To the Editor:
Preterm birth (PTB) (1) and childhood asthma (2) are major health problems that disproportionally affect urban, poor minorities in the United States. Growing evidence suggests that children born preterm are at increased risk for asthma, but findings are inconsistent across studies (3–5). Of the 30 major studies on the association published worldwide, nearly a third reported null effects, whereas the others reported significant associations, with odds ratios ranging from 1.2 to 4.9 (4). We suspected that inconsistencies in the PTB–asthma association in part reflected differences among studies in three key domains: definitions of asthma (6), degree of prematurity, and the age when asthma was assessed.
We therefore investigated whether the association between PTB and asthma varied by definition of asthma used (e.g., recurrent wheezing vs. asthma, physician diagnosis vs. prescribed medication), degree of prematurity, and age at assessment (0–5 yr vs. 6–9 yr) in the Boston Birth Cohort (BBC). Our study is the first to simultaneously address these three sources of ambiguity in a large, prospective U.S. birth cohort. As an urban, poor, predominantly minority cohort with a high burden of PTB and asthma, the BBC is well-suited to this investigation.
Methods
Our analysis included 2,540 children (age 5.0 ± 2.8 yr) recruited at Boston Medical Center after excluding 161 children because of postterm birth or incomplete data. BBC data collection protocols are described elsewhere (7, 8) and were approved by the appropriate institutional review boards.
Asthma measures were created using electronic medical record data on physician diagnoses and prescriptions documented between October 2003 and September 2013. Eleven measures were created for children assessed between ages 0 and 5 years, and eight measures for children assessed between ages 6 and 9 years (see Table E1 in the online supplement).
Gestational age was measured by an algorithm combining the first day of the last menstrual period and data from the <20 weeks’ ultrasound (9). Prematurity was defined in conventional categories (PTB [<37 wk, 28.3% of sample] vs. term birth [TB, 37–41 wk, 71.7%]) and refined categories (early preterm [22–31 wk, 8.1%], late preterm [32–36 wk, 20.2%], early term [37–38 wk, 25.7%], and full term [39–41 wk, 44.1%]) (Table E2).
We used multivariate logistic regression to examine the associations between categories of PTB and the asthma measures at ages 0–5 (n = 2,540) and 6–9 (n = 1,072) years. All analyses were performed using Stata 12.0 (College Station, TX).
Results
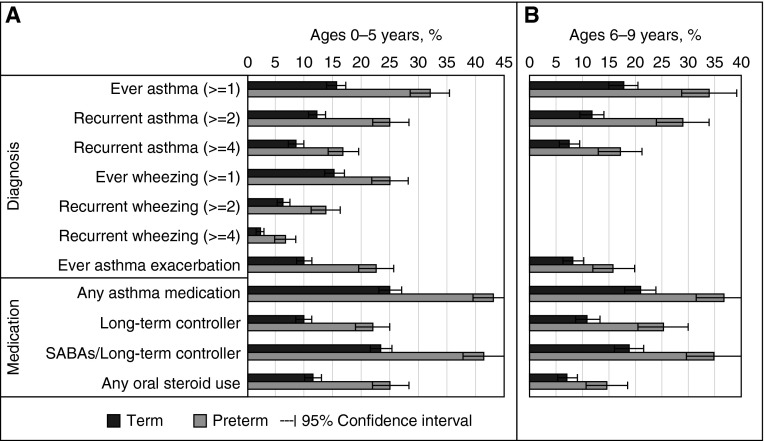
The prevalence of asthma varied substantially across the asthma measures in both age ranges (7–43% in PTB; 2–25% in TB). However, children born preterm had a significantly higher prevalence (P < 0.001) of asthma than children born term on all asthma measures in both age ranges (Figure 1). Models adjusting for covariates showed the same results, with adjusted odds ratios (AORs) ranging from 1.8 (95% confidence interval [CI], 1.5–2.3) to 2.9 (95% CI, 2.1–4.1) (P < 0.001) in both age ranges (Table 1).
Figure 1.
Percentage of childhood asthma among preterm births versus term births, stratified by age group. (A) Childhood asthma measures assessed between ages 0 and 5 years. (B) Childhood asthma measures assessed between ages 6 and 9 years. The preterm group had a higher percentage of each asthma measure than the term group (P < 0.001). For asthma measures: ever asthma (≥1) = had at least one asthma diagnosis from the electronic medical record during the specified ages; recurrent asthma (≥2) = had two or more asthma diagnoses during the specified ages; recurrent asthma (≥4) = had four or more asthma diagnoses during follow-up time; ever wheezing (≥1) = had at least one wheezing diagnosis during the specified ages; recurrent wheezing (≥2) = had two or more wheezing diagnoses during the specified ages; recurrent wheezing (≥4) = had four or more wheezing diagnoses during the specified ages; ever asthma exacerbation = had an asthma exacerbation diagnosis during the specified ages. Please see Table E1 for more detailed description of asthma measures. SABAs = short-acting β-agonists.
Table 1.
Adjusted Odds Ratios of Asthma-related Outcomes across Preterm Birth Categories*
| Preterm Birth (<37 wk); Ref = Term (37–41 wk) |
Degree of Prematurity; Ref = Full Term (39–41 wk) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Preterm (22–31 wk) |
Late Preterm (32–36 wk) |
Early Term (37–38 wk) |
||||||||||
| AOR | 95% CI | P Value | AOR | 95% CI | P Value | AOR | 95% CI | P Value | AOR | 95% CI | P Value | |
| Outcome at ages 0–5 yr (N = 2,540) | ||||||||||||
| Diagnosis | ||||||||||||
| Ever asthma (≥1) | 2.45 | (1.98–3.04) | <0.001 | 5.90 | (4.19–8.31) | <0.001 | 1.70 | (1.30–2.23) | <0.001 | 1.05 | (0.80–1.38) | 0.718 |
| Recurrent asthma (≥2) | 2.28 | (1.81–2.88) | <0.001 | 4.13 | (2.88–5.93) | <0.001 | 1.70 | (1.27–2.28) | <0.001 | 0.95 | (0.70–1.29) | 0.763 |
| Recurrent asthma (≥4) | 2.00 | (1.52–2.61) | <0.001 | 4.09 | (2.72–6.13) | <0.001 | 1.54 | (1.09–2.19) | 0.015 | 1.17 | (0.83–1.66) | 0.364 |
| Ever wheezing (≥1) | 1.85 | (1.48–2.31) | <0.001 | 3.15 | (2.21–4.49) | <0.001 | 1.67 | (1.26–2.20) | <0.001 | 1.27 | (0.97–1.66) | 0.084 |
| Recurrent wheezing (≥2) | 2.29 | (1.71–3.09) | <0.001 | 4.16 | (2.66–6.51) | <0.001 | 1.92 | (1.31–2.82) | <0.001 | 1.21 | (0.82–1.79) | 0.339 |
| Recurrent wheezing (≥4) | 2.85 | (1.83–4.43) | <0.001 | 6.17 | (3.30–11.55) | <0.001 | 2.12 | (1.17–3.82) | 0.013 | 1.20 | (0.64–2.26) | 0.567 |
| Ever asthma exacerbation | 2.48 | (1.94–3.17) | <0.001 | 4.49 | (3.08–6.55) | <0.001 | 2.05 | (1.50–2.79) | <0.001 | 1.14 | (0.82–1.57) | 0.435 |
| Medication | ||||||||||||
| Any asthma medication | 2.22 | (1.83–2.68) | <0.001 | 5.74 | (4.14–7.96) | <0.001 | 1.68 | (1.33–2.12) | <0.001 | 1.22 | (0.97–1.52) | 0.088 |
| Long-term controller | 2.42 | (1.89–3.09) | <0.001 | 5.82 | (4.03–8.39) | <0.001 | 1.54 | (1.12–2.12) | 0.008 | 1.00 | (0.72–1.39) | 0.988 |
| SABAs/long-term controller | 2.26 | (1.87–2.74) | <0.001 | 5.60 | (4.03–7.76) | <0.001 | 1.73 | (1.36–2.19) | <0.001 | 1.21 | (0.96–1.52) | 0.109 |
| Any oral steroid use | 2.45 | (1.95–3.08) | <0.001 | 5.35 | (3.76–7.60) | <0.001 | 1.87 | (1.39–2.51) | <0.001 | 1.18 | (0.87–1.59) | 0.292 |
| Outcome at ages 6–9 yr (N = 1,072)† | ||||||||||||
| Diagnosis | ||||||||||||
| Ever asthma (≥1) | 2.28 | (1.66–3.12) | <0.001 | 4.92 | (2.91–8.31) | <0.001 | 2.04 | (1.38–3.01) | <0.001 | 1.46 | (0.98–2.16) | 0.062 |
| Recurrent asthma (≥2) | 2.94 | (2.08–4.15) | <0.001 | 5.68 | (3.27–9.86) | <0.001 | 2.46 | (1.60–3.80) | <0.001 | 1.22 | (0.76–1.95) | 0.409 |
| Recurrent asthma (≥4) | 2.40 | (1.57–3.67) | <0.001 | 3.92 | (2.05–7.47) | <0.001 | 1.97 | (1.16–3.36) | 0.013 | 1.10 | (0.61–1.98) | 0.744 |
| Ever asthma exacerbation | 2.05 | (1.35–3.12) | <0.001 | 3.91 | (2.04–7.50) | <0.001 | 2.09 | (1.21–3.60) | 0.008 | 1.83 | (1.07–3.15) | 0.029 |
| Medication | ||||||||||||
| Any asthma medication | 2.05 | (1.51–2.77) | <0.001 | 3.82 | (2.28–6.41) | <0.001 | 1.87 | (1.29–2.71) | <0.001 | 1.31 | (0.90–1.90) | 0.163 |
| Long-term controller | 2.64 | (1.84–3.77) | <0.001 | 3.57 | (2.03–6.28) | <0.001 | 2.26 | (1.47–3.49) | <0.001 | 0.99 | (0.61–1.61) | 0.972 |
| SABAs/long-term controller | 2.17 | (1.59–2.96) | <0.001 | 4.00 | (2.37–6.73) | <0.001 | 2.02 | (1.38–2.96) | <0.001 | 1.35 | (0.91–1.99) | 0.133 |
| Any oral steroid use | 2.16 | (1.39–3.36) | <0.001 | 3.21 | (1.68–6.15) | <0.001 | 1.60 | (0.93–2.75) | 0.092 | 0.90 | (0.50–1.65) | 0.743 |
Definition of abbreviations: AOR = adjusted odds ratio; CI = confidence interval; Ref = reference; SABAs = short-acting β-agonists.
See Table E1 for description of asthma measures.
Adjusted for maternal age, race/ethnicity, marital status, education, history of asthma, maternal persistent smoking during the index pregnancy; child sex and age, followed years, and family member smoking.
Wheezing measures were excluded because of low prevalence during ages 6–9 years.
Compared with full-term children, early preterm children had the highest odds of asthma (AORs 3.2 [95% CI, 2.2–4.5] to 6.2 [95% CI, 3.3–11.6]; P < 0.001), and late preterm children had the second highest (AORs 1.5 [95% CI, 1.1–2.2] to 2.5 [95% CI, 1.6–3.8]; P < 0.05) (Table 1). These associations were consistent across all asthma measures in both age ranges. Early term children’s odds of asthma were not significantly higher than those for full-term children, with the exception of asthma exacerbation at ages 6–9 years (AOR = 1.8 [95% CI, 1.1–3.2]; P = 0.029). Given the variable length of follow-up resulting from our rolling enrollment design, we performed an analysis of the subset of children with continuous electronic medical record data from birth (n = 1973). Those analyses demonstrated similar or even higher PTB–asthma associations after adjusting for length of follow-up (Table E3).
Discussion
We found that, although the prevalence of asthma varied by asthma definition, children born preterm were at a higher risk of asthma compared with term children on all measures at ages 0–5 and 6–9 years. We also observed a robust dose–response association between degree of prematurity and all asthma measures in both age ranges. Our results provide strong evidence that PTB is an important risk factor for asthma.
Despite the strengths of the BBC data, this study has potential limitations. First, although electronic medical record data are not affected by recall bias, they may suffer from other errors, such as underdiagnosing because of transcriber/coder’s experience. In addition, the BBC sample may limit generalizability of our results; however, the results are highly relevant to urban, poor, minority populations, who have a high prevalence of both asthma and PTB.
About one in nine of all children and one in six of black infants were born preterm in the United States in 2013 (1). PTB is not included in the Asthma Predictive Index (10), but our findings underscore the important role it plays in the development of childhood asthma. Prevention of PTB is thus important not only for reducing well-known PTB-associated mortality and morbidity but also for reducing the burden of asthma, especially the disproportionate burden among urban, poor Americans.
Acknowledgments
Acknowledgment
We gratefully acknowledge the individuals who participated in the studies and contributed to this work.
Footnotes
The parent study is supported in part by grants from March of Dimes (20-FY02-56; Principal Investigator [PI]: X.W.), the National Institute of Environmental Health Sciences (R21 ES011666; PI: X.W.), and the National Institute of Child Health and Human Development (R01 HD041702; PI: X.W.). The follow-up study is supported in part by the Bunning family and their foundations, National Institute of Allergy and Infectious Diseases (U01AI090727 and R21AI079872; PI: X.W.), and the Maternal and Child Health Bureau (R40MC27443; PI: X.W.). C.A.K. is supported by the National Institute of Allergy and Infectious Diseases (K23AI103187). H.H. has been supported by the China Scholarship Council PhD Scholarship, Bernard Jane Guyer Scholarship, and Cheryl Alexander Memorial Fund Award in her doctoral degree training.
Author Contributions: X.W. is the principal investigator of the Boston Birth Cohort and has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis in this report. The subject recruitment, follow-up, and data collection were overseen and managed by X.W. and B.S.Z. and were conducted by a team of investigators including C.P., X.H., and D.M.C. H.H., M.E.H., and X.W. conceptualized this study. H.H. assumed primary responsibility for data cleaning and statistical analyses and drafted this manuscript. All the other coauthors provided critical inputs on the study design, data analyses, and interpretation of the study findings. All the authors reviewed the final version of the manuscript and approved submission to the journal for publication.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Matthews TJ Division of Vital Statistics. Births: final data for 2013. National Vital Statistics Reports. Vol. 64, No. 1. Hyattsville, MD: National Center for Health Statistics; 2015. [PubMed] [Google Scholar]
- 2.Bloom B, Cohen RA, Freeman G. Hyattsville, MD: National Center for Health Statistics; 2012. Summary health statistics for US children: National Health Interview Survey, 2011. Vital Health Statistics. Vol. 10, No. 254. [PubMed] [Google Scholar]
- 3.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, Basterrechea M, Bisgaard H, Chatzi L, Corpeleijn E, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–1329. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaakkola JJK, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 6.Van Wonderen KE, Van Der Mark LB, Mohrs J, Bindels PJE, Van Aalderen WMC, Ter Riet G. Different definitions in childhood asthma: how dependable is the dependent variable? Eur Respir J. 2010;36:48–56. doi: 10.1183/09031936.00154409. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311:587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robison RG, Kumar R, Arguelles LM, Hong X, Wang G, Apollon S, Bonzagni A, Ortiz K, Pearson C, Pongracic JA, et al. Maternal smoking during pregnancy, prematurity and recurrent wheezing in early childhood. Pediatr Pulmonol. 2012;47:666–673. doi: 10.1002/ppul.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Rodriguez JA. The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol. 2010;126:212–216. doi: 10.1016/j.jaci.2010.06.032. [DOI] [PubMed] [Google Scholar]