showed a significant loss (88.5%) of ciliated HBCs after 4 weeks of eating a dox-containing diet (Fig. 3C,D; N = 5). Interestingly, loss of cilia did not result in a change in the number of HBCs (Fig. 3E; N = 5). Immunostaining with the mature OSN marker OMP and acetylated α-tubulin (Acet-Tub) revealed no changes in OSN composition (Fig. 3F). An increase in cilia loss on HBCs (95.6%) was detected after 8 weeks of dox administration (Fig. 4A,B), with no effect on the number of HBCs (Fig. 4C), GBCs (Fig. 4D,E), and OSNs (Fig. 4F,G; control mice, N = 2; iHBC-IFT88 mice, N = 4). These data demonstrate that genetic ablation of Ift88 efficiently removes cilia from HBCs, without altering HBC quiescence or the usual maintenance of the OE.
Figure 3.
Cell-specific deletion of Ift88 in HBCs results in the loss of HBC cilia with no effects on OSN cilia. Control and iHBC-IFT88 mutant mice (referred to as iHBC-IFT88) were administered a dox-containing diet at P28 for 1 or 4 weeks, and immunofluorescence staining of the olfactory epithelium was performed. A, After 1 week of the dox-containing diet, Cre is present in K5-labeled HBCs of mice with K5rtTA and TetOCre alleles. B, After 1 week of the dox-containing diet, Cre is absent in mice lacking the TetOCre allele. C, After 4 weeks of the dox-containing diet, in control mice but not in iHBC-IFT88 mice, K5-labeled HBCs possess Arl13b-labeled cilia. D, E, Quantified data show that the percentage of HBCs that are ciliated in control mice is significantly reduced by ∼88.5% in iHBC-IFT88 mice, with no significant difference in the number of HBCs per millimeter of OE in control and iHBC-IFT88 mice after 4 weeks of the dox-containing diet. F, Acet-Tub-labeled cilia are still present in OMP-labeled mature OSNs (see short arrows). Scale bars, 10 μm. Dashed lines, Basement membrane. N = 5 for both groups. ****p < 0.0001. n.s., No significance by Student's t test. Data are shown as the mean ± SEM.
Figure 4.
Loss of HBC cilia has no homeostatic effects on the cell composition of the OE. Control and iHBC-IFT88 mice were administered a doxycycline-containing diet at P28 for 8 weeks. A, In control mice, but not in iHBC-IFT88 mice, K5-labeled HBCs possess ARL13B-labeled cilia (see arrows; inset, magnified image). B, C, Quantified data show a significant loss of ciliated HBCs in iHBC-IFT88 mice, with no change in the number of HBCs per millimeter of OE. D, SEC8-labeled GBCs in the OE of control and iHBC-IFT88 mice. E, Quantified data show no significant difference in the number of GBCs per millimeter of OE in both groups. F, OMP-labeled mature OSNs in control and iHBC-IFT88 mice. G, Quantified data show no significant difference in the number of mature OSNs per millimeter of OE between both groups. Scale bars, 10 μm. Dashed lines, Basement membrane. N = 2 for control mice, N = 4 for iHBC-IFT88 mice. ****p < 0.0001. n.s., No significance by Student's t test. Data are shown as the mean ± SEM.
Loss of cilia in HBCs results in the improper regeneration of the olfactory epithelium following injury
HBCs are believed to be a quiescent population of cells unless activated by severe injury to participate in OE regeneration (Leung et al., 2007). MMI is an olfactory toxicant whose metabolites induce cell death of OSNs, SUS cells, and GBCs, but spares HBCs in the mouse OE (Brittebo, 1995; Packard et al., 2011). Four weeks after dox administration, iHBC-IFT88 and control mice were given an intraperitoneal injection of MMI (75 mg/kg) to chemically lesion the OE, and were analyzed at 8 weeks after recovery (Genter et al., 1995; Bergman and Brittebo, 1999). The presence of cilia on HBCs remained significantly reduced in iHBC-IFT88 mice after recovery (Fig. 5A,C; N = 5 for both groups), while no difference in the number of HBCs was observed between iHBC-IFT88 and control mice, as indicated by the number of K5+ cells (Fig. 5A,D; N = 5 for both groups). Surprisingly, there was a significant reduction in the number of SEC8+ GBCs in iHBC-IFT88 mice compared with control mice (Fig. 5B,E; N = 5 for both groups). In addition, the number of OMP+ mature OSNs was reduced by ∼75% in iHBC-IFT88 mice (Fig. 5F,G; N = 5 for both groups). Consequently, the thickness of the OE was also reduced by ∼50% (Fig. 5H; N = 5 for both groups). Interestingly, no change in the number of cleaved caspase-3+ cells was observed between control and iHBC-IFT88 mice 8 weeks after recovery, suggesting that the reduction in the number of mature OSNs and the thickness of the OE were not due to increased cell death (Fig. 5I,J; N = 5 for both groups). While the regeneration of neurons was not completely eliminated, the OSNs that returned appeared, in some areas of the OE, to possess acetylated α-tubulin-labeled cilia (Fig. 5F). Since IFT88 is required for OSN cilia (McIntyre et al., 2012), the presence of ciliated OSNs in the regenerated OE suggests that these OSNs were derived from progenitors other than HBCs lacking IFT88. In our iHBC-IFT88 mice that were administered dox for 4 weeks, ∼12% of HBCs still possessed cilia (see Results). It is therefore likely that this remaining population of ciliated HBCs, in which IFT88 is present, contributed to regeneration of the OE and not HBCs lacking cilia. Another possible explanation is that OSNs in the regenerated OE were derived from residual GBCs, not from HBCs lacking IFT88. This is consistent with the MMI-induced lesion, which can leave residual GBCs (Xie et al., 2013). However, further studies involving lineage tracing are required to conclusively determine whether HBCs lacking cilia contribute to OE regeneration. Nonetheless, such significant reduction of OSNs should coincide with a decline in synaptic input into the OB, which can be measured by the expression of TH in the dopaminergic interneurons of the OB (Baker et al., 1993, 1999). Following recovery, there was a significant reduction in the intensity of TH in the bulb of iHBC-IFT88 mice compared with that in control mice (Fig. 5K,L; p < 0.0001, N = 4 for both groups), and the glomerular perimeter was significantly reduced (p < 0.0001, N = 4 for both groups; data not shown). Together, these data indicate that, without cilia, HBCs are unable to contribute to the regeneration of the OE, potentially due to disrupted HBC proliferation, resulting in impaired OSN recovery and olfactory function.
Figure 5.
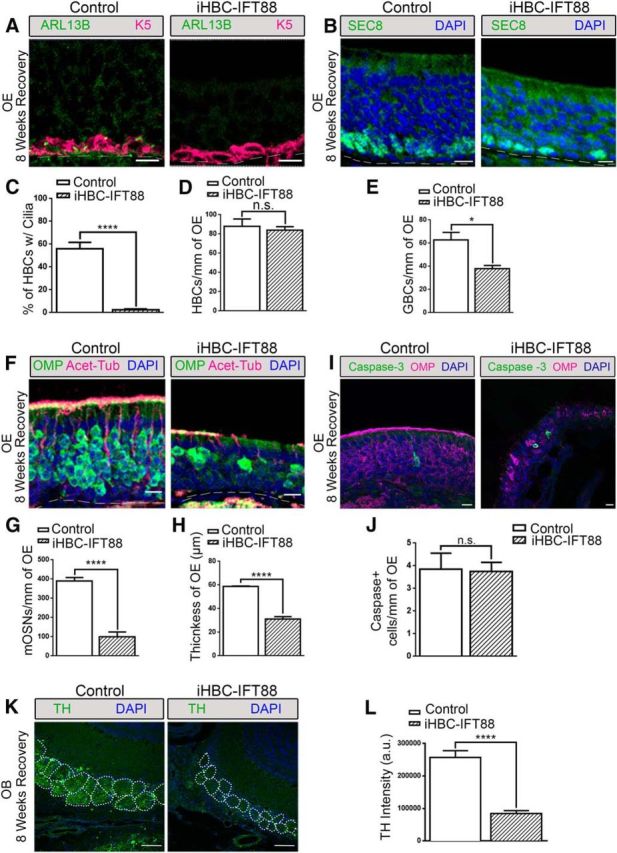
Loss of HBC cilia results in the improper regeneration of the OE and loss of TH expression in the OB. Control and iHBC-IFT88 mice were administered a dox-containing diet at P28 for 4 weeks and then were given an intraperitoneal injection of 75 mg/kg methimazole to ablate the OE. Following 8 weeks of recovery, immunofluorescence staining was performed. A, In the OE of control mice, but not of iHBC-IFT88 mice, K5-labeled HBCs possess ARL13B-labeled cilia. B, SEC8-labeled GBCs in the OE of control and iHBC-IFT88 mice. C, D, Quantified data show that the percentage of HBCs that are ciliated in control mice is significantly reduced in iHBC-IFT88 mice with no difference in the number of HBCs per millimeter of OE between both groups. E, Quantified data show a significant reduction in the number of GBCs per millimeter of OE. F, OMP-labeled mature OSNs and Acet-Tub-labeled cilia in the OE of control and iHBC-IFT88 mice. G, Quantified data show a significant reduction in the number of mature OSNs per millimeter of OE. H, A significantly thinner OE in iHBC-IFT88 mice. I, J, No difference in the number of cleaved caspase-3-labeled apoptotic cells was observed in the OE of control and iHBC-IFT88 mice. K, TH expression within glomeruli (dotted circles) in the OBs of control and iHBC-IFT88 mice. L, Quantified data show that the intensity of TH measured in arbitrary units is significantly reduced in the OBs of iHBC-IFT88 mice. Scale bars, 10 μm. Dashed lines, Basement membrane. N = 4 for both groups. *p < 0.05, ****p < 0.0001. n.s., No significance by Student's t test. Data are shown as the mean ± SEM.
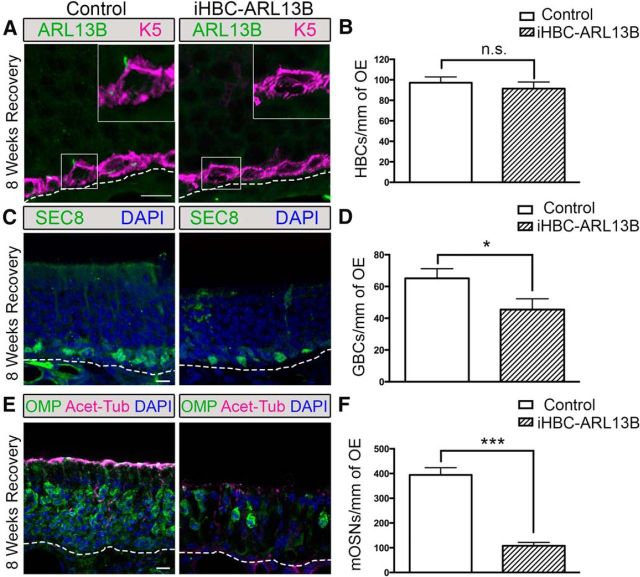
To link the observed effects to cilia function, and not to off-target effects of IFT88 loss, we repeated these experiments using a cell-specific knockout of Arl13b (Caspary et al., 2007; Larkins et al., 2011). ARL13B is specifically localized to the cilia membrane and is required for proper ciliogenesis (Caspary et al., 2007). Moreover, mutations in Arl13b lead to loss of cilia phenotypes as well as hedgehog-related phenotypes due to the disruption in the localization and ciliary targeting of hedgehog signaling proteins (Caspary et al., 2007; Duldulao et al., 2009; Larkins et al., 2011). iHBC-ARL13B mice (K5rtTA;TetOcre;Arl13bfl/fl) and control mice (K5rtTA;TetOcre;Arl13bfl/wt) underwent the same MMI lesion paradigm as stated above. After 8 weeks of recovery, ARL13B+ HBC cilia were observed in control mice but not in iHBC-ARL13B mice (Fig. 6A). Similar to results observed in iHBC-IFT88 mice, the number of HBCs did not differ between control and iHBC-ARL13B mice (Fig. 6B; N = 3 for both groups). In addition, there was a significant reduction (∼30%) in the number of SEC8+ GBCs (Fig. 6C,D; N = 3 for both groups) as well an ∼75% reduction in the number of OMP+ mature OSNs (Fig. 6E,F; N = 3 for both groups) in iHBC-ARL13B mice compared with controls. Interestingly, the apical surface of the OE of iHBC-ARL13B mice had a reduced number of acetylated α-tubulin-labeled OSN cilia. Together, these results not only recapitulate those observed in the iHBC-IFT88 mice but also suggest that Arl13b is required for HBC cilia function and regeneration of the OE.
Figure 6.
Loss of Arl13b in HBCs results in the improper regeneration of the OE. Control and iHBC-ARL13B mice were administered a doxycycline-containing diet at P28 for 4 weeks to induce the deletion of Arl13b. Mice were then given an intraperitoneal injection of 75 mg/kg methimazole to ablate the OE and were allowed 8 weeks of recovery. A, K5-labeled HBCs in the OE of iHBC-ARL13B mice do not possess ARL13B-labeled cilia. B, The number of HBCs per millimeter of OE in both control and iHBC-ARL13B mice. C, SEC8-labeled GBCs in the OE of control and iHBC-ARL13B mice. D, Quantified data show a significant reduction in the number of GBCs per millimeter of OE. E, OMP-labeled mature OSNs and Acet-Tub-labeled cilia in the OE of control and iHBC-ARL13B mice. F, Quantified data show a significant reduction in the number of mature OSNs per millimeter of OE. Scale bars, 10 μm. Dashed lines, Basement membrane. N = 3 for both groups. *p < 0.05, ***p < 0.001. n.s., No significance by Student's t test. Data are shown as the mean ± SEM. ARL13B, ADP-ribosylation factor-like protein 13b; mOSN, mature OSN.
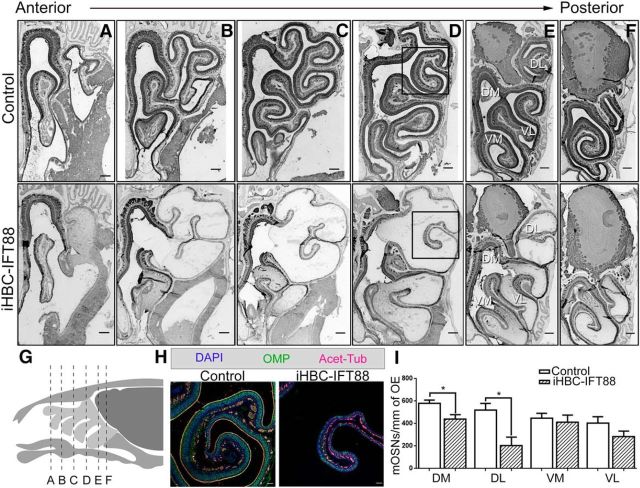
Depletion of HBC cilia results in the impaired development of the OE
In mice, the OE begins to form at E10 (Murdoch and Roskams, 2007). Small numbers of p63-expressing cells first appear at E14, after the emergence of all other cell types (Packard et al., 2011). Therefore, HBCs are not believed to participate in the establishment of the OE. HBCs settle to the basal lamina, begin to express K5, and become dormant between ∼E16 and P10 (Packard et al., 2011). Exploiting this late development, iHBC-IFT88 mice were administered dox starting at E16 for 4.5–5 weeks to ablate cilia during OE maturation. At P28, iHBC-IFT88 mice had a near complete loss of cilia on HBCs across all regions of the OE compared with control mice (control mice, 55.47 ± 5.02%; iHBC-IFT88 mice, 5.67 ± 2.91%; p < 0.01, N = 5 for both groups), with no difference in the total number of HBCs (control mice, 89.72 ± 5.69%; iHBC-IFT88 mice, 87.86 ± 2.83%; p > 0.05, N = 5 for both groups). Interestingly, the dorsal-lateral region associated with ectoturbinates 2 and 2′ exhibited a significantly reduced number of OMP+ mature OSNs in iHBC-IFT88 mice (Fig. 7A–H). While other regions of the OE also exhibit a reduction in the number of mature OSNs, this reduction was variable along the anterior–posterior axis of the OE and was most pronounced near the midpoint of the anterior–posterior axis. Quantification of OMP+ neurons in the posterior OE revealed a reduction in the number of mature OSNs in the dorsal-medial and dorsal-lateral regions, but not the ventral-medial and ventral-lateral OE (Fig. 7E,I; N = 5 for both groups). These data show that cilia on HBCs are important for proper neurogenesis during the development of the OE, providing evidence that HBCs participate in the establishment of this tissue that was thought to be limited to GBCs (Treloar et al., 2010).
Figure 7.
Loss of HBC cilia results in the impaired development of regions in the OE. Control and iHBC-IFT88 mice were treated with a doxycycline-containing diet at E16 for 4.5–5 weeks and were analyzed at P28 with immunofluorescence staining of the OE. A–F, OMP-labeled mature OSNs in anterior–posterior sections of control and iHBC-IFT88 mice. Scale bars, 200 μm. G, Illustration depicting the location of sections A–F in a sagittal view of the mouse olfactory organ. H, Magnified view of the boxed region in D with OMP-labeled mature OSNs and Acet-Tub-labeled cilia in the OE of control and iHBC-IFT88 mice. Scale bars, 50 μm. I, Quantified data show that the number of mature OSNs per millimeter of OE in iHBC-IFT88 mice is significantly reduced specifically in dorsal-lateral and medial regions of the OE, but not in ventral regions. N = 5 for both groups. *p < 0.05 by Student's t test. Data are shown as the mean ± SEM. DL, Dorsal-lateral; DM, dorsal-medial; VL, ventral-lateral; VM, ventral-medial.
Discussion
Within the olfactory system, it is well known that cilia from individual OSNs create a large receptive field in which an OSN can be activated by an odorant (for review, see DeMaria and Ngai, 2010). Until now it was widely believed that neurons were the sole ciliated cell type in the OE. Here, we show for the first time that primary cilia exist on a reserve population of olfactory basal stem cells, the HBCs. Furthermore, using the genetic deletion of two cilia-specific genes in HBCs, we demonstrate that primary cilia are critical for activation of the regenerative capacity of HBCs following chemical lesion. While Ift88 is well established in playing a critical role in ciliogenesis and maintenance, it has also been implicated in G1-S transition in ciliated cells (Robert et al., 2007). However in other studies, targeted deletion of Kif3a, a kinesin motor important for cilia function, shows similar phenotypic effects observed with the loss of Ift88, suggesting a common mechanism of action through the cilium (Wong et al., 2009; Berbari et al., 2013). In our study, we have validated the results observed through the deletion of Ift88 by the targeted removal of Arl13b. The loss of Arl13b has previously been demonstrated to affect neural development, and mutations in Arl13b underlie human ciliopathies (Caspary et al., 2007; Cantagrel et al., 2008; Higginbotham et al., 2012; Zhang et al., 2013). As Ift88 and Arl13b serve different roles in cilia function, the similar phenotypes observed in two different mutant lines support the conclusion that the loss in regenerative capacity of HBCs is due to dysfunctional primary cilia.
HBCs are long-lived quiescent progenitors that are activated only after extensive lesioning of the OE in which SUS cells and GBCs are depleted (Leung et al., 2007). The mechanism by which HBCs become activated remains unknown, however, SUS cell end feet have been previously shown to terminate on HBCs (Holbrook et al., 1995). Given that the present findings demonstrate an anatomical localization of the HBC cilia relative to SUS cell end feet, it is possible that HBC cilia may act to sense cues from the SUS cells regarding the health of the OE. This signaling paradigm may be analogous to the immunological synapse that forms between a T cell and an antigen-presenting cell functioning to orchestrate signals that drive T-cell activation (for review, see Bromley et al., 2001). Following injury to the OE, signaling is conceivably altered between SUS cells and HBCs, resulting in activation of the quiescent HBCs. Without cilia, HBCs are unlikely to detect signals necessary for proliferation and/or differentiation. Additional support for an SUS cell–HBC connection in regenerating the OE comes from different lesion models. In OSN-specific lesions, where the deaths of SUS cells and GBCs are spared, HBCs remain quiescent, and the GBCs are the leading contributor to regeneration (Leung et al., 2007; Makino et al., 2009). However, when lesions result in SUS cell and GBC death, HBCs become active and contribute to regeneration (Leung et al., 2007; Jia et al., 2010). These findings suggest that the death of SUS cells stimulates HBCs to proliferate and contribute to regeneration. However, an alternative explanation that cannot be ruled out based on current lesion models is whether signaling occurs between GBCs and HBCs, and how such signaling may contribute to HBC proliferation and OE regeneration. Interestingly, negative-feedback signaling is known to occur in the OE, as is observed between neuronal and stem/progenitor cells, to control self-replication and differentiation of the stem progenitor cell population (Beites et al., 2005; Gokoffski et al., 2010). It is therefore plausible that such feedback signaling may occur between GBCs and HBCs via HBC cilia, and that this signaling is lost when a lesion results in GBC death, resulting in HBC activation. In either case, whether the injury results in the release of a stimulatory signal or the loss of a tonic inhibitory cue between HBCs and other cell types remains to be determined.
Quiescent adult stem cells typically forgo cell cycle decisions to respond to transient environment signals sensed through the cilia (Plotnikova et al., 2009). These signals include the Notch, Wnt, and hedgehog pathways, each of which are strongly linked to neurogenesis in the brain (Kumamoto et al., 2012; Tong et al., 2014). The Wnt pathway has been previously implicated in OE proliferation and neurogenesis during development and regeneration (Wang et al., 2011; Chen et al., 2014). In contrast, Notch signaling appears to participate in the development of supporting cells but not neurons in the OE (Manglapus et al., 2004; Krolewski et al., 2012). Currently, there is no evidence for the role of the hedgehog pathway in OE development. However, canonical hedgehog signaling is strongly linked to cilia in numerous other systems (for review, see Goetz and Anderson, 2010). Numerous hedgehog pathway components dynamically localize to cilia, while the disruption of cilia results in aberrant Hedgehog signal transduction and impaired morphogenesis in specific organ systems (for review, see Berbari et al., 2009).
While the signaling pathways may be shared, the phenotypic effects of cilia loss differ between ciliated cell types, including various stem cells. In the adult skin, loss of cilia on basal cells resulted in the proliferation of cells in the interfollicular epidermis with subsequent perturbation of epidermal homeostasis (Croyle et al., 2011). In contrast, the conditional ablation of primary cilia postnatally from NSCs in the hippocampus led to a reduction in amplifying progenitor cells without altering the number of quiescent NSCs (Amador-Arjona et al., 2011). In addition, primary cilia in the cerebellum are required for the expansion of the neural progenitor pool (Chizhikov et al., 2007). Similarly, a recent report (Tong et al., 2014) suggested that localized ablation of cilia in NSCs in the ventricular SVZ (V-SVZ) decreases neurogenesis only in the ventral V-SVZ. These data in NSCs are similar to what we observed with a loss of cilia from HBCs in the OE: no change in the number of HBCs, but a reduction in the neurogenesis of the OE. Together, these data suggest the convergence of a common cilia ablation phenotype between neural stem cells as opposed to epidermis.
During development, neural stem cells are important for early neurogenesis and morphogenesis of the OE (Gokoffski et al., 2010). While maturing GBCs that migrate from the apical surface clearly contribute to organogenesis (Cau et al., 2002), the precise role of HBCs is unclear. Evidence from a lineage-trace mouse model suggests that upon their establishment in the OE at P10, HBCs do participate in neurogenesis (Iwai et al., 2008). Our data support the notion of HBC-driven neurogenesis in early postnatal development of the OE and suggest that cilia on HBCs are required for normal OE development. Interestingly, the loss of OSNs observed in the developing OE was regional, whereas in the recovering OE it was global. We speculate that these differences can be attributed to the temporal and spatial release of morphogens during development that are synchronized after severe injury to the OE (Vedin et al., 2009). Nevertheless, our data demonstrate a clear role for HBCs and their cilia in the developing OE.
Our finding of cilia on HBCs and their importance in neurogenesis reveals a potential novel mechanism for the etiology of olfactory dysfunction. It is now well recognized that anosmia is a clinical manifestation of ciliopathies, a growing class of pleiotropic genetic disorders that are traditionally associated with the loss of cilia on OSNs. However, the ability to maintain a functional olfactory system throughout life depends on the presence of a stem cell population. While the OE undergoes constant neurogenesis, there is a net loss of neurons over time due to reduced stem cell function (Ducray et al., 2002; Conley et al., 2003). Changes that alter cilia function on HBCs could hasten this decline and contribute to age-related olfactory loss. Moreover, ciliopathy patients may show increased susceptibility to chemical insult to the OE. Work here suggests that impaired neurogenesis may be a new unforeseen mechanism for olfactory dysfunction in congenital anosmias.
Footnotes
This work was funded by National Institutes of Health Grants R01-DC-009606 (to J.R.M.); T32-DC-00011 and F31-DC-013496 (to A.M.J.); and K99-DC-013555 (to J.C.M.). B.L.A. and J.R.M. were supported by a Research Team Grant from the University of Michigan Center for Organogenesis. A.M.J. was also supported by the Rackham Merit Fellowship from the Rackham Graduate School, University of Michigan. We thank Drs. Andrzej Dlugosz, Bradley Yoder, and David Clapham for their mouse lines. We also thank Corey Williams for the contribution of graphic art.
The authors declare no competing financial interests.
References
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, Enikolopov G, Roberts AJ, Terskikh AV. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci. 2011;31:9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/A:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-L. [DOI] [PubMed] [Google Scholar]
- Baker H, Cummings DM, Munger SD, Margolis JW, Franzen L, Reed RR, Margolis FL. Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice. J Neurosci. 1999;19:9313–9321. doi: 10.1523/JNEUROSCI.19-21-09313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17:113–122. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber PC. Neurogenesis and regeneration in the primary olfactory pathway of mammals. Bibl Anat. 1982:12–25. [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Sharma N, Malarkey EB, Pieczynski JN, Boddu R, Gaertig J, Guay-Woodford L, Yoder BK. Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease. Cytoskeleton. 2013;70:24–31. doi: 10.1002/cm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman U, Brittebo EB. Methimazole toxicity in rodents: covalent binding in the olfactory mucosa and detection of glial fibrillary acidic protein in the olfactory bulb. Toxicol Appl Pharmacol. 1999;155:190–200. doi: 10.1006/taap.1998.8590. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Brittebo EB. Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol Toxicol. 1995;76:76–79. doi: 10.1111/j.1600-0773.1995.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attié-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chen M, Tian S, Yang X, Lane AP, Reed RR, Liu H. Wnt-responsive Lgr5(+) globose basal cells function as multipotent olfactory epithelium progenitor cells. J Neurosci. 2014;34:8268–8276. doi: 10.1523/JNEUROSCI.0240-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley DB, Robinson AM, Shinners MJ, Kern RC. Age-related olfactory dysfunction: cellular and molecular characterization in the rat. Am J Rhinol. 2003;17:169–175. [PubMed] [Google Scholar]
- Costanzo RM. Regeneration of olfactory receptor cells. Ciba Found Symp. 1991;160:233–242. doi: 10.1002/9780470514122.ch12. [DOI] [PubMed] [Google Scholar]
- Croyle MJ, Lehman JM, O'Connor AK, Wong SY, Malarkey EB, Iribarne D, Dowdle WE, Schoeb TR, Verney ZM, Athar M, Michaud EJ, Reiter JF, Yoder BK. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development. 2011;138:1675–1685. doi: 10.1242/dev.060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria S, Ngai J. The cell biology of smell. J Cell Biol. 2010;191:443–452. doi: 10.1083/jcb.201008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Khan M, Cunningham AM. Expression of the intermediate filament protein nestin by sustentacular cells in mature olfactory neuroepithelium. J Comp Neurol. 2001;437:186–195. doi: 10.1002/cne.1278. [DOI] [PubMed] [Google Scholar]
- Ducray A, Bondier JR, Michel G, Bon K, Millot JL, Propper A, Kastner A. Recovery following peripheral destruction of olfactory neurons in young and adult mice. Eur J Neurosci. 2002;15:1907–1917. doi: 10.1046/j.1460-9568.2002.02044.x. [DOI] [PubMed] [Google Scholar]
- Duldulao NA, Lee S, Sun Z. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development. 2009;136:4033–4042. doi: 10.1242/dev.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge AS, Chen ZY. Hair cell regeneration. Curr Opin Neurobiol. 2008;18:377–382. doi: 10.1016/j.conb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Rosenbaum J, Baldari CT. Intraflagellar transport: a new player at the immune synapse. Trends Immunol. 2011;32:139–145. doi: 10.1016/j.it.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Genter MB, Deamer NJ, Blake BL, Wesley DS, Levi PE. Olfactory toxicity of methimazole: dose-response and structure-activity studies and characterization of flavin-containing monooxygenase activity in the Long-Evans rat olfactory mucosa. Toxicol Pathol. 1995;23:477–486. doi: 10.1177/019262339502300404. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokoffski KK, Kawauchi S, Wu HH, Santos R, Hollenbeck PLW, Lander AD, Calof AL. Feedback regulation of neurogenesis in the mammalian olfactory epithelium: new insights from genetics and systems biology. In: Menini A, editor. The neurobiology of olfaction. Boca Raton, FL: CRC; 2010. pp. 241–266. [PubMed] [Google Scholar]
- Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, Wang A, Wilbert D, Patel RM, Ferris J, Diener J, Allen M, Lim S, Syu LJ, Verhaegen M, Dlugosz AA. Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest. 2011;121:1768–1781. doi: 10.1172/JCI46307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Guadiana SM, Semple-Rowland S, Daroszewski D, Madorsky I, Breunig JJ, Mykytyn K, Sarkisian MR. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci. 2013;33:2626–2638. doi: 10.1523/JNEUROSCI.2906-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Bielas S, Tanaka T, Gleeson JG. Transgenic mouse line with green-fluorescent protein-labeled Centrin 2 allows visualization of the centrosome in living cells. Transgenic Res. 2004;13:155–164. doi: 10.1023/B:TRAG.0000026071.41735.8e. [DOI] [PubMed] [Google Scholar]
- Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. 2012;23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, Anton ES. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci. 2013;16:1000–1007. doi: 10.1038/nn.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. doi: 10.1002/(SICI)1096-9861(19981102)400:4<469::AID-CNE3>3.0.CO%3B2-8. [DOI] [PubMed] [Google Scholar]
- Irigoín F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Chen X, Flis D, Harris M, Schwob JE. Label-retaining, quiescent globose basal cells are found in the olfactory epithelium. J Comp Neurol. 2014;522:731–749. doi: 10.1002/cne.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Hegg CC. NPY mediates ATP-induced neuroproliferation in adult mouse olfactory epithelium. Neurobiol Dis. 2010;38:405–413. doi: 10.1016/j.nbd.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia C, Roman C, Hegg CC. Nickel sulfate induces location-dependent atrophy of mouse olfactory epithelium: protective and proliferative role of purinergic receptor activation. Toxicol Sci. 2010;115:547–556. doi: 10.1093/toxsci/kfq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Miyoshi K, Murakami S, Miyazaki I, Asanuma M. Visualization of astrocytic primary cilia in the mouse brain by immunofluorescent analysis using the cilia marker Arl13b. Acta Med Okayama. 2014;68:317–322. doi: 10.18926/AMO/53020. [DOI] [PubMed] [Google Scholar]
- Krolewski RC, Packard A, Jang W, Wildner H, Schwob JE. Ascl1 (Mash1) knockout perturbs differentiation of nonneuronal cells in olfactory epithelium. PLoS One. 2012;7:e51737. doi: 10.1371/journal.pone.0051737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Packard A, Schwob JE. Global expression profiling of globose basal cells and neurogenic progression within the olfactory epithelium. J Comp Neurol. 2013;521:833–859. doi: 10.1002/cne.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci. 2012;15:399–405. S1. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 2011;22:4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Makino N, Ookawara S, Katoh K, Ohta Y, Ichikawa M, Ichimura K. The morphological change of supporting cells in the olfactory epithelium after bulbectomy. Chem Senses. 2009;34:171–179. doi: 10.1093/chemse/bjn074. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- McIntyre JC, Davis EE, Joiner A, Williams CL, Tsai IC, Jenkins PM, McEwen DP, Zhang L, Escobado J, Thomas S, Szymanska K, Johnson CA, Beales PL, Green ED, Mullikin JC, Mullikin JC, Sabo A, Muzny DM, Gibbs RA, Attié-Bitach T, et al. Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nat Med. 2012;18:1423–1428. doi: 10.1038/nm.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007;38:581–599. doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J Neurosci. 2011;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Plotnikova OV, Pugacheva EN, Golemis EA. Primary cilia and the cell cycle. Methods Cell Biol. 2009;94:137–160. doi: 10.1016/S0091-679X(08)94007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A, Margall-Ducos G, Guidotti JE, Brégerie O, Celati C, Bréchot C, Desdouets C. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J Cell Sci. 2007;120:628–637. doi: 10.1242/jcs.03366. [DOI] [PubMed] [Google Scholar]
- Sharma N, Malarkey EB, Berbari NF, O'Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK. Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J Am Soc Nephrol. 2013;24:456–464. doi: 10.1681/ASN.2012020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Su CY, Bay SN, Mariani LE, Hillman MJ, Caspary T. Temporal deletion of Arl13b reveals that a mispatterned neural tube corrects cell fate over time. Development. 2012;139:4062–4071. doi: 10.1242/dev.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A. 2014;111:12438–12443. doi: 10.1073/pnas.1321425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HB, Miller AM, Ray A, Greer CA. Development of the olfactory system. In: Menini A, editor. The neurobiology of olfaction. Boca Raton, FL: CRC; 2010. pp. 131–156. [Google Scholar]
- Vedin V, Molander M, Bohm S, Berghard A. Regional differences in olfactory epithelial homeostasis in the adult mouse. J Comp Neurol. 2009;513:375–384. doi: 10.1002/cne.21973. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Y, Adamson CL, Valdez G, Guo W, Hsu SC. The mammalian exocyst, a complex required for exocytosis, inhibits tubulin polymerization. J Biol Chem. 2004;279:35958–35966. doi: 10.1074/jbc.M313778200. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao T, Hamad S, Lott P, Schnittke N, Schwob JE, Zhou CJ. Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci. 2011;124:1553–1563. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Fang C, Schnittke N, Schwob JE, Ding X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: effects on stem cells and noninvolvement of acute induction of the inflammatory cytokine IL-6. Toxicol Appl Pharmacol. 2013;272:598–607. doi: 10.1016/j.taap.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res. 2013;319:2316–2322. doi: 10.1016/j.yexcr.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]