Abstract
Rationale: The mechanisms that underlie the pathogenesis of chronic rhinosinusitis without nasal polyps (CRSsNP), chronic rhinosinusitis with nasal polyps (CRSwNP), and aspirin-exacerbated respiratory disease (AERD) are not clear.
Objectives: To first evaluate the inflammatory profiles of CRSsNP and CRSwNP tissues and then to investigate whether clinical differences observed between CRSwNP and AERD are in part secondary to differences in inflammatory mediator expression within nasal polyp (NP) tissues.
Methods: Expression levels of numerous inflammatory mediators were determined by quantitative real-time polymerase chain reaction, ELISA, and multiplex immunoassay.
Measurements and Main Results: CRSwNP NP had increased levels of type 2 mediators, including IL-5 (P < 0.001), IL-13 (P < 0.001), eotaxin-2 (P < 0.001), and monocyte chemoattractant protein (MCP)-4 (P < 0.01), compared with sinonasal tissue from subjects with CRSsNP and control subjects. Expression of IFN-γ messenger RNA or protein was low and not different among the chronic rhinosinusitis subtypes examined. Compared with CRSwNP, AERD NP had elevated protein levels of eosinophil cationic protein (ECP) (P < 0.001), granulocyte–macrophage colony–stimulating factor (GM-CSF) (P < 0.01), and MCP-1 (P = 0.01), as well as decreased gene expression of tissue plasminogen activator (tPA) (P = 0.02). Despite the higher eosinophilia in AERD, there was no associated increase in type 2 mediator protein levels observed.
Conclusions: CRSwNP was characterized by a predominant type 2 inflammatory environment, whereas CRSsNP did not reflect a classic type 1 milieu, as has been suggested previously. AERD can be distinguished from CRSwNP by elevated ECP levels, but this enhanced eosinophilia is not associated with elevations in traditional type 2 inflammatory mediators associated with eosinophil proliferation and recruitment. However, other factors, including GM-CSF, MCP-1, and tPA, may be important contributors to AERD pathogenesis.
Keywords: CRSwNP, CRSsNP, eosinophil
At a Glance Commentary
Scientific Knowledge on the Subject
The mechanisms that underlie the pathogenesis of chronic rhinosinusitis without nasal polyps (CRSsNP), chronic rhinosinusitis with nasal polyps (CRSwNP), and aspirin-exacerbated respiratory disease (AERD) are not well understood.
What This Study Adds to the Field
Chronic rhinosinusitis is a highly heterogeneous disease. CRSwNP displays a predominant type 2 inflammatory signature, whereas CRSsNP does not reflect a specific T-cell cytokine inflammatory environment. AERD also has type 2 characteristics and can be distinguished from CRSwNP by enhanced tissue eosinophilia, but this does not appear to be driven by an excess of traditional eosinophilic inflammatory mediators. However, other factors, including granulocyte–macrophage colony–stimulating factor, monocyte chemoattractant protein-1, and tissue plasminogen activator, may be important contributors to AERD pathogenesis.
Chronic rhinosinusitis (CRS) is associated with chronic inflammation of the sinonasal tissue. It affects approximately 31 million people in the United States and is estimated to result in $5.8 billion in direct medical costs annually (1–3). CRS is a heterogeneous disease and can be classified into subtypes based upon the presence or absence of nasal polyps (NP) (4). Although chronic rhinosinusitis without nasal polyps (CRSsNP) is more prevalent, chronic rhinosinusitis with nasal polyps (CRSwNP) accounts for approximately 20% of all CRS and has been associated with more severe clinical disease (1, 5). An estimated 8.7% of patients with CRS also have asthma and a hypersensitivity to medications that inhibit the cyclooxygenase 1 enzyme (6, 7). This clinical triad is known as aspirin-exacerbated respiratory disease (AERD) (8, 9) and may represent a more severe form of CRSwNP or possibly even a distinct disease entity. Studies have shown that patients with AERD tend to have severe asthma, and they also undergo more revision sinus surgeries and have a higher rate of symptom recurrence after surgery than patients with CRSwNP (10–12).
The mechanisms of CRS pathogenesis in general are not well understood and are most likely multifactorial. Disruptions in epithelial barrier function, dysregulation of innate and adaptive immune responses, and production of antibodies against Staphylococcus aureus enterotoxins are thought to be important contributors (13–15). The role of specific inflammatory mediators in CRS pathogenesis has also been evaluated. It has been suggested that CRSsNP is driven by a predominant type 1 inflammatory response, whereas CRSwNP is characterized by an enhanced type 2 response (16–18). However, there are conflicting reports regarding expression levels of specific mediators in CRS, and thus a more comprehensive analysis is warranted (19). Dysregulation of arachidonic acid metabolism and altered platelet function are thought to specifically contribute to AERD pathogenesis, but additional studies are needed to further investigate potential roles that other mediators may play in this severe form of polypoid sinus disease (20, 21).
The present study was designed to characterize more extensively the inflammatory signature profiles of CRS subtypes by simultaneously measuring numerous mediators within the same specimen and then comparing levels among healthy controls and patients with CRSsNP or CRSwNP. We also wanted to evaluate expression patterns of various mediators in NP of patients with AERD versus CRSwNP as a means to further investigate the potential mechanisms that could drive the enhanced clinical disease typically observed in AERD. Some of the results of these studies have been reported previously in the form of an abstract (22).
Methods
Study Population
All control subjects and subjects with CRSsNP, CRSwNP, or AERD signed informed consent forms and were recruited from the Division of Allergy/Immunology and the Department of Otolaryngology clinics at the Northwestern University Feinberg School of Medicine. Additional information and details of the subjects’ characteristics are listed in Tables 1 and 2 and in Tables E1 and E2 in the online supplement. The Institutional Review Board of Northwestern University Feinberg School of Medicine approved this study.
Table 1.
Demographics of Subjects Evaluated in the Characterization of Sinonasal Tissue Inflammatory Mediator Profiles
| Control UT | CRSsNP UT | CRSwNP UT | CRSwNP Polyp | P Value | |
|---|---|---|---|---|---|
| Luminex protein assay, n | 17 | 9 | 15 | 15 | |
| Male sex, n (%) | 8 (47%) | 5 (56%) | 11 (73%) | 13 (87%) | 0.09 |
| White, n (%) | 11 (65%) | 7 (78%) | 12 (80%) | 13 (87%) | 0.51 |
| Mean ± SEM age, yr | 47 ± 4 | 38 ± 2 | 47 ± 3 | 39 ± 3 | 0.16 |
| Atopic, n (%) | 0 (0%) | 7 (78%) | 9 (60%) | 10 (67%) | <0.001 |
| Asthmatic, n (%) | 0 (0%) | 2 (22%) | 6 (40%) | 6 (40%) | 0.03 |
| Oral steroid use, n (%) | 1 (6%) | 0 (0%) | 4 (27%) | 5 (33%) | 0.08 |
| Intranasal steroid use, n (%) | 0 (0%) | 4 (44%) | 7 (47%) | 7 (47%) | <0.01 |
| Leukotriene antagonist use, n (%) | 0 (0%) | 1 (11%) | 2 (13%) | 2 (13%) | 0.49 |
| Preoperative antibiotic use, n (%) | 0 (0%) | 1 (11%) | 3 (20%) | 1 (7%) | 0.25 |
| Average ± SEM number surgeries | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.1 | 0.43 |
| Microarray, n | 6 | 6 | 6 | 6 | |
| Male sex, n (%) | 2 (33%) | 2 (33%) | 4 (67%) | 4 (67%) | 0.45 |
| White, n (%) | 4 (67%) | 5 (83%) | 6 (100%) | 6 (100%) | 0.24 |
| Mean ± SEM age, yr | 36 ± 6 | 47 ± 6 | 38 ± 5 | 38 ± 5 | 0.62 |
| Atopic, n (%) | 0 (0%) | 1 (17%) | 1 (17%) | 1 (17%) | 0.77 |
| Asthmatic, n (%) | 0 (0%) | 0 (0%) | 1 (17%) | 1 (17%) | 0.54 |
| Oral steroid use, n (%) | 0 (0%) | 2 (33%) | 1 (17%) | 1 (17%) | 0.49 |
| Intranasal steroid use, n (%) | 0 (0%) | 0 (0%) | 1 (17%) | 1 (17%) | 0.54 |
| Leukotriene antagonist use, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1.00 |
| Preoperative antibiotic use, n (%) | 0 (0%) | 1 (17%) | 2 (33%) | 2 (33%) | 0.43 |
| Average ± SEM number of surgeries | 1.0 ± 0.0 | 1.3 ± 0.2 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.10 |
| RT-PCR for IFN family members, n | 12 | 15 | 13 | 14 | |
| Male sex, n (%) | 7 (58%) | 3 (20%) | 10 (77%) | 11 (79%) | <0.01 |
| White, n (%) | 10 (83%) | 13 (87%) | 9 (69%) | 10 (71%) | 0.62 |
| Mean ± SEM age, yr | 47 ± 5 | 43 ± 4 | 39 ± 3 | 47 ± 4 | 0.52 |
| Atopic, n (%) | 0 (0%) | 9 (60%) | 9 (69%) | 8 (57%) | <0.01 |
| Asthmatic, n (%) | 0 (0%) | 5 (33%) | 7 (54%) | 6 (43%) | 0.03 |
| Oral steroid use, n (%) | 0 (0%) | 0 (0%) | 1 (8%) | 2 (14%) | 0.29 |
| Intranasal steroid use, n (%) | 0 (0%) | 3 (20%) | 7 (54%) | 10 (71%) | <0.001 |
| Leukotriene antagonist use, n (%) | 0 (0%) | 0 (0%) | 3 (23%) | 1 (7%) | 0.08 |
| Preoperative antibiotic use, n (%) | 0 (0%) | 1 (7%) | 2 (15%) | 3 (21%) | 0.31 |
| Average ± SEM number of surgeries | 1.0 ± 0.0 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 0.36 |
Definition of abbreviations: CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic sinusitis with nasal polyps; RT-PCR = real-time polymerase chain reaction; UT = uncinate tissue.
Table 2.
Demographics of Subjects with CRSwNP or AERD Evaluated in the Comparison of Inflammatory Mediator Profiles in Nasal Polyp Tissue
| CRSwNP Polyp | AERD Polyp | P Value | |
|---|---|---|---|
| Luminex protein assay, n | 15 | 15 | |
| Male sex, n (%) | 13 (87%) | 6 (40%) | 0.02 |
| White, n (%) | 13 (87%) | 8 (53%) | 0.11 |
| Mean ± SEM age, yr | 39 ± 3 | 43 ± 3 | 0.41 |
| Atopic, n (%) | 10 (67%) | 14 (93%) | 0.17 |
| Asthmatic, n (%) | 6 (40%) | 15 (100%) | <0.001 |
| Oral steroid use, n (%) | 5 (33%) | 10 (67%) | 0.14 |
| Intranasal steroid use, n (%) | 7 (47%) | 13 (87%) | 0.05 |
| Leukotriene antagonist use, n (%) | 2 (13%) | 7 (47%) | 0.11 |
| Preoperative antibiotic, use n (%) | 1 (7%) | 3 (20%) | 0.60 |
| Average ± SEM number of surgeries | 1.1 ± 0.1 | 3.3 ± 0.5 | <0.001 |
| RT-PCR for tPA, n | 20 | 19 | |
| Male sex, n (%) | 16 (80%) | 8 (42%) | 0.02 |
| White, n (%) | 14 (70%) | 14 (74%) | 1.00 |
| Mean ± SEM age, yr | 48 ± 3 | 44 ± 2 | 0.51 |
| Atopic, n (%) | 9 (45%) | 16 (84%) | 0.02 |
| Asthmatic, n (%) | 7 (35%) | 19 (100%) | <0.001 |
| Oral steroid use, n (%) | 4 (20%) | 14 (74%) | <0.01 |
| Intranasal steroid use, n (%) | 11 (55%) | 13 (68%) | 0.51 |
| Leukotriene antagonist use, n (%) | 2 (10%) | 9 (47%) | 0.01 |
| Preoperative antibiotic use, n (%) | 3 (15%) | 2 (11%) | 1.00 |
| Average ± SEM number of surgeries | 1.3 ± 0.1 | 2.5 ± 0.4 | <0.01 |
Definition of abbreviations: AERD = aspirin-exacerbated respiratory disease; CRSwNP = chronic sinusitis with nasal polyps; RT-PCR = real-time polymerase chain reaction; tPA = tissue plasminogen activator.
Nasal Lavage Fluid Sampling
Nasal lavage fluid was obtained and processed as previously described, with further details provided in the online supplement (23). As nasal lavage fluid could not be isolated from specific anatomic structures, data are presented as representative of the entire nasal cavity for each group.
Extraction and Measurement of Total Protein from Nasal Tissue
Protein was extracted and measured from surgical uncinate tissue (UT) or NP samples as previously described, with additional details provided in the online supplement (23).
Identification of Eosinophils in Sinonasal Tissues
Eosinophils were identified and counted in hematoxylin and eosin–stained sinonasal tissue sections as previously described, with further details provided in the online supplement (24).
Eosinophil Cationic Protein Measurement
Eosinophil cationic protein (ECP) was measured using the MESACUP ECP TEST per the manufacturer’s instructions (MBL International, Woburn, MA). Tissue ECP concentrations were corrected for total protein concentration, and the results are reported as nanograms of ECP per milligram of total protein. ECP levels in nasal lavage fluid are reported as nanograms of ECP per milliliter.
Microarray Analysis
Relative expression of IFN-α2, IFN-α4, IFN-α5, IFN-α6, IFN-α7, IFN-α8, IFN-α10, IFN-α13, IFN-α14, IFN-α16, IFN-α17, IFN-α21, IFN-β1, IFN-ε, IFN-κ, IFN-γ, IFN-ω, IFN-λ1, IFN-λ2, and IFN-λ3 was obtained from a previous comprehensive microarray analysis deposited with the Gene Expression Omnibus under accession number GSE36830 (25).
Quantitative Real-Time Polymerase Chain Reaction
RNA was isolated from nasal tissue samples, and cDNA was prepared as previously described (23, 26). TaqMan or SYBR Green–based real-time polymerase chain reactions (RT-PCRs; Applied Biosystems, Foster City, CA) were run; for details, see the online supplement. All expression values were normalized to β-glucuronidase and expressed as 2−ΔCt values.
Cytokine and Chemokine Determinations
The following inflammatory mediators were examined: IL-4, IL-5, IL-6, IL-10, IL-13, IL-33, IFN-α2, IFN-γ, stem cell factor (SCF), granulocyte–macrophage colony–stimulating factor (GM-CSF), eotaxin-1/chemokine (C-C motif) ligand 11 (CCL11), eoxtain-2/CCL24, eotaxin-3/CCL26, regulated upon activation, normal T-cell expressed and secreted protein (RANTES)/CCL5, monocyte chemoattractant protein (MCP)-1/CCL2, MCP-4/CCL13, thymus and activation–regulated chemokine (TARC)/CCL17, B cell–attracting chemokine (BCA)-1/chemokine (C-X-C motif) ligand 13 (CXCL13), and stromal cell–derived factor protein (SDF)-1αand SDF-1β/CXCL12. Analytes were measured using a custom MILLIPLEX MAP kit per the manufacturer’s instructions (EMD Millipore, Billerica, MA), run on a MAGPIX clinical diagnostics instrument (Luminex, Austin, TX). Data were analyzed with xPONENT software (Luminex).
Statistics
All data are reported as mean ± SEM. A P value less than 0.05 was considered significant. Further details are provided in the online supplement.
Results
Levels of Mediators in Sinonasal Tissue
The first aim of this study was to characterize the inflammatory signature profiles of sinonasal tissues from healthy control subjects and from patients with CRSsNP and patients with CRSwNP. To do so, a multiplex bead-based immunoassay was performed to measure numerous mediators simultaneously in control UT, CRSsNP UT, CRSwNP UT, and CRSwNP polyp tissue. Additionally, ECP was measured in each sample by ELISA and served as a surrogate marker for tissue eosinophilia.
The levels of ECP and mediators measured in control and CRS tissues were first compared using nonparametric Kruskal–Wallis analysis of variance (ANOVA) to determine whether there were any overall significant differences in expression between the groups (Table 3, left column). In this analysis, significant differences in 65% of the mediators assayed were observed. These mediators included ECP (P < 0.001) and the classic type 2 mediators IL-4 (P < 0.001), IL-5 (P < 0.001), IL-13 (P < 0.001), eotaxin-1 (P < 0.01), eotaxin-2 (P < 0.001), eotaxin-3 (P < 0.001), and MCP-4 (P < 0.01). Other growth factors, including GM-CSF (P < 0.05) and SCF (P < 0.05), as well as the chemokine RANTES (P < 0.001), were also found to differ significantly among the sinonasal tissues. There was a trend for levels of MCP-1 to differ, but this did not reach statistical significance (P = 0.05).
Table 3.
Inflammatory Mediators in Sinonasal Tissue Extracts
| Control UT (Mean ± SEM) | CRSsNP UT (Mean ± SEM) | CRSwNP UT (Mean ± SEM) | CRSwNP Polyp (Mean ± SEM) | |
|---|---|---|---|---|
| ECP* | 113,000 ± 64,900 | 49,600 ± 13,700 | 258,000 ± 89,500 | 763,000 ± 210,000†‡ |
| IL-4* | 8.4 ± 3.2 | 0.7 ± 0.4† | 2.1 ± 1.0 | 4.1 ± 1.1‡ |
| IL-5* | 0.0 ± 0.0 | 0.6 ± 0.4 | 0.6 ± 0.2 | 11.3 ± 4.4†‡§ |
| IL-10* | 0.4 ± 0.1 | 1.3 ± 0.5 | 0.5 ± 0.1 | 1.5 ± 0.2†§ |
| IL-13* | 0.7 ± 0.3 | 0.7 ± 0.5 | 3.5 ± 1.2 | 14.7 ± 4.7†‡ |
| Eotaxin-2* | 150 ± 74.7 | 259 ± 80.1 | 319 ± 92.4 | 1,460 ± 475†‡ |
| Eotaxin-3* | 1.5 ± 0.8 | 194 ± 136 | 378 ± 93.5† | 400 ± 138† |
| RANTES* | 3,432 ± 703 | 2,233 ± 856 | 1,982 ± 307 | 983 ± 353† |
| Eotaxin-1‖ | 20.0 ± 6.9 | 32.7 ± 10.3 | 17.3 ± 3.4 | 75.5 ± 18.3†§ |
| MCP-4‖ | 11.5 ± 4.1 | 11.2 ± 4.4 | 15.7 ± 4.5 | 109 ± 53.9†‡ |
| TARC‖ | 2.6 ± 0.8 | 3.8 ± 1.1 | 4.6 ± 1.4 | 9.9 ± 3.5† |
| SCF¶ | 43.3 ± 11.8 | 29.7 ± 9.9 | 32.1 ± 5.3 | 14.5 ± 4.6† |
| GM-CSF¶ | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.5 ± 0.2 | 0.8 ± 0.5 |
| MCP-1 | 352 ± 81.0 | 338 ± 135 | 279 ± 52.1 | 531 ± 92.4 |
| IL-6 | 2.3 ± 0.7 | 4.5 ± 3.4 | 1.8 ± 0.6 | 5.3 ± 1.7 |
| IL-33 | 195 ± 67.2 | 149 ± 24.7 | 154 ± 22.4 | 136 ± 49.7 |
| IFN-α2 | 15.2 ± 7.4 | 3.8 ± 0.5 | 6.2 ± 1.5 | 2.9 ± 0.7 |
| IFN-γ | 0.9 ± 0.3 | 0.6 ± 0.10 | 0.9 ± 0.2 | 0.5 ± 0.1 |
| BCA-1 | 42.4 ± 24.8 | 85.7 ± 40.6 | 136 ± 109 | 221 ± 110 |
| SDF-1α and SDF-1β | 101 ± 40.6 | 61.8 ± 28.1 | 52.2 ± 11.2 | 29.7 ± 13.0 |
Definition of abbreviations: BCA = B cell–attracting chemokine; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic sinusitis with nasal polyps; ECP = eosinophil cationic protein; MCP = monocyte chemoattractant protein; GM-CSF = granulocyte–macrophage colony–stimulating factor; RANTES = regulated upon activation, normal T-cell expressed and secreted protein; SCF = stem cell factor; SDF = stromal cell–derived factor; TARC = thymus and activation–regulated chemokine; UT = uncinate tissue.
All values are given in picograms per milligram of total protein. Bold indicates mediators of particular interest.
P < 0.001, Kruskal–Wallis test for statistical significance.
Significantly different (P < 0.05) compared with control UT accordng to post hoc analysis using Dunn’s multiple-comparisons test.
Significantly different (P < 0.05) compared with CRSsNP UT accordng to post hoc analysis using Dunn’s multiple-comparisons test.
Significantly different (P < 0.05) compared with CRSwNP UT accordng to post hoc analysis using Dunn’s multiple-comparisons test.
P < 0.01, Kruskal–Wallis test for statistical significance.
P < 0.05, Kruskal–Wallis test for statistical significance.
Next, we investigated whether the significant differences in mediator levels observed were driven by differential expression in one particular sinonasal tissue subset relative to the other groups. Thus, a post hoc analysis for statistical significance using Dunn’s correction was performed, adjusting for multiple comparisons. Of the mediators that were significantly altered above, only GM-CSF did not reach statistically significant differences when we compared any two subsets of sinonasal tissues (Table 3). However, we found 11 mediators to be significantly different between CRSwNP NP and control UT, 6 to be significantly different between CRSwNP NP and CRSsNP UT, and 3 to be significantly different between CRSwNP NP and CRSwNP UT (Table 3). One mediator, IL-5, was consistently elevated in NP of CRSwNP compared with all other groups. With the exception of eotaxin-3, there were no differences in mediator levels of CRSwNP UT and control UT. Interestingly, IL-4 was the only mediator significantly different between CRSsNP UT and control UT, with the higher level of expression measured in the control group (Table 3).
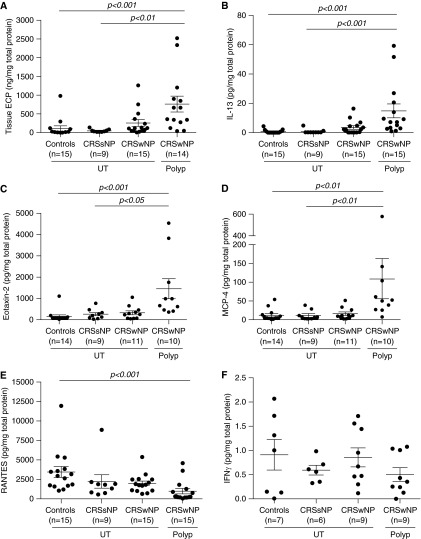
Notably, the majority of mediators significantly elevated in NP were associated with type 2 inflammation. For example, levels of ECP were significantly elevated in NP of CRSwNP (763 ng/mg total protein) compared with CRSsNP UT (49.6 ng/mg total protein; P < 0.01) and control UT (113 ng/mg total protein; P < 0.001) (Figure 1A). The mean eosinophil count was also significantly elevated in CRSwNP NP (287 ± 71 eosinophils per square millimeter of tissue) and in CRSwNP UT (72 ± 20 eosinophils per square millimeter of tissue) compared with control UT (1 ± 0.4 eosinophil per square millimeter of tissue) (P < 0.001 and P < 0.01 respectively) (Figure E1A). Eosinophil counts correlated well with ECP levels in the entire cohort of patients and controls (r = 0.72; P < 0.001) (Figure E1B). Other type 2 inflammatory mediators found to be elevated in CRSwNP NP compared with control UT and to CRSsNP UT included IL-13 (Figure 1B), eotaxin-2 (Figure 1C), and MCP-4 (Figure 1D). This is in contrast to RANTES, which was significantly decreased in CRSwNP NP compared with control UT (P < 0.001) (Figure 1E). Also, gene expression levels of the inflammatory cell markers CD45 (Figure E2A), CD68 (Figure E2B), and tryptase (Figure E2C), representing total leukocytes, macrophages, and mast cells, respectively, were significantly elevated in CRSwNP compared with healthy UT.
Figure 1.
Protein expression of select inflammatory mediators in sinonasal tissues. Protein levels of (A) eosinophil cationic protein (ECP), (B) IL-13, (C) eotaxin-2, (D) monocyte chemoattractant protein (MCP)-4, (E) regulated upon activation, normal T-cell expressed and secreted protein (RANTES), and (F) IFN-γ were measured in uncinate tissue (UT) of healthy controls, UT of patients with chronic rhinosinusitis without nasal polyps (CRSsNP), and UT and nasal polyps of patients with chronic sinusitis with nasal polyps (CRSwNP). Statistical significance (P < 0.05) was determined by Kruskal-Wallis test with post hoc analysis using Dunn’s test for multiple comparison. Dot plots illustrate individual data points, and solid lines represent the mean ± SEM. Concentrations of ECP were expressed as nanograms per milligram of tissue, and all other mediators are expressed as picograms per milligram of tissue.
Interferon Expression in Sinonasal Tissue
Van Zele and coworkers have advanced the paradigm of a type 2 T helper cell (Th2)-predominant environment in CRSwNP and a Th1-predominant environment in CRSsNP, reflecting increased levels of IL-5 and IFN-γ, respectively, measured in the sinonasal tissues (16, 17). Whereas our results support a type 2 inflammatory environment in CRSwNP NP, they do not support the concept of CRSsNP having a type 1–predominant inflammatory milieu. Protein levels of IFN-γ were very low and were not significantly different among healthy UT, CRSsNP UT, CRSwNP UT, and CRSwNP polyp tissues (Figure 1F). To test this further, we performed a comprehensive analysis of IFN-γ gene expression using microarray (Table 4) and real-time PCR (Figure 2A) data, and in both cases we failed to find evidence of Th1 bias in CRSsNP. IFN-γ is only one member of a larger family of IFNs, which together play important roles in innate and adaptive immunity. Similarly to IFN-γ, there were no significant differences noted in relative gene expression of IFN-α2, IFN-α4, IFN-α5, IFN-α6, IFN-α7, IFN-α8, IFN-α10, IFN-α13, IFN-α14, IFN-α16, IFN-α17, IFN-α21, IFN-β1, IFN-ε, IFN-κ, IFN-ω, IFN-λ1, IFN-λ2, or IFN-λ3 (Table 4) among all subtypes of sinonasal tissue, as determined by Kruskal–Wallis ANOVA. The microarray findings were subsequently confirmed by RT-PCR, which again revealed no significant difference in gene expression of IFN-α2 (P = 0.05) (Figure 2B), IFN-α8 (P = 0.09) (Figure 2C), or IFN-β1 (P = 0.19) (Figure 2D) among all sinonasal tissue groups.
Table 4.
IFN Relative Gene Expression in Sinonasal Tissues
| Control UT (Mean ± SEM) | CRSsNP UT (Mean ± SEM) | CRSwNP UT (Mean ± SEM) | CRSwNP Polyp (Mean ± SEM) | |
|---|---|---|---|---|
| IFN-α2 | 8.5 ± 3.5 | 7.5 ± 3.3 | 5.3 ± 1.6 | 8.3 ± 3.2 |
| IFN-α4 | 2.7 ± 0.9 | 4.8 ± 3.3 | 2.3 ± 1.4 | 4.3 ± 3.2 |
| IFN-α5 | 8.3 ± 2.6 | 9.0 ± 3.5 | 15.5 ± 6.3 | 5.2 ± 1.7 |
| IFN-α6 | 7.8 ± 3.8 | 7.7 ± 4.1 | 19.8 ± 9.4 | 9.7 ± 3.4 |
| IFN-α7 | 7.5 ± 3.6 | 8.8 ± 3.7 | 15.5 ± 6.3 | 9.5 ± 4.3 |
| IFN-α8 | 1.0 ± 0.3 | 2.5 ± 1.3 | 6.8 ± 2.7 | 6.5 ± 1.6 |
| IFN-α10 | 5.2 ± 3.6 | 2.3 ± 0.6 | 6.3 ± 2.6 | 1.7 ± 0.3 |
| IFN-α13 | 9.3 ± 2.5 | 4.5 ± 1.0 | 5.1 ± 1.9 | 5.3 ± 2.1 |
| IFN-α14 | 4.7 ± 3.5 | 8.7 ± 2.9 | 4.0 ± 1.7 | 1.7 ± 0.3 |
| IFN-α16 | 5.3 ± 2.7 | 8.5 ± 4.0 | 9.0 ± 5.5 | 3.8 ± 2.2 |
| IFN-α17 | 6.3 ± 5.1 | 2.3 ± 0.7 | 5.0 ± 2.2 | 3.2 ± 1.0 |
| IFN-α21 | 8.0 ± 2.0 | 16.0 ± 4.6 | 10.5 ± 4.1 | 8.2 ± 2.4 |
| IFN-β1 | 10.3 ± 3.3 | 2.2 ± 0.4 | 1.3 ± 0.2 | 1.7 ± 0.7 |
| IFN-ε | 19.2 ± 5.2 | 24.0 ± 6.2 | 24.8 ± 8.0 | 24.3 ± 9.6 |
| IFN-κ | 4.5 ± 2.6 | 7.2 ± 3.0 | 7.7 ± 3.7 | 6.0 ± 1.8 |
| IFN-γ | 76.5 ± 31.9 | 129 ± 58.8 | 131 ± 46.3 | 78.2 ± 10.2 |
| IFN-ω | 8.0 ± 3.2 | 10.3 ± 3.4 | 8.3 ± 2.0 | 10.2 ± 4.0 |
| IFN-λ1 | 24.8 ± 2.2 | 15.7 ± 6.0 | 25.5 ± 6.3 | 36.8 ± 6.8 |
| IFN-λ2 | 7.3 ± 3.3 | 7.3 ± 2.4 | 13.8 ± 3.6 | 8.8 ± 3.1 |
| IFN-λ3 | 13.5 ± 2.8 | 11.2 ± 1.6 | 20.8 ± 7.9 | 10.5 ± 3.2 |
Definition of abbreviations: CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic sinusitis with nasal polyps; UT = uncinate tissue.
Figure 2.
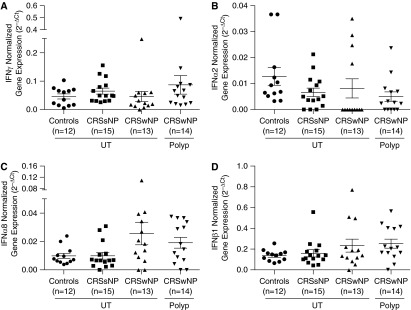
Relative gene expression of select type I interferons in sinonasal tissue. Total RNA was extracted from uncinate tissue (UT) isolated from controls, UT from patients with chronic rhinosinusitis without nasal polyps (CRSsNP) or chronic sinusitis with nasal polyps (CRSwNP), or nasal polyps from patients with CRSwNP. Expression of messenger RNA for (A) IFN-γ, (B) IFN-α2, (C) IFN-α8, and (D) IFN-β1 was measured by real-time polymerase chain reaction. Statistical significance (P < 0.05) was calculated by Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Dot plots illustrate individual data points, and solid lines represent the mean ± SEM. Ct = cycle threshold.
Levels of Inflammatory Mediators in Nasal Lavage Fluid
Nasal lavage fluid can serve as an alternative and more convenient way to study CRS disease pathogenesis, as specimens can be readily obtained without requiring surgical excision. However, it is not known whether mediator levels in lavage fluid accurately represent the levels in the tissue itself. To this end, a multiplex bead-based immunoassay was used to measure the same inflammatory mediators in nasal lavage fluid of healthy control subjects and patients with CRSsNP or CRSwNP. We measured ECP levels by ELISA.
In contrast to what we observed in sinonasal tissue, only 40% of mediators were found to be significantly different in nasal lavage fluid among the subgroups, as determined by Kruskal–Wallis ANOVA (Table 5, left column). On further analysis controlled for multiple comparisons, six mediators were found to be significantly different in CRSwNP versus control lavage fluid. Similar to sinonasal tissue, the type 2 inflammatory mediators ECP, IL-5, IL-13, eotaxin-2, eotaxin-3, and MCP-4 were elevated in lavage fluid of CRSwNP compared with that of control subjects (Table 5). Additionally, IL-4, IL-13, eotaxin-2, eotaxin-3, MCP-4, and TARC were significantly increased in CRSwNP compared with CRSsNP lavage fluid (Table 5). There were no significant differences in mediator levels in lavage fluid of CRSsNP and control subjects (Table 5).
Table 5.
Inflammatory Mediators in Nasal Lavage Fluid
| Control (Mean ± SEM) | CRSsNP (Mean ± SEM) | CRSwNP (Mean ± SEM) | |
|---|---|---|---|
| ECP* | 61,000 ± 28,000 | 280,000 ± 106,000 | 598,000 ± 194,000† |
| IL-5‡ | 0.7 ± 0.3 | 1.0 ± 0.3 | 26.2 ± 11.2† |
| IL-13‡ | 2.6 ± 1.1 | 1.4 ± 0.3 | 12.6 ± 5.4†§ |
| Eotaxin-2‡ | 198 ± 34.6 | 195 ± 33.2 | 839 ± 268†§ |
| Eotaxin-3‡ | 12.2 ± 6.7 | 81.9 ± 34.8 | 559 ± 142†§ |
| MCP-4* | 4.0 ± 0.4 | 3.7 ± 0.6 | 8.0 ± 1.3†§ |
| IL-4‖ | 4.7 ± 1.3 | 3.0 ± 1.5 | 7.7 ± 2.4§ |
| TARC‖ | 10.6 ± 2.3 | 7.0 ± 1.4 | 36.8 ± 20.6§ |
| RANTES | 787 ± 211 | 734 ± 308 | 863 ± 367 |
| Eotaxin-1 | 27.2 ± 4.2 | 25.6 ± 4.8 | 31.8 ± 5.3 |
| MCP-1 | 480 ± 162 | 422 ± 150 | 685 ± 192 |
| GM-CSF | 0.9 ± 0.3 | 0.7 ± 0.2 | 0.9 ± 0.3 |
| SCF | 3.9 ± 1.5 | 1.2 ± 0.4 | 2.7 ± 0.9 |
| IL-6 | 26.1 ± 8.7 | 12.0 ± 3.0 | 230 ± 144 |
| IL-10 | 3.3 ± 0.8 | 2.1 ± 0.4 | 13.7 ± 7.9 |
| IL-33 | 186 ± 72.2 | 62.3 ± 15.9 | 74.0 ± 20.2 |
| IFN-α2 | 2.0 ± 0.7 | 2.5 ± 0.5 | 2.4 ± 0.5 |
| IFN-γ | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.7 ± 0.3 |
| BCA-1 | 102 ± 62.8 | 73.7 ± 22.7 | 305 ± 65.5 |
| SDF-1α and SDF-1β | 0.0 ± 0.0 | 0.0 ± 0.0 | 18.0 ± 10.9 |
Definition of abbreviations: BCA = B cell–attracting chemokine; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic sinusitis with nasal polyps; ECP = eosinophil cationic protein; MCP = monocyte chemoattractant protein; GM-CSF = granulocyte–macrophage colony–stimulating factor; RANTES = regulated upon activation, normal T-cell expressed and secreted protein; SCF = stem cell factor; SDF = stromal cell–derived factor; TARC = thymus and activation–regulated chemokine.
All values are given as picograms per milliliter. Bold indicates mediators of particular interest.
P < 0.01, Kruskal–Wallis test for statistical significance.
Significantly different (P < 0.05) compared with control accordng to post hoc analysis using Dunn’s multiple-comparisons test.
P < 0.001, Kruskal–Wallis test for statistical significance.
Significantly different (P < 0.05) compared with CRSsNP accordng to post hoc analysis using Dunn’s multiple-comparisons test.
P < 0.05, Kruskal–Wallis test for statistical significance.
Because an enhanced signature of type 2 inflammation was observed in both nasal lavage fluid and NP tissue in CRSwNP, we next investigated if there was a correlation of mediator expression between the two specimen types. To do so, we compared mediator expression levels in nasal lavage fluid and NP tissue that had been collected from the same individual. Overall, no significant correlation was found between nasal lavage and NP for the majority of mediators assessed, including those associated with type 2 inflammation (Table E3).
Levels of Inflammatory Mediators in AERD versus CRSwNP Nasal Polyp Tissue
We next investigated if the inflammatory signature of NP from patients with AERD differed from that observed in NP from patients with CRSwNP (Table 2). In total, only five mediators were significantly elevated in NP of AERD compared with CRSwNP, as determined by Kruskal–Wallis ANOVA (Table 6).
Table 6.
Comparison of Inflammatory Mediators in Nasal Polyps of CRSwNP and AERD
| CRSwNP Polyp Mean ± SEM | AERD Polyp Mean ± SEM | P Value | |
|---|---|---|---|
| ECP* | 763,000 ± 210,000 | 3,937,000 ± 943,000 | <0.001 |
| GM-CSF† | 0.8 ± 0.5 | 1.2 ± 0.3 | <0.01 |
| SDF-1α and SDF-1β‡ | 29.7 ± 13.0 | 8.9 ± 7.0 | 0.01 |
| MCP-1‡ | 531 ± 92.4 | 1,187 ± 211 | 0.01 |
| IL-10‡ | 1.5 ± 0.2 | 0.9 ± 0.2 | 0.03 |
| SCF | 14.5 ± 4.6 | 21.5 ± 3.6 | 0.05 |
| Eotaxin-3 | 400 ± 138 | 235 ± 82.2 | 0.05 |
| Eotaxin-1 | 75.5 ± 18.3 | 97.9 ± 18.4 | 0.14 |
| RANTES | 983 ± 353 | 933 ± 181 | 0.14 |
| TARC | 9.9 ± 3.5 | 10.4 ± 1.2 | 0.17 |
| IL-13 | 14.7 ± 4.7 | 22.5 ± 6.0 | 0.19 |
| IFN-γ | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.27 |
| Eotaxin-2 | 1,460 ± 475 | 1514 ± 241 | 0.30 |
| MCP-4 | 109 ± 53.9 | 89.9 ± 18.4 | 0.39 |
| IFN-α2 | 2.9 ± 0.7 | 4.1 ± 1.0 | 0.58 |
| IL-4 | 4.1 ± 1.1 | 5.6 ± 1.3 | 0.59 |
| IL-6 | 5.3 ± 1.7 | 4.4 ± 0.9 | 0.59 |
| IL-33 | 136 ± 49.7 | 112 ± 27.2 | 0.68 |
| BCA-1 | 221 ± 110 | 175 ± 94.4 | 0.71 |
| IL-5 | 11.3 ± 4.4 | 10.7 ± 3.3 | 0.73 |
Definition of abbreviations: AERD = aspirin-exacerbated respiratory disease; BCA = B cell–attracting chemokine; CRSwNP = chronic sinusitis with nasal polyps; ECP = eosinophil cationic protein; GM-CSF = granulocyte–macrophage colony–stimulating factor; MCP = monocyte chemoattractant protein; RANTES = regulated upon activation, normal T-cell expressed and secreted protein; SCF = stem cell factor; SDF = stromal cell–derived factor; TARC = thymus and activation–regulated chemokine.
All values are given as picograms per milligram of total protein. Bold indicates mediators of particular interest.
P < 0.001, Mann–Whitney U test for statistical significance.
P < 0.01, Mann–Whitney U test for statistical significance.
P < 0.05, Mann–Whitney U test for statistical significance.
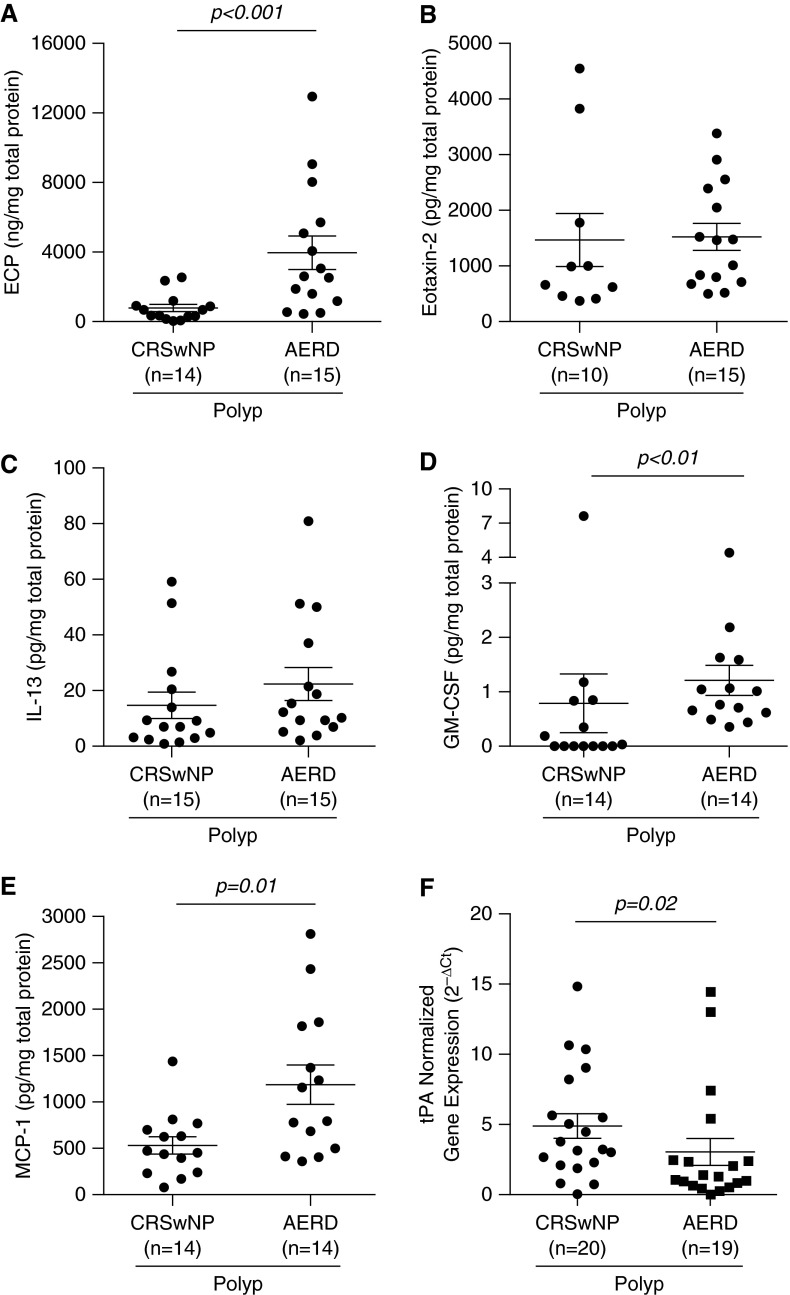
Confirming reports of increased eosinophils in NP derived from AERD compared with CRSwNP (24, 27), we found ECP to be significantly elevated in AERD by approximately fivefold (3,937 ng/mg total protein) versus CRSwNP (763 ng/mg total protein) (P < 0.001) (Figure 3A). In contrast, the total number of eosinophils quantified by immunohistochemistry was not significantly higher in AERD (375 ± 65 eosinophils per square millimeter of tissue) compared with CRSwNP NP (287 ± 71 eosinophils per square millimeter of tissue (P < 0.24) (Figure E1C). With regard to other inflammatory cell types, we observed no difference in gene expression levels of CD45 and tryptase when we compared CRSwNP and AERD NP, whereas CD68 was reduced in the AERD subgroup (Figures E2D–E2F).
Figure 3.
Expression of select inflammatory mediators in nasal polyps of aspirin-exacerbated respiratory disease (AERD) compared with chronic sinusitis with nasal polyps (CRSwNP). Protein levels of (A) eosinophil cationic protein (ECP), (B) eotaxin-2, (C) IL-13, (D) granulocyte–macrophage colony–stimulating factor (GM-CSF), and (E) monocyte chemoattractant protein (MCP)-1 were measured in nasal polyp tissue from patients with either CRSwNP or AERD. Concentrations of ECP were measured as nanograms per milligram of tissue, and all other mediators are expressed as picograms per milligram of tissue. (F) Expression of messenger RNA for tissue plasminogen activator (tPA) was measured by real-time polymerase chain reaction. Statistical significance (P < 0.05) was determined by Mann–Whitney U test. Dot plots illustrate individual data points, and solid lines represent the mean ± SEM. Ct = cycle threshold.
Despite the significant elevation of ECP in AERD compared with CRSwNP, there were no significant differences in levels of type 2 inflammatory mediators, including eotaxin-2 (Figure 3B) and IL-13 (Figure 3C), or of the type 1 inflammatory mediator IFN-γ (Table 6) in AERD and CRSwNP polyps. In contrast, GM-CSF was significantly elevated in AERD (1.2 pg/mg total protein) compared with CRSwNP (0.8 pg/mg total protein) (P = 0.005) (Figure 3D), although levels were somewhat low. MCP-1 was another mediator elevated in AERD compared with CRSwNP (P = 0.01) (Figure 3E), whereas IL-10, SDF1-α, and SDF1-β were significantly reduced in AERD (Table 6).
As mentioned above, patients with AERD tend to require more revision surgeries than those with CRSwNP. Our group has recently shown that NP from CRSwNP have decreased levels of tissue plasminogen activator (tPA) compared with healthy UT, which may contribute to polyp growth (26, 28). Thus, we sought to determine if there were any further differences in tPA expression between NP of AERD and CRSwNP. Relative gene expression of tPA was significantly reduced in NP of AERD compared with CRSwNP (P = 0.02) (Figure 3F). There was no difference in relative expression of factor XIIIa, another factor that we have previously hypothesized may play a role in polyp growth (28), between the two groups (P = 0.97; data not shown).
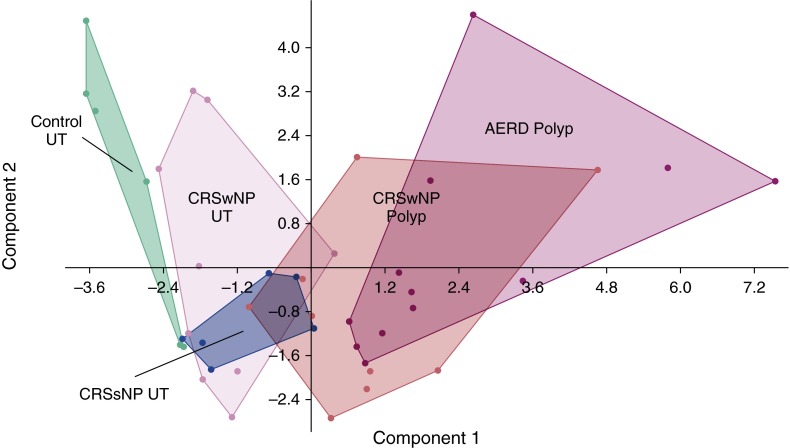
Principal Component Analysis of Sinonasal Tissues
Given the large number of mediators and various sinonasal tissues assessed, we asked if expression patterns of inflammatory mediators could broadly discriminate sinonasal tissue types or subsets of CRS disease. To do so, principal component analysis (PCA) was used to aid in the interpretation of the entire multivariate immunoplex data. Principal component 1 (PC1) accounted for 29.9% of the variance in the dataset and was helpful in discriminating between NP and control UT (Figure 4). NP samples, as a whole, had higher expression of eotaxin-1, eotaxin-2, eotaxin-3, MCP-1, MCP-4, TARC, IL-5, IL-10, and IL-13 relative to controls. However, protein levels of these same mediators could not distinguish NP of AERD from CRSwNP. In contrast, control UT was best characterized by increased RANTES expression, as this cytokine was the greatest discriminator in PC2. Other mediators measured, including SDF-1, SCF, GM-CSF, BCA-1, IFN-γ, IFN-α, IL-4, and IL-33, were not helpful in defining any signature pattern for the study groups.
Figure 4.
Principal component analysis (PCA) of inflammatory mediator data. The PCA plot illustrates the first versus second principal component of inflammatory mediator levels in sinonasal tissue. Dots represent individual patients, and distinct clusters of the different disease states and/or tissue types are outlined. The colors represent the various disease states and tissue types. Green = control uncinate tissue (UT); pink = chronic sinusitis with nasal polyps (CRSwNP) UT; blue = chronic rhinosinusitis without nasal polyps (CRSsNP) UT; red = CRSwNP nasal polyp; purple = aspirin-exacerbated respiratory disease (AERD) nasal polyp.
Discussion
Elucidating the mechanisms driving CRS pathogenesis has been the subject of numerous studies, with some focused on the role various cytokines and chemokines may play in the disease process. However, as recently summarized, there are conflicting reports regarding expression levels of specific inflammatory mediators in CRS, which may in part be secondary to different types of specimens and/or methodology used for analysis (19). As a result, the first goal of our present study was to measure mediators simultaneously in a given type of sinonasal tissue specimen and characterize these inflammatory signatures in controls, CRSsNP, and CRSwNP.
We found 12 mediators to be differentially expressed among the various sinonasal tissues, driven predominantly by elevations in various type 2 inflammatory cytokines and chemokines in NP of CRSwNP compared with UT from CRSsNP or controls. Increased levels of IL-5, IL-13, eotaxin-1, eotaxin-2, eotaxin-3, and MCP-4 can together contribute to the recruitment and proliferation of eosinophils, which in turn can promote the enhanced tissue eosinophilia that we and others have observed in NP of CRSwNP (29, 30). Interestingly, in prior studies, investigators have reported no significant difference in protein levels of another CCR3 ligand, RANTES, in NP compared with control sinonasal tissue (29, 31, 32). We similarly did not find RANTES to be significantly elevated in NP, suggesting that this mediator is not critical in promoting tissue eosinophilia. However, why RANTES is significantly increased in control UT compared with NP remains unclear. In summary, our findings altogether support other published work in suggesting that NP, in general, have an increased type 2 inflammatory signature compared with sinonasal tissue of healthy subjects or patients with CRSsNP (17, 27, 29, 30, 33, 34).
In contrast, our data do not support the converse hypothesis that CRSsNP is composed of a predominant type 1 inflammation characterized by elevated IFN-γ (16–18, 33). We did not detect any changes in gene expression of IFN family members, including IFN-γ, IFN-α2, IFN-α8, and IFN-β1. We also found no significant difference in IFN-γ protein levels in any sinonasal tissues examined, which is similar to the findings of some studies (27, 34, 35), but different from others (17, 18, 33).
The discrepancy between our findings, which show very low and stable levels of IFN-γ expression across sinonasal tissues, and the work of others who have demonstrated enhanced IFN-γ in CRSsNP UT may reside in the sinonasal tissue selected for assessment. Researchers in prior studies have used inferior turbinate, ethmoidal mucosal, or unspecified nasal tissue as controls for NP (17, 18, 33), whereas in our present study we used UT, as this tissue is taken from the middle turbinate near the site at which polyps form. Previous work has shown that there are regional differences in expression of various host defense molecules between inferior turbinate tissue and UT (36). Taken together, the local sinonasal environment appears to influence specific inflammatory mediator expression, but a more comprehensive evaluation of type 1 cytokine production in various anatomic locations within the nasal cavity of patients with CRSwNP and patients with CRSsNP is needed. Additionally, although we cannot exclude that the cytokine profiles observed may have been influenced by an underlying bacterial infection or colonization, our records show that less than 10% of all the sinonasal tissue samples used in the multiplex bead-based immunoassay were associated with pus at the time of sinus surgery, as documented by the operating otolaryngologist.
Because they are surgically extracted, sinonasal tissue specimens may not always be readily available for study, and nasal lavage fluid has been used for some analyses. This prompted us to ask if nasal lavage fluid serves as a good surrogate for estimating polyp tissue inflammation in CRSwNP. We found that some, but not all, of the mediators elevated in NP were also elevated in lavage fluid compared with controls. Although the same basic pattern of changes was observed in lavage as in tissues, correlations between specific mediators in matched nasal lavage and polyp samples were largely insignificant, possibly owing to the pronounced regional variability of inflammation in the sinonasal cavity. This suggests that mediator levels in nasal lavage may not be reliably representative of the local inflammatory environment adjacent to NP. A more directed approach to measuring nasal secretions, such as the use of nasal sponges, may be needed to better approximate NP inflammation (37 and unpublished observations).
We also investigated if variations in inflammatory mediators could contribute to the clinical and histologic differences observed between AERD and CRSwNP. Principal component analysis using a large panel of markers could not distinguish between NP of AERD and CRSwNP; this finding is consistent with other large microarray-based studies in which researchers investigated differences in various sinonasal tissues (38, 39). However, some significant differences between the two polyp subtypes were identified upon a more focused analysis of specific mediators.
Levels of ECP were elevated approximately fivefold in AERD compared with CRSwNP, reflective of the known enhanced tissue eosinophilia characteristic of the former condition (24, 40). However, we found no concomitant elevation in the number of eosinophils quantified by immunohistochemistry or levels of various prominent mediators of eosinophil proliferation, recruitment, and migration (i.e., IL-4, IL-5, IL-13, eotaxin-1, eotaxin-2, and MCP-4). Our finding supports those of Olze and colleagues, who also found no difference in eotaxin-1 and eotaxin-2 protein levels when they compared NP from aspirin-intolerant patients with those of allergic patients with CRS (41). Unfortunately, owing to specimen availability limitations, additional studies aimed at evaluating a potential correlation between levels of various inflammatory mediators and numbers of eosinophils in NP tissue could not be performed. Such assessments could be the focus of future studies.
Although we found a significant correlation between ECP levels and eosinophil numbers when we evaluated all sinonasal tissue samples together (Figure E1B), when we compared only NP from AERD with NP from CRSwNP, a discrepancy in ECP levels and eosinophil numbers was observed (Figure 3A and Figure E1C). This difference might be explained by the activation and degranulation of eosinophils in NP tissue, leading to accumulation of extracellular ECP. Because degranulated eosinophils would not have the same morphologic characteristics as resting eosinophils, this could potentially explain the inability to detect them by traditional hematoxylin and eosin staining.
In regard to other inflammatory cell types, we additionally did not observe any differences in gene expression levels of a marker for leukocytes (CD45) or mast cells (tryptase) in NP of patients with AERD compared with NP from patients with CRSwNP. This latter observation is in support of previous findings showing similar numbers of mast cells identified in NP of patients with AERD and patients with CRSwNP by immunohistochemical staining (24). However, it is possible that the strong trend toward elevated levels of SCF observed in AERD compared with CRSwNP NP could promote mast cell activation and possibly impact the ability to count these cells. Gene expression levels of CD68, a marker for monocytes, were reduced in AERD NP compared with CRSwNP. This finding is unexpected and worthy of further studies of macrophage number and function in NP of patients with AERD compared with patients with CRSwNP.
Some of our findings are in contrast to other studies that have shown, for example, increased levels of IL-5 (30) or IFN-γ (42) in patients with AERD compared with those with CRSwNP. Explanations for these discrepancies might include differences in systemic corticosteroid use and/or types of assays utilized for analysis. Our study population was not restricted from using oral steroids, whereas systemic steroids were withheld from participants for at least 4 weeks before surgery in the study by Perez-Novo and colleagues (30). However, it should be noted that not all of our patients were taking corticosteroids at the time of the study. Also, even in the presence of some oral corticosteroid use, ECP continued to be elevated in AERD, suggesting that excessive eosinophil recruitment was still occurring. Additionally, our results showing no differences in IFN-γ expression may reflect our measurement of mRNA and total protein levels in frozen tissue homogenates rather the assessment of intracellular protein levels on a per-leukocyte basis from fresh polyp tissue, as performed by Steinke and colleagues (42). Whereas the diagnosis of AERD was made to the best of our clinical abilities, a graded dose aspirin challenge to confirm the diagnosis was not performed in all study patients with CRSwNP. Thus, we cannot completely exclude the possibility that a patient in our CRSwNP group may have actually had AERD or vice versa.
Altogether, our data suggest that mechanisms and factors besides traditional type 2 mediators of eosinophil proliferation and chemotaxis might contribute to the pronounced local eosinophilia in AERD NP. Because arachidonic acid metabolism is dysregulated in AERD, it is possible that enhanced levels of certain metabolites, including cysteinyl leukotrienes, may be contributing to the enhanced eosinophilia observed (20). Previous work has shown that, upon recruitment to the airways, eosinophils can respond to GM-CSF but downregulate the IL-5 receptor α, becoming less responsive to IL-5 (43, 44). Additionally, in a separate study, GM-CSF was found to augment eosinophil survival, even in the presence of glucocorticoids (45). In our present study, patients with AERD were more likely to be taking oral corticosteroids than patients with CRSwNP and were also found to have elevated levels of GM-CSF in NP compared with subjects with CRSwNP. Taken together, GM-CSF may play a role in enhanced eosinophil survival in AERD. Further studies to address whether the levels we observed are physiologically relevant and capable of promoting such eosinophil survival effects are critical.
Weber and colleagues reported that a cleaved variant of MCP-1 containing a deletion of the NH2-terminal residue became a potent activator of eosinophils compared with the full-length MCP-1 (46). Additionally, it has been reported that the chemokine CCL23 can be cleaved by NP tissue into a product with higher receptor binding activity (47). Unfortunately, the multiplex immunoassay used in our study to measure MCP-1 would not be able to discriminate between the full-length and cleaved forms. Nevertheless, it is interesting to speculate that, in AERD NP, MCP-1 could potentially be cleaved into a variant that would promote the enhanced eosinophil infiltration observed in NP tissue. However, additional experiments are necessary to further evaluate this hypothesis.
We found the relative gene expression of tPA to be reduced in NP from patients with AERD compared with those with CRSwNP. In previous work in CRSwNP, investigators hypothesized that a reduction in tPA, an important mediator in fibrinolysis, could in turn contribute to the excessive fibrin deposition and tissue remodeling seen in this disease (26). Such further reductions in tPA in AERD could potentially lead to more enhanced tissue remodeling and thus help explain the clinical observation that AERD NP are more recalcitrant than others to medical and surgical therapies (10–12).
In conclusion, CRS is a heterogeneous disease with varying clinical and inflammatory signature profiles that are dependent not only on the specific CRS subtype but also on the type of sinonasal tissue examined. Altogether, CRSwNP is characterized by a predominant type 2 inflammatory environment, whereas CRSsNP does not necessarily reflect enhanced type 1 inflammation. AERD can be distinguished from CRSwNP by elevated levels of eosinophils, as detected by the eosinophil granule protein ECP, but not by concomitant elevations in traditional type 2 inflammatory mediators involved in eosinophil proliferation and recruitment. However, other factors, including GM-CSF, MCP-1, and tPA, may be important contributors to the eosinophilia as well as to other features of AERD pathogenesis. Further investigation of the cellular and molecular mechanisms involved in AERD pathogenesis could lead to the discovery of novel therapeutic targets that in turn could advance current AERD treatment strategies.
Footnotes
Supported by National Institutes of Health grants T32 AI083216, K12 HD055884, R37 HL068546, R01 HL0788860, and R01 AI104733; the Ernest S. Bazley Foundation; and the Chronic Rhinosinusitis Integrative Studies Program (U19-AI106683).
Author Contributions: C.J.O. and W.W.S.: contributed to data acquisition, data analysis, and authorship of the manuscript; M.S. and M.M.: contributed to data acquisition and analysis; S.B., K. E. Hulse, A.K., T.T., and S.F.: contributed to the data analysis and interpretation; L.S., J.E.N., R.G.C., K. E. Harris, D.B.C., R.K.C., B.K.T., A.T.P., L.C.G., and R.C.K.: involved in patient recruitment, specimen procurement, and specimen processing; R.P.S.: contributed to the conception and design of the work as well as interpretation of the data. All authors provided critical review of the manuscript and approved the final version for publication.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201412-2278OC on June 11, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dykewicz MS, Hamilos DL. Rhinitis and sinusitis. J Allergy Clin Immunol. 2010;125(2) Suppl 2:S103–S115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;144:440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 4.Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86:427–443. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, Grammer LC, Avila PC, Kern RC, Stewart WF, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang JE, White A, Simon RA, Stevenson DD. Aspirin-exacerbated respiratory disease: burden of disease. Allergy Asthma Proc. 2012;33:117–121. doi: 10.2500/aap.2012.33.3541. [DOI] [PubMed] [Google Scholar]
- 7.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: a meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–81.e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Samter M, Beers RF., Jr Concerning the nature of intolerance to aspirin. J Allergy. 1967;40:281–293. doi: 10.1016/0021-8707(67)90076-7. [DOI] [PubMed] [Google Scholar]
- 9.White AA, Stevenson DD. Aspirin-exacerbated respiratory disease: update on pathogenesis and desensitization. Semin Respir Crit Care Med. 2012;33:588–594. doi: 10.1055/s-0032-1325618. [DOI] [PubMed] [Google Scholar]
- 10.Robinson JL, Griest S, James KE, Smith TL. Impact of aspirin intolerance on outcomes of sinus surgery. Laryngoscope. 2007;117:825–830. doi: 10.1097/MLG.0b013e3180333121. [DOI] [PubMed] [Google Scholar]
- 11.Awad OG, Lee JH, Fasano MB, Graham SM. Sinonasal outcomes after endoscopic sinus surgery in asthmatic patients with nasal polyps: a difference between aspirin-tolerant and aspirin-induced asthma? Laryngoscope. 2008;118:1282–1286. doi: 10.1097/MLG.0b013e318170af1e. [DOI] [PubMed] [Google Scholar]
- 12.Kim JE, Kountakis SE. The prevalence of Samter’s triad in patients undergoing functional endoscopic sinus surgery. Ear Nose Throat J. 2007;86:396–399. [PubMed] [Google Scholar]
- 13.Stevens WW, Schleimer RP, Chandra RK, Peters AT. Biology of nasal polyposis. J Allergy Clin Immunol. 2014;133:1503–, e1–e4. doi: 10.1016/j.jaci.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–38. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, Bachert C. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–148. doi: 10.1111/j.1398-9995.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 16.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 18.Derycke L, Eyerich S, Van Crombruggen K, Pérez-Novo C, Holtappels G, Deruyck N, Gevaert P, Bachert C. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, Castells MC, Chhay H, Boyce JA. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–3798. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens WW, Ocampo CJ, Norton J, Carter RG, Suh L, Grammer LC, Hulse KE, Peters AT, Chandra RK, Conley DB, et al. Investigation of molecular characteristics of aspirin exacerbated respiratory disease [abstract] J Allergy Clin Immunol. 2015;135(2 Suppl):AB170. [Google Scholar]
- 23.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, Conley D, Grammer LC, Kern R, Schleimer RP. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121:1385–1392.e2. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, Kern RC, Conley DB, Chandra R, Hulse KE, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133:1759–1763. doi: 10.1016/j.jaci.2013.12.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, Kato A, Chandra RK, Harris KE, Chu HW, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67:920–928. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, Norton J, Grammer LC, Cho SH, Tan BK, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, Norton J, Grammer LC, Tan BK, Chandra RK, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–592.e4. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–1196. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Beck LA, Stellato C, Beall LD, Schall TJ, Leopold D, Bickel CA, Baroody F, Bochner BS, Schleimer RP. Detection of the chemokine RANTES and endothelial adhesion molecules in nasal polyps. J Allergy Clin Immunol. 1996;98:766–780. doi: 10.1016/s0091-6749(96)70126-4. [DOI] [PubMed] [Google Scholar]
- 32.Meyer JE, Bartels J, Görögh T, Sticherling M, Rudack C, Ross DA, Maune S. The role of RANTES in nasal polyposis. Am J Rhinol. 2005;19:15–20. [PubMed] [Google Scholar]
- 33.Van Bruaene N, Pérez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, Schmidt-Weber C, Akdis C, Van Cauwenberge P, Bachert C, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–1441.e3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, Suh LA, Norton J, Harris KE, Grammer LC, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Bruaene N, C PN, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, Gevaert P, Bachert C. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy. 2012;42:883–890. doi: 10.1111/j.1365-2222.2011.03898.x. [DOI] [PubMed] [Google Scholar]
- 36.Seshadri S, Rosati M, Lin DC, Carter RG, Norton JE, Choi AW, Suh L, Kato A, Chandra RK, Harris KE, et al. Regional differences in the expression of innate host defense molecules in sinonasal mucosa. J Allergy Clin Immunol. 2013;132:1227–1230.e5. doi: 10.1016/j.jaci.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123:E72–E78. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 38.Stankovic KM, Goldsztein H, Reh DD, Platt MP, Metson R. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope. 2008;118:881–889. doi: 10.1097/MLG.0b013e31816b4b6f. [DOI] [PubMed] [Google Scholar]
- 39.Berdnikovs S, Kato A, Norton J, Suh L, Kern RC, Conley D, Chandra R, Peters AT, Grammer LC, Harris KE, et al. Meta-analysis of gene expression microarrays reveals novel biomarkers consistent with altered functionality of mucosal barrier in patients with chronic rhinosinusitis [abstract] J Allergy Clin Immunol. 2014;133(2 Suppl):AB236. [Google Scholar]
- 40.Mullol J, Picado C. Rhinosinusitis and nasal polyps in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:163–176. doi: 10.1016/j.iac.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Olze H, Förster U, Zuberbier T, Morawietz L, Luger EO. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006;44:145–150. [PubMed] [Google Scholar]
- 42.Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-γ in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013;132:856–865.e3. doi: 10.1016/j.jaci.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor α on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169:6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 44.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor α on human eosinophils: I. Loss of membrane IL-5 receptor α on airway eosinophils and increased soluble IL-5 receptor α in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 45.Lamas AM, Leon OG, Schleimer RP. Glucocorticoids inhibit eosinophil responses to granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:254–259. [PubMed] [Google Scholar]
- 46.Weber M, Uguccioni M, Baggiolini M, Clark-Lewis I, Dahinden CA. Deletion of the NH2-terminal residue converts monocyte chemotactic protein 1 from an activator of basophil mediator release to an eosinophil chemoattractant. J Exp Med. 1996;183:681–685. doi: 10.1084/jem.183.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato A, Keswani A, Kim J, Poposki J, Peterson S, Suh L, Norton J, Peters AT, Hulse KE, Grammer LC, et al. Post-translational modification by serine proteases controls the CCL23 activity in nasal polyps of chronic rhinosinusitis [abstract] J Allergy Clin Immunol. 2014;133(2 Suppl):AB129. [Google Scholar]