Abstract
Rationale: National trends in tracheostomy for mechanical ventilation (MV) patients are not well characterized.
Objectives: To investigate trends in tracheostomy use, timing, and outcomes in the United States.
Methods: We calculated estimates of tracheostomy use and outcomes from the National Inpatient Sample from 1993 to 2012. We used hierarchical models to determine factors associated with tracheostomy use among MV patients.
Measurements and Main Results: We identified 1,352,432 adults who received tracheostomy from 1993 to 2012 (9.1% of MV patients). Tracheostomy was more common in surgical patients, men, and racial/ethnic minorities. Age-adjusted incidence of tracheostomy increased by 106%, rising disproportionately to MV use. Among MV patients, tracheostomy rose from 6.9% in 1993 to 9.8% in 2008, and then it declined to 8.7% in 2012 (P < 0.0001). Increases in tracheostomy use were driven by surgical patients (9.5% in 1993; 15.0% in 2012; P < 0.0001), with little change among nonsurgical patients (5.8% in 1993; 5.9% in 2012; P < 0.0001). Over time, tracheostomies were performed earlier (median, 11 d in 1998; 10 d in 2012; P < 0.0001), whereas hospital length of stay declined (median, 39 d in 1993; 26 d in 2012; P < 0.0001), discharges to long-term facilities increased (40.1% vs. 71.9%; P < 0.0001), and hospital mortality declined (38.1% vs. 14.7%; P < 0.0001).
Conclusions: Over the past two decades, tracheostomy use rose substantially in the United States until 2008, when use began to decline. The observed dramatic increase in discharge of tracheostomy patients to long-term care facilities may have significant implications for clinical care, healthcare costs, policy, and research. Future studies should include long-term facilities when analyzing outcomes of tracheostomy.
Keywords: tracheostomy, epidemiology, United States, mechanical ventilation
At a Glance Commentary
Scientific Knowledge on the Subject
Evidence about the optimal timing of tracheostomy for patients expected to require prolonged mechanical ventilation has evolved, with studies showing conflicting results about the benefits of early versus later tracheostomy. To our knowledge, no prior studies have examined how practice patterns or outcomes of tracheostomy among patients requiring mechanical ventilation may have changed nationally in association with an evolving evidence base.
What This Study Adds to the Field
Our study shows a doubling in population-based use of tracheostomy over the past two decades in the United States, with a peak in 2008 and declining use subsequently. Over time, tracheostomies have been placed earlier among mechanically ventilated patients, with dramatically increased rates of discharge to long-term care facilities, shorter lengths of stay, and lower in-hospital mortality rates among patients receiving tracheostomy. The changing use and outcomes of tracheostomy have implications for clinical care, healthcare costs, and research.
Nearly 800,000 U.S. residents require invasive mechanical ventilation (MV) annually (1), accounting for at least 25% of intensive care admissions in many hospitals (2). Use of MV has increased over time and is expected to continue to rise with the aging of the population (3, 4). More than 30% of patients who require MV for at least 2 days require prolonged MV (5, 6) often defined as MV for at least 21 days or continued MV following hospital discharge. Patients who cannot readily be weaned from MV, their families, and their physicians often face choices of whether to proceed with tracheostomy to facilitate prolonged MV.
The choice to proceed with tracheostomy has substantial implications for cost, resource use, and outcomes during the acute hospitalization and following hospital discharge (5, 7, 8). Yet little is known about population-based trends in use of tracheostomy for prolonged MV in the United States. Although one study showed a near-doubling in use of tracheostomy among MV patients in North Carolina from 1993 to 2002 (9), it is unknown whether this trend is generalizable to the rest of the country, and if so, whether rapid growth in tracheostomy has continued into contemporary practice.
Determining the optimal timing for tracheostomy for patients anticipated to require prolonged MV is a challenging clinical decision (10). Although small studies published in 1995 and 2004 suggested that tracheostomy within 48 hours of intubation decreased both ventilator-associated pneumonia and 30-day mortality (11, 12), subsequent trials of early tracheostomy (within ∼1 wk of intubation) have not replicated these benefits (13–16). Studies have also suggested other benefits to early tracheostomy including improved patient comfort (17), reduced sedative use (14, 16, 17), early mobility (18), improved physiology (19), and early oral feeding (14, 17). Despite potential benefits, performing tracheostomy earlier may result in more patients receiving a potentially unnecessary procedure with complication rates as high as 39% (13, 16); in randomized trials, roughly twice as many patients in the early tracheostomy arm underwent tracheostomy, because patients in the late tracheostomy arm were able to be successfully liberated from MV before the scheduled tracheostomy date (13, 16).
In light of these controversies, we sought to examine how use, timing, and outcomes of tracheostomy for anticipated prolonged MV have changed over the last two decades in the United States, and to determine factors associated with receipt of tracheostomy. Taking into consideration early studies favoring shorter time to tracheostomy and secular trends that facilitate and incentivize tracheostomy placement (e.g., development of bedside percutaneous tracheostomy techniques, high hospital reimbursement associated with tracheostomy diagnosis-related groups [DRGs] [7], pressures to reduce hospital length of stay [LOS], and increased availability of long-term acute care hospitals [LTACHs]) (10, 20), we hypothesized that MV patients would show a trend toward earlier and increased tracheostomy use during the study period. Some of the results of these studies have been previously reported in the form of an abstract. (21)
Methods
Study Design
We used the U.S. Agency for Healthcare and Research Quality’s Healthcare Cost and Utilization Project’s (HCUP) National Inpatient Sample (NIS) from 1993 to 2012. The NIS is a 20% stratified probability sample of all nonfederal U.S. acute care hospitalization that contains deidentified administrative claims data from 5 to 8 million hospital discharges per year (22).
We identified adult (≥18 yr) patients receiving MV during an acute hospitalization via International Classification of Disease, Ninth Revision, Clinical Modification (ICD9-CM) code 96.7x. We identified tracheostomies with ICD9-CM codes 31.1x, 31.21, and 31.29. Patients with a Major Therapeutic ICD9-CM procedure code (excluding tracheostomy) according to the HCUP classification system (23) were considered “surgical.” All others were defined as “nonsurgical.”
Our primary outcome was yearly rates of tracheostomy use among MV patients. Secondary analyses included hospital LOS, hospital mortality, discharge location (see Table E1 in the online supplement), and factors associated with receipt of tracheostomy. We used previously described methods related to time-stamped procedure codes in the NIS (24) to conduct an exploratory analysis into tracheostomy timing, defined as first MV procedure day subtracted from the tracheostomy procedure day (limited to availability of procedure timing data, 1998–2012 and excluding records with missing timing data). We performed prespecified subgroup analyses stratified by surgical status.
Statistical Analysis
We used U.S. Census Bureau yearly population estimates (25) to determine age-standardized rates per 100,000 U.S. adults. We derived national estimates using survey-weighted methods. We determined average annual percentage change in tracheostomy rates among MV patients using Joinpoint Software version 4.1 (Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD) with tests for significant changes in average annual percentage change using a Monte Carlo permutation method (26, 27). We used logistic and Poisson regression models to assess for changes in patient and hospital characteristics as well as count variables, such as LOS and tracheostomy timing with time as a continuous variable. We used the presence of multisystem organ failure (two or more acute organ failures other than respiratory failure) (see Table E2) (28) as a measure of severity of illness.
Using hierarchical multivariable logistic regression models with hospital-level random intercepts, adjusting for patient demographics (age, sex, race/ethnicity, primary insurance payer, median income of patient zip code), patient comorbidities (HCUP Comorbidity Software, Version 3.7) (29, 30), and hospital characteristics (bed size, location/teaching status, U.S. region, control/ownership status), we identified patient and hospital factors associated with tracheostomy among MV patients in 2012.We excluded hospitals with fewer than 25 MV cases for the regression analysis for 2012 (31).
Our primary goal was to evaluate patterns of tracheostomy use among patients with anticipated prolonged MV for respiratory failure, as opposed to patients who received an emergent or prophylactic tracheostomy for acute airway injury or facial or airway pathology. Thus, we conducted a sensitivity analysis excluding patients with DRGs for tracheostomies related to face, head, or neck conditions (DRG 482 before and including 2007; DRG 011, 012, and 013 after 2007) and an analysis excluding patients with time to tracheostomy less than or equal to 0 days.
Statistical testing was two-tailed and performed with α = 0.05 with SAS v9.3 (Cary, NC) and Joinpoint v4.1 software (Bethesda, MD). This study was deemed exempt from review by the Boston University Medical Campus Institutional Review Board.
Results
Cohort Characteristics
Among 14,937,014 (3,128,913 unweighted) hospitalizations with MV from 1993 to 2012, 1,352,432 patients (9.1%) received tracheostomy. For the study period, 55.4% of tracheostomy patients were male, 55.8% were white, and 48.9% were surgical. The mean age of the group was 61.8 years (SE = 0.03).
Temporal Trends in Tracheostomy Use
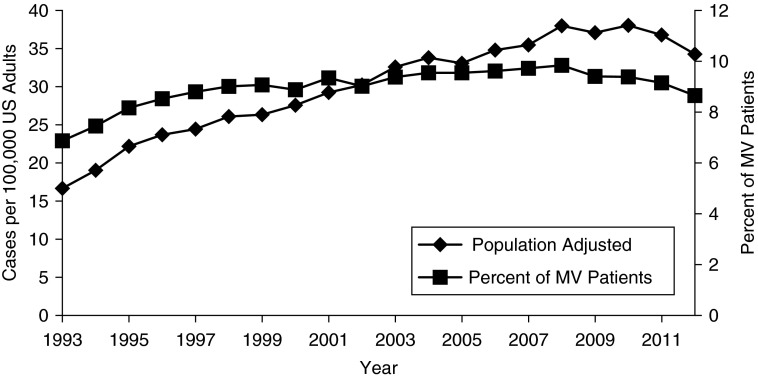
Age-adjusted, population-based rates of tracheostomy increased by 106% over the study period, rising from 16.7 to 34.3 tracheostomies per 100,000 U.S. adults in 1993 versus 2012 (P < 0.0001). This represents 36,401 tracheostomies placed for MV patients in 1993 compared with 82,350 in 2012. The rise in tracheostomy was disproportionate to the age-adjusted growth in MV over the same time period, which rose 63%, from 242.8 to 396.2 episodes of MV per 100,000 U.S. adults. Among MV patients, tracheostomy use increased from 6.9% in 1993 to a peak of 9.8% in 2008, with subsequent decline to 8.6% in 2012 (P < 0.0001) (Table 1, Figure 1). Severity of illness, as evidenced by the presence of multisystem organ failure, steadily increased among all MV patients throughout the study period and did not mirror changes in tracheostomy use (see Figure E1).
Table 1.
Characteristics of Patients Receiving Tracheostomy in Selected Study Years
| 1993 (n = 36,401) | 1999 (n = 60,104) | 2006 (n = 78,077) | 2012 (n = 82,350) | P Value* | |
|---|---|---|---|---|---|
| Tracheostomies per 100,000 U.S. adults | 18.9 | 29.1 | 34.7 | 34.3 | <0.0001 |
| % of mechanically ventilated patients (No. of mechanically ventilated patients) | 6.9 (529,960) | 9.0 (678,456) | 9.6 (811,961) | 8.7 (951,660) | <0.0001 |
| Surgical admission, % | 42.7 | 45.5 | 49.6 | 52.0 | 0.02 |
| Female, % | 46.1 | 46.4 | 44.0 | 43.0 | <0.0001 |
| Age | |||||
| Mean (SE), yr | 63.2 (0.2) | 63.3 (0.2) | 61.4 (0.1) | 60.3 (0.1) | <0.0001 |
| <65 yr, % | 42.3 | 44.9 | 52.4 | 56.3 | <0.0001 |
| 65–84 yr, % | 52.2 | 48.1 | 41.9 | 38.6 | |
| ≥85 yr, % | 5.6 | 7.0 | 5.7 | 5.1 | |
| Race/ethnicity, % | <0.0001 | ||||
| White | 63.9 | 57.4 | 51.0 | 59.9 | |
| Black | 14.0 | 13.9 | 13.5 | 18.7 | |
| Hispanic | 5.8 | 4.7 | 8.3 | 9.1 | |
| Other† | 16.3 | 24.0 | 27.2 | 12.3 | |
| Primary payer, % | <0.0001 | ||||
| Medicare | 57.1 | 54.2 | 52.6 | 50.7 | |
| Medicaid | 11.4 | 12.9 | 15.0 | 17.4 | |
| Private insurance | 23.7 | 25.9 | 23.9 | 22.9 | |
| Self-pay | 4.4 | 3.2 | 4.2 | 4.7 | |
| Other† | 3.4 | 3.8 | 4.4 | 4.3 | |
| Median income for patient zip code, %‡ | Not available | Not available | 0.03 | ||
| Level 1 | 31.1 | 33.5 | |||
| Level 2 | 25.7 | 25.2 | |||
| Level 3 | 23.2 | 22.8 | |||
| Level 4 | 20.1 | 18.5 | |||
| Comorbidities, median (IQR) | 1.3 (2.0) | 1.7 (2.1) | 2.2 (2.4) | 3.6 (2.9) | <0.0001 |
| Hospital location and teaching status, % | <0.0001 | ||||
| Rural | 6.4 | 5.3 | 3.6 | 3.2 | |
| Urban nonteaching | 41.7 | 35.0 | 32.4 | 28.4 | |
| Urban teaching | 52.0 | 59.3 | 64.0 | 68.4 | |
| Hospital size, % | <0.0001 | ||||
| Small | 7.9 | 10.3 | 9.2 | 9.8 | |
| Medium | 27.5 | 28.3 | 24.7 | 25.3 | |
| Large | 64.6 | 62.4 | 67.7 | 64.9 | |
| Hospital region, % | <0.0001 | ||||
| Northeast | 23.1 | 21.6 | 20.7 | 18.7 | |
| Midwest | 15.7 | 20.2 | 21.1 | 21.4 | |
| South | 41.9 | 39.1 | 37.8 | 39.8 | |
| West | 19.3 | 19.2 | 20.4 | 20.1 |
Definition of abbreviation: IQR = interquartile range.
Sample sizes represent survey-weighted estimates.
P value for trend for 1993–2012.
Includes values listed as missing.
For 2006, Level 1 = $1–37,999; Level 2 = $38,000–46,999; Level 3 = $47,000–61,999; Level 4 > $62,000. For 2012, Level 1 = $1–38,999; Level 2 = $39,000–47,999; Level 4 = $48,000–62,999; Level 4 > $63,000.
Figure 1.
Tracheostomy use rates in the United States, 1993–2012. Left y-axis: Age-adjusted U.S. population incidence, cases of tracheostomy per 100,000 U.S. adults. Right y-axis: Tracheostomy use rates as percentage of all patients receiving invasive mechanical ventilation (MV).
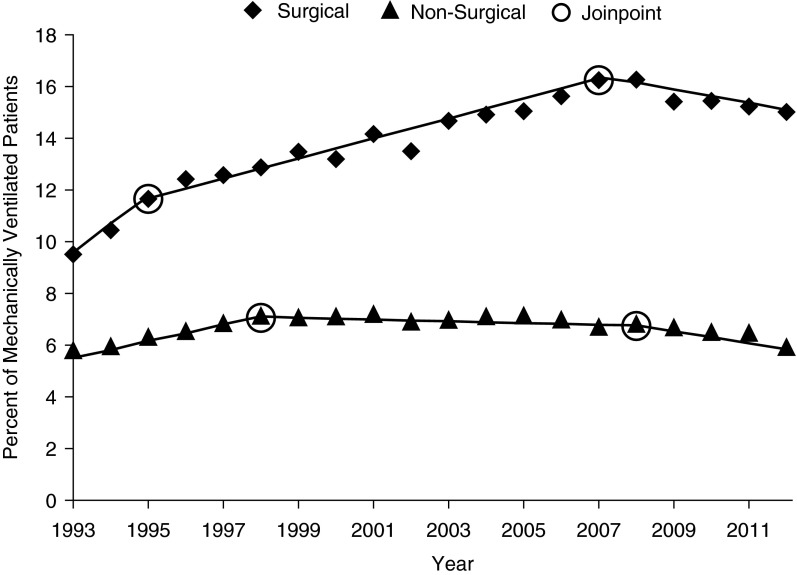
Trends in usage rates for tracheostomy differed significantly based on surgical status (P = 0.0002); surgical patients were more likely to receive tracheostomy compared with nonsurgical patients (unadjusted odds ratio, 2.29; 95% confidence interval, 2.27–2.31). Tracheostomy rates for surgical MV patients increased from 9.5% in 1993 to 15.0% in 2012 (P < 0.0001), outpacing the increase in MV among surgical patients that occurred during the same time period. By contrast, among nonsurgical MV patients, tracheostomy rates were relatively stable (5.7% in 1993 to 5.9% in 2009; P < 0.0001), keeping pace with the rise in MV among nonsurgical patients during this time period. Joinpoint analysis demonstrated two significant inflection points for changing rates of tracheostomy use (1995 and 2007 for surgical patients, P < 0.0001; 1998 and 2009 for nonsurgical patients, P < 0.0001). Tracheostomy among surgical patients rose from 1993 to 2007 before beginning to decline, whereas tracheostomy among nonsurgical patients rose from 1993 to 1998 and then began to decline more rapidly beginning in 2009 (Figure 2; see Table E3).
Figure 2.
Tracheostomy use rates in the United States by surgical status, 1993–2012. Tracheostomy rates among patients receiving invasive mechanical ventilation based on underlying surgical status, with trendlines and relevant Joinpoint findings for change in average annual percentage change.
Patient Characteristics Associated with Tracheostomy
Across all study years, white male patients with Medicare insurance accounted for most tracheostomies (Table 1). However, over time, patients receiving tracheostomy were younger (63.2 in 1993 vs. 60.3 yr in 2012; P < 0.0001), less likely to be female (46.1% in 1993 vs. 43.0% in 2012; P < 0.0001), more likely to belong to a racial/ethnic minority (36.1% vs. 40.1%; P < 0.0001), and more likely to have Medicaid insurance (11.4% vs. 17.4%; P < 0.0001). These trends outpaced smaller changes in the overall MV population (e.g., racial/ethnic minorities, 35.9% in 1993 vs. 35.6% in 2012; Medicaid, 9.3% vs. 13.4%). Surgical patients accounted for 42.7% of tracheostomies in 1993, and increased to 52.0% in 2012 (P < 0.0001). Patients undergoing tracheostomy showed an increasing number of comorbidities over time (median, 1.3 in 1993 vs. 3.6 in 2012; P < 0.0001).
From 1993 to 2012, the characteristics of hospitals where patients received tracheostomies evolved (Table 1), with tracheostomies more likely to be performed in urban, teaching hospitals in later years. Additionally, we identified wide regional variation in distribution of tracheostomy. Across all study years, most tracheostomies were performed in the South, and over time tracheostomy increased in the Midwest and decreased in the Northeast (P < 0.0001).
Factors Associated with Receipt of Tracheostomy in 2012
We examined factors associated with receipt of tracheostomy among all patients who received MV in 2012 (Table 2). Among MV patients, older and female patients were less likely to receive a tracheostomy. In addition, racial/ethnic minorities had higher odds of receiving a tracheostomy compared with white patients. Patients with Medicaid and private insurance also had higher odds of tracheostomy compared with Medicare patients, whereas self-pay patients were less likely to receive a tracheostomy. Surgical admission was strongly associated with tracheostomy, whereas increasing number of comorbidities was inversely associated with tracheostomy (see Table E4 for individual comorbidities). Patients admitted to private/for-profit, larger, urban, teaching hospitals were more likely to receive tracheostomy.
Table 2.
Factors Associated with Receiving Tracheostomy in 2012 among Mechanically Ventilated Patients
| Odds Ratio | 95% CI | |
|---|---|---|
| Age | ||
| <65 yr | Ref | — |
| 65–84 yr | 0.94 | 0.89–0.98 |
| >85 yr | 0.59 | 0.54–0.64 |
| Female | 0.93 | 0.90–0.96 |
| Race/ethnicity | ||
| White | Ref | — |
| Black | 1.17 | 1.11–1.24 |
| Hispanic | 1.11 | 1.04–1.19 |
| Other | 1.10 | 1.03–1.17 |
| Primary payer | ||
| Medicare | Ref | — |
| Medicaid | 1.30 | 1.16–1.35 |
| Private insurance | 1.26 | 1.16–1.34 |
| Self-pay | 0.79 | 0.72–0.86 |
| Other | 1.10 | 1.00–1.21 |
| Median income for patient zip code | ||
| $1–38,999 | Ref | — |
| $39,000–47,999 | 0.99 | 0.95–1.04 |
| $48,000–62,999 | 0.99 | 0.94–1.04 |
| >$63,000 | 0.99 | 0.94–1.05 |
| Surgical admission | 2.60 | 2.51–2.70 |
| Number of comorbidities | 0.94 | 0.90–0.99 |
| Hospital size | ||
| Small | Ref | — |
| Medium | 1.18 | 1.07–1.30 |
| Large | 1.35 | 1.23–1.47 |
| Hospital location/teaching status | ||
| Rural | Ref | — |
| Urban nonteaching | 1.50 | 1.32–1.71 |
| Urban teaching | 1.97 | 1.73–2.24 |
| Hospital region | ||
| Northeast | Ref | — |
| Midwest | 0.78 | 0.72–0.84 |
| South | 0.88 | 0.81–0.95 |
| West | 0.81 | 0.75–0.88 |
| Hospital ownership | ||
| Private, nonprofit | Ref | — |
| Government, nonfederal | 1.17 | 1.08–1.27 |
| Private, invest-own/profit | 1.15 | 1.06–1.24 |
Definition of abbreviations: CI = confidence interval; Ref = reference.
Tracheostomy Timing
Tracheostomy timing was available for 74.5% of our cohort from 1998 to 2012. Median time to tracheostomy decreased over the study period from a median of 11 days in 1998 to 10 days in 2012 (P < 0.0001) with similar decreases for surgical and nonsurgical patients (Table 3). Over the study period, both surgical and nonsurgical patients experienced fewer late tracheostomies (>14 d), with shifts to the first and second week of MV (P < 0.0001).
Table 3.
Timing of Tracheostomy among Mechanically Ventilated Patients
| 1998 | 2006 | 2012 | P Value* | |
|---|---|---|---|---|
| Nonsurgical | ||||
| n | 32,657 | 39,335 | 39,560 | |
| Days to tracheostomy,† median (IQR) | 11 (10) | 10 (9) | 10 (8) | <0.0001 |
| Time to tracheostomy, % | <0.0001 | |||
| 0–7 d | 32.5 | 32.7 | 33.7 | |
| 8–14 d | 36.3 | 38.3 | 41.5 | |
| >14 d | 31.3 | 29.1 | 24.7 | |
| Surgical | ||||
| n | 27,446 | 38,741 | 42,790 | |
| Days to tracheostomy,† median (IQR) | 11 (11) | 11 (11) | 10 (9) | <0.0001 |
| Time to tracheostomy, % | <0.0001 | |||
| 0–7 d | 35.0 | 34.6 | 37.8 | |
| 8–14 d | 34.7 | 34.1 | 37.7 | |
| >14 d | 30.4 | 31.3 | 24.6 |
Definition of abbreviation: IQR = interquartile range.
Tracheostomy timing was available for 74.5% of our cohort from 1998 to 2012.
P value for trend for 1998–2012.
Days to tracheostomy were calculated as the difference between the procedure day for tracheostomy and the first procedure day for mechanical ventilation. Data only available after 1998.
Patient Outcomes
Hospital mortality for patients receiving tracheostomies decreased from 38.1% in 1993 to 14.7% in 2012 (P < 0.0001) with similar changes for surgical and nonsurgical patients (Table 4). Hospital LOS among tracheostomy survivors decreased from a median of 39 days (interquartile range, 37) in 1993 to 26 days (interquartile range, 21) in 2012 (P < 0.0001). Declining hospital mortality and shorter LOS were accompanied by changing sites of discharge for tracheostomy patients, with a significant increase in discharges to long-term facilities (LTACH and skilled nursing facilities, 40.1% in 1993, 71.9% in 2012) and decreasing discharges home (21.4% in 1993, 13.1% in 2012; P < 0.0001).
Table 4.
Outcomes of Patients Receiving Tracheostomy
| 1993 (n = 36,401) | 1999 (n = 60,104) | 2006 (n = 78,077) | 2012 (n = 82,350) | P Value* | |
|---|---|---|---|---|---|
| LOS among survivors, d, median (IQR) | 39 (37) | 31 (26) | 29 (24) | 26 (21) | <0.0001 |
| Discharge status, % | <0.0001 | ||||
| Died | 38.1 | 27.7 | 21.2 | 14.7 | |
| Home | 21.4 | 14.8 | 14.8 | 13.1 | |
| LTACH/SNF | 40.1 | 57.2 | 63.7 | 71.9 |
Definition of abbreviations: IQR = interquartile range; LOS = length of stay; LTACH = long-term acute care hospital; SNF = skilled nursing facility.
P value for trend for 1993–2012.
Sensitivity Analysis
When we excluded patients with a DRG code for tracheostomy related to a face, head, or neck condition (2.1%) or patients recorded as having a tracheostomy time of less than or equal to 0 days (10.7%), we observed similar trends in tracheostomy use over time (see Figure E2). When we excluded DRGs for tracheostomy related to face, head, or neck condition, 6.7% of MV patients received tracheostomy in 1993, increasing to a peak of 9.7% in 2008, and decreasing to 8.5% in 2012 (P < 0.0001). Under both exclusion criteria, multivariable modeling for factors associated with tracheostomy trends remained similar to the primary analysis (see Table E5).
Discussion
We report trends in tracheostomy practices and outcomes among MV patients in the United States across two decades from 1993 to 2012. Tracheostomy use increased rapidly through 2008, at which point we began to observe yearly declines in tracheostomy use. Among MV patients, increases in tracheostomy were primarily driven by surgical patients, with little change in the proportion of nonsurgical MV patients who received tracheostomy across the study period. We observed shifts toward earlier tracheostomy, shorter hospital LOS, more frequent discharges to long-term care facilities, and fewer discharges from hospital to home. We also observed shifting demographics of tracheostomy patients out of proportion to MV patients, with tracheostomy in later study years performed more often among younger men, surgical hospitalizations, racial/ethnic minorities, and Medicaid beneficiaries than in earlier years. Our findings were robust to several sensitivity analyses aimed at increasing the likelihood that we were identifying patients who received tracheostomies for anticipated prolonged MV.
Few studies have investigated the epidemiology of tracheostomy use in the United States. Our national findings of increases in tracheostomy rates during the 1990s, accompanied by decreasing time to tracheostomy, shorter hospital LOS, and increase in discharge to long-term facilities, are similar to those that Cox and coworkers (9) identified when examining trends in tracheostomy use in North Carolina from 1993 to 2002. However, with the benefit of 10 additional years of data, we found that tracheostomy use peaked in 2008 nationally and has begun to decline thereafter. Our findings of a decrease in median time to tracheostomy are similar to those observed internationally (32), but have not previously been described at the national level in the United States.
Several factors likely contributed to the rise in tracheostomy use over the past 20 years. Greater recognition of complications from prolonged endotracheal intubation may have influenced the decision to opt for tracheostomy over continued endotracheal intubation. The dissemination of percutaneous dilational tracheostomy (33), which can often be performed by intensivists at the bedside, may have lowered the threshold to perform tracheostomy. Furthermore findings in some studies that tracheostomy may improve aspects of care of MV patients, such as sedation requirements, mobility, and oral feeding, may have contributed to a push toward tracheostomy to avoid complications of prolonged MV (12, 14, 15, 17, 18). Although increasing severity of illness of MV patients could account for some degree of the increase in tracheostomy use, we found that tracheostomy use began to decrease after 2008 even as severity of illness continued to rise, suggesting that changes in tracheostomy use were not driven exclusively by changes in severity of illness. It is unclear whether changes in hospital reimbursement for tracheostomy may have contributed to changes in use.
The disproportionate rise in tracheostomy rates among surgical patients may in part result from a growing number of studies focusing on surgical patients that demonstrate improvements in outcomes for early tracheostomies (e.g., days on MV and hospital LOS) (14, 34, 35). Because surgeons are often the ones who perform tracheostomies, some of the disproportionate rise observed in surgical patients may be explained by the fact that surgeons are able to perform their own procedures. Additionally, surgical patients may have higher tracheostomy use because patients with underlying conditions that may require prolonged MV, such as intracranial bleeding and trauma, are often managed surgically.
Although many factors may have contributed to rising use of tracheostomy from 1993 to 2008, it is less clear why rates of tracheostomy subsequently have begun to decline. We speculate that more recent randomized trials (13, 16) and metaanalyses that have showed no difference in mortality or ventilator-associated pneumonia with early tracheostomy (36–38) may have decreased enthusiasm for early tracheostomy, resulting in fewer tracheostomy procedures. Alternatively, it is possible that with increasing use of advance directives (39) and better data on long-term outcomes of chronic critical illness (40, 41), fewer families are opting to pursue prolonged MV and are instead choosing to pursue less invasive, more comfort-based care. Finally, it is possible that emerging evidence and guidelines encouraging reduced use of sedatives and improved processes for ventilator weaning (42) have resulted in fewer cases of prolonged MV and less need for tracheostomy in later study years.
We identified shifting demographics with an increasing percentage of racial/ethnic minorities receiving tracheostomies over time relative to the overall MV population, as well as higher adjusted odds of tracheostomy for racial/ethnic minorities in 2012. Greater use of tracheostomies among racial/ethnic minorities is consistent with studies demonstrating that minorities are less likely to use hospice services and more likely to use aggressive care, such as intensive care unit procedures, at the end of life compared with white patients (43–45).
In our exploratory analysis of tracheostomy timing, we demonstrate a shift toward earlier tracheostomy over the study period. Many of the factors driving the increase in tracheostomy use have likely also contributed to shift toward earlier tracheostomy. Studies from the 1990s and 2000s that suggested multiple clinical benefits of early tracheostomy (11, 12, 14, 15, 17, 18) may have influenced the observed trends in tracheostomy timing. Moreover, secular trends and financial incentives favor earlier tracheostomy. Pressures to reduce hospital LOS have risen over time, and concurrently, the availability of LTACHs to care for patients requiring prolonged MV has expanded dramatically (20). Tracheostomy DRGs receive high reimbursement (7, 46), and earlier tracheostomy allows hospitals to maximize efficiency by reducing LOS and turning over intensive care beds for new admissions sooner. Indeed, we observed both reduced LOS and dramatic increases in proportions of patients discharged to LTACHs over the study period.
Our study highlights the shifting burden of care for patients with a tracheostomy toward Medicaid and long-term care facilities. This increase in percentage of Medicaid patients receiving tracheostomy over time suggests a shift from federal to state payment. Because Medicaid also tends to have lower reimbursement rates for long-term care facilities compared with Medicare and private insurance, Medicaid patients are typically less likely to transfer to LTACHs (47). Although current payment structures are financially beneficial to LTACHs (40), it is unclear if the change in payer-mix could affect the financial viability of long-term care facilities. Perhaps more concerning is the finding of lower use of tracheostomy among self-pay patients, a group that likely represents the uninsured. Confronted with the potential to bear a larger share of the cost of care for prolonged MV and long-term care, many families may forego the decision to proceed with tracheostomy.
We identified striking changes in discharge status over time, with fewer tracheostomy patients dying in the hospital, fewer patients discharged to home, and most (>70%) patients discharged to long-term facilities by the end of the study period. Although hospital mortality may be reduced, we cannot conclude this represents a true improvement in outcomes. Indeed, other studies have shown 1- and 2-year mortality is similar whether patients receive care for prolonged MV in the hospital or LTACH (8, 40, 48), suggesting that the location of death may merely have shifted to LTACHs in more recent years. Moreover, even patients who survive the first year after tracheostomy have remarkably poor outcomes, with multiple readmissions to acute care hospitals and very few patients (<10%) ultimately returning to their homes with full functional capacity (8). To capture more patient-centered outcomes, future studies should use time-defined (e.g., 60-d or 1-yr) mortality rates and plan to track tracheostomy patients as they transfer to long-term care facilities, because much of their morbidity and mortality occurs outside the acute care setting.
Our study had several limitations. We used administrative data, which relies on ICD9-CM and DRG coding and lacks markers of disease severity, such as physiologic parameters, ventilator settings, advance directives, and other factors that may affect tracheostomy use. Moreover, over the course of the 20-year study period, there may have been changes in coding practices. However, we attempted to identify and account for all relevant changes to ICD9-CM and DRG codes in use during the study period. In addition, because MV and tracheostomy were associated with high levels of reimbursement throughout the study period, it is unlikely that these procedures would be inaccurately coded (49); indeed, sensitivity and specificity of these codes have been documented to be high dating back to the 1990s (50). Nonetheless, we believe the ability to generate nationally representative estimates of tracheostomy use among all payers is a strength of our study. Our exploratory analysis of time to tracheostomy was limited by missing data and should be confirmed in subsequent studies with more complete data on procedure timing.
In summary, we found substantial changes to tracheostomy practices and outcomes over the past two decades in the United States. We identified a doubling in the incidence of tracheostomy, a rate far outpacing the rise in use of MV. Interestingly, the rising rates of tracheostomy use over the 1990s and early 2000s may now be slowing, with a decline in use identified beginning in 2008. Additionally, we found significant decreases in time to tracheostomy and hospital LOS with large increases in discharge to long-term care facilities, perhaps explained in part by the overall push for faster hospital discharge and promising initial findings in the literature regarding the benefits of early tracheostomy. The near doubling in discharge to long-term facilities represents a major shift in the site of care for tracheostomy patients over time and has significant implications for clinical care, healthcare costs, and research related to prolonged MV in the United States. Future studies should consider morbidity and mortality in long-term facilities when investigating outcomes related to tracheostomy.
Footnotes
Supported in part by National Institutes of Health (NIH) grant T32 86308 (A.B.M.), Agency for Healthcare and Research Quality grant K08HS020672 (C.R.C.), NIH NHLBI grant K01HL116768 (A.J.W.), and NIH grant K07 CA138772 (R.S.W.). This study was also supported by resources from the VA Boston Healthcare System (S.N.S.) and the Edith Nourse Rogers Memorial VA Hospital (R.S.W.).
Author Contributions: A.B.M., study and database design, statistical analysis, and principal manuscript preparation. S.N.S., study and database design and manuscript preparation. L.B. and C.R.C., manuscript preparation. A.J.W., study design, statistical analysis, manuscript preparation, and study supervision. R.S.W., study and database design, statistical analysis, manuscript preparation, and study supervision.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201502-0239OC on May 8, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Lam S, Ridley S. Critically ill medical patients, their demographics and outcome. Anaesthesia. 1999;54:845–852. doi: 10.1046/j.1365-2044.1999.00966.x. [DOI] [PubMed] [Google Scholar]
- 3.Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, Lindenauer PK. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8:76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: preparing for the aging baby boomers. Crit Care Med. 2005;33:574–579. doi: 10.1097/01.ccm.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 5.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18:461–476. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 6.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35:1918–1927. doi: 10.1097/01.CCM.0000275391.35834.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004;125:220–227. doi: 10.1378/chest.125.1.220. [DOI] [PubMed] [Google Scholar]
- 8.Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, Clay AS, Chia J, Gray A, Tulsky JA, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit Care Med. 2004;32:2219–2226. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 10.Angus DC. When should a mechanically ventilated patient undergo tracheostomy? JAMA. 2013;309:2163–2164. doi: 10.1001/jama.2013.6014. [DOI] [PubMed] [Google Scholar]
- 11.Blot F, Guiguet M, Antoun S, Leclercq B, Nitenberg G, Escudier B. Early tracheotomy in neutropenic, mechanically ventilated patients: rationale and results of a pilot study. Support Care Cancer. 1995;3:291–296. doi: 10.1007/BF00335304. [DOI] [PubMed] [Google Scholar]
- 12.Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32:1689–1694. doi: 10.1097/01.ccm.0000134835.05161.b6. [DOI] [PubMed] [Google Scholar]
- 13.Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, Miletto A, Mangione S, Sinardi AU, Pastorelli M, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303:1483–1489. doi: 10.1001/jama.2010.447. [DOI] [PubMed] [Google Scholar]
- 14.Trouillet JL, Luyt CE, Guiguet M, Ouattara A, Vaissier E, Makri R, Nieszkowska A, Leprince P, Pavie A, Chastre J, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med. 2011;154:373–383. doi: 10.7326/0003-4819-154-6-201103150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Sui F, Chen XK, Zhang GC, Wang XW, Zhao S, Song Y, Liu W, Xin X, Li WX. Early versus late percutaneous dilational tracheostomy in critically ill patients anticipated requiring prolonged mechanical ventilation. Chin Med J (Engl) 2012;125:1925–1930. [PubMed] [Google Scholar]
- 16.Young D, Harrison DA, Cuthbertson BH, Rowan K TracMan Collaborators. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309:2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 17.Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, Chastre J. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33:2527–2533. doi: 10.1097/01.ccm.0000186898.58709.aa. [DOI] [PubMed] [Google Scholar]
- 18.Clum SR, Rumbak MJ. Mobilizing the patient in the intensive care unit: the role of early tracheotomy. Crit Care Clin. 2007;23:71–79. doi: 10.1016/j.ccc.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Diehl JL, El Atrous S, Touchard D, Lemaire F, Brochard L. Changes in the work of breathing induced by tracheotomy in ventilator-dependent patients. Am J Respir Crit Care Med. 1999;159:383–388. doi: 10.1164/ajrccm.159.2.9707046. [DOI] [PubMed] [Google Scholar]
- 20.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syeda SN, Wiener RS. National trends in the utilization of tracheostomy among United States adults, 1997–2008 [abstract] Am J Respir Crit Care Med. 2011;183:A2594. [Google Scholar]
- 22.Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Overview of the National (Nationwide) Inpatient Sample (NIS) [accessed 2014 Dec 18]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp
- 23.Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Procedure Classes 2015 [accessed 2014 Dec 18]. Available from: http://www.hcup-us.ahrq.gov/toolssoftware/procedure/procedure.jsp
- 24.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000-2009: a population-based study. Ann Am Thorac Soc. 2013;10:10–17. doi: 10.1513/AnnalsATS.201206-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Census BureauPopulation estimates [accessed 2014 Dec 18]. Available from: http://www.census.gov/popest/data/historical/index.html
- 26.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for Joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer InstituteJoinpoint Regression Program, Version 4.1.1.1. Available from: http://surveillance.cancer.gov/joinpoint/
- 28.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 31.Center for Medicare & Medicaid ServicesHospital Compare. 30-day unplanned readmission and death measures [accessed 2014 Dec 30]. Available from: http://www.medicare.gov/hospitalcompare/Data/30-day-measures.html
- 32.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F, Cide D, Goldwaser R, Soto L, Bugedo G, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med. 2000;161:1450–1458. doi: 10.1164/ajrccm.161.5.9902018. [DOI] [PubMed] [Google Scholar]
- 33.Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412–1418. doi: 10.1378/chest.118.5.1412. [DOI] [PubMed] [Google Scholar]
- 34.Alali AS, Scales DC, Fowler RA, Mainprize TG, Ray JG, Kiss A, de Mestral C, Nathens AB.Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study J Trauma Acute Care Surg 20147670–76.discussion 76–78 [DOI] [PubMed] [Google Scholar]
- 35.Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Crit Care. 2004;8:R347–R352. doi: 10.1186/cc2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes Silva BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2012;3:CD007271. doi: 10.1002/14651858.CD007271.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS One. 2014;9:e92981. doi: 10.1371/journal.pone.0092981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CC, Livingstone D, Dixon E, Dort JC. Early versus late tracheostomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;152:219–227. doi: 10.1177/0194599814561606. [DOI] [PubMed] [Google Scholar]
- 39.Silveira MJ, Wiitala W, Piette J. Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62:706–710. doi: 10.1111/jgs.12736. [DOI] [PubMed] [Google Scholar]
- 40.Kahn JM, Werner RM, David G, Ten Have TR, Benson NM, Asch DA. Effectiveness of long-term acute care hospitalization in elderly patients with chronic critical illness. Med Care. 2013;51:4–10. doi: 10.1097/MLR.0b013e31826528a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 43.Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169:493–501. doi: 10.1001/archinternmed.2008.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end-of-life treatment. J Gen Intern Med. 2009;24:695–701. doi: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139:1025–1033. doi: 10.1378/chest.10-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewar DM, Kurek CJ, Lambrinos J, Cohen IL, Zhong Y. Patterns in costs and outcomes for patients with prolonged mechanical ventilation undergoing tracheostomy: an analysis of discharges under diagnosis-related group 483 in New York State from 1992 to 1996. Crit Care Med. 1999;27:2640–2647. doi: 10.1097/00003246-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Lane-Fall MB, Iwashyna TJ, Cooke CR, Benson NM, Kahn JM. Insurance and racial differences in long-term acute care utilization after critical illness. Crit Care Med. 2012;40:1143–1149. doi: 10.1097/CCM.0b013e318237706b. [DOI] [PubMed] [Google Scholar]
- 48.Hall WB, Willis LE, Medvedev S, Carson SS. The implications of long-term acute care hospital transfer practices for measures of in-hospital mortality and length of stay. Am J Respir Crit Care Med. 2012;185:53–57. doi: 10.1164/rccm.201106-1084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.RAND CorporationEvaluations of severity-adjusted DRG systems: report WR-434-CMS [accessed 2014 Aug 21]. Available from: www.rand.org/pubs/working_papers/WR434/
- 50.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]