Abstract
Controversial results of the association between household physical activity and cancer risk were reported among previous epidemiological studies. We conducted a meta-analysis to investigate the relationship of household physical activity and cancer risk quantitatively, especially in dose-response manner. PubMed, Embase, Web of science and the Cochrane Library were searched for cohort or case-control studies that examined the association between household physical activity and cancer risks. Random–effect models were conducted to estimate the summary relative risks (RRs), nonlinear or linear dose–response meta-analyses were performed to estimate the trend from the correlated log RR estimates across levels of household physical activity quantitatively. Totally, 30 studies including 41 comparisons met the inclusion criteria. Total cancer risks were reduced 16% among the people with highest household physical activity compared to those with lowest household physical activity (RR = 0.84, 95% CI = 0.76–0.93). The dose-response analyses indicated an inverse linear association between household physical activity and cancer risk. The relative risk was 0.98 (95% CI = 0.97–1.00) for per additional 10 MET-hours/week and it was 0.99 (95% CI = 0.98–0.99) for per 1 hour/week increase. These findings provide quantitative data supporting household physical activity is associated with decreased cancer risk in dose-response effect.
Cancer has been the leading cause of disease burden worldwide, and the increase rates of morbidity and mortality are faster than before in global population. It is estimated that 12.7 million cancer cases and 7.6 million cancer deaths occurred in 20081. Around 90% of cancers have been related to environmental exposures and lifestyle. Physical activity is one of the important known lifestyle-related factors. The World Health Organization (WHO) states that, compared to less active adults, more active individuals have lower rates of all-cause mortality, coronary heart disease, high blood pressure, stroke, type 2 diabetes, metabolic syndrome, colon and breast cancer, and depression. Accordingly, the WHO recommends at least 2.5 h of moderate intensity, 1.25 h of vigorous intensity or an equivalent combination of moderate and vigorous intensity aerobic physical activity throughout the week to reduce the risk of chronic non-communicable diseases (NCDs)2. Household physical activity might make a larger contribution to total physical activity, especially among women3. It is important whether household physical activity could affect health benefits. In recent years, there is growing evidence suggesting an association between household physical activity and cancer risk4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. Nevertheless, epidemiological evidence on the relationship has not been systematically assessed. Moreover, many of the individual studies have grouped participants into quantitatively designated categories of household physical activity based on energy expenditure6,9,18,25,26,28,29,31,33 or time expenditure5,7,8,10,13,15,16,17,18,22,24, making it possible to quantify the association between household physical activity and cancer risk in a dose-dependent manner.
Therefore, we conducted a meta-analysis of observation comparisons assessing the association between household physical activity and cancer risk quantitatively to provide more detailed and useful evidence for public health guidelines.
Results
Study Selection
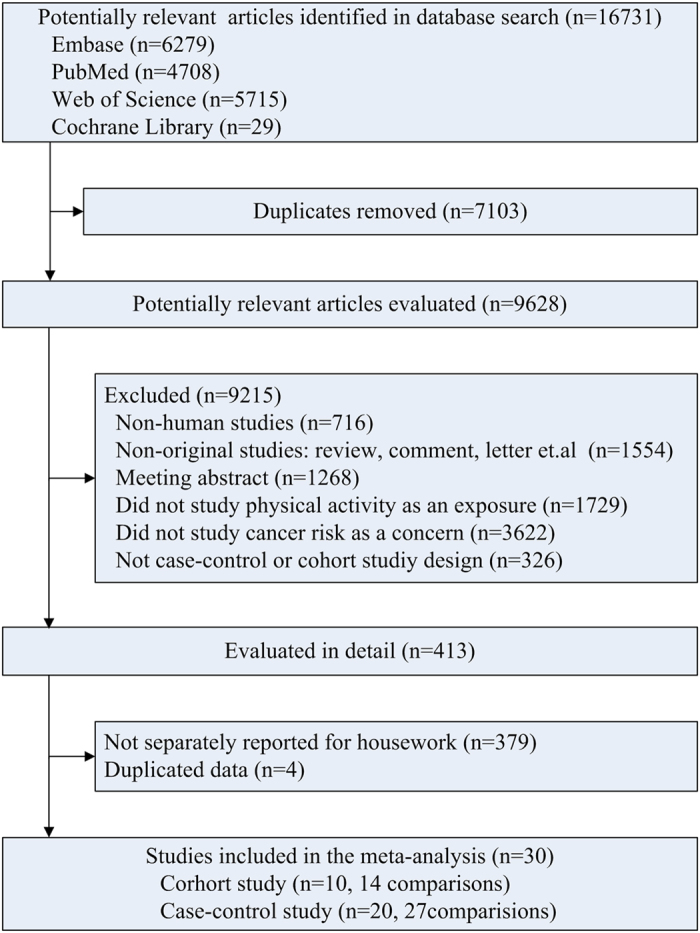
Figure 1 shows study selection process and results from the literature search. We identified 16,731 potentially relevant articles by the search strategy from the four databases. After exclusion of papers that did not meet the inclusion criteria based on information included in the abstracts, we obtained 413 full articles of potentially relevant studies. After full text evaluation, 4 studies were excluded due to duplicated data. 379 studies were further excluded because they reported total physical activity or other physical activity subgroup but not separately reported for household physical activity. Finally, 30 studies4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 were included in the primary meta-analysis.
Figure 1. Flowchart of study selection.
Study Characteristics
General characteristics of the 30 included studies4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 which totaled 41 comparisons had been shown in Supplementary Table S1. Briefly, 14 comparisons14,15,16,17,19,24,26,27,28,29 were cohort study design, and 27 comparisons4,5,6,7,8,9,10,11,12,13,18,20,21,22,23,25,30,31,32,33 were case-control study design. 7 comparisons5,11,14,16,21,27,32 presented the estimates for males, 29 comparisons4,5,6,7,8,9,10,13,14,15,17,18,19,20,22,26,27,28,29,30,31,32,33 for females. 27 comparisons4,5,8,9,12,14,15,16,17,18,19,20,21,24,25,27,28,29,32 were constructed in Europe, 7 comparisons7,13,22,23,26 in Asia, 6 comparisons6,11,30,31 in America and 1 comparisons33 in Africa. Among those comparisons on relationship between household physical activity and cancer risk, breast cancer was estimated mostly, which included 21 comparisons6,7,8,9,10,15,18,20,22,26,28,29,30,31,33, and endometrial cancer was estimated in 4 comparisons4,13,17,19. In addition, colorectal cancer5,16, lung cancer14,25, lymphoid neoplasma12,27, pancreatic cancer32, prostate cancer11,21, gastric cancer23,24, and esophageal carcinoma24 were estimated in the rest comparisons. 20 and 16 comparisons provided quantitative estimates of household physical activity categories in the form of energy expenditure (MET-hour/week)6,9,18,25,26,28,29,31,33 and time expenditure (hour/week)5,7,8,10,13,15,16,17,18,22,24, respectively. Results of study quality assessment yielded an average score of 7 for all studies. The proportions of low, moderate, and high quality were 0.0% (0/30), 30% (9/30), and 70% (21/30).
Highest versus lowest analysis
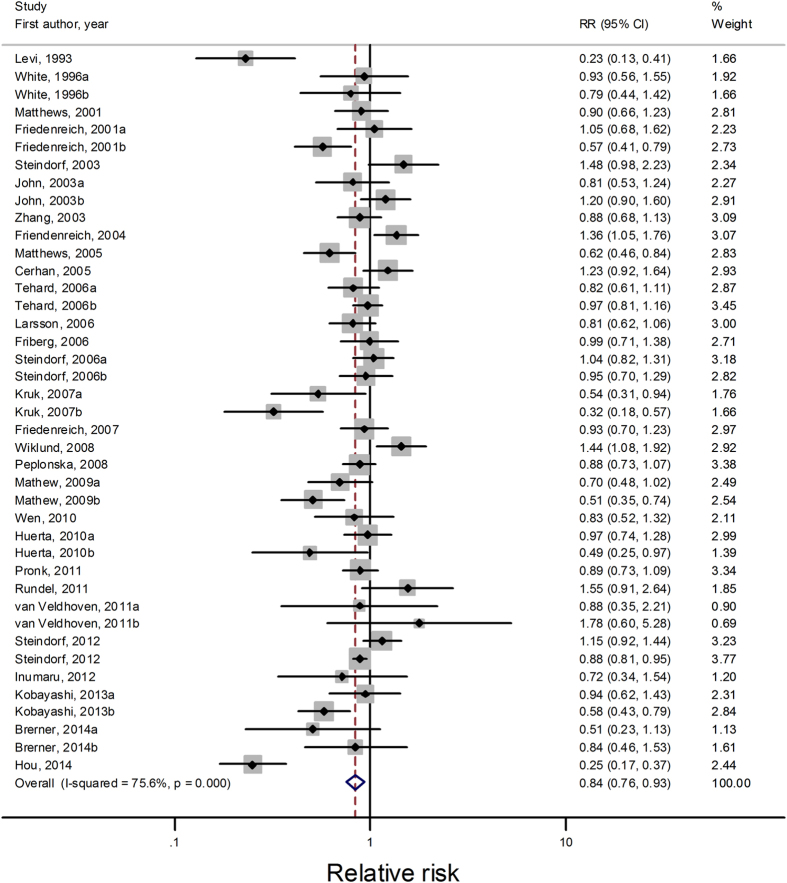
All studies with 41 comparisons4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33 were included for highest versus lowest analysis, which consisted of 2,242,789 participants and 33,949 cancer cases. Compared with lowest household physical activity level, the highest level had a summary RR of 0.84 (95% CI = 0.76–0.93, I2 for heterogeneity = 75.6%; Fig. 2). Table 1 presents the results of subgroup analyses. A significant inverse association between household physical activity and cancer risk was found in both cohort studies and case-control studies. However, pooled estimate from cohort comparisons was more conservative with the summary relative risks of 0.92 (95% CI = 0.87–0.97, I2 for heterogeneity = 0.1%; Table 1), compared with 0.77 (95% CI = 0.65–0.92, I2 for heterogeneity = 82.4%; Table 1) from case-control studies. Geographically, active household physical activity resulted in cancer risk reduction in Asia (RR = 0.76, 95% CI = 0.65–0.90), but not in America (RR = 0.83, 95% CI = 0.59–1.18) or Europe (RR = 0.92, 95% CI = 0.82–1.02). A significant inverse association between household physical activity and cancer risk was observed in women (RR = 0.78, 95% CI = 0.69–0.88) but not in men (RR = 1.04, 95% CI = 0.84–1.30). When further stratified sex by study design and location, the results also showed significant association for women but non-significant association for men. Besides, we examined whether obesity mediated the inverse relation of household physical activity with cancer risk in subgroup analyses. The inverse association of household physical activity to cancer risk was statistically significant in studies adjusting for BMI/weight (RR = 0.80, 95% CI = 0.71–0.90) but it was not significant in studies without adjustment for BMI/weight (RR = 0.93, 95% CI = 0.78–1.10).
Figure 2. Forest plots of highest versus lowest meta-analysis on the relationship between household physical activity and cancer risk.
Table 1. Summary results from subgroup analyses for the relationship between highest versus lowest categories of household physical activity and cancer risk.
| Subgroups | No. of comparisons | Relative Risk (95% CI) | P for heterogeneity | I2(%) | Begg’s test, Egger’s test |
|---|---|---|---|---|---|
| All studies | 41 | 0.84 (0.76–0.93) | <0.001 | 75.6 | 0.052, 0.173 |
| Sex | |||||
| Male | 7 | 1.04 (0.84–1.30) | 0.019 | 60.6 | 0.764, 0.344 |
| Female | 29 | 0.78 (0.69–0.88) | <0.001 | 77.1 | 0.058, 0.093 |
| Study location | |||||
| Europe | 27 | 0.92 (0.82–1.02) | <0.001 | 67.0 | 0.182, 0.614 |
| Asia | 7 | 0.76 (0.65–0.90) | 0.089 | 45.4 | 0.230, 0.205 |
| America | 6 | 0.83 (0.59–1.18) | <0.001 | 80.6 | 1.000, 0.767 |
| Study design | |||||
| Cohort study | 14 | 0.92 (0.87–0.97) | 0.447 | 0.1 | 1.000, 0.550 |
| Case-control Study | 27 | 0.77 (0.65–0.92) | <0.001 | 82.4 | 0.055, 0.094 |
| Mean age | |||||
| ≥50 | 27 | 0.85 (0.75–0.96) | <0.001 | 74.3 | 0.182, 0.332 |
| <50 | 7 | 0.81 (0.59–1.11) | <0.001 | 87.4 | 1.000, 0.535 |
| No. of cases | |||||
| ≥500 | 26 | 0.89 (0.82–0.98) | <0.001 | 63.1 | 0.402, 0.735 |
| <500 | 15 | 0.72 (0.54–0.95) | <0.001 | 84.8 | 0.060, 0.161 |
| PA measures | |||||
| MET-h/wk | 20 | 0.90 (0.77–1.04) | <0.001 | 80.2 | 0.928, 0.938 |
| h/wk | 16 | 0.80 (0.71–0.92) | 0.002 | 57.9 | 0.053, 0.022 |
| No quantitive | 4 | 0.66 (0.32–1.34) | <0.001 | 88.3 | 0.308, 0.271 |
| Cancer type | |||||
| Breast Cancer | 21 | 0.78 (0.69–0.89) | <0.001 | 79.1 | 0.05, 0.125 |
| Endometrial cancer | 4 | 0.64 (0.40–1.03) | <0.001 | 86.4 | 0.308, 0.154 |
| Study quality | |||||
| score ≥ 8 | 14 | 0.91 (0.81–1.03) | 0.015 | 50.6 | 0.324, 0.556 |
| score < 8 | 27 | 0.80 (0.69–0.92) | <0.001 | 80.8 | 0.037, 0.202 |
| Adjustment for BMI/Weight | |||||
| Yes | 27 | 0.80 (0.71–0.90) | <0.001 | 80.3 | 0.002, 0.057 |
| No | 14 | 0.93 (0.78–1.10) | 0.002 | 59.7 | 0.827, 0.474 |
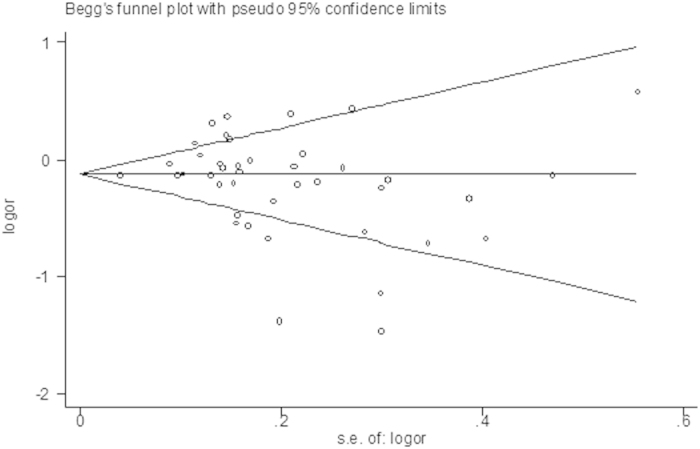
Sensitivity analysis found that the summary RR was not substantially influenced by any of the individual studies when recalculating the overall results by omitting one study each time, with a range from 0.82 (95% CI = 0.75–0.91) to 0.87 (95% CI = 0.80–0.95). Marginal publication bias was indicated by Begg’s test (P = 0.052, Fig. 3) but not Egger’s tests (P = 0.173).
Figure 3. Funnel plots of highest versus lowest meta-analysis on the relationship between household physical activity and cancer risk.
Dose-response analysis
By MET-hour/week
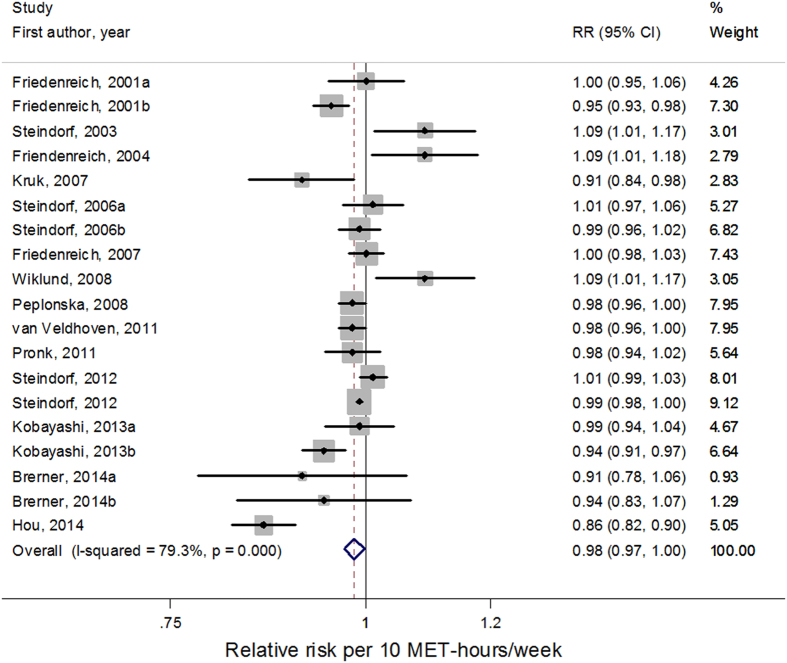
Among the 20 comparisons6,9,18,25,26,28,29,31,33 estimating household physical activity categories quantitatively in the form of energy expenditure, a total of 19 comparisons6,9,18,26,28,29,31,33 were included in the dose-response analysis by MET-hour/week. One study25 was excluded due to lack of category-specific number of cases and person-year/non-cases. Restricted cubic splines model indicated linear association between household physical activity and cancer risk (P for non-linearity = 0.89). In the linear dose-response meta-analysis, the summary relative risk for each 10 MET-hours/week increase in household physical activity was 0.98 (95% CI = 0.97–1.00, I2 for heterogeneity = 79.3%; Fig. 4). The summary relative risk of a 10 MET-hours/week increment of household physical activity was 0.99 (95% CI = 0.99–1.00, I2 for heterogeneity = 5.1%) for cohort studies and 0.98 (95% CI = 0.94–1.01, I2 for heterogeneity = 83.3%) for case-control studies, respectively. The pooled relative risk of a 10 MET-hours/week increment of household physical activity was 1.03 (95% CI = 0.97–1.09, I2 for heterogeneity = 58.5%) for men and 0.98 (95% CI = 0.96–0.99, I2 for heterogeneity = 82.7%) for women, respectively. By study location, the relative risk of a 10 MET-hours/week increment of household physical activity was 0.99 (95% CI = 0.98–1.01, I2 for heterogeneity = 58.9%) and 0.98 (95% CI = 0.95–1.02, I2 for heterogeneity = 74.9%) for cancer in Europe and America, respectively.
Figure 4. Forest plots of linear dose–response meta-analysis by MET-hour/week on the relationship between household physical activity and cancer risk.
Sensitivity analysis found the summary relative risk was not substantially influenced by any of the individual studies when omitting one study each time. Publication bias was not indicated statistically both with Begg’s test (P = 0.972) and Egger’s tests (P = 0.577).
By hour/week
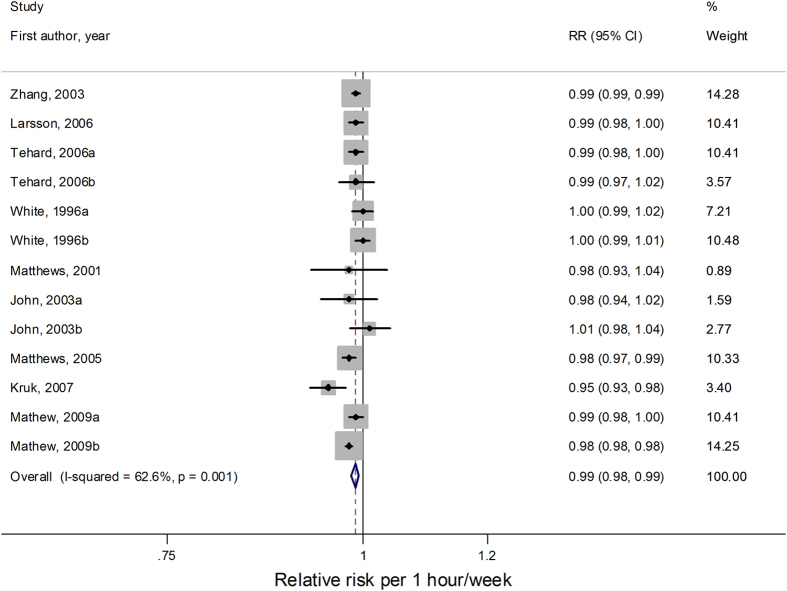
Among the 16 comparisons5,7,8,10,13,15,16,17,18,22,24 estimating quantitatively household physical activity categories in the form of time expenditure, a total of 13 comparisons5,7,8,10,13,15,16,18,22 were included in the dose-response analysis by hour/week. One comparison17 was excluded because its quantitative measures were less than 3 levels. Other two comparisons from Huerta et al.’ study24 which didn’t provide cut-off points for categories were also excluded. From the pooled results of 13 comparisons, we detected a linear association between household physical activity and cancer risk (P for non-linearity = 0.41). In the linear dose-response meta-analysis, the summary relative risk for each 1 hour/week increase in household physical activity was 0.99 (95% CI = 0.98–0.99, I2 for heterogeneity = 62.6%; Fig. 5). The summary relative risk of 1 hour/week increment was 0.99 (95% CI = 0.98–1.00,) for both cohort studies and case-control studies. Stratifying by geographic region, the relative risk of 1 hour/week increment was 0.98 (95% CI = 0.97–1.00, I2 for heterogeneity = 66.4%) for Europe, 1.00 (95% CI = 0.99–1.01, I2 for heterogeneity = 0.0%) for America and 0.99 (95% CI = 0.98–0.99, I2 for heterogeneity = 58.8%) for Asia, respectively.
Figure 5. Forest plots of linear dose–response meta-analysis by hour/week on the relationship between household physical activity and cancer risk.
Sensitivity analysis found the summary relative risk was not substantially influenced by any of the individual studies when omitting one study each time and recalculated the overall results. Publication bias was not indicated statistically both with Begg’s test (P = 0.393) and Egger’s tests (P = 0.761).
Discussion
To our knowledge, this meta-analysis is the first study to summarize the inverse association between household physical activity and cancer risk. A 16% lower overall cancer risk was detected by comparing the most active with the least active household physical activity. The dose-response meta-analyses revealed an inverse linear relationship between household physical activity and cancer risk. Increment of household physical activity by every 10 MET-hour/week or 1 hour/week was associated with a 1% reduction of cancer risk.
The previous reviews have indicated the important role of physical activity in cancer prevention34. And the relationships between physical activity and risk of some types of cancer have been revealed in dose-response manners. A meta-analysis of observational studies by Keum et al.35 found an increase in leisure-time physical activity by 3 MET-hours/week was associated with a 2% reduced risk of endometrial cancer and an increase by an hour/week was associated with a 5% reduced risk of endometrial cancer. Wu et al.’s meta-analysis36 revealed that the risk of breast cancer decreased by 2% for every 25 MET-hour/week increment in non-occupational activity, and 3% for every 10 MET-hour/week increment in recreational activity, respectively. Besides, dose-response meta-analyses were performed to detect the association of non-occupational physical activity with ovarian cancer37, and moderate to vigorous physical activity with gastroesophageal cancer38. Although previous meta-analyses indicated the potential relationship of various domains of activity and cancer risk, few had focused on the association between household physical activity and cancer risk in a dose-response manner. This meta-analysis first indicated the significantly decreased risk of cancer consistently along with the increase of energy expenditure and time expenditure of household physical activity.
Furthermore, we explored the potential inconsistency among different subgroups and revealed some meaningful phenomenons. The inverse association between household physical activity and cancer risk appeared to be more pronounced in case-control studies than cohort studies for binary meta-analysis. However, no obvious gap between the two study designs was found in the linear dose-response analyses. In general, case-control studies are more susceptible to recall and selection bias. And population-based case-control studies are generally less prone to selection bias than hospital-based case-control studies. As we removed hospital-based case-control studies from subgroup analyses by study design, we found the relationship between household physical activity and cancer risk for case-control studies was significantly weakened in highest versus lowest analysis, but was little changed in both of the linear dose-response analyses. The bias caused by hospital-based studies could be an important source for the different results between case-control studies and cohort studies in binary analysis. In addition, we noticed that the inverse association between household physical activity and cancer risk was only found in women but not in men. And further subgroup analyses by study design and location for it suggested the result was robust. Apart from chance, one possible explanation for the finding is the difference in life style between males and females. The proportion of household physical activity accounted for total moderate to vigorous physical activity was much higher among women than that among men39. Besides, sex hormone, a mediator between physical activity and cancer risk, may be another possible explanation for the gender difference. Furthermore, the relatively insufficient studies conducted in men may obstacle the detection of the linkage. The inverse association between household physical activity and cancer risk was modestly enhanced when the analysis was restricted to comparisons those were adjusted for BMI/weight. Accumulating evidence suggested that obesity may increase risk of a variety of cancers40. It has been estimated that about 20% of all cancers were caused by excess weight41. The inverse relationship between household physical activity and cancer risk may be attenuated by the positive relationship between obesity and cancer when studies without adjustment for obesity included.
A number of plausible mechanisms have been proposed to support the cancer prevention role of household physical activity. Hyperinsulinemia and insulin resistance have been associated with increased risk of cancer42,43. Hyperglycemia indirectly influences cancer cells through upregulation of insulin/IGF-1 and inflammatory cytokines in circulation. However, physical activity could reduce insulin resistance and lower fasting insulin levels, thus inhibited cell proliferation and cellular transformations43,44. Another explanation is that physical activity could decreases the concentration of inflammatory adipocytokines and increases anti-inflammatory adipocytokines levels alone or by avoidance of adiposity45,46. And that lowers production of inflammatory markers have been linked with decreased cancer risk47. In addition, physical activity might enhance innate and acquired immune response, increase number and activity of macrophages, natural killer and lymphokine-activated killer cells and cytokines, and promote tumor surveillance48,49. It is also suggested that physical activity could play a role in reducing cancer risk through regulating sex hormones, which have been associated with alterations in cancer risk, especially in breast, endometrial, ovarian and prostate cancer35,37,50.
Several limitations of our study should be acknowledged. Firstly, moderate to high heterogeneity was observed in the overall analysis, which may limit our understanding of the association in various settings and restricts the generalisability of our findings. Therefore, the results should be interpreted with caution. It should be noticed that significant heterogeneity only existed in case-control studies but not in cohort studies, which implied that study design was an important source of the heterogeneity. In addition, subgroup analyses showed that the number of case, measure units of household physical activity and study quality could also bring heterogeneity. Secondly, we failed evaluate household physical activity levels in each study using uniform and accurate measurement due to the heterogeneity in measurement and reporting of physical activity from different studies, which might result in biased results. Thirdly, as this meta-analysis was based on observational studies, although the adjusted estimates were used to pool the results, because they were not fully adjusted, the potential effect from residual confounding could not be ruled out. A primary source of potential residual confounding is likely to stem from confounding variables which were either unmeasured or insufficiently measured in the individual comparisons themselves. Finally, physical activity was assessed by self-report in most included studies, thus misclassification of activity levels is probable and quantitative characterizations should therefore be considered approximate in nature.
Our meta-analysis has several strengths as well. To the best of our knowledge, this is the first meta-analysis that identified the inverse dose–response relationship between household physical activity and cancer risk, and quantized the association within a homogeneous measure of physical activity. We chose to quantify physical activity in units of metabolic equivalent-hours per week and hours per week as they were more frequently reported in studies, which makes the results easier to understand and more conductive. By conducting dose–response analyses in two different measures, intensity of household physical activity itself was shown related to confer a benefit on cancer risk.
In conclusions, the present meta-analysis suggests that household physical activity is associated with a decreased risk of cancer. Approximately, every 10 MET-hours/week or 1 hour/week increase is associated with a 1% reduction in cancer risk. However, caution is needed in interpreting the finding from our meta-analysis because of the inevitable heterogeneity. Further well-designed studies are warranted to confirm the results.
Methods
Literature Search
The meta-analysis was performed following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines51. PubMed, Embase, Web of Science and the Cochrane Library were searched for eligible studies published up to June 18, 2015. Search terms including “cancer”, “carcinoma” or “neoplasm” combined with “physical activity”, “exercise”, “household chores” or “housework” and with “risk”, “incidence” or “mortality” were applied in the literature search. No restrictions were imposed on language of publication. References of any related studies and reviews were further screened for potential missing studies.
Selection criteria
Studies were included if they met the following criteria: (1) was an original study; (2) had a cohort or case-control study design; (3) participants were healthy people at baseline for cohort studies and the outcome was cancer, while in case-control studies, the participants were the patients with primary cancer in cases groups and were healthy people without personal history of cancer in the control group. (4) studied household physical activity as an exposure and cancer risk as an concern; (5) provided the estimate of relative risk (RR) and its 95% confidence interval (CI) or sufficiently data to drive these numbers. Studies were excluded if they were: (1) a case report, review or meeting abstract; (2) cohorts of patients with basic chronic disease (for example, cardiovascular disease). For the dose-response analysis, a quantitative measure of household physical activity for at least three levels, the level-specific number of cases and the level-specific number of either person-years or non-cases had to be provided. Containment relationship in separate publications would be filtered carefully to pick up the one with largest sample size. Two authors (SY and LT) independently read the full text of all articles to determine whether the study met the eligibility criteria outlined above. Disagreements were resolved by discussion.
Data Extraction
Data were extracted by one author (SY) using a data extraction form and entered into a database. A second author (LT) independently checked these data, and all disagreements were resolved by discussion. For each study, we extracted the effect estimate (reported as a RR or odds ratio [OR]) and its associated 95% CI for the association of household physical activity with cancer risk. If available, we extracted the risk estimates with the greatest adjustment. If a study reported the effect of physical activity at multiple periods or ages and over the lifetime, we used the lifetime result. If a study reported results for males and females separately, both risk estimates were included in the primary analysis. For all comparisons, we abstracted OR/RR and its 95% CI for comparison between the most active group and the least active group. The effect size and 95% CI were inverted for comparisons in which the most active group was used as the reference group. For studies reported household physical activity in the unit of MET-hour/week or hour/week, the quantitative measure range of household physical activity, effect size, 95% CI, the number of cases and person-years or non-cases were abstracted for each activity group. Other extracted data included the first author’s name, the publication year, the category of cancer, the study design (eg. case-control or cohort), the sex of the participants and the location in which the study took place.
Quality assessment
Two reviewers (SY and LT) completed the quality assessment independently by using Newcastle-Ottawa Scale52. This scale awards a maximum of nine points to each study: selection of the study groups (maximum 4 points), comparability of the study populations (maximum 2 points) and ascertainment of the outcome of interest (maximum 3 points). For each point, 1 score indicates higher quality, whereas, 0 representatives lower quality. We assigned scores of 0–3, 4–6 and 7–9 for low, moderate and high quality of studies, respectively.
Statistical Analysis
Two (highest vs. lowest, dose-response) types of meta-analyses were performed. We combined the case-control and cohort comparisons in the primary meta-analysis because ORs and RRs provide similar estimates of risk when the outcome is rare53. The RR was used as a measure of the association between household physical activity and cancer risk. The highest versus lowest analysis was conducted using random-effect model54.
Dose-response meta-analyses were performed to estimate the trend from the correlated log RR estimates across levels of household physical activity. First we examined a potential nonlinear association between household physical activity and cancer risk, using restricted cubic splines with four knots at the 5th, 35th, 65th and 95th percentiles of the levels of household physical activity55. A P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. If a liner relationship was found, a summary risk was derived for a 10 MET-hours/week and 1 hour/week increment in household physical activity respectively. The method used to estimate the study-specific RRs was described by Greenland and Longnecker56. Then the study-specific risk increments were combined in random-effect meta-analysis. Forest plots of the linear dose–response results were presented for RRs for per 10 MET-hours/week increment and for per 1 hour/week increment. Only comparisons with three or more quantitative exposure levels and using MET-hour/week or hour/week to describe household physical activity dose were included in these analyses. For each study, the median or mean level in each category of household physical activity was assigned to the corresponding relative risk. We assigned the mid-point of the upper and lower boundaries in each category if median or mean were not reported. For studies reported open upper boundaries or uppest boundaries closed with extreme value, we set the half width of this category was the same with nearest category and used the sum of half width and lower boundary as mid-point.
Heterogeneity between comparisons was assessed by Q statistics (P < 0.10)57 and I2 was used to quantify the proportion of the total variation due to the heterogeneity58. To identify the sources of heterogeneity and explore the potential effects of specific study characteristics on association between household physical activity and cancer risk, subgroup analyses were conducted according to a priori selected variables. Sensitivity analysis was also performed to explore the potential influence of individual study on overall results by omitting one study each time and recalculated the combined RR. Publication bias was assessed by visual inspection of funnel plots as well as statistically with the use of the Begg’s test59 and the Egger’s test60 (significant level P < 0.10). Except where otherwise specified, statistical tests were two-sided and a P-value less than 0.05 was considered statistically significant. All analyses were conducted with Stata software (version 12.0; StataCorp, College Station, TX).
Additional Information
How to cite this article: Shi, Y. et al. Household physical activity and cancer risk: a systematic review and dose-response meta-analysis of epidemiological studies. Sci. Rep. 5, 14901; doi: 10.1038/srep14901 (2015).
Supplementary Material
Acknowledgments
This work is supported by the National Natural Science Foundation of China (NSFC-81172752, and NSFC-81302491).
Footnotes
Author Contributions Conceived and designed the study strategy: S.F.N. and L.L.; Acquire the data: Y.S., T.T.L., Y.W. and L.L.Z.; Statistical analysis Y.S.; Wrote the manuscript: Y.S.; Prepared the tables and figures: Y.S. and T.T.L.; Revise of the manuscript: Y.W., Q.Q., J.Y.Y and S.W.; All authors reviewed the manuscript.
References
- Jemal A. et al. Global cancer statistics. CA Cancer J Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- WHO. Global Strategy on Diet, Physical Activity and Health (2015) Available at: http://www.who.int/dietphysicalactivity/factsheet_adults/en/. (Accessed: 1th July 2015).
- Phongsavan P., Merom D., Marshall A. & Bauman A. Estimating physical activity level: the role of domestic activities. J Epidemiol Community Health 58, 466–467 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F., La Vecchia C., Negri E. & Franceschi S. Selected physical activities and the risk of endometrial cancer. Br J Cancer 67, 846–851 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Jacobs E. J. & Daling J. R. Physical activity in relation to colon cancer in middle-aged men and women. Am J Epidemiol 144, 42–50 (1996). [DOI] [PubMed] [Google Scholar]
- Friedenreich C. M., Bryant H. E. & Courneya K. S. Case-control study of lifetime physical activity and breast cancer risk. Am J Epidemiol 154, 336–347 (2001). [DOI] [PubMed] [Google Scholar]
- Matthews C. E. et al. Lifetime physical activity and breast cancer risk in the Shanghai Breast Cancer Study. Br J Cancer 84, 994–1001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- John E. M., Horn-Ross P. L. & Koo J. Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomarkers Prev 12, 1143–1152 (2003). [PubMed] [Google Scholar]
- Steindorf K., Schmidt M., Kropp S. & Chang-Claude J. Case-control study of physical activity and breast cancer risk among premenopausal women in Germany. Am J Epidemiol 157, 121–130 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang Z., Gao E., Wu J. & Li D. Relationship between Physical Activity and Breast Cancer Risk in China. Reproduction and Contraception 23, 291–298 (2003). [Google Scholar]
- Friedenreich C. M., McGregor S. E., Courneya K. S., Angyalfi S. J. & Elliott F. G. Case-control study of lifetime total physical activity and prostate cancer risk. Am J Epidemiol 159, 740–749 (2004). [DOI] [PubMed] [Google Scholar]
- Cerhan J. R. et al. Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control 16, 1203–1214 (2005). [DOI] [PubMed] [Google Scholar]
- Matthews C. E. et al. Physical activity and risk of endometrial cancer: a report from the Shanghai endometrial cancer study. Cancer Epidemiol Biomarkers Prev 14, 779–785 (2005). [DOI] [PubMed] [Google Scholar]
- Steindorf K. et al. Physical activity and lung cancer risk in the European Prospective Investigation into Cancer and Nutrition Cohort. Int J Cancer 119, 2389–2397 (2006). [DOI] [PubMed] [Google Scholar]
- Tehard B., Friedenreich C. M., Oppert J. M. & Clavel-Chapelon F. Effect of physical activity on women at increased risk of breast cancer: results from the E3N cohort study. Cancer Epidemiol Biomarkers Prev 15, 57–64 (2006). [DOI] [PubMed] [Google Scholar]
- Larsson S. C., Rutegard J., Bergkvist L. & Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer 42, 2590–2597 (2006). [DOI] [PubMed] [Google Scholar]
- Friberg E., Mantzoros C. S. & Wolk A. Physical activity and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev 15, 2136–2140 (2006). [DOI] [PubMed] [Google Scholar]
- Kruk J. Lifetime physical activity and the risk of breast cancer: a case-control study. Cancer Detect Prev 31, 18–28 (2007). [DOI] [PubMed] [Google Scholar]
- Friedenreich C. et al. Physical activity and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer 121, 347–355 (2007). [DOI] [PubMed] [Google Scholar]
- Peplonska B. et al. Adulthood lifetime physical activity and breast cancer. Epidemiology 19, 226–236 (2008). [DOI] [PubMed] [Google Scholar]
- Wiklund F. et al. Lifetime total physical activity and prostate cancer risk: a population-based case-control study in Sweden. Eur J Epidemiol 23, 739–746 (2008). [DOI] [PubMed] [Google Scholar]
- Mathew A. et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: a case-control study. Eur J Cancer Prev 18, 368–376 (2009). [DOI] [PubMed] [Google Scholar]
- Wen X. Y. Salt taste sensitivity, physical activity and gastric cancer. Asian Pac J Cancer Prev 11, 1473–1477 (2010). [PubMed] [Google Scholar]
- Huerta J. M. et al. Prospective study of physical activity and risk of primary adenocarcinomas of the oesophagus and stomach in the EPIC (European Prospective Investigation into Cancer and nutrition) cohort. Cancer Causes Control 21, 657–669 (2010). [DOI] [PubMed] [Google Scholar]
- Rundle A. et al. Physical activity and lung cancer among non-smokers: a pilot molecular epidemiological study within EPIC. Biomarkers 15, 20–30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk A. et al. Physical activity and breast cancer risk in Chinese women. Br J Cancer 105, 1443–1450 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veldhoven C. M. et al. Physical activity and lymphoid neoplasms in the European Prospective Investigation into Cancer and nutrition (EPIC). Eur J Cancer 47, 748–760 (2011). [DOI] [PubMed] [Google Scholar]
- Steindorf K. et al. Physical activity and risk of breast cancer overall and by hormone receptor status: The European prospective investigation into cancer and nutrition. Int J Cancer 132, 1667–1678 (2012). [DOI] [PubMed] [Google Scholar]
- Steindorf K. et al. Prospective Study on Physical Activity and Risk of In Situ Breast Cancer. Cancer Epidemiol Biomarkers Prev 21, 2209–2219 (2012). [DOI] [PubMed] [Google Scholar]
- Inumaru L. E., Irineu Gomes Duarte Quintanilha M., Aparecida da Silveira E. & Veloso Naves M. M. Risk and protective factors for breast cancer in Midwest of Brazil. J Environ Public Health 2012, 356851 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi L. C. et al. Moderate-to-vigorous intensity physical activity across the life course and risk of pre- and post-menopausal breast cancer. Breast Cancer Res Treat 139, 851–861 (2013). [DOI] [PubMed] [Google Scholar]
- Brenner D. R. et al. Physical activity and risk of pancreatic cancer in a central European multicenter case-control study. Cancer Causes Control 25, 669–681 (2014). [DOI] [PubMed] [Google Scholar]
- Hou N. et al. An epidemiologic investigation of physical activity and breast cancer risk in Africa. Cancer Epidemiol Biomarkers Prev 23, 2748–2756 (2014). [DOI] [PubMed] [Google Scholar]
- Clague J. & Bernstein L. Physical activity and cancer. Curr Oncol Rep 14, 550–558 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum N. et al. Leisure-time physical activity and endometrial cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer 135, 682–694 (2014). [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang D. & Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 137, 869–882 (2013). [DOI] [PubMed] [Google Scholar]
- Zhong S. et al. Nonoccupational physical activity and risk of ovarian cancer: a meta-analysis. Tumour Biol 35, 11065–11073 (2014). [DOI] [PubMed] [Google Scholar]
- Behrens G. et al. The association between physical activity and gastroesophageal cancer: systematic review and meta-analysis. Eur J Epidemiol 29, 151–170 (2014). [DOI] [PubMed] [Google Scholar]
- Murphy M. H., Donnelly P., Breslin G., Shibli S. & Nevill A. M. Does doing housework keep you healthy? The contribution of domestic physical activity to meeting current recommendations for health. BMC Public Health 13, 966 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T., Nothlings U. & Boeing H. Obesity and cancer. The Proceedings of the Nutrition Society 67, 128–145 (2008). [DOI] [PubMed] [Google Scholar]
- De Pergola G. & Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013, 291546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. & Balkau B. Diabetes mellitus, hyperglycaemia and cancer. Diabetes & metabolism 36, 182–191 (2010). [DOI] [PubMed] [Google Scholar]
- Tsugane S. & Inoue M. Insulin resistance and cancer: epidemiological evidence. Cancer Sci 101, 1073–1079 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. & Tsugane S. Insulin resistance and cancer: epidemiological evidence. Endocr Relat Cancer 19, F1–8 (2012). [DOI] [PubMed] [Google Scholar]
- Bradley R. L., Jeon J. Y., Liu F. F. & Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab 295, E586–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluco C. et al. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol 7, 133–138 (2000). [DOI] [PubMed] [Google Scholar]
- Il’yasova D. et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev 14, 2413–2418 (2005). [DOI] [PubMed] [Google Scholar]
- McTiernan A. Mechanisms linking physical activity with cancer. Nature reviews. Cancer 8, 205–211 (2008). [DOI] [PubMed] [Google Scholar]
- Woods J. A., Davis J. M., Smith J. A. & Nieman D. C. Exercise and cellular innate immune function. Med Sci Sports Exerc 31, 57–66 (1999). [DOI] [PubMed] [Google Scholar]
- Christine M. Friedenreich M. R. O. Physical Activity and Cancer Prevention: Etiologic Evidence and Biological Mechanisms. J. Nutr. 132, 3456S–3464S (2002). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9, 1–30 (1987). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Robins J. M. Estimation of a common effect parameter from sparse follow-up data. Biometrics 41, 55–68 (1985). [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.