Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease. Although the pathogenesis is poorly understood, evidence suggests that genetic and epigenetic alterations, such as DNA methylation, may play a key role. Bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGF-β) superfamily and are important regulators in IPF. Here we identified BMP endothelial cell precursor-derived regulator (BMPER) as a key regulator of fibroblast activation. BMPER is a secreted glycoprotein that binds directly to BMPs and may regulate TGF-β/BMP signaling, but its role in lung fibrosis is not clear. BMPER is highly expressed in human IPF lung fibroblasts compared to normal lung fibroblasts. Demethylation agent 5′-azacytidine decreased BMPER expression in fibroblasts, and attenuated the invasion and migration of IPF lung fibroblasts. Furthermore, siRNA-mediated reduction of BMPER in the human lung fibroblasts impaired cell migration and invasion. 5′-azacytidine treatment additionally regulated BMPER expression and reduced lung fibrosis in mice in vivo. These findings demonstrate that methylation of specific genes in fibroblasts may offer a new therapeutic strategy for IPF by modulating fibroblast activation.
Progressive lung fibrosis is an increasing cause of morbidity and mortality worldwide with limited therapeutic options. IPF, a particularly severe form of lung fibrosis, is a chronic, progressive, and often fatal interstitial lung disease of unknown etiology with an average survival of 2–3 years from diagnosis1,2,3. The current treatment options for IPF are very limited, therefore the development of novel treatment strategies that inhibited fibroblast activation, reduced epithelial apoptosis, or induced alveolar repaired is needed3,4,5. Epigenetic alterations can contribute to the pathogenesis of IPF6. Both DNA hypermethylation and hypomethylation have been observed in IPF6,7,8,9. Methylation of DNA occurs on cytosine residues that precede a guanosine in the DNA sequence (the CpG dinucleotide)10. Demethylating agent 5′-azacytidine normalized the phenotype of fibrotic fibroblasts in vitro and ameliorated experimental kidney fibrosis in mice11. Even though the effect of 5′-azacytidine is not limited to fibrosis, methylation of specific genes in fibroblasts is pivotal for perpetuating fibrogenesis11,12.
Both TGF-β and BMP signaling have been shown to play a role in lung fibrogenesis13. The balance between TGF-β and BMP signaling is crucial for lung homeostasis and fibrogenesis13,14. TGF-β signaling has been implicated to play an important role in fibrosis through stimulating matrix deposition15,16. In lung development, BMP signaling was shown to be essential for the growth and maintenance of lung epithelium17,18. BMP signaling plays a role in animal models of lung injury and in human lung disease19,20,21,22 by regulating fibroblast proliferation and transdifferentiation into myofibroblasts at the injury site23,24,25.
BMPER is a secreted glycoprotein that contains five cysteine-rich domains followed by a von Willebrand D domain and a trypsin-inhibitor domain26. BMPER is known to bind and modulate at least three BMPs (BMP-2, -4 and -6) and was originally identified in a screen for differentially expressed proteins in embryonic endothelial precursor cells26. High levels of BMPER antagonized BMP2-Smad5-Id1 signaling and prevented BMP2-mediated decrease of E-cadherin and hyperpermeability27. BMP activity controlled by BMPER regulates the proinflammatory phenotype of the endothelium28. BMPER can both promote and repress BMP signaling activity29. Mutations in the BMPER gene cause Diaphanospondylodysostosis in humans, possibly by disrupting the tight regulation of BMP signaling that governs development of the skeleton and other organs30, suggesting a critical role for BMPER in BMP signaling-mediated mesenchymal development. BMPER is highly expressed in malignant tumors and controls invasive cell behavior31. Our previous studies showed that increased invasion and migration capacities of fibroblasts contribute to severe lung fibrosis32,33. Inhibition of fibroblast migration can attenuate pulmonary fibrosis34. However, the role of BMPER in lung fibrosis has not been investigated. Interestingly, we found that the expression of several BMP binding proteins, including Gremlin1 and BMPER, was decreased in IPF fibroblasts after 5′-azacytidine treatment. The expression of Gremlin is increased in IPF lungs35. In this study, we tested the hypothesis that methylation-mediated expression of BMPER might be involved in lung fibrogenesis. We found that BMPER was up regulated in lung tissue and fibroblasts from IPF patients. Demethylation with 5′-azacytidine treatment resulted in decreased BMPER expression in primary lung fibroblasts, attenuated fibroblast activation in vitro and lung fibrosis in vivo. Furthermore, BMPER regulated lung fibrosis through TGF-β/BMP signaling.
Results
Up-Regulation of BMPER in IPF lung fibroblasts and tissues
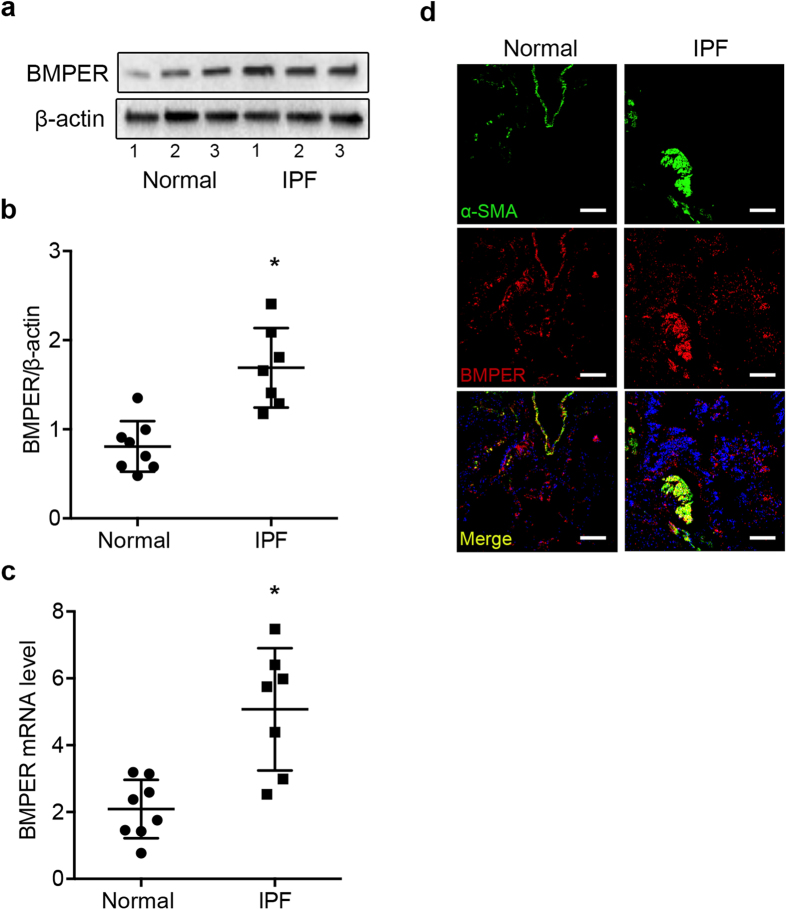
First we examined whether BMPER expression was aberrant between IPF and normal lung fibroblasts by Western blotting and qRT-PCR. Both the protein (Fig. 1a,b) and mRNA levels (Fig. 1c) of BMPER were elevated in IPF lung fibroblasts compared to normal lung fibroblasts. The increased BMPER protein levels were confirmed by immunofluorescence of human lung tissue. BMPER and α-SMA staining was increased in the fibrotic foci of IPF lung tissue (Fig. 1d). These findings indicate that BMPER levels are increased in IPF lung fibroblasts and lung tissues. It validates the need for further mechanistic insight into what role of BMPER may play in IPF.
Figure 1. Increased BMPER expression in IPF lung fibroblasts and tissues.
(a,b) BMPER protein levels were compared in primary lung fibroblasts derived from normal subjects and IPF patients with Western blot analysis (3 samples are shown). β-actin was used as a loading control. (b) Quantification of BMPER protein levels normalized to β-actin in normal and IPF lung fibroblasts by densitometry analysis (normal n = 8 and IPF n = 7; *P < 0.05, data are mean and SEM, unpaired t test). (c) BMPER mRNA expression was compared in primary lung fibroblasts derived from normal subjects and IPF patients with qRT-PCR analysis (normal n = 8 and IPF n = 7, *P < 0.05, data are mean and SEM, unpaired t test). GAPDH was used as an internal control. (d) BMPER was co-localized with α-SMA in fibrotic foci. Representative confocal photomicrographs of immunofluorescence labeling for α-SMA (green), BMPER (red) and merged (yellow) images were from normal subjects and IPF patients. Scale bars, 20 μm. The experiments were repeated three times. The full size blots were shown in the Supplementary Fig. S1.
BMPER expression is decreased after 5′-azacytidine treatment in normal and IPF lung fibroblasts
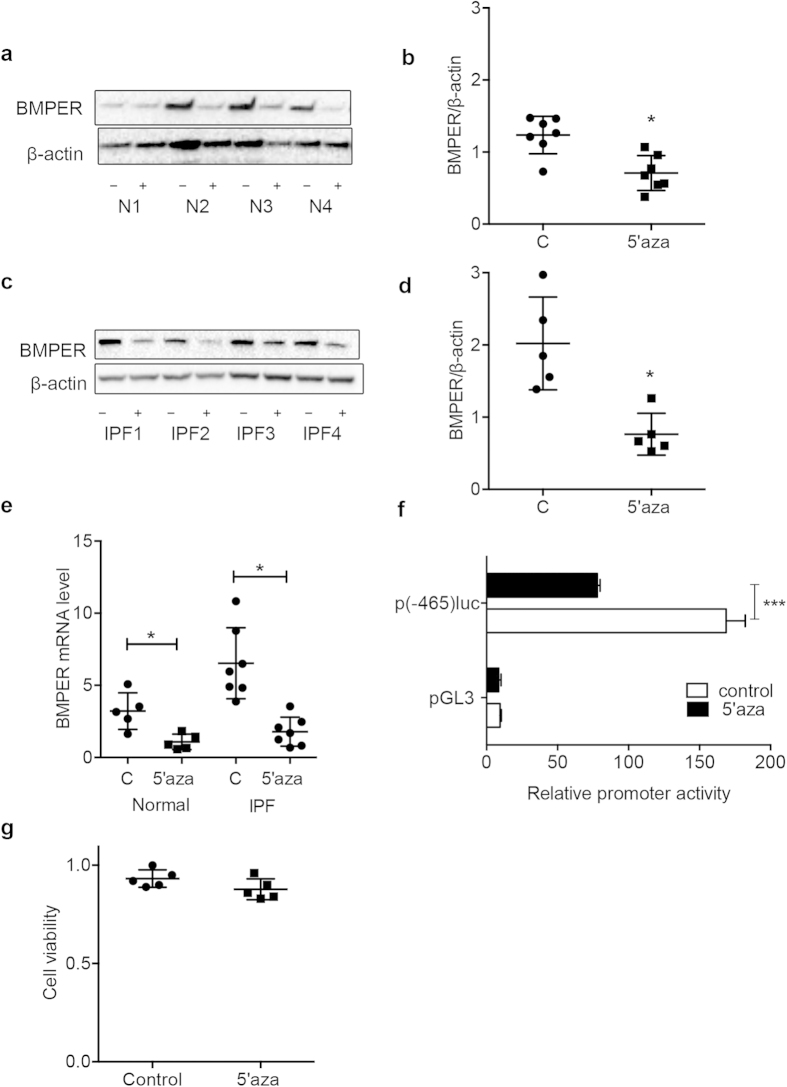
DNA methylation has been suggested to play a role in fibrotic diseases11. In kidney, demethylating agent 5′-azacytidine ameliorated experimental renal fibrosis by mediating RASAL1 gene expression in mice in vivo11. In order to define the epigenetic regulation of lung fibrotic fibroblasts, a gene array was used to compare the gene expression changed in human lung fibroblasts from IPF patients after demethylation with 5′-azacytidine. It showed that after 5′-azacytidine treatment, the expression of several BMP binding proteins such as Gremlin135, thrombospondin 1, SPARC-like 1, and BMPER were decreased compared to that of control fibroblasts. To directly investigate if elevated BMPER expression in IPF fibroblasts is associated with DNA methylation, we treated fibroblasts from IPF and normal lungs with the demethylation agent 5′-azacytidine. BMPER expression was decreased at both the protein and mRNA levels in the 5’-azacytidine treated normal and IPF fibroblasts (Fig. 2a–e).
Figure 2. Demethylation decreased BMPER expression in normal and IPF fibroblasts and regulated the BMPER promoter activity.
Primary normal (a,b) and IPF lung fibroblasts (c,d) were treated with 5′-azacytidine at 5 μM for 24 hours. BMPER protein levels in normal and IPF lung fibroblasts were analyzed with Western blotting (4 samples are shown). β-actin was used as a loading control. The relative amount was shown with densitometry analysis (normal n = 7 and IPF n = 5; N, normal; C, control; 5′aza, 5′-azacytidine; *P < 0.05; paired t test). (e) BMPER mRNA expression was assessed in human normal and IPF lung fibroblasts after 5 μM 5′-azacytidine treatment for 24 hours with qRT-PCR (normal n = 5 and IPF n = 7; C, control; 5′aza, 5′-azacytidine; *P < 0.05, paired t test). (f) Luciferase activity of human BMPER promoter was measured in HEK293 cells treated with 5′-azacytidine at 5 μM for 24 hours (***P < 0.001, data are mean and SEM, unpaired t test). (g) Cell viability of human lung fibroblasts with and without 5′-azacytidine treatment was measured with MTT assay. (n = 5, P > 0.05, paired t test). The full size blots were shown in the Supplementary Fig. S2.
Next we wanted to determine if the regulation of BMPER expression by demethylation was under the transcriptional level. A comparison of the 2000 base pairs upstream of the BMPER transcriptional start site in human identified positive regulatory cis-elements contained in the proximal promoter (−465 to +1)36. To determine possible regulation of BMPER by demethylation, we generated the BMPER promoter construct containing −465 to +1 to drive luciferase as a reporter. The BMPER promoter construct was transfected into HEK293 cells. The cells were treated with 5′-azacytidine 24 hours after transfection, and then harvested for luciferase activity measurement 48 hours after transfection. Consistent with a previous report36, we found that the proximal promoter (−465 to +1) contained an activating regulatory element (Fig. 2f). The promoter activity was significantly reduced after 5′-azacytidine treatment (Fig. 2f), indicating that BMPER expression is regulated by demethylation at the transcriptional level.
Additionally, cell viability was not significantly changed with 5′-azacytidine treatment as determined by MTT staining (Fig. 2g), suggesting that the decreased expression of BMPER by demethylation was not due to cytotoxic effects of 5′-azacytidine.
5′-azacytidine treatment diminishes the matrix production of IPF lung fibroblasts
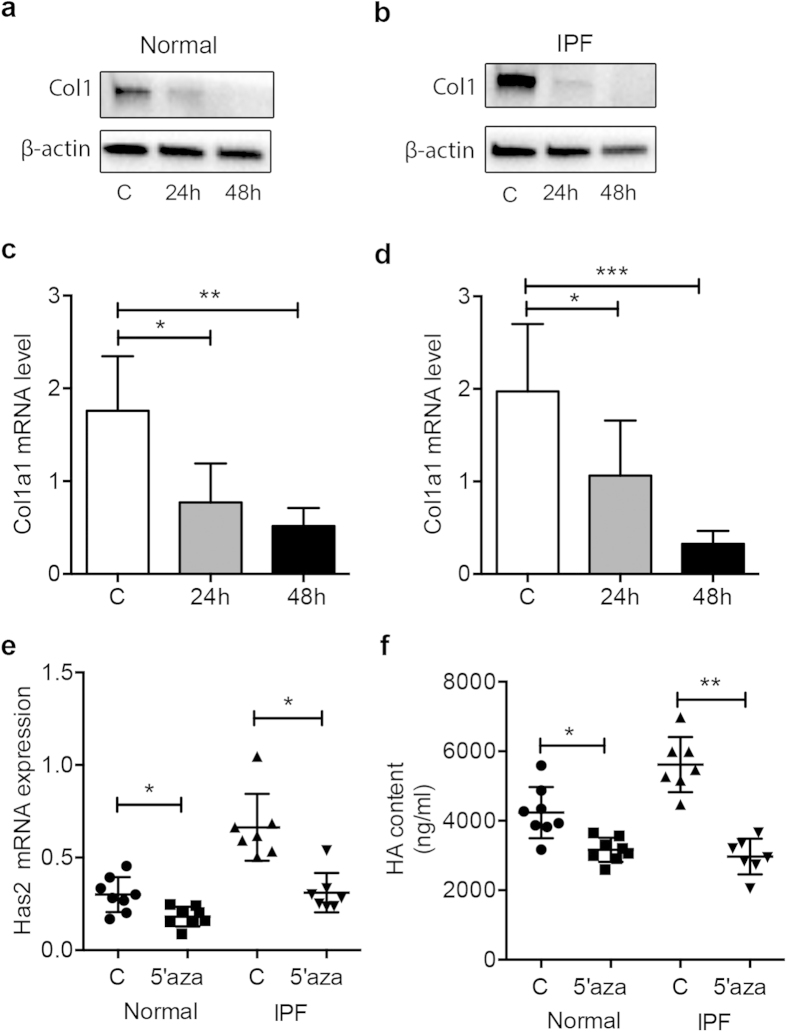
IPF is a terminal illness characterized by unremitting matrix deposition in the lung33. These matrices include Collagen I (Col1), hyaluronan (HA) and hyaluronan synthase 2 (Has2)5,37. To gain insight into the role of BMPER in the progression of lung fibrosis, we next investigated if and how 5′-azacytidine treatment affects matrix production. After normal and IPF fibroblasts were treated with 5′-azacytidine, western blotting analysis showed that Col1 protein (Fig. 3a,b) and mRNA levels (Fig. 3c,d) were decreased in both normal and IPF lung fibroblasts. Also Has2 mRNA expression was decreased after 5′-azacytidine treatment (Fig. 3e). HA ELISA confirmed that after 5′-azacytidine treatment, the production of HA in the supernatant was decreased (Fig. 3f). Taken together, these data imply that down-regulated BMPER expression correlates with the decreased extracellular matrix production in lung fibroblasts.
Figure 3. Demethylation correlated with the extracellular matrix production of primary lung fibroblasts.
Extracellular matrix production of primary normal and IPF fibroblasts treated with 5′-azacytidine at 5 μM or control medium for 24 hours or 48 hours was assessed with Western, RT-PCR, and ELISA. Col1 protein levels in normal (a) and IPF (b) lung fibroblasts were analyzed with Western blotting. β-actin was used as a loading control (normal n = 3 and IPF n = 3). Col1 mRNA levels in normal (c) and IPF (d) lung fibroblasts were assessed with RT-PCR. GAPDH was used as a loading control. (Col1, Collagen 1, C, control, normal n = 4 and IPF n = 6, *P < 0.05; **P < 0.01; ***P < 0,001; data are mean and SEM, unpaired t test) (e). Has2 mRNA expression in primary normal and IPF fibroblasts treated with 5′-azacytidine were assessed with RT-PCR (C, control, normal n = 8 and IPF n = 7. *P < 0.05, paired t test). (f) The levels of HA in the supernatant of normal and IPF lung fibroblasts treated with 5′-azacytidine at 5 μM for 24 hours (C, control, HA, Hyaluronan, Normal n = 8 and IPF n = 7, *P < 0.05; **P < 0.01, paired t test). The full size blots were shown in the Supplementary Fig. S3.
5′-azacytidine treatment reduces the invasion and migration capacities of IPF fibroblasts
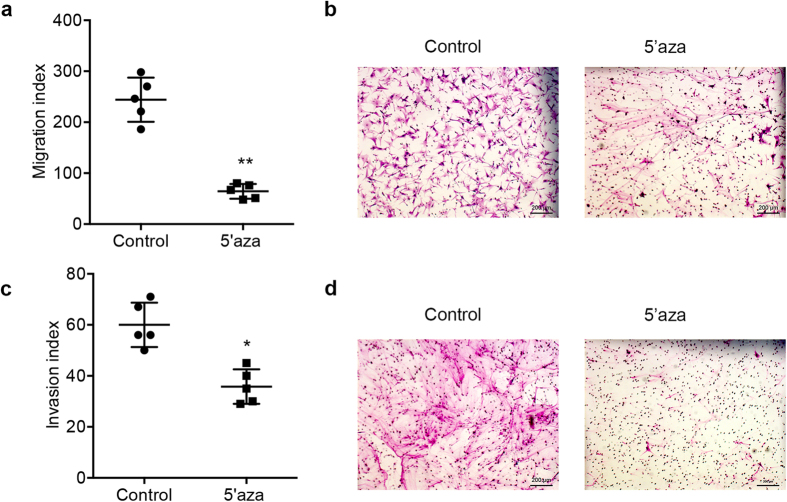
To investigate whether demethylation would affect IPF fibroblast behaviors, we measured the migration and invasion capacities of IPF fibroblasts after 5′-azacytidine treatment. Our results showed that after 5′-azacytidine treatment, both the migration (Fig. 4a,b) and invasion capacities (Fig. 4c,d) of the cells were decreased compared to untreated cells. These data suggest that demethylation may not only affect matrix production, but also change the fibroblast behavior.
Figure 4. Reduced invasion and migration capacities of IPF fibroblasts treated with 5′-azacytidine.
The invasion and migration capacities of primary IPF fibroblasts treated with 5′-azacytidine at 5 μM for 24 hours were assessed. (a) Migration capacity of IPF fibroblasts untreated and treated with 5′-azacytidine was assayed with a Boyden chamber (**P < 0.01, Data are mean and SEM, unpaired t test, n = 5 patients). (b) Representative images of migration from IPF fibroblasts untreated and treated with 5′-azacytidine (Scale bars, 200 μm). (c) Invasion capacity of IPF fibroblasts untreated and treated with 5′-azacytidine (*P < 0.05, Data are mean and SEM, unpaired t test, n = 5 patients). (d) Representative images of invasive IPF fibroblasts from IPF fibroblasts untreated and treated with 5′-azacytidine (Scale bars, 200 μm).
TGF-β/BMP signaling is regulated by BMPER
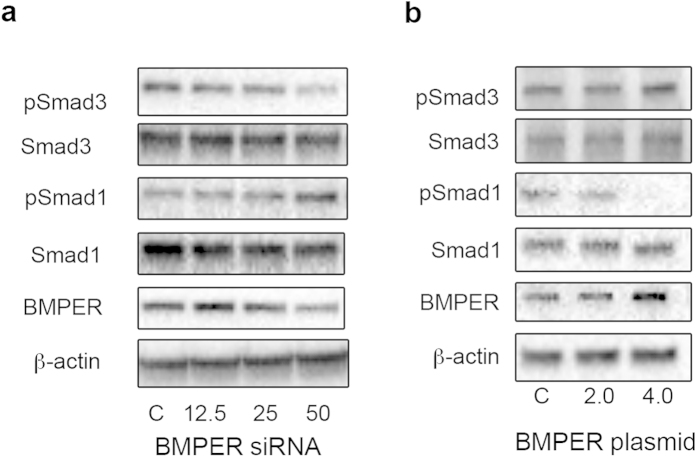
Both TGF-β and BMP signaling have been shown to play a role in lung fibrogenesis. The balance between TGF-β and BMP signaling is crucial for lung homeostasis and lung fibrosis13,14. In general, Smad2/3 transmit TGF-β signaling, while BMPs signal through Smad1/5/813. We hypothesized that BMPER may mediate TGF-β/BMP signaling through Smad phosphorylation during demethylation. We therefore used siRNA interference (loss-of-function) and overexpression of BMPER (gain-of-function) in MRC5 cells to examine Smad-mediated TGF-β/BMP signaling that occurs downstream of BMPER. When BMPER was knocked down with BMPER specific siRNA, BMPER expression was reduced (Fig. 5a). The phosphorylation levels of Smad3 were attenuated, whereas the phosphorylation levels of Smad1 were increased (Fig. 5a). In contrast, the phosphorylation levels of Smad3 were increased and the phosphorylation levels of Smad1 were decreased in MRC5 cells with BMPER overexpression (Fig. 5b). These results show that BMPER can modulate TGF-β/BMP signaling, in which BMPER may activate TGF-β signaling and inhibit the BMP signaling pathway.
Figure 5. BMPER modulates fibroblast activation by facilitating TGF-β/BMP signaling.
Western blot analysis was used to assess p-Smad3, Smad3, p-Smad1, Smad1 and BMPER expression levels in MRC5 cells (a) Cells were transfected with either 12.5–50 pmol BMPER siRNA or control siRNA 48 hours and (b) Cells were transfected with either 2–4 μg BMPER plasmid or control empty plasmid 48 hours. β-actin was used as a loading control. The experiments were repeated three times (C, control). The full size blots were shown in the Supplementary Fig. S4.
BMPER regulates cell migration and invasion in fibroblasts
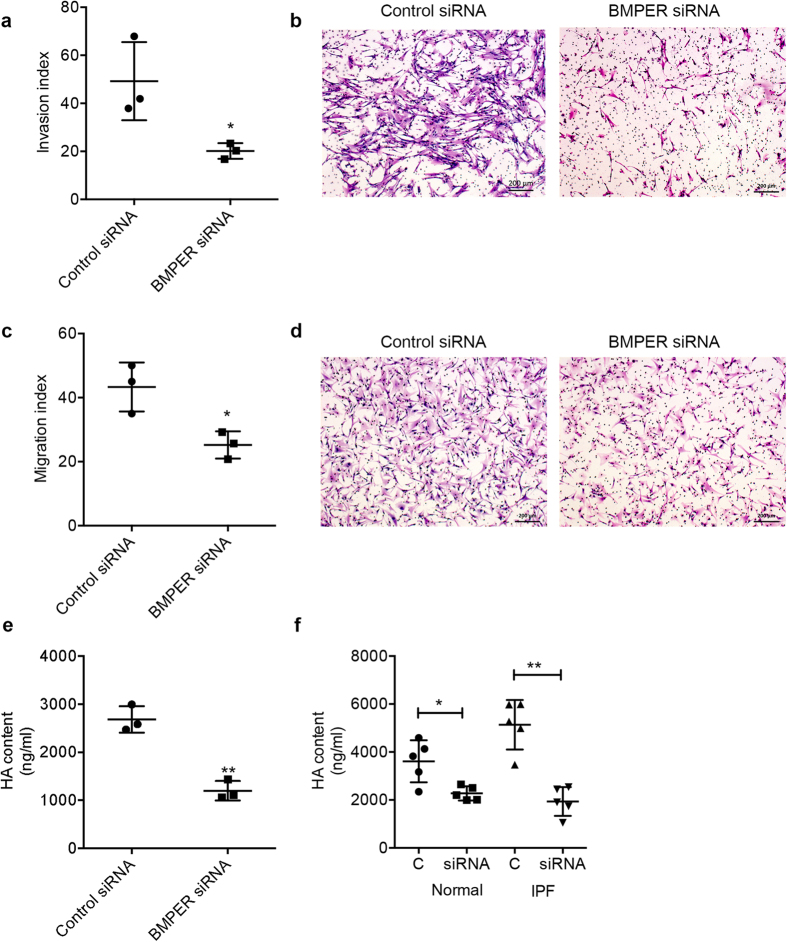
We next determined the functional role of BMPER in lung fibroblasts. Knowing that BMPER is a crucial regulator of TGF-β/BMP pathway activity and can regulate tumor cell migration and invasion31, we investigated if BMPER expression can regulate cell invasion and migration in fibroblasts. Therefore, BMPER was knocked down using BMPER-specific siRNA in MRC5 cells, primary normal and IPF lung fibroblasts. Downregulation of BMPER with specific siRNA markedly reduced cell invasion (Fig. 6a,b) and migration (Fig. 6c,d). To investigate the potential mechanism of increased cell invasion and migration, we measured matrix production after knocking down BMPER. Generally, matrix facilitates migratory38 and invasive processes of fibrotic fibroblasts in IPF33. When BMPER was knocked down in fibroblasts, the HA expression was significantly diminished (Fig. 6e,f). These data indicate that BMPER regulates matrix production and fibroblast behavior.
Figure 6. Knocking down BMPER with siRNA reduced migration and invasion capacities and matrix production of fibroblasts.
(a) Cell invasion capacity of MRC5 cells transfected with BMPER siRNA or control siRNA (*P < 0.05, data are mean and SEM, unpaired t test, n = 3 times). (b) Representative images of Invasion (Scale bars, 200 μm). (c) Cell migration capacity of MRC5 cells transfected with BMPER siRNA or control siRNA was assayed with a Boyden chamber (*P < 0.05; data are mean and SEM, unpaired t test, n = 3 times). (d) Representative images of migration (Scale bars, 200 μm). (e) HA concentration of MRC5 cells transfected with BMPER siRNA or control siRNA (HA, Hyaluronan; **P < 0.01; data are mean and SEM, unpaired t test; n = 3 times). (f) HA concentration of normal and IPF lung fibroblasts transfected with BMPER siRNA or control siRNA (HA, Hyaluronan; *P < 0.05; **P < 0.01; data are mean and SEM, unpaired t test, normal n = 5 subjects and IPF n = 5 patients).
5′-azacytidine attenuates lung fibrosis in mice in vivo
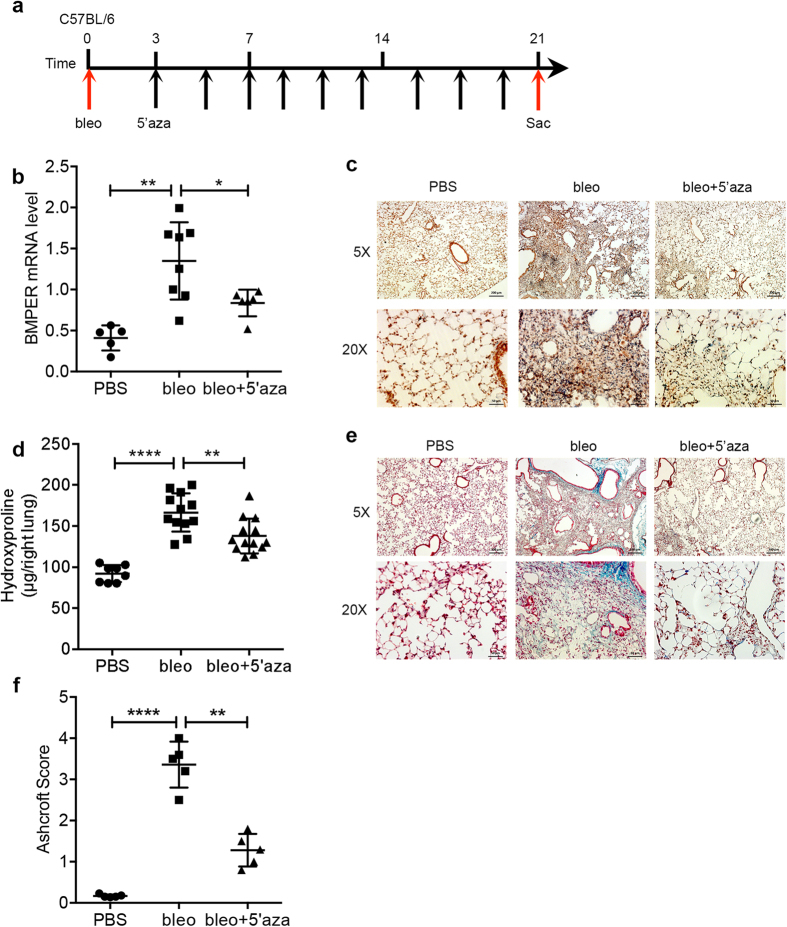
Given our in vitro results that BMPER expression was regulated by 5′-azacytidine at the transcriptional level in human lung fibroblasts, we treated C57BL/6 mice with bleomycin either with or without the demethylation agent 5′-azacytidine (Fig. 7a). Please note that we used 1 mg/kg 5′-azacytidine intraperitoneally, and the dose was much lower compared with the dose of 10 mg/kg used in the Bechtel’s study11. We observed a significant increase of BMPER in the lung fibroblasts of mice that received bleomycin-treated compared to that of PBS controls. BMPER expression was decreased in the lung fibroblasts of the mice received additional 5′-azacytidine treatment compared to bleomycin only, the expression of BMPER was significantly different (Fig. 7b,c). Similar to human BMPER regulation, mouse BMPER expression was also regulated by 5′-azacytidine at transcriptional level (Fig. S5). Collagen accumulation was significantly reduced in 5′-azacytidine treated lung tissue as determined by hydroxyproline assay (Fig. 7d) and Masson’s trichrome staining (Fig. 7e). Lung fibrosis was attenuated as assessed with histopathological Ashcroft score (Fig. 7f). Taken together, demethylation with 5′-azacytidine regulates BMPER expression and reduces lung fibrosis in mice in vivo.
Figure 7. Decreased BMPER expression with 5′-azacytidine attenuated lung fibrosis in mouse in vivo.
(a) Experimental strategy for in vivo treatments. Mice were given bleomycin (2.5 U/kg) at day 0, and followed by intraperitoneal injection of with 5′-azacytidine (1 mg/kg) every other day from day 3 to day 21. Mice were sacrificed at day 21 and lung tissues were harvested for analysis. (b) BMPER mRNA expression in mouse lung fibroblasts from mice treated with PBS (n = 5), bleomycin only (n = 8) or bleomycin and 5′-azacytidine (n = 6), 3 weeks after bleomycin treatment (*P < 0.05; **P < 0.01; data are mean and SEM, one-way ANOVA analysis). (c) Immunohistochemical staining of BMPER in lung tissues of mice treated with PBS, bleomycin only, or bleomycin with 5′-azacytidine (scale bars, 5×, 200 μm; 20×, 50 μm). (d) Hydroxyproline content from mice treated with PBS (n = 8), bleomycin only (n = 12) or bleomycin and 5′-azacytidine (n = 13) (**P < 0.01; ****P < 0.0001; data are mean and SEM, one-way ANOVA analysis). (e) Thichrome staining of lungs from mice treated with PBS, bleomycin only, or bleomycin and 5′-azacytidine (scale bars, 5×, 200 μm; 20×, 50 μm). (f) Ashcroft score from mice treated with PBS (n = 5), bleomycin only (n = 5) or bleomycin and 5′-azacytidine (n = 5), 3 weeks after bleomycin treatment (**P < 0.01; ****P < 0.0001; data are mean and SEM, one-way ANOVA analysis. bleo, bleomycin; 5′aza, 5′azacytidine; Sac, sacrifice).
Discussion
Our studies demonstrated the profibrotic role of BMPER in pulmonary fibrosis. First, BMPER mRNA and protein levels were highly expressed in IPF lung fibroblasts compared to normal lung fibroblasts. Second, down-regulation of BMPER caused a decrease in cell invasion and migration capacities in IPF lung fibroblasts. Third, BMPER expression correlated with the extracellular matrix production, since reduced expression of BMPER, either through demethylation or siRNA inhibition, was accompanied by a decrease of the matrix production. Our previous study showed that cell invasion is increased in IPF fibroblasts33. Moreover, IPF lung fibroblasts have an increased migratory capacity compared to normal lung fibroblasts4,38. But the mechanism of fibroblast activation in IPF is not completely clear. We showed here that BMPER in IPF lung fibroblasts might be responsible for the activation of fibrotic fibroblasts. At the molecular level, we showed that BMPER regulates fibrosis through mediating TGF-β/BMP signaling pathway, in which BMPER may activate TGF-β signaling and inhibit BMP signaling pathway. Both TGF-β and BMP4 have been shown to play roles in regulating tissue fibrosis18. For example, TGF-β is a key cytokine in the pathogenesis of IPF and its signaling components have been shown to play a pivotal role in regulating tissue fibrosis15,39,40. And in bleomycin-induced lung fibrosis, TGF-β and BMP signaling followed an inverse course, with dynamic activation of TGF-β signaling and repression of BMP signaling activity. Modulating the balance between BMP and TGF-β may be a therapeutic target in fibrotic lung disease41.
Epigenetic regulation has been shown to play a role in fibrogenesis42. We previously showed non-coding RNAs play a role in lung injury and fibrosis43,44. In the kidney, RASAL1 was reportedly associated with the perpetuation of fibroblast activation and fibrogenesis11. In the lung, altered DNA methylation of Thy-1, prostaglandin E receptor 2, chemokine CXCL10 (IP-10), p14ARF and α-SMA has been shown in IPF and bleomycin-induced lung fibrosis7,8,39,40,45,46. Extensive DNA methylation changed in CpG islands in IPF lungs have been reported6,9. 5′-azacytidine which is a potent inhibitor of DNA methylation has been shown to have two distinct mechanisms: cytotoxicity and demethylation12. Treatment with 5′-azacytidine in mice in vivo has been shown a global demethylation event11. In this study, much lower dose of 5′-azacytidine was used to minimize the cytotoxicity and retain its ability as a potent inhibitor of DNA methylation.
We further demonstrated that the demethylation agent 5′-azacytidine regulated BMPER expression through the transcriptional level in vitro and attenuated lung fibrosis in mice in vivo. It is consistent with previous reports that epigenetic mechanisms control gene expression and are likely to regulate the IPF transcriptome47. In vivo experiment, we saw that global demethylation with 5′-azacytidine decreased the expression of BMPER and extracellular matrix, but did not completely abrogate lung fibrosis following bleomycin-induced lung fibrosis in mice. We tried high-dose (10 mg/kg) of 5′-azacytidine in mouse models in vivo as in a previous report11 and it caused severe cytotoxic effect in our experimental conditions. Thus, the use of low-dose may take advantage of the demethylating effects of the drug in the current study, allowing more frequent or long-term administrations while minimizing cytotoxic side effects. For the demethylating agents currently being used in mice in lung fibrosis models, the important issue that must be carefully evaluated is the ideal dose of the 5′-azacytidine and the efficiency of the drug.
However, we also recognized that there are a few limitations with the current investigation. Although we showed that 5′-azacytidine regulated BMPER promoter activity, more detailed analysis such as the promoter sequence requirement, the possible methylation/demethylation enzymes mediated methylation, and the methylation of BMPER promoter in IPF fibroblasts are needed. We showed that BMPER is upregulated in IPF lung fibroblasts, however the role of BMPER in alveolar type II cells was not investigated. The regulation of BMPER, and the role of BMPER in mediating TGF-β/BMP signaling may differ in alveolar type II cells compared to lung fibroblasts. We recently showed that Fstl1 promoted TGF-β signaling and inhibits BMP signaling in epithelial cells, whereas in fibroblasts Fstl1 promotes TGF-β signaling without altering BMP signaling13. Additional studies are required to gain the mechanisms that cause BMPER promoter be methylated and whether BMPER promoter only is sufficient to induce lung fibrosis. These questions are currently being studied in our laboratory.
Therefore, further investigation into the mechanisms under which BMPER regulates lung fibrosis could provide a novel target for the development of therapeutics for patients with pulmonary fibrosis.
Materials and Methods
Fibroblast isolation and culture
Human lung fibroblasts were isolated from surgical lung biopsies or lung transplant explants obtained from patients with IPF and healthy donors as previously reported48. And mouse lung fibroblasts were obtained as previously reported13. Cells from passage 4 to 7 were used. Human lung fibroblast cell line (MRC5) and human embryonic kidney cell line (HEK293) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). All experiments were approved by the Cedars-Sinai Medical Center Institutional Review Board (CR00008648) and in accordance with the guidelines outlined by the board.
Western blotting
The expression of BMPER, Collagen1 (Col1), Phospho-Smad1 (p-Smad1), Smad1, Phospho-Smad3 (p-Smad3) and Smad3 were evaluated by western blotting essentially as described33. For western blotting analysis, the following antibodies were used: BMPER (ab75183, 1:1000, Abcam, Cambridge, MA, USA), Collagen1 (ab21285, 1:250, Abcam), p-Smad1 (5753s, 1:1000, Cell Signaling, Danvers, MA, USA), Smad1 (9743s, 1:1000, Cell Signaling), p-Smad3 (9520p, 1:1000, Cell Signaling), Smad3 (9523s, 1:1000, Cell Signaling) and β-actin (12620, 1:1000, Cell Signaling). The secondary antibodies used were Peroxidase AffiniPure F(ab’)2 Fragment Donkey Anti-Rabbit IgG (H+L) (Code: 711-036-152, 1:5000, Jackson ImmunoResearch, West Grove, PA, USA) and Peroxidase AffiniPure Goat Anti-Mouse IgG, Fcγ Subclass 1 Specific, (Code: 115-035-205, 1:5000, Jackson ImmunoResearch,). Pierce ECL Western Blotting Substrate (Cat. # 32106, Life Technologies, Grand Island, NY, USA) was used to visualize on a ChemiDoc MP Imaging System (Bio-RAD, Philadelphia, PA, USA). Quantitative densitometric analysis relative to β-actin was used to aid clarity.
Quantification of mRNA
Total RNA was purified from lung fibroblasts after various treatments using RNeasy mini kit (Qiagen, Valencia, CA, USA). The quality of RNA was verified.
Real-time RT-PCR was used to quantify the relative mRNA levels in human and mouse lung fibroblasts using gene-specific primers. The relative expression levels of the gene were determined against GAPDH levels in the samples. The following primers were used: human HAS2 (GenBank accession no. NM_005328) forward, 5′-TCGCAACACGTAACGCAAT-3′; human HAS2 reverse, 5′-ACTTCTCTTTTTCCACCCCATTT-3; human GAPDH (GenBank accession no. NM_002046) forward, 5′-CCCATGTTCGTCATGGGTGT-3′; human GAPDH reverse, 5′-TGGTCATGAGTCCTTCCACGATA-3; human BMPER (GenBank accession no. NM_133468) forward, 5′-CTGCACAGCTTGTACCTGCA-3′, human BMPER reverse 5′-TTGCAAACCACAGTAGAGTC-3′; human COL1 (GenBank accession no. NM_000088) forward, 5′-GTGTTGTGCGATGACG-3′, human COL1 reverse, 5′-TCGGTGGGTGACTCTG-3′; Mouse BMPER (GenBank accession no. NM_028472) forward, 5′-GGTGCGCTGTGTTGTTCATT-3′, mouse BMPER reverse, 5′-TTCTCTCACGCACTGTGTCC-3′; mouse GAPDH (GenBank accession no. NM_001289726) forward, 5′-ATCATCTCCGCCCCTTCTG-3′, mouse GAPDH reverse, 5′-GGTCATGAGCCCTTCCACAAC-3′.
Histology and immunohistochemistry
Mice were sacrificed at various time points after bleomycin treatment under anesthesia. The trachea was cannulated, and the left lungs were inflated with 0.5 mL of 10% neutral buffered formalin. Mouse lung tissue was fixed, embedded in paraffin, sectioned to 5 μm slices for Masson’s trichrome13,49 and BMPER staining. Anti-BMPER (ab75183, 1:200, Abcam) and anti-rabbit HRP-DAB cell and tissue staining kit were used according to the manufacturer’s instructions (R&D systems, Minneapolis, MN, USA). The semiquantitative Ashcroft score was used to score pulmonary fibrosis50. In short, upon 200× magnification, each successive field was given a score ranging from 0 (normal) to 8 (total fibrous obliteration of the field). All scores from 5 sections were averaged41.
Paraffin-embedded sections of normal and IPF lungs were used for immunofluorescence staining. Antibodies specific for BMPER (ab75183, 1:200, Abcam) and α-SMA (F3777, 1:250, Sigma, St. Louis, MO, USA) were used for staining. Alexa Fluor-546-conjugated secondary antibody was from Life Technologies. The nucleus were labeled with DAPI (Vector lab, Burlingame, CA, USA), photographed with a Leica TCS SP5 confocal microscope, and analyzed with Leica confocal software.
Small Interference RNA Transfection
Pre-designed siRNA against human BMPER (Cat. # AM16708, 127801, Life Technologies) and Negative Control siRNA (Cat. # 1022076, Qiangen) were used. MRC-5 cells, primary human normal and IPF lung fibroblasts growing at 50–60% confluence in the complete medium in 6-well plates were transfected with 12.5–50 pmol of either BMPER siRNA or control siRNA using the Lipofectamine RNAiMAX transfection reagent (Cat. # 13778, Life Technologies) according to the manufacture’s instructions. Cell culture supernatants and proteins were harvested 48 hours after transfection.
Plasmid transfection
BMPER expression plasmid was purchased from Genecoppiea (EX-T6437-M61) in which BMPER cDNA (NM_133468.4) was under control of a CMV promoter. One day before transfection, MRC-5 cells, primary human normal and IPF lung fibroblasts (2.5 × 105 per well) were plated in 6-well plates in 2.5 mL growth medium without antibiotics. The cells were transfected with 2–4 μg BMPER plasmid and control plasmid using Lipofectamine 2000 (Cat. # 11668, Life Technologies) according to the manufacture’s instructions. Six hours after transfection, the transfectants were changed to the growth medium and 48 hours later, cells were harvested for proteins.
Demethylation treatment
5′-azacytidine (A2385, Sigma) was dissolved in serum free DMEM and given to mice at 1 mg/kg intraperitoneally from day 3 after bleomycin treatment (2.5 U/kg) every other day until day 21. Mice were anesthetized and lungs were harvested for RNA preparation, protein isolation, or fibroblast isolation on day 21.5 μM of 5′-azacytidine was used for cell culture.
Matrigel invasion assay
The characterization of the invasive behavior of fibroblasts isolated from IPF lungs was performed as described previously33,51. Equal numbers of fibroblasts (0.5 × 105 cells) were plated into the top BioCoat Matrigel Invasion Chamber (BD Biosciences), and DMEM containing 10 ng/mL PDGF (R&D, Minneapolis, MN, USA) was loaded into the bottom chamber. After 24 hours incubation at 37 °C with 5% CO2, the polycarbonate filters with the invaded cells were fixed and stained with the Protocol Hema 3 stain set (Fisher Healthcare, Pittsburgh, PA, USA). Matrigel matrix and non-invading cells on the upper surface of the filter were removed by wiping with a cotton swab, and the filters were removed from the insert using a scalpel blade and were mounted onto glass slides. The invading cells of each sample were counted in five randomly selected fields under a microscope at 400× magnification. The data from 5 fields were averaged. To ensure consistency, each sample from a specific patient has performed in triplicate filters. The average of triplicates was determined for each patient.
Fibroblast migration
Modified Boyden chambers containing 8 μm pores (24-well Transwell plate, Sigma) were used to measure the chemotaxis of lung fibroblasts to PDGF as previously described32. The chambers were coated with 10 μg/ml fibronectin (R&D) in PBS for 2 hours at 37 °C with 5% CO2. Primary lung fibroblasts (5 × 104 cells) diluted in serum-free DMEM containing 1% BSA were loaded into the top chamber, and 500 μL of serum-free DMEM containing 10 ng/ml PDGF was loaded into the bottom chamber. After 4 hours incubation at 37 °C with 5% CO2, the fibroblasts that migrated across the fibronectin-coated filter were stained with Protocol Hema3 stain set and non-migrating cells on the upper surface of the filter were removed by wiping with a cotton swab, and the filters were removed from the insert using a scalpel blade and were mounted onto glass slides. The cells of each sample were counted in five randomly selected fields under a microscope at 400× magnification. The data from 5 fields were averaged. To ensure consistency, each sample from a specific patient has performed in triplicate filters. The average of triplicates was determined for each patient.
Promoter constructs and luciferase assay
The BMPER promoter from −465 to +1 was amplified by PCR from mouse and human genomic DNA with the following primers: mouse (−465 to +1) forward: 5′-ATCCCTCGAGCCCGCGAGTGAA-3′, mouse (−465 to +1) reverse: 5′-CGACAAGCTTGGCGCACGCGAA-3′; human (−465 to +1): forward: 5′-AGCTCTCGAGATGAGCGGTGGG-3′, human (−465 to +1): reverse: 5′-CGAGAAGCTTGCGCGTCTCCAC-3′. The amplified product was inserted into the HindIII/XhoI sites of the luciferase expression vector PGL3 immediately downstream of the luciferase gene.
HEK 293 cells were transfected with the BMPER promoter luciferase report vectors and empty vector, as well as Renilla-luciferase as an internal control by using Lipofectamine 2000 according to the manufacture’s protocol. Six hours after transfection, the transfectants were changed to DMEM + 2% FBS medium overnight. Then the cells were treated with 5′-azacytidine at 5 μM for 24 hours. For luciferase assay, Firefly and Renilla luciferase activities were measured consecutively by using dual luciferase assays (Promega, Madison, WI, USA) 48 hours after transfection according to manufacture’s suggestions.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of HA was determined by HA ELISA-like assay (Hyaluronan DuoSet ELISA kit, DY3614, R&D Systems) following the manufacturer’s instructions.
Assessment of cell viability
Cell viability was evaluated using a colorimetric assay with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Human lung fibroblasts were seeded into a 24-well plate and starved with DMEM containing 2% FBS for 24 hours. The cells were then treated with 5′-azacytidine at 5 μM for additional 24 hours. The MTT staining was performed and the optical density of each well was measured at 595 nm. The OD of control wells was taken as 100% cell viability.
Bleomycin lung injury and fibrosis model
All experiments were carried out using 8–12 weeks old C57BL/6 female mice (The Jackson Laboratory, Bar Harbor, Maine, USA). Bleomycin (bleo) (Hospira, San Jose, CA, USA) was injected intratracheally at a dose of 2.5 U/kg body weight as described previously33,49. At designated time points after bleomycin injection, mice were anesthetized by ketamine (Vedco) and xylazine (Anased, Shenandoah, IA, USA) injection, and lungs were harvested for RNA preparation, protein isolation, or fibroblast isolation. All mouse experiments were approved by IACUC at Duke University (A134-12-05) and Cedars-Sinai Medical Center (004751).
Hydroxyproline assay
Collagen content in the right lung tissue was measured with the conventional hydroxyproline method33.
Affymetrix cDNA microarray
Cultured fibroblasts (at passage 4) from 3 IPF patients were treated with 5′-azacytidine 5 μM for 24 hours. RNA were extracted and hybridized on Affymetrix microarrays. Differentially expressed genes between groups (IFP treated vs non-treated) selected with following cutoff P <= 0.05 and fold change >= 1.5. The array data have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus database (accession no: GSE69764).
Statistical analysis
Data are presented as means ± SEM. We assessed differences in measured variables using the paired or unpaired two-sided Student t test. Data among groups were compared by One-way ANOVA with Tukey post test. Statistical difference was accepted at P < 0.05. Prism version 5.0 (GraphPad) was used to generate graphs and statistical analyses.
Additional Information
How to cite this article: Huan, C. et al. Methylation-mediated BMPER expression in fibroblast activation in vitro and lung fibrosis in mice in vivo. Sci. Rep. 5, 14910; doi: 10.1038/srep14910 (2015).
Supplementary Material
Acknowledgments
This work was supported by NIH grants P01 HL108793 and R01 HL122068 (to P.W.N. and D.J.).
Footnotes
Author Contributions Conceived and designed the experiments: P.W.N. and D.J. Performed the experiments: C.H., T.Y., J.L., T.X., L.C., N.L., A.K., C.W., H.D. and J.M.M. Analyzed the data: C.H., T.Y. and J.L. Wrote the paper: C.H., P.W.N. and D.J.
References
- ATS/ERS. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161, 646–664 (2000). [DOI] [PubMed] [Google Scholar]
- Olson A. L. et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 176, 277–284 (2007). [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Zhou Y., Gaggar A. & Duncan S. R. Fibrosis: ultimate and proximate causes. J Clin Invest 124, 4673–4677 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q. et al. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis. Drugs 71, 981–1001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble P. W., Barkauskas C. E. & Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest 122, 2756–2762 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders Y. Y. et al. Altered DNA methylation profile in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186, 525–535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. K. et al. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol 177, 2245–2255 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros J. et al. Hypermethylation-mediated silencing of p14(ARF) in fibroblasts from idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303, L295–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich E. I. et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One 7, e33770 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. & Wolffe A. P. Methylation-induced repression--belts, braces, and chromatin. Cell 99, 451–454 (1999). [DOI] [PubMed] [Google Scholar]
- Bechtel W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16, 544–550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini V., Kantarjian H. M. & Issa J. P. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med 134, 573–586 (2001). [DOI] [PubMed] [Google Scholar]
- Dong Y. et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med 212, 235–252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello C. M., Cahill E., Martin F., Gaine S. & McLoughlin P. Role of gremlin in the lung: development and disease. Am J Respir Cell Mol Biol 42, 517–523 (2010). [DOI] [PubMed] [Google Scholar]
- Sime P. J., Xing Z., Graham F. L., Csaky K. G. & Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100, 768–776 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Bonniaud P., Sime P., Ask K. & Kolb M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans 35, 661–664 (2007). [DOI] [PubMed] [Google Scholar]
- Bellusci S., Henderson R., Winnier G., Oikawa T. & Hogan B. L. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 122, 1693–1702 (1996). [DOI] [PubMed] [Google Scholar]
- Geng Y. et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A 108, 7058–7063 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam H. H. et al. Basal expression of bone morphogenetic protein receptor is reduced in mild asthma. Am J Respir Crit Care Med 177, 1074–1081 (2008). [DOI] [PubMed] [Google Scholar]
- Koli K. et al. Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol 169, 61–71 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson J. C. et al. Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal 23, 398–406 (2011). [DOI] [PubMed] [Google Scholar]
- Rosendahl A. et al. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol 27, 160–169 (2002). [DOI] [PubMed] [Google Scholar]
- Leask A. & Abraham D. J. TGF-beta signaling and the fibrotic response. FASEB J 18, 816–827 (2004). [DOI] [PubMed] [Google Scholar]
- Selman M., Pardo A. & Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 5, e62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. & Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans 37, 849–854 (2009). [DOI] [PubMed] [Google Scholar]
- Moser M. et al. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol 23, 5664–5679 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbing T. et al. Inhibition of BMP activity protects epithelial barrier function in lung injury. J Pathol 231, 105–116 (2013). [DOI] [PubMed] [Google Scholar]
- Helbing T. et al. BMP activity controlled by BMPER regulates the proinflammatory phenotype of endothelium. Blood 118, 5040–5049 (2011). [DOI] [PubMed] [Google Scholar]
- Dyer L., Wu Y., Moser M. & Patterson C. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev Biol 386, 385–394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funari V. A. et al. BMPER mutation in diaphanospondylodysostosis identified by ancestral autozygosity mapping and targeted high-throughput sequencing. Am J Hum Genet 87, 532–537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke J. et al. Bone morphogenetic protein modulator BMPER is highly expressed in malignant tumors and controls invasive cell behavior. Oncogene 31, 2919–2930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D. et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 120, 2049–2057 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208, 1459–1471 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager A. M. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14, 45–54 (2008). [DOI] [PubMed] [Google Scholar]
- Farkas L. et al. Transient overexpression of Gremlin results in epithelial activation and reversible fibrosis in rat lungs. Am J Respir Cell Mol Biol 44, 870–878 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbing T. et al. Kruppel-like factor 15 regulates BMPER in endothelial cells. Cardiovasc Res 85, 551–559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Liang J. & Noble P. W. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat Rec (Hoboken) 293, 982–985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G. Q. et al. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res 316, 1600–1609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward W. R., Watts K., Feghali-Bostwick C. A., Jenkins G. & Pang L. Repression of IP-10 by interactions between histone deacetylation and hypermethylation in idiopathic pulmonary fibrosis. Mol Cell Biol 30, 2874–2886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Gharaee-Kermani M., Wu Z. & Phan S. H. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am J Pathol 177, 21–28 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe E. et al. Enhanced endogenous bone morphogenetic protein signaling protects against bleomycin induced pulmonary fibrosis. Respir Res 16, 38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg E. M. & Zeisberg M. The role of promoter hypermethylation in fibroblast activation and fibrogenesis. J Pathol 229, 264–273 (2013). [DOI] [PubMed] [Google Scholar]
- Xie T. et al. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics 43, 479–487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T. et al. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcgamma receptor I. J Immunol 188, 2437–2444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders Y. Y. et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol 39, 610–618 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Gharaee-Kermani M., Wu Z. & Phan S. H. Essential role of MeCP2 in the regulation of myofibroblast differentiation during pulmonary fibrosis. Am J Pathol 178, 1500–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I. V. et al. Relationship of DNA methylation and gene expression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190, 1263–1272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer E. B. et al. Bayesian probit regression model for the diagnosis of pulmonary fibrosis: proof-of-principle. BMC Med Genomics 4, 70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D. et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest 114, 291–299 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft T., Simpson J. M. & Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41, 467–470 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. S. et al. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 168, 436–442 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.