Abstract
Rationale: Transmission is driving the global tuberculosis epidemic, especially in congregate settings. Worldwide, natural ventilation is the most common means of air disinfection, but it is inherently unreliable and of limited use in cold climates. Upper room germicidal ultraviolet (UV) air disinfection with air mixing has been shown to be highly effective, but improved evidence-based dosing guidelines are needed.
Objectives: To test the efficacy of upper room germicidal air disinfection with air mixing to reduce tuberculosis transmission under real hospital conditions, and to define the application parameters responsible as a basis for proposed new dosing guidelines.
Methods: Over an exposure period of 7 months, 90 guinea pigs breathed only untreated exhaust ward air, and another 90 guinea pigs breathed only air from the same six-bed tuberculosis ward on alternate days when upper room germicidal air disinfection was turned on throughout the ward.
Measurements and Main Results: The tuberculin skin test conversion rates (>6 mm) of the two chambers were compared. The hazard ratio for guinea pigs in the control chamber converting their skin test to positive was 4.9 (95% confidence interval, 2.8–8.6), with an efficacy of approximately 80%.
Conclusions: Upper room germicidal UV air disinfection with air mixing was highly effective in reducing tuberculosis transmission under hospital conditions. These data support using either a total fixture output (rather than electrical or UV lamp wattage) of 15–20 mW/m3 total room volume, or an average whole-room UV irradiance (fluence rate) of 5–7 μW/cm2, calculated by a lighting computer-assisted design program modified for UV use.
Keywords: tuberculosis transmission, infection control, air disinfection, ultraviolet irradiation, tuberculosis prevention
At a Glance Commentary
Scientific Knowledge on the Subject
The efficacy of upper room air disinfection is no longer in doubt, but so far there has been only one other hospital-based field study involving humans as the source of transmission and infection (of guinea pigs) as the endpoint. For 60 years, the application of upper room ultraviolet (UV) germicidal irradiation has been hampered by inadequate application guidelines, primarily based on fixture input wattage, not UV output. This is the first hospital-based study to have characterized conditions sufficiently well to provide a basis for international application guidelines.
What This Study Adds to the Field
Beyond demonstrating an approximately 80% reduction in transmission, this is the first field study to attempt to completely define the conditions responsible. Moreover, it highlights the need for basing UV fixture number and location on fixture UV output, not electrical input, as has been done in the past. The proposed application guidelines are based on the entire room volume, either total fixture output per cubic meter volume or, using a recently characterized computer-assisted design program, the average UV irradiance (fluence rate) for the entire room volume. These application guidelines should provide a firm scientific basis for a critically important technology that is often poorly applied, especially in high-burden settings in which institutional airborne tuberculosis transmission is a key factor driving the global tuberculosis epidemic.
Ultraviolet (UV) germicidal irradiation (UVGI) has long been a standard method of water disinfection, but although rigorously studied, UVGI air disinfection has been less well accepted and is poorly defined in terms of evidence-based international application guidelines (1). The most important global application of upper room UVGI is to prevent the spread of Mycobacterium tuberculosis (Mtb) in health care and other congregate spaces, especially in high-burden, resource-limited settings (2). The high volume of mechanical ventilation (6–12 air changes per hour [ACH]) recommended for the prevention of airborne infection is often not feasible in these settings. Facilities in suitable climates often depend on natural ventilation, which can be highly effective in well-designed buildings under optimal outside conditions, but can also be inadequate when conditions are suboptimal, and at night when windows may be closed for thermal comfort, pest control, or security (3). In cold climates natural ventilation is often not a practical option, but even in hot climates, as air conditioning becomes more widely used for thermal comfort, windows are usually closed. In these and other settings, upper room UVGI, often combined with natural or mechanical ventilation, may be the most cost-effective method for providing effective air disinfection.
Wall- or ceiling-mounted UVGI fixtures are designed to produce an intensive air disinfecting zone in the upper room, above people’s heads, while limiting irradiation in the lower, occupied room to prevent eye and skin irritation of room occupants (4–6). Air mixing between the upper and lower room, commonly ensured by low-velocity ceiling fans, results in effective air disinfection in the occupied breathing zone (7–9). Efficacy of upper room UVGI depends on three principal factors: (1) the average UVGI fluence rate (irradiance from multiple sources) produced in the upper room and its distribution, (2) the rate of vertical air mixing, and (3) the UV susceptibility of the organism under ambient conditions (10–12). Using aerosolized surrogate test organisms in room-size test chambers, and microbiologic air sampling, various researchers have reported upper room UVGI air disinfection equivalent to 10, 20, and even higher ACH (7, 12–15). In contrast to the upper room application, except in very small rooms, most UVGI-based room air cleaners (air moving devices) are greatly limited by their clean air delivery rate, short-circuiting of processed air, and resulting low equivalent ACH (16).
Despite extensive laboratory evidence of efficacy over many years, the paucity of convincing field trials in clinically relevant settings has been a major barrier to full acceptance, technology development, and wider implementation of upper room UVGI (17). Here we report the results of a controlled field trial of the effectiveness of upper room UVGI with air mixing to reduce transmission of Mtb from patients with multidrug-resistant (MDR) or extensively drug-resistant tuberculosis (TB) in a provincial referral hospital in South Africa. Because mechanical microbiologic air sampling is not feasible for naturally generated Mtb, we used highly susceptible guinea pigs as biologic air samplers, using infection (detected by the tuberculin skin test [TST]) as the most clinically relevant endpoint (18). We also report on the application of a recently validated computer-assisted design (CAD) program (Visual-UV; Acuity Brands, Connors, GA) to better define critical UVGI parameters, such as average room germicidal UV fluence rates (19). Finally, based on the results of this trial, we propose two new dosing strategies using total UVGI fixture output for determining the germicidal irradiation required in designing highly effective UVGI room installations. Some of the results of these studies have been previously reported in the form of an oral abstract presentation (20).
In their classic 1958–1962 experiments, Riley and colleagues (21–24) quantified the airborne transmission of human-source Mtb using hundreds of highly susceptible sentinel guinea pigs exposed to the air exhausted from an experimental six-bed TB ward in a Baltimore hospital. The investigators had also intended to test the efficacy of upper room UVGI, as evident in the 1959 paper describing the experimental ward where a UVGI wall fixture is illustrated in a patient room (21). But, after 4 years of experiments, the hospital reclaimed the ward for other purposes, and upper room UVGI was never tested. In 1996, however, Riley’s longtime research collaborator, the late Solbert Permutt, suggested a novel design for air disinfection experiments, using two guinea pig chambers to control for variable patient infectiousness (25, S. Permutt, personal communication). His concept called for one guinea pig exposure chamber (intervention chamber) to receive exhaust air from the ward only every other day, the days when upper room UVGI was turned on in the ward; and the other exposure chamber (control chamber) to receive exhaust air from the same ward only on the alternate days, when upper room air disinfection was turned off. This experimental design ensured that patient infectiousness would be equivalent during the cumulative intervention and control periods. After months of exposure to ward air, any differences in the TST reaction rates between the two guinea pig chambers would be a direct measure of the effectiveness of UVGI.
In the last decade two research groups have reestablished experimental TB hospital wards similar to Riley’s for the purpose of studying TB transmission and control strategies, and have adopted Permutt’s alternate-day experimental design (17). Thus far, upper room UVGI, room air ionizers, masks on patients, portable room air cleaning (filtration) machines, and inhaled antibiotics have been subjected to controlled testing in very different clinical settings in Peru and South Africa (17, 26). Escombe and coworkers (17) reported the effectiveness of upper room UV in a TB/HIV ward occupied by patients with mostly drug-susceptible TB in Lima, Peru. The present study tests the effectiveness of upper room UVGI with air mixing in an MDR-TB referral hospital in South Africa as a basis for proposed new dosing guidelines.
Methods
The Airborne Infections Research Facility
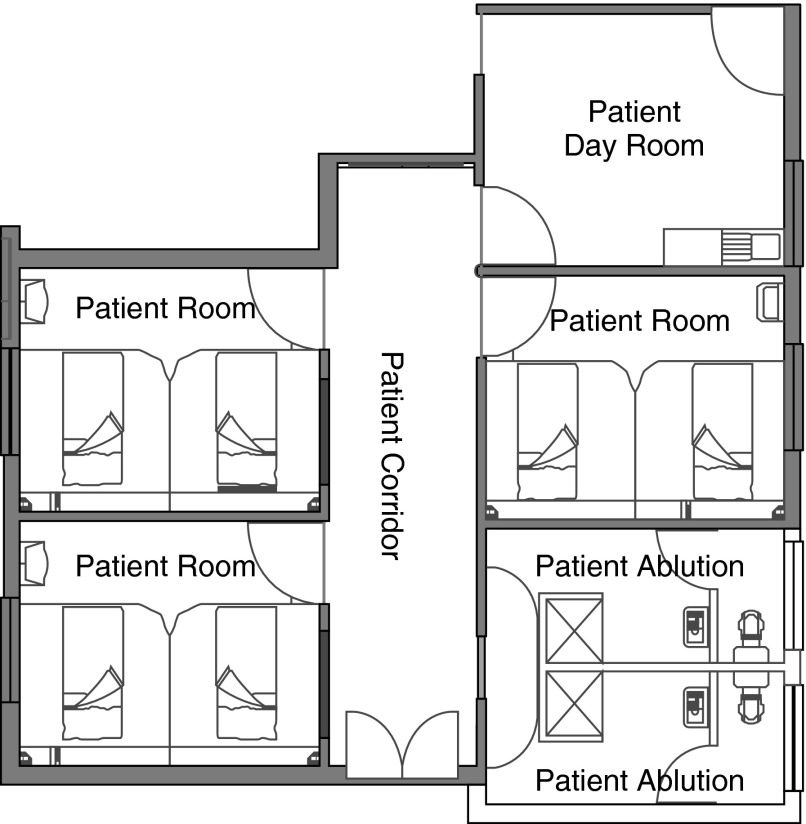
This study was performed at the airborne infections research (AIR) facility in eMahlahleni, South Africa. Like Riley’s facility, the AIR facility was designed to expose hundreds of susceptible sentinel guinea pigs to airborne TB (infectious droplet nuclei) generated by patients with active, culture-proven, mostly sputum smear-positive, cavitary, pulmonary TB. The facility is physically part of the Mpumalanga Provincial MDR-TB Referral Hospital, and the patients recruited for this study were among those newly admitted with highly drug-resistant TB and just started on standardized South African MDR-TB treatment. The facility consists of a six-bed inpatient MDR-TB ward connected by an airtight ventilation system to two identical guinea pig exposure chambers (Figure 1). The study methods (human and animal protocols) were identical to our previously published study of the effectiveness of surgical masks on patients, but instead of masks on patients, upper room UVGI was turned on in the three patient rooms and corridor every other day (27). Human subjects in this study served only to generate infectious aerosols while on the ward. They received exactly the same care and treatment as on the main MDR wards. Subject inclusion and exclusion criteria, recruitment, consent, and TB treatment were the same as in the published surgical mask study (23). This study was approved by the human and animal ethics committees of all participating organizations.
Figure 1.
Layout of the airborne infections research facility showing three two-bed patient rooms, corridor, and patient day room.
Selecting the UVGI Fixtures to be Tested
The goals of this research were to test the effectiveness of upper room UVGI air disinfection with air mixing in a real hospital setting, under defined conditions, using the best available UVGI fixtures, but not to test any one manufacturer’s product. Based on our published test chamber experiments and fixture testing, technical specifications for wall fixtures to be used in this study were developed and candidate fixtures solicited in the United States and South Africa (see the online supplement). Unless previously characterized at the Harvard School of Public Health, samples of stock or custom manufactured fixtures claiming to meet these specifications were tested for compliance.
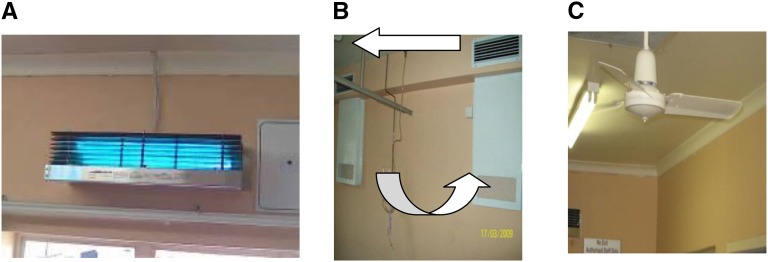
Among those submitted for consideration, only two fixtures approximated our specifications. Both were made in the United States. Each manufacturer donated six wall fixtures for the study, one each for the three patient rooms, the hallway, common room, and one of each to be kept in reserve should a fixture fail. Spare lamps for each fixture were also on hand. The fixtures were mounted 2.1 m from the ground, on opposite walls, but staggered to produce as uniform a UVGI distribution as possible from two sources, and the longest (estimated) average UV ray length possible in the room (Figures 2A and 3).
Figure 2.
(A) Ultraviolet germicidal irradiation wall fixture, (B) room ventilation arrangement, and (C) ceiling paddle fan model in use during experiments. Note that the photograph in A was taken wearing ultraviolet protective goggles because even brief direct exposure at close range to upper room ultraviolet germicidal irradiation lamps can cause corneal inflammation. The arrows in B indicate air movement.
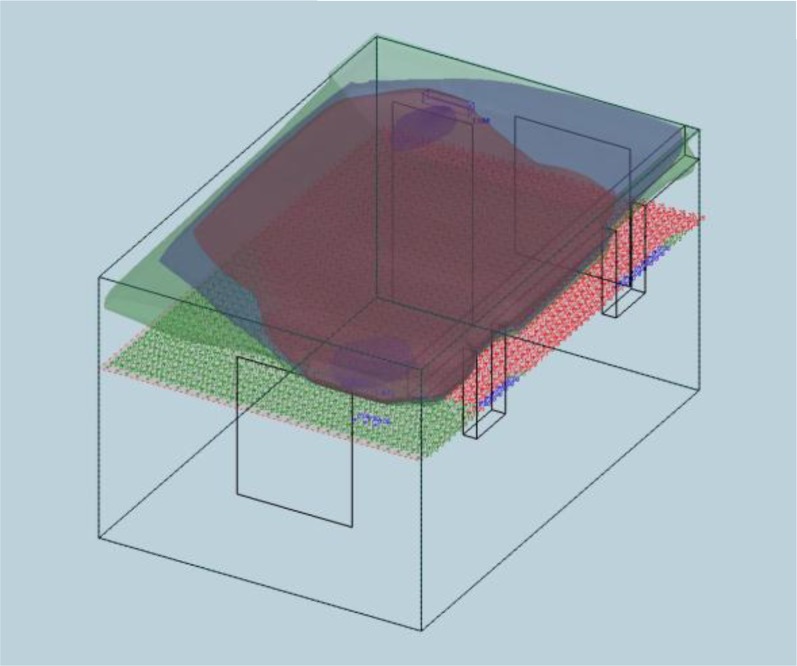
Figure 3.
A computer-assisted design–generated illustration estimating the ultraviolet (UV) germicidal irradiation distribution in one of the patient rooms in the airborne infections research facility. The fixtures are represented by the blue ovoid structures on the near and far upper walls. The upper tricolor distribution represents three levels of ultraviolet germicidal irradiation flux in the “kill zone,” whereas the lower “safety” distribution indicates peak UV fluence rates 1.7 m (5.5 ft) from the floor.
The commonly available ceiling fans used in the three patient rooms and the corridor (Figure 2C) had three blades 0.46-m length, and operated at all times at 100 rpm in the upward direction. Air was also mixed by the heating, ventilation, and air conditioning system, which ran at six ACH (outside air) during this 7-month exposure experiment. Details on ventilation and average humidity are discussed in the online supplement.
Results
The Experiments
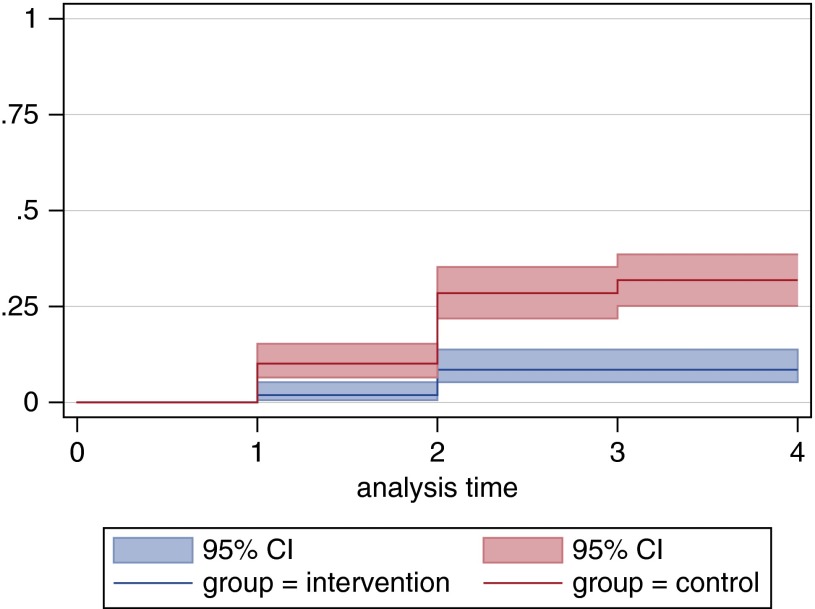
When an initial 4-month exposure study resulted in too few guinea pig infections in the control chamber to meet our power calculation requirements, a nearly identical second 3-month experiment was subsequently conducted with fresh animals and additional human subjects. The only difference between the two experiments was a change in the location of the exhaust ducts from the patient rooms. When the first UV study showed only nine guinea pig TST conversions, all under control conditions (Table 1), we questioned whether the upper room location of the exhaust ducts in the patient rooms might be contributing to this preliminary 100% efficacy estimate by selectively sampling irradiated air. Exhaust ports were relocated to the breathing zone positions indicated in Figure 2B. However, as predicted by our engineers, subsequent tracer gas studies and air flow modeling indicated that the ceiling fans produced well-mixed conditions, that is, exhaust air was not disproportionately exhausted from the irradiated zone (28). There was no scientific rationale not to combine the results of the two experiments in the analysis, as presented in the Kaplan-Meier plot (Figure 4) showing the proportion getting infected, at least once, as a function of time, accommodating the killed animals as censored. This does not depict the multiple infections of a single animal.
Table 1.
Crude TST Conversion Rate for Animals under Control and Ultraviolet Intervention Conditions by Month of Exposure for the Two Sequential Experiments (Combined in the Analysis)
| TST (>6 mm) | Experiment 1 |
Experiment 2 |
||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| Month 1 | 0 | 1 | 4 | 17 |
| Month 2 | 0 | 3 | 12 | 31 |
| Month 3 | 0 | 5 | 0 | 1 |
| Month 4 | 0 | 0 | — | — |
| Total | 0 | 9 | 16 | 49 |
Definition of abbreviation: TST = tuberculin skin test.
Figure 4.
Kaplan-Meier risk estimates of combined tuberculin skin test conversions (y-axis) of exposed guinea pigs in control and intervention chambers by month (x-axis) of exposure. P < 0.0005; combined hazard ratio, 4.9 (95% CI, 2.8–8.6). CI = confidence interval.
To incorporate the possibility of multiple hits we use a Poisson model and estimate the monthly rate of infection using maximum likelihood. Even though we cannot accurately count the number of infections, we can count the numbers that escape infection each month. We calculate the probability of not getting infected as a function of the rate of infection to provide the likelihood equations. Using this method, we estimate that over the 3 months for the first study, there were a total of zero observed and zero estimated infections for the intervention group and a total of nine observed and 10.61 estimated for the control group. For the second study, over the 4 months, there were a total of 15 infections observed and 16.41 estimated for the intervention group, and 49 observed and 68.59 estimated for the control group. The Poisson correction method was similar to that routinely applied in mechanical air sampling (29). Uncorrected, an estimated 74% protection was calculated, whereas corrected for multiple hits, the estimated protection increased to approximately 80%.
Safety measurements were made at eye level with a sensitive 254-nm photometer (Gigahertz Optik, Newburyport, MA) before any patients entered the ward, and confirmed peak values of no more than 0.4 μW/cm2, values within local Medical Research Council guidelines and published recommendations, and highly unlikely to lead to eye or skin irritation among room occupants (30, 31). There were no eye or skin complaints registered by staff or patients during the study.
Discussion
Optimal application guidelines for upper room UVGI require evidence of safe and effective air disinfection in high-risk facilities, and careful characterization of the key parameters responsible. Our study demonstrates that two very well-characterized UVGI fixtures per room with ceiling fans provided an estimated 80% protection, statistically corrected for multiple hits. This is protection equivalent to adding approximately 24 ACH of ventilation. Escombe and colleagues (17) found 72% protection, uncorrected for multiple hits, almost identical to our uncorrected 74%, using very different fixtures and air mixing fans in a very different hospital setting in Peru. Moreover, the equivalent air changes added in this study are similar to what has been previously reported in several experimental room-scale studies, suggesting that upper room UVGI is a robust technology, not critically dependent on fine details as long as adequate average UVGI fluence rates are produced in the upper room and there is good vertical air mixing.
Implications for Designing Upper Room UVGI Air Mixing Systems
At present, the number, location, and performance characteristics of UV fixtures required to reduce TB transmission (or other airborne infections) in rooms are poorly defined. A single room study by Riley and coworkers (32) over 30 years ago had long been the basis for the widely used guideline of one 30-W (nominal electrical power) UV fixture for 18.6 m2 (200 sq ft). However, neither the total UVGI output (efficiency) of the 17-W unlouvered fixture, nor the air mixing in the unventilated test room (radiator but no fan), were defined. Based on the more recent room studies of Miller and colleagues (13), the National Institute for Occupational Safety and Health (NIOSH) has suggested two alternative dosing strategies: either six lamp watts (UV) per cubic meter irradiated zone, or 30–50 μW/cm2 average UV irradiance in the upper room (1). However, like the Riley guideline, the first NIOSH dosing strategy does not consider fixture efficiency, which can vary greatly, as detailed in the online supplement. Although the second NIOSH guideline is based on fixture germicidal UV output, there is no standard method for measuring average upper room UVGI fluence rates and no practical way for planning an installation to achieve the recommended average.
UVGI room irradiance (fluence rates)
To predict average germicidal UV fluence rates anywhere in rooms, we adapted and validated a commercially available CAD lighting tool called Visual (Acuity Brands) for upper room UVGI design purposes (Visual-UV) (19). With full gonioradiometeric input data for the two fixture models used in this study, Visual-UV software calculated UV fluence rates for the irradiated AIR facility areas. The average fluence rate in the upper irradiated room (above 2.1 m, 24% of total room volume) was 19.5 μW/cm2. Rather than the average UV fluency rates for a single fixture-level horizontal plane, reported in Miller and colleagues' study (13), or for the entire upper irradiated zone, Rudnick and First (33) proposed average fluency rates for the entire room volume as a preferable dosing parameter. Their reasoning was that disinfecting larger room air volumes requires additional UVGI fluence rates, not accounted for by the guidelines based on either single plane or upper irradiated zone average fluence rates. Visual UV calculated an average fluency rate for the three AIR facility patient rooms and corridor as 5.88 μW/cm2, or approximately 6 μW/cm2.
Rudnick and First (33) also suggested that under well-mixed conditions, the UVGI fluence parameter determining germicidal UV efficacy is approximated by the total UV fixture output (W/m3) per room volume. They made two other assumptions: that fluence rates are distributed as evenly as possible in the upper room; and that wall fixtures are positioned for maximum average ray length (including premature impact of rays on ceilings caused by any upward angle above the horizontal) (33). Under well-mixed conditions, all airborne organisms have an equal chance of exposure in the upper room until they are exhausted from the room. UVGI ray length is important because photons, although diverging, remain fully germicidal until they are absorbed by a surface. From this perspective, wall-mounted fixtures generally have longer average ray lengths compared with fixtures designed to be mounted in the center of rooms. To calculate total UV fixture output per room volume used in this study, we directly measured total fixture UV output for both fixture models using the integrating sphere method (courtesy of Professor W. Leuschner, University of Pretoria). In each patient room containing two fixtures, one of each design, the total combined UVGI fixture output (fluence rate) was 0.71 W, and the total fixture output for the entire treated space (volume 171 m3) was 2.9 W. Therefore, the total fixture UVGI output applied was 17 mW/m3 room volume.
By comparison with our 17 mW/m3 total room dose, a recalculation of Miller and colleagues' room study (13) (using aerosolized Mycobacterium parafortuitum) based on estimates of total fixture output of the fixtures used (nominal 216 W) results in a total dose of 12.6 mW/m3. Although differences in methods (including test organism susceptibility) preclude direct comparison, the similarity of outcomes is striking. In the two studies, a total average fixture dose of 13–17 mW/m3 resulted in an estimated 16–24 Eq ACH. This agreement, while requiring confirmation, suggests that the total UV fixture dose per room volume may be more useful than older dosing guidelines. We do not know enough about the germicidal output of the fixtures used in Escombe and colleagues' hospital study (17) (the only other human to guinea pig Mtb transmission UVGI experiment) to make a similar estimate. With adequate mixing, higher doses than those used in the study reported here would likely have produced greater air disinfection, but also a potentially greater risk of occupant UVGI exposure. For that reason we suggest a target range of 15–20 mW/m3. Although 16–24 added Eq ACH is much higher (and less costly) than can be routinely accomplished by other means of air disinfection, further studies are needed to determine the upper practical limits of safe upper room UVGI air disinfection in occupied spaces.
Dosing strategy 1 (practical method, not requiring CAD)
Based on this hospital-based study we suggest providing at least 15–20 mW/m3 total fixture wattage to each room. To calculate the number of fixtures needed, total germicidal UV output for each fixture model must be provided by the manufacturer, based on measurements in a qualified lighting laboratory using the integrating sphere method or full gonioradiometry. Upper room fixtures should be positioned to keep the beam at least 2.1 m above the floor. Fixture should be located to fulfill two further goals: to produce as even an upper room distribution of irradiance as possible, and to achieve maximum estimated average ray length in the upper room. Sample calculations are found in the online supplement. A simple direct measurement method for estimating total UV fixture output for louvered fixtures has been submitted for publication by Dr. Steven Rudnick, Harvard School of Public Health.
Dosing strategy 2 (requiring CAD [Visual-UV])
Visual-UV can calculate the average UV fluency rate for the entire room. The advantage of this dosing method is that average ray length is accounted for by the room specifications required as input by the CAD program. Using dosing strategy 1 (discussed previously) as a first approximation, Visual-UV can be used by trial and error to verify the number, design, and optimal location of fixtures required to produce an average room fluency rate of at least 5–7 μW/cm2.
Beyond the total fixture UV output measurement required for dosing strategy 1, use of dosing strategy 2 requires full gonioradiometric data of each fixture model as input for the Visual-UV program. In our view, fixture manufacturers should provide full output data (both measurements) for the fixture models that they sell. Ideally, a few high-quality lighting laboratories around the world will be used to measure fixture output for manufacturers using standardized methods. Both the laboratory and the specific methods used should be identified with the output measurements for each fixture model.
Closely spaced louvers greatly reduce fixture efficiency, but have been necessary for rooms with low ceilings (below 2.7 m) to prevent occupant overexposure (31). However, the same fixture with the louvers removed delivers up to seven times the UV output. Fixtures with less restrictive louvers can be used in rooms with very high ceilings (over 2.7 m) if eye-level photometry is performed to ensure safety (31). Another approach where ceiling heights permit is to use the same wall fixtures without louvers above an “eggcrate” ceiling, which interferes little with airflow but intercepts stray or reflected UV ray that would otherwise reach the occupied lower room (34).
Air Mixing
Ceiling fan performance is characterized by the airflow rate generated, which is measured by a method prescribed by the U.S. Environmental Protection Agency (35). To normalize this airflow rate, it is divided by room volume, resulting value in h−1, called the fan’s air turnover rate. Although no actual airflow measurements were made for our study, the air turnover rate of the fans was calculated to be 57 h−1 using a computational fluid dynamics method similar to that published by Zhu and coworkers (36).
The Miller and colleagues and Escombe and colleagues studies cited used different approaches to air mixing and also obtained good germicidal efficacy (13, 17, 37). Although room air stagnation needs to be avoided, recent studies have confirmed earlier impressions that, within a wide range easily achieved with conventional low-velocity ceiling fans, neither the direction of airflow nor the exact air turnover rate between the upper and lower room seem to be critical application parameters (38). Patient comfort under cool and warm conditions may also determine fan speed and direction. For rooms with very high ceilings, downward flow direction is more likely to avoid air stagnation in the lower room.
Limitations
Although sampling exhaust air from the ward accurately reflects the average infectiousness of well-mixed ward air, it may not reflect transient higher local concentrations before mixing occurs and the associated risk to those working in close proximity to patients. However, in this study, tracer gas studies and airflow modeling did reveal well-mixed conditions from the ceiling fans, making high local concentrations less likely. Although the engineering parameters described can easily be achieved with commercially available equipment, regular maintenance of upper room UVGI equipment is essential to ensure the predicted equivalent air changes (see online supplement).
Conclusions
This study confirms the high effectiveness of upper room UVGI with air mixing under realistic hospital conditions and defines parameters for its safe and effective application. It shows that commercially available upper room fixtures all generate useful germicidal irradiation, but vary greatly in efficiency. Because most dosing formulae have been based on nominal lamp wattage, or UV wattage, rather than total fixture output, rooms using inefficient fixtures may be greatly underdosed. Total fixture germicidal UV output must be known and incorporated into any dosing formula. For ceilings over 2.6 m, tightly louvered fixtures may not be needed and much more efficient fixtures can be used. Eggcrate UV is a promising, more efficient approach to upper room UVGI (34). With fixtures of any design, we recommend using either of two dosing strategies based on total fixture output: 15–20 mW/m3 total fixture UV wattage delivered per room volume (with fixtures arranged for maximum ray length and upper room coverage), or 5–7 μW/cm2 average UV fluence rate in the entire room calculated by a validated CAD program (Visual is available for UV dose calculations at no charge through Richard.Vincent@mountsinai.org). Either method requires that fixture manufacturers supply users with detailed data on the UV output of their fixtures.
Acknowledgments
Acknowledgment
This article is dedicated to the memory of Drs. Melvin First, Phillip Brickner, and Sidney Parsons, who contributed substantially to the application of germicidal ultraviolet air disinfection to prevent tuberculosis spread. The authors thank Kobus Venter and Willem Lubbe for their technical support; Atlantic Ultraviolet Corporation (Haupauge, NY) and Lumalier, Inc. (Memphis, TN) for ultraviolet fixtures; and the South African patients who generously contributed their time to this study.
Footnotes
Supported by CDC/National Institute for Occupational Safety and Health grant 1RO1 OH009050 and National Institutes of Health/Fogarty International grant 1D43TW009379.
Author Contributions: Conception and design, M.M., A.S.D., P.A.J., K.W., and E.A.N. Study management, M.M., A.S.D., P.A.J., M.v.d.W., K.W., and E.A.N. Provision of key expertise and measurements, P.A.J., S.N.R., T.H.v.R., W.L., T.A.S., S.P.M., A.C.S., K.W., and E.A.N. Analysis and interpretation, A.S.D., S.N.R., T.H.v.R., M.A.P., S.P.M., and E.A.N. Drafting the manuscript for important intellectual content, M.M., A.S.D., and E.A.N. Review and editing of manuscript, M.M., A.S.D., P.A.J., S.N.R., T.H.v.R., S.P.M., and E.A.N.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201501-0060OC on April 30, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Institute for Occupational Safety and Health. Atlanta, GA: National Institute for Occupational Safety and Health; 2009. Environmental control of tuberculosis: basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings. [Google Scholar]
- 2.Nardell E, Dharmadhikari A. Turning off the spigot: reducing drug-resistant tuberculosis transmission in resource-limited settings. Int J Tuberc Lung Dis. 2010;14:1233–1243. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Geneva, Switzerland: World Health Organization; 2009. Natural ventilation for infection control in health care settings. [PubMed] [Google Scholar]
- 4.Nardell E, Riley RL.A new ultraviolet germicidal irradiation (UVGI) fixture design for upper room air disinfection with low ceilingsPresented at the World Congress on Tuberculosis, November 16–19, 1992, Bethesda, MD [Google Scholar]
- 5.Rudnick SN. Predicting the ultraviolet radiation distribution in a room with multilouvered germicidal fixtures. AIHAJ. 2001;62:434–445. doi: 10.1080/15298660108984645. [DOI] [PubMed] [Google Scholar]
- 6.Riley RL, Nardell EA. Clearing the air. The theory and application of ultraviolet air disinfection. Am Rev Respir Dis. 1989;139:1286–1294. doi: 10.1164/ajrccm/139.5.1286. [DOI] [PubMed] [Google Scholar]
- 7.Riley RL, Permutt S. Room air disinfection by ultraviolet irradiation of upper air: air mixing and germicidal effectiveness. Arch Environ Health. 1971;22:208–219. doi: 10.1080/00039896.1971.10665834. [DOI] [PubMed] [Google Scholar]
- 8.Riley RL, Permutt S, Kaufman JE. Convection, air mixing, and ultraviolet air disinfection in rooms. Arch Environ Health. 1971;22:200–207. doi: 10.1080/00039896.1971.10665833. [DOI] [PubMed] [Google Scholar]
- 9.Riley RL, Permutt S, Kaufman JE. Room air disinfection by ultraviolet irradiation of upper air: further analysis of convective air exchange. Arch Environ Health. 1971;23:35–39. doi: 10.1080/00039896.1971.10665951. [DOI] [PubMed] [Google Scholar]
- 10.First M, Nardell E, Chaission W, Riley R. Guidelines for the application of upper-room ultraviolet germicidal irradiation for preventing transmission of airborne contagion. Part II: Design and operations guidance. ASHRAE Transactions. 1999;105:877–887. [Google Scholar]
- 11.First M, Nardell E, Chaission W, Riley R. Guidelines for the application of upper-room ultraviolet germicidal irradiation for preventing transmission of airborne contagion. Part I: Basic principles. ASHRAE Transactions. 1999;105:869–876. [Google Scholar]
- 12.First M, Rudnick SN, Banahan KF, Vincent RL, Brickner PW. Fundamental factors affecting upper-room ultraviolet germicidal irradiation. Part I. Experimental. J Occup Environ Hyg. 2007;4:321–331. doi: 10.1080/15459620701271693. [DOI] [PubMed] [Google Scholar]
- 13.Miller SLHM, Fennelly K, Martyny J, Macher J.Efficacy of ultraviolet irradiation in controlling the spread of tuberculosis 2002. [accessed 2002 Oct 19]. Available from: http://www.cdc.gov/niosh/reports/contract/pdfs/ultrairrTB.pdf
- 14.Ko G, First MW, Burge HA. The characterization of upper-room ultraviolet germicidal irradiation in inactivating airborne microorganisms. Environ Health Perspect. 2002;110:95–101. doi: 10.1289/ehp.0211095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDevitt JJ, Milton DK, Rudnick SN, First MW. Inactivation of poxviruses by upper-room UVC light in a simulated hospital room environment. PLoS One. 2008;3:e3186. doi: 10.1371/journal.pone.0003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nardell EA. Fans, filters, or rays? Pros and cons of the current environmental tuberculosis control technologies. Infect Control Hosp Epidemiol. 1993;14:681–685. doi: 10.1086/646669. [DOI] [PubMed] [Google Scholar]
- 17.Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, Noakes C, Martínez C, Sheen P, Ramirez R, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6:e43. doi: 10.1371/journal.pmed.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardell EA. Air sampling for tuberculosis: homage to the lowly guinea pig. Chest. 1999;116:1143–1145. doi: 10.1378/chest.116.4.1143. [DOI] [PubMed] [Google Scholar]
- 19.Rudnick SN, First MW, Sears T, Vincent RL, Brickner PW, Ngai P, Zhang J, Levin RE, Chin K, Rahn RO, et al. Spatial distribution of fluence rate from upper room ultraviolet germicidal irradiation: experimental validation of a computer-aided design tool. HVAC&R Research. 2012;18:774–794. [Google Scholar]
- 20.Mphahlele M.Highly effective upper room ultraviolet air disinfection on a MDR-TB ward in Sub-Saharan Africa [abstract]. Presented at the International Union Against Tuberculosis and Lung Disease Annual Scientific Meeting. December 3–6, 2009, Cancun, Mexico [Google Scholar]
- 21.Riley RL, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc. 1957;75:420–431. doi: 10.1164/artpd.1957.75.3.420. [DOI] [PubMed] [Google Scholar]
- 22.Riley RL, Mills CC, O’Grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 23.Riley RL. What nobody needs to know about airborne infection. Am J Respir Crit Care Med. 2001;163:7–8. doi: 10.1164/ajrccm.163.1.hh11-00. [DOI] [PubMed] [Google Scholar]
- 24.Riley RLMC, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, Riley MC, Wells WF. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995;142:3–14. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 25.van Geuns HA. Tuberculosis control in the eradication phase in The Netherlands. Bull Int Union Tuberc Lung Dis. 1989;64:31–32. [PubMed] [Google Scholar]
- 26.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011;91:329–338. doi: 10.1016/j.tube.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmadhikari AS, Mphahlele M, Stoltz A, Venter K, Mathebula R, Masotla T, Lubbe W, Pagano M, First M, Jensen PA, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185:1104–1109. doi: 10.1164/rccm.201107-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Reenen T. Pretoria, South Africa: Council for Scientific and Industrial Research; 2013. Assessment of room air mixing due to low velocity ceiling fans in the AIR facility [internal report] [Google Scholar]
- 29.Macher JM. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am Ind Hyg Assoc J. 1989;50:561–568. doi: 10.1080/15298668991375164. [DOI] [PubMed] [Google Scholar]
- 30.Coker I, Nardell E, Fourie B, Brickner P, Parsons S, Bhagwandin N, Onyebujoh P. Pretoria: Medical Research Council; 2000. Guidelines for the utilisation of ultraviolet germicidal irradiation (UVGI) technology in controlling transmission of tuberculosis in health care facilities in South Africa. [Google Scholar]
- 31.Nardell EA, Bucher SJ, Brickner PW, Wang C, Vincent RL, Becan-McBride K, James MA, Michael M, Wright JD. Safety of upper-room ultraviolet germicidal air disinfection for room occupants: results from the Tuberculosis Ultraviolet Shelter Study. Public Health Rep. 2008;123:52–60. doi: 10.1177/003335490812300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley RL, Knight M, Middlebrook G. Ultraviolet susceptibility of BCG and virulent tubercle bacilli. Am Rev Respir Dis. 1976;113:413–418. doi: 10.1164/arrd.1976.113.4.413. [DOI] [PubMed] [Google Scholar]
- 33.Rudnick SN, First MW. Fundamental factors affecting upper-room ultraviolet germicidal irradiation. Part II. Predicting effectiveness. J Occup Environ Hyg. 2007;4:352–362. doi: 10.1080/15459620701298167. [DOI] [PubMed] [Google Scholar]
- 34.Linnes JC, Rudnick SN, Hunt GM, McDevitt JJ, Nardell EA. Eggcrate UV: a whole ceiling upper-room ultraviolet germicidal irradiation system for air disinfection in occupied rooms. Indoor Air. 2014;24:116–124. doi: 10.1111/ina.12063. [DOI] [PubMed] [Google Scholar]
- 35.EPA. Washington, DC: Environmental Protection Agency; 2011. Energy star laboratory guidance manual: building a testing facility and performing the solid state test method for energy star qualification of ceiling fans. Version 1.2. [Google Scholar]
- 36.Zhu S, Srebric J, Rudnick SN, Vincent RL, Nardell EA. Numerical modeling of indoor environment with a ceiling fan and an upper-room ultraviolet germicidal irradiation system. Build Environ. 2014;72:116–124. doi: 10.1016/j.buildenv.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng X, Peccia J, Fabian P, Martyny J, Fennelly K, Hernandez M, Miller S. Efficacy of ultraviolet germicidal irradiation of upper-room air in inactivating airborne bacterial spores and mycobacteria in full-scale studies. Atmos Environ. 2003;37:405–419. [Google Scholar]
- 38.Rudnick SN, McDevitt JJ, Hunta GM, Stawnychya MT, Brickner PW.Influence of ceiling fan’s speed and direction on efficacy of upper-room ultraviolet germicidal irradiation—Part I. Experimental, building and environment 2014Build Environ [online ahead of print] 12 Apr 2014; DOI: 10.1016/j.buildenv.2014.03.025 [DOI] [PMC free article] [PubMed]