To the Editor:
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition (1). The identification of COPD phenotypes may allow stratified treatment approaches that modulate discrete disease mechanisms. Peripheral blood eosinophilia is both a common and repeatable finding in COPD (2). In addition, the presence of a blood/sputum eosinophilia is associated with a significant proportion of COPD exacerbations (3, 4) and a favorable response to systemic steroids (5). However, the role of blood eosinophils in stratifying treatment response to inhaled corticosteroid/long-acting β-agonist combinations is poorly understood.
The FORWARD (Foster 48-Week Trial to Reduce Exacerbations in COPD) study was a randomized, double-blind, parallel group trial that compared 48 weeks of treatment with extrafine beclomethasone dipropionate plus formoterol fumarate (BDP/FF), 100/6 μg pressurized metered-dose inhaler, two inhalations twice a day, versus FF 12 μg pressurized metered-dose inhaler, one inhalation twice a day, in patients with severe COPD with a history of exacerbations (clinical trial registered with www.clinicaltrials.gov [NCT 00929851]). The results of the study have been reported (6) and showed a significant reduction in exacerbation rate (28%) and improvement in lung function with BDP/FF compared with FF treatment.
Here we evaluate the hypothesis that these treatment differences differ according to the baseline blood eosinophil count by performing a post hoc analysis on the FORWARD study data.
Methods
The median (quartile 1; quartile 3) baseline blood eosinophil count was 181.6 (110.4; 279.8), and the distribution of counts is shown in Figure E1 in the online supplement. The patients (n = 1,184) were stratified into quartile groups on the basis of the baseline eosinophil count. The clinical characteristics of the study population across the quartiles of baseline blood eosinophils are reported in Table E1. The following endpoints were analyzed: COPD exacerbation rate over the course of 48 weeks, using a negative binomial model for adjusted exacerbation rates, Kaplan-Meier analysis, and Cox proportional hazard model for time to first exacerbation event; change from baseline in predose morning FEV1 at 48 weeks, using a linear mixed model for repeated measurements; and change from baseline in St. George’s Respiratory Questionnaire total score at 48 weeks, using an analysis of covariance model. Further details of the models are provided in the online supplement. Additional analyses using percentage eosinophil count thresholds and considering absolute counts as a continuous variable were also performed. A predictive model (see online supplement for details) for future COPD exacerbation rate was estimated, accounting for a variety of baseline factors that may influence exacerbations (7). The effect of baseline blood eosinophil count on adverse events, and in particular pneumonia, was also evaluated.
Results
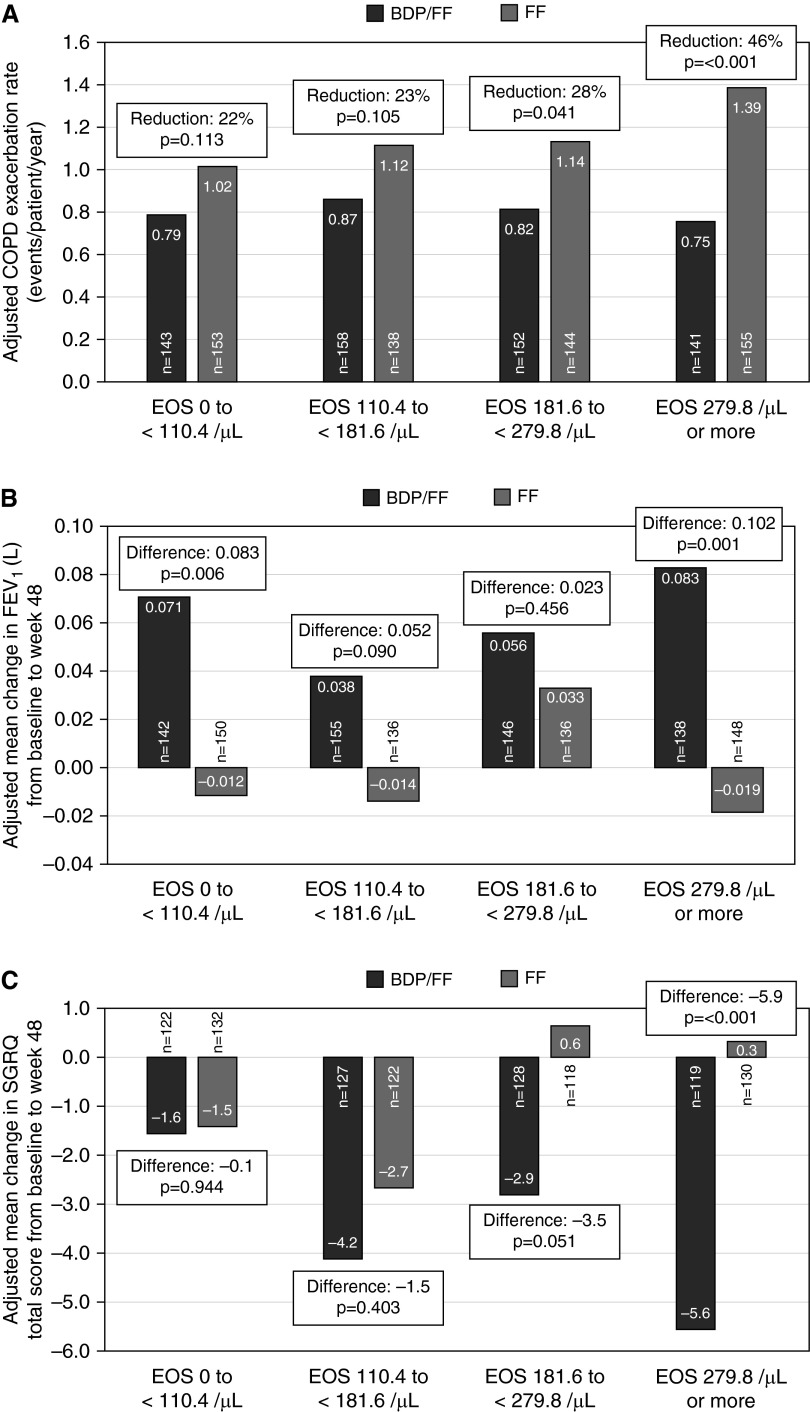
The adjusted exacerbation rate in patients receiving BDP/FF was similar across the quartiles, ranging from 0.75 to 0.87 events/patient/year. However, there was a pattern of increasing exacerbation frequency with increasing eosinophil count in patients treated with FF, with 1.39 events/patient/year within the highest quartile (≥279.8/μl); a 46% reduction in adjusted exacerbation rate caused by BDP/FF was found in this quartile (P < 0.001), with numerically smaller treatment effects in the other quartiles (Figure 1A). These results were supported by a similar trend when evaluating the time to first exacerbation event (Figure E2 and Table E2). Exploratory predictive modeling supported these observations and suggested that in patients treated with BDP/FF, the risk for future exacerbations was not influenced by baseline blood eosinophils, in contrast to in patients treated with FF alone (Figure E3).
Figure 1.
(A) Adjusted chronic obstructive pulmonary disease (COPD) exacerbation rate (events/patient/year) with beclomethasone dipropionate plus formoterol fumarate (BDP/FF) (black) and FF (gray) stratified by baseline blood eosinophil (EOS) quartile. (B) Adjusted mean difference from baseline to 48 weeks with BDP/FF (black) and FF (gray) in predose morning FEV1 (L) at 48 weeks stratified by baseline blood EOS quartile. (C) Adjusted mean difference from baseline to 48 weeks with BDP/FF (black) and FF (gray) in total St. George’s Respiratory Questionnaire (SGRQ) at 48 weeks, stratified by baseline blood EOS quartile.
The treatment difference for the adjusted mean change in predose FEV1 from baseline to 48 weeks within the highest blood eosinophil quartile was 0.102 L in favor of BDP/FF (P = 0.001) (Figure 1B). The treatment differences were lower in the other quartiles and retained in the lowest quartile (0.083 L; P = 0.006).
Patients receiving BDP/FF within the highest blood eosinophil quartile demonstrated an adjusted mean change in St. George’s Respiratory Questionnaire total score from baseline to 48 weeks of −5.6 units compared with +0.3 units in the FF-only group; Δ = 5.9 units in favor of BDP/FF (P < 0.001) (Figure 1C). Smaller differences were seen in the other quartiles.
Analyses of these outcomes according to percentage baseline eosinophils and eosinophils as a continuous variable identified a similar trend (see Tables E3–E5 and online supplement).
No significant differences were observed between BDP/FF and FF alone in adverse events, including pneumonia, across the blood eosinophil quartiles (Table E6).
Discussion
Greater treatment differences in the FORWARD study were observed in patients with eosinophil counts ≥ 279.8/μl compared with lower eosinophil counts. Patients in the highest eosinophil quartile experienced the highest exacerbation rate with FF treatment, and the benefit of additional inhaled corticosteroid therapy was most evident in these patients. For both prebronchodilator FEV1 and St. George’s Respiratory Questionnaire, the lack of inhaled corticosteroid treatment in the highest quartile led to a worsening, and consequently a much larger treatment difference, compared with other quartiles.
The FORWARD study was not powered for the post hoc analyses reported here. Our approach was to look for overall trends in the analyses, and we saw a consistent pattern for the largest treatment effects in patients with the highest blood eosinophils, whether defined by eosinophil percentage or absolute counts.
Peripheral blood eosinophilia in COPD may identify patients with a particularly favorable response to inhaled corticosteroid/long-acting β-agonist therapy, perhaps because of the profile of inflammation that responds well to inhaled corticosteroid treatment. Prospective studies are required to evaluate the role of blood eosinophils as a biomarker of inhaled therapy response in COPD.
Acknowledgments
Acknowledgment
The authors thank Drs. Stefano Petruzzelli and Rino Costanza from Chiesi Farmaceutici for their help in preparing and critically reviewing the study report.
Footnotes
This work was partly funded through research collaborations with Chiesi Farmaceutici S.p.A. Pharmaceuticals. This paper also presents independent research funded by the National Institute for Health Research. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Author Contributions: S.H.S. conceived the study, prepared the study manuscript, and provided a scientific critique of the data; A.G. performed the statistical analyses and revised the study manuscript; J.V., P.J., A.A., P.P., and D.S. revised the study manuscript and provided a scientific critique of the data; and J.A.W. was the primary investigator for the FORWARD Study, revised the study manuscript, and provided a scientific critique of the data.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Agusti A. The path to personalised medicine in COPD. Thorax. 2014;69:857–864. doi: 10.1136/thoraxjnl-2014-205507. [DOI] [PubMed] [Google Scholar]
- 2.Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R ECLIPSE investigators. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44:1697–1700. doi: 10.1183/09031936.00162414. [DOI] [PubMed] [Google Scholar]
- 3.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 4.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 5.Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186:48–55. doi: 10.1164/rccm.201108-1553OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Singh D, Vestbo J, Paggiaro PL, Jones PW, Bonnet-Gonod F, Cohuet G, Corradi M, Vezzoli S, Petruzzelli S, et al. FORWARD Investigators. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108:1153–1162. doi: 10.1016/j.rmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, Ortega H, Chanez P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]