Abstract
Rationale: Microbiologically based criteria such as sputum culture conversion to negative have traditionally been used to define treatment success for mycobacterial diseases. There are, however, limited data regarding whether nontuberculous mycobacterial sputum culture conversion or semiquantitative culture analysis correlates with subjective or nonmicrobiologic objective indices of treatment response.
Objectives: To determine whether a semiquantitative mycobacterial culture scale correlated with clinical disease status and was predictive of long-term sputum mycobacterial culture conversion to negative in a cohort of patients with nodular/bronchiectatic Mycobacterium avium complex lung disease undergoing therapy.
Methods: One hundred and eighty patients undergoing standard macrolide-based therapy for M. avium complex lung disease were monitored at standard frequent intervals with symptomatic, radiographic, and microbiologic data collected, including semiquantitative mycobacterial culture analysis. Analyses were used to evaluate clinical and microbiologic predictors of long-term sputum conversion to culture negative.
Measurements and Main Results: After 12 months of therapy, 148 (82%) patients had sputum conversion to culture negative. Baseline semiquantitative sputum culture scores did not differ between patients with sputum conversion and those without. The change in sputum culture semiquantitative score from baseline to Month 3 was highly predictive of subsequent sputum long-term conversion status indicative of treatment success, as was improvement in cough, and especially early radiographic improvement.
Conclusions: Early semiquantitative sputum agar plate culture results can be used to predict symptomatic and radiographic improvement as well as long-term sputum culture conversion to negative in this population. We suggest that semiquantitative sputum culture scores can be a useful tool for evaluating new nontuberculous mycobacterial lung disease therapies.
Keywords: Mycobacterium avium complex, mycobacterial cultures, mycobacterial therapy
At a Glance Commentary
Scientific Knowledge on the Subject
Microbiologic endpoints have generally been used to define treatment success for nontuberculous mycobacterial lung diseases, although the relation of microbiologic results to other subjective and objective endpoints is not well defined.
What This Study Adds to the Field
Semiquantitative culture analysis during antibiotic therapy for Mycobacterium avium complex lung disease predicts sputum culture conversion and correlates with symptom and radiographic improvement. Semiquantitative sputum analysis may be a useful tool for evaluating nontuberculous mycobacterial therapies in the future.
Treatment of Mycobacteria avium complex (MAC) lung disease is lengthy and complex. Recommended treatment regimens for MAC include a macrolide, ethambutol, and rifamycin administered daily for cavitary disease or three times weekly for nodular/bronchiectatic disease until patients are culture-negative for sputum acid-fast bacilli (AFB) for at least 12 months while on therapy (1). These microbiologic criteria or other similar microbiologically based criteria have traditionally been used to define treatment success for both nontuberculous mycobacterial (NTM) disease as well as tuberculosis (TB) (1–3). In addition, a few studies, primarily from one center, have used semiquantitative sputum AFB culture analysis as an indicator of clinical response to therapy (4–7). However, data are limited regarding whether sputum AFB culture conversion or semiquantitative AFB culture analysis correlates with either subjective indices of treatment response such as patient symptoms or nonmicrobiologic measures of treatment response such as radiographic appearance. The predictive value of semiquantitative sputum AFB culture analysis for successful treatment outcomes as defined by microbiologic criteria has also not been established.
Hales and colleagues noted that the U.S. Food and Drug Administration (FDA) was aware of a lack of attention to the association between microbiologically defined TB treatment outcomes and TB symptoms, and that this observation has implications for the future assessment and approval of new antituberculosis medications (3). Even for TB therapy, the answers are not necessarily self-evident or intuitive as residual symptoms and pulmonary impairments have been reported with microbiologically cured pulmonary TB (3). This shift in focus by the FDA has implications relevant to the assessment and approval of drugs to treat NTM pathogens. Patient-based subjective endpoints tied to objective microbiologic endpoints may be critical components for future NTM treatment trials.
We evaluated whether sputum AFB cultures assessed using a semiquantitative scale in a cohort of 180 patients with nodular/bronchiectatic MAC lung disease undergoing therapy correlated with clinical disease status and if the semiquantitative sputum AFB culture scores were predictive of long-term sputum AFB culture conversion and treatment success.
Methods
The University of Texas Health Science Center (Tyler, TX; UTHSCT) is a referral center for patients with NTM lung disease. The clinical treatment outcome studies, retrospective chart reviews, and maintenance of a database were approved by the UTHSCT Institutional Review Board (IRB #760, #11-009).
For this report we included 180 patients with nodular/bronchiectatic MAC lung disease at the UTHSCT, treated according to standard macrolide-containing treatment regimens as defined by the American Thoracic Society/Infectious Diseases Society of America guidelines with at least 12 months of follow-up (1). Specific treatment regimens and outcomes for these patients have been reported (8). Patients were assessed at regular intervals: monthly for the first 6 months of therapy and then every 2–3 months for the duration of therapy. For each patient, demographic data on age, sex, and race/ethnicity were recorded. For each clinical examination visit, body mass index was recorded; symptoms including cough, hemoptysis, subjective or documented fever greater than 100°F, fatigue, and sputum production were assessed as present or absent, and when present as same, better, or worse relative to the prior assessment.
Mycobacterial cultures were obtained every 1–2 months during the first 12 months of follow-up, with at least six sputum AFB cultures collected from each patient. Three routine expectorated sputum samples for AFB culture were collected at initiation of therapy. For patients unable to produce sputum by spontaneous expectoration, sputum induction was performed with hypertonic (usually 7%) saline administered by nebulizer with instructions on how to expectorate and directions for home use. Patients initially diagnosed by bronchoscopy did not routinely undergo repeat bronchoscopies to obtain specimens as most of these patients were successful in submitting sputum samples after induction.
Sputum samples were processed in the UTHSCT clinical laboratory, using standard decontamination procedures, fluorochrome microscopy, solid medium culture on a biplate of Middlebrook 7H10 agar with and without antibiotics, and a broth culture (BACTEC 960 [Becton Dickinson, Sparks, MD] or ESP [TREK Diagnostic Systems, Cleveland, OH]) as previously described (4, 5). MAC isolates were identified with AccuProbe (Hologic-GenProbe, San Diego, CA).
Semiquantitative AFB smear and culture results for each submitted clinical specimen during and after therapy were recorded as previously described (4, 5). Briefly, a negative culture exhibited no mycobacterial growth. Cultures were reported as positive if growth occurred in broth medium only; growth on broth medium plus solid medium cultures with countable colonies were reported as 0–49 colonies, 1+; solid medium growth with 50–99 colonies, 2+; solid medium growth with 100–199 colonies; 3+, solid medium growth with 200–299 colonies; and 4+, solid medium growth with at least 300 colonies. For data analysis, each culture was scored as follows: 0, no growth in broth or solid medium; 1, broth medium growth only; 2, countable colonies (<50 colonies) on solid medium; and 3–6, 1+ to 4+ growth on solid medium, respectively. Patients who demonstrated no mycobacterial growth (a score of “0”) for three or more consecutive cultures over a minimum of 3 months were labeled as “converters.”
Routine chest radiographs (CXR) and high-resolution computed tomography (HRCT) chest scans were performed at the start of therapy and then at 1- to 2-month intervals for chest radiographs and 6-month intervals for chest CT scans, with more frequent chest CT scans obtained at the discretion of the provider. Baseline and follow-up chest radiographs and/or CT scans were reviewed by the clinician caring for the patient and a radiologist experienced in reviewing radiographs from MAC patients with nodular/bronchiectatic disease. A second radiologist reviewed the patient radiographs in a blinded manner. The radiographs were classified as improved (score, 1), no change (score, 0), or worse (score, –1) when compared with the prior radiograph. The specific score assignment was based on a global or overall impression of the radiographic appearance, which frequently included a “mixed” response with some areas of improvement and some areas of increased density compared with the prior radiographic study. Discrepancies between the radiologist’s and clinician’s interpretation of the radiographs were resolved in favor of the second radiologist blinded to the clinical status of the patient.
Descriptive statistics and Student's t tests were used to compare differences between converters and nonconverters and to describe the time to culture conversion. Because the percentage of converters is expected to exceed 10% (and would therefore represent a common outcome event), both univariate generalized linear models (with a log link and Poisson distribution), which produce more conservative risk ratio (RR) estimates, and logistic regression models (9) were used to identify predictors of early (within 3 mo of treatment initiation) culture conversion. Variables that were significant (P < 0.05) in univariate models were assessed for correlation. Uncorrelated significant variables were evaluated in multivariate models that also controlled for sex and age. Odds ratios (ORs) and RRs and 95% confidence intervals (CIs) were reported for significant variables; adjusted ORs (aORs) and adjusted RRs (aRRs) were reported for multivariate models. Additional correlations among semiquantitative culture data with reported symptom and radiographic data at various time points throughout the follow-up period were assessed. All analyses were conducted with Statistical Analysis Software (SAS) version 9.3 (SAS Institute, Cary, NC).
Results
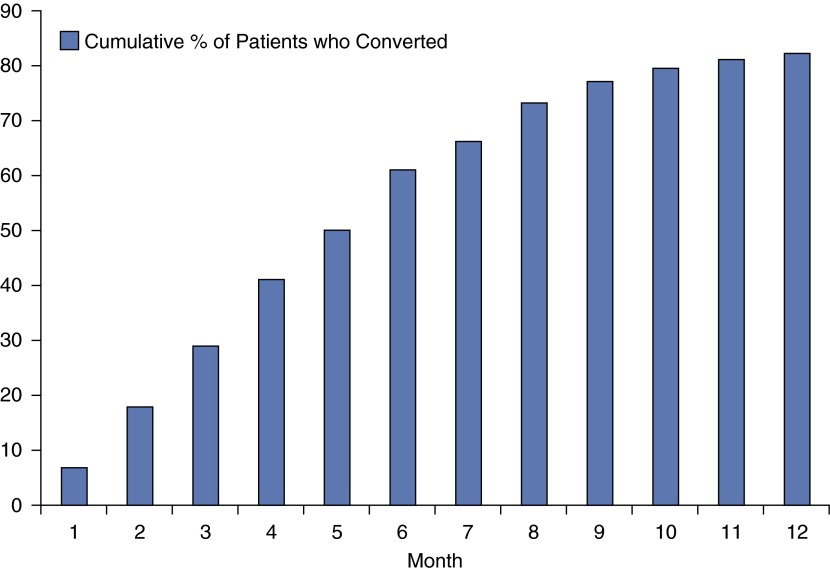
Of 180 patients (mean age at diagnosis, 75 ± 14 yr old; 93% female), 148 (82%) met the definition of sputum conversion within 12 months of initiating antibiotic therapy (Figure 1). Converters and nonconverters were similar with respect to age, race/ethnicity, body mass index, and sex (P > 0.4) at baseline. Patients who converted from positive to negative had their first negative sputum AFB culture in a median of 110 days (range, 4–355 d) after treatment initiation. One hundred and twenty-three patients (83%) who had sputum conversion had their first negative culture within 6 months of starting therapy. Of these 123 patients, 52 (42%) had at least one episode of respiratory deterioration attributed to bronchiectatic exacerbation requiring additional antibiotic coverage (usually a fluoroquinolone) compared with 38 of 47 (81%) patients who had sputum conversion more than 6 months after starting MAC therapy or who did not convert their sputum to negative after 12 months of therapy (P < 0.0001). Twenty-eight of 38 (74%) of these latter patients had at least two exacerbations versus 17 of 52 patients (33%) who converted within the first 6 months of therapy (P < 0.0001). The bronchiectatic exacerbations occurred serendipitously throughout the study, with no discernable pattern related to their MAC treatment course.
Figure 1.
Cumulative percentage of patients who converted from positive to negative cultures by month of follow-up time.
Semiquantitative Culture Scores
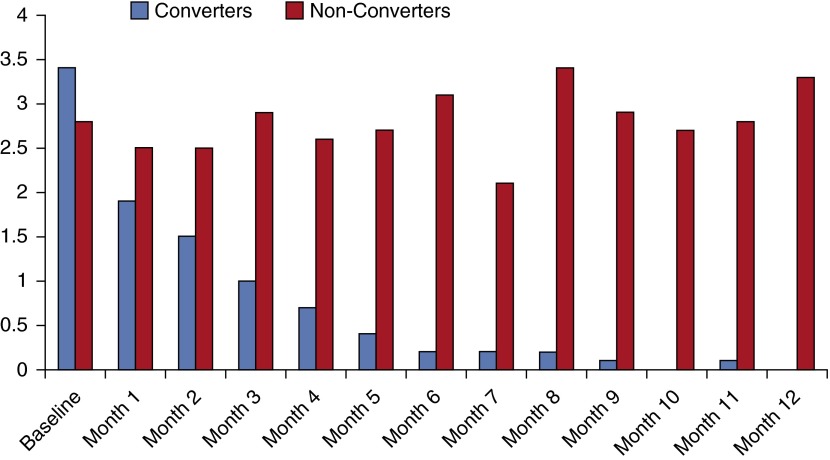
At baseline, mean semiquantitative mycobacterial culture scores did not differ significantly between converters and nonconverters, and were in fact slightly higher among converters (3.4 vs. 2.8; P = 0.09) (Figure 2). However, by 1 month after treatment initiation, converters were already scoring lower on average than nonconverters (1.9 vs. 2.5), and this difference was significant by Month 2 (1.5 vs. 2.5; P = 0.01). Over the 1-year period, the scores of converters consistently dropped each consecutive month, whereas the scores of nonconverters never averaged less than 2.1; by 12 months, nonconverters had an average score of 3.3, which was even greater than the mean baseline values.
Figure 2.
Mean semiquantitative culture scores (vertical axis) for Mycobacterium avium complex by patient conversion status at baseline, 1 year after treatment initiation.
The change in semiquantitative culture scores from baseline to Month 2 was significantly predictive of conversion status by 12 months in all models (univariate results shown in Table 1; multivariate results controlling for age, sex, and including significant, uncorrelated variables were similar). Specifically, with each additional one-point improvement (i.e., a lower value) in culture scores from baseline to Month 2, the odds of a patient converting were seven times greater in both univariate and multivariate models (OR = 6.9, 95% CI = 2.9–16.6, P < 0.0001; aOR = 7.2, 95% CI = 2.3–22.0, P = 0.0006); in more conservative risk ratio models, with each one-point decrease in culture score a patient was 20% more likely to convert to negative (RR = 1.2, 95% CI = 1.1–1.3; P < 0.0001) by 1 year of follow-up time.
Table 1.
Predicted Odds and Risk of Converting from Positive to Negative for Mycobacterium avium Complex by Early Semiquantitative Cultures, Symptom Presentation, and Radiologic Scores*
| Univariate Generalized Linear Models |
Univariate Logistic Regression Models |
|||||
|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | P Value | OR | 95% CI | P Value |
| Culture results | ||||||
| Baseline culture | 1 | 0.90–1.02 | 0.2 | 0.8 | 0.6–1.0 | 0.2 |
| Culture Month 2 | 1.1 | 1.0–1.2 | 0.02 | 1.4 | 1.1–2.0 | 0.01 |
| Culture Month 3 | 1.2 | 1.1–1.4 | <0.0001 | 2.1 | 1.5–3.0 | <0.0001 |
| Change between baseline and Month 2 | 1.2 | 1.1–1.3 | <0.0001 | 6.9 | 2.9–16.6 | <0.0001 |
| Change between baseline and Month 3 | 1.2 | 1.1–1.3 | <0.0001 | 3.9 | 2.2–6.9 | <0.0001 |
| Symptoms | ||||||
| Improvement in cough between baseline and next clinical assessment | 1 | 1.002–1.004 | <0.0001 | 9.0 | 3.2–25.8 | <0.0001 |
| Radiology | ||||||
| Improvement in CXR between baseline and next clinical assessment | 1.3 | 1.2–1.5 | <0.0001 | 6.6 | 2.5–17.4 | 0.0001 |
| Improvement in CT between baseline and next clinical assessment | 2.3 | 1.5–3.5 | <0.0001 | 8.0 | 3.6–18.0 | <0.0001 |
Definition of abbreviations: CI = confidence interval; CT = computed tomography; CXR = chest X-ray; OR = odds ratio; RR = relative risk.
Results presented here are similar to those observed in multivariate models that included significant, uncorrelated variables from univariate models and were controlled for age and sex (multivariate results reported in text only and are not shown here for the sake of brevity).
Represents the risk of converting with each one-unit decrease in semiquantitative culture scores.
Symptom Presentation
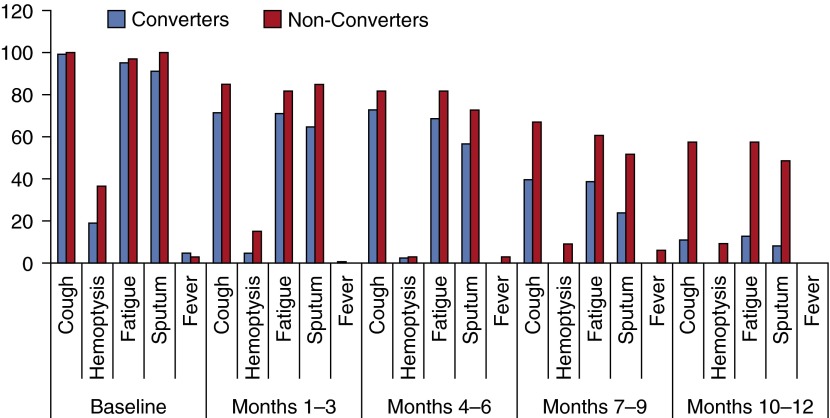
At baseline, converters and nonconverters were similar regarding their symptom presentation (Figure 3; Table 1). Virtually all patients presented initially with cough, sputum and fatigue, although a significantly greater percentage of nonconverters (36%) presented with hemoptysis than did converters (19%) (P = 0.04). Few patients (<5%) had a subjective or documented fever greater than 100°F at baseline or any visit thereafter.
Figure 3.
Percentage of patients reporting symptoms (vertical axis) at baseline, and from 1–3, 4–6, 7–9, and 10–12 months of follow-up time, by long-term sputum conversion status. Number of patients observed for symptoms varies by month of follow-up.
Patients who showed clinical improvement in their cough between baseline and their next assessment were more likely (P < 0.0001) to ultimately convert in all models, although the actual risk difference was minimal (Table 1). Even regardless of conversion status, patients with a lingering cough throughout the study period had a higher semiquantitative culture score than those who no longer had a cough present by the end of the study (mean score among patients with no cough present in Month 12, 0.7 ± 1.2; mean score among patients with cough present in Month 12, 2.2 ± 2.0). With the exception of sputum production, the presence of all other symptoms evaluated (fatigue, hemoptysis, fever) was significantly although weakly correlated with the semiquantitative culture result when evaluated at monthly time intervals throughout the follow-up study period (P < 0.003; correlation coefficient range, 0.1–0.2).
Radiologic Evaluations
Converters demonstrated significantly (P < 0.0001) greater radiologic improvement from their baseline to next radiologic assessment than did nonconverters in both CT scans (mean improvement score, 0.9 vs. –0.04) and CXRs (mean improvement score, 0.5 vs. 0.03), as well as over the entire study (mean CT improvement score, 0.9 vs. –0.09; mean CXR improvement score, 0.6 vs. 0.1). When assessing correlations among semiquantitative culture results and radiologic improvement across the entire study period, the change in culture scores from baseline through Month 2 was significantly associated with clinical improvement in CT scans (r = 0.5, P < 0.0001) and CXRs (r = 0.2, P < 0.02) during that same time period.
For patients demonstrating early radiologic improvement by their second assessment, which on average occurred 59 (±34) days postbaseline, the odds of converting to negative during the study period ranged from seven to eight times greater than for those who did not (Table 1); although a lower magnitude of effect was observed, conservative risk ratio models demonstrated similar findings, with early radiologic improvement doubling the likelihood of conversion by 12 months out (CT scans: RR = 2.3, 95% CI = 1.5–3.5, P < 0.0001; CXRs: RR = 1.3, 95% CI = 1.2–1.5, P < 0.0001). Multivariate models demonstrated similar results, although the magnitude of effect of radiologic improvement increased slightly across all models (CT: aOR = 10.0, 95% CI = 3.2–31.1, P < 0.0001; aRR = 2.4, 95% CI = 1.5–3.6, P < 0.0001; CXR: aOR = 3.7, 95% CI = 1.1–12.3, P = 0.03; aRR = 1.4, 95% CI = 1.2–1.6, P < 0.0001).
Discussion
In this study of 180 patients undergoing therapy for nodular/bronchiectatic MAC lung disease we found that changes in early semiquantitative sputum AFB culture scores were significantly predictive of subsequent sputum AFB culture conversion to negative. In addition, change in sputum AFB semiquantitative culture score and symptom response were significantly correlated, and in particular, improvement in patient cough was significantly predictive of subsequent sputum AFB culture conversion. Sputum AFB semiquantitative sputum culture scores were also significantly associated with radiographic improvement on therapy.
Among clinical experts treating MAC lung disease, confidence in MAC treatment success is variable with estimated values for sputum culture conversion ranging from 70 to 84% (8, 10). In contrast, treatment success rates for drug-susceptible pulmonary TB are significantly higher than that for NTM lung disease in general and MAC lung disease specifically (11). Admittedly, there are significant demographic and socioeconomic differences between TB and MAC lung disease populations that might also influence these outcomes. However, the experience with TB, a disease for which potent antimicrobials are available and that is almost universally cured microbiologically, still offers some insights into the evaluation of NTM lung disease.
Hales and colleagues published the results of a retrospective analysis of four clinical trials in which participants had culture-positive TB, standardized symptom assessment, and follow-up mycobacterial cultures (3). The presence of concurrent productive cough during treatment was consistently associated with sputum culture positivity. Productive cough and fever were also associated with culture-confirmed treatment failure and recurrence. Whereas fever and sweats improved rapidly with treatment, productive cough decreased more slowly and was present in 20% of visits after treatment completion. Therefore, although sputum culture conversion to negative was significantly associated with symptom improvement there was less than universal symptomatic resolution with treatment (3).
For patients with MAC lung disease who are treated with less potent drugs than patients with TB and who, without exception, have a comorbid lung condition, the complexity and difficulty of symptom analysis are compounded. Bronchiectasis specifically presents a formidable impediment to analysis of treatment response because of the waxing and waning of bronchiectasis-related symptoms. Because some patients were comanaged by physicians outside our facility, we cannot be certain we captured all bronchiectatic exacerbation episodes. In that context, bronchiectatic exacerbations occurred frequently for patients with sputum conversion within 6 months of starting MAC therapy and were almost universal for patients who took longer than 6 months for sputum conversion or who did not have sputum conversion. These latter patients were also more likely to have multiple bronchiectatic exacerbations, which is almost certainly a consequence of their longer observation period. Bronchiectatic exacerbations occurred for many patients at the time of clinical assessment for MAC disease, thus obfuscating any symptomatically beneficial effect of the MAC therapy.
Even at baseline, bronchiectasis-related symptoms overlap significantly with MAC lung disease symptoms and a variety of measures, especially airway clearance strategies, can impact the bronchiectasis-related symptoms (12–19). With the exception of universal macrolide administration, however, the approach to bronchiectasis management in this patient cohort was not uniform because many patients were treated by referring physicians for their bronchiectasis. The coexistence of the two conditions and the frequent bronchiectatic exacerbations make attribution of symptoms at a specific point in time difficult.
In spite of the confounding effect of waxing and waning bronchiectasis-related symptoms we still found a significant correlation between improved sputum AFB semiquantitative sputum scores and improvement in cough as well as sputum conversion. We also found significant, albeit relatively weak, associations between fatigue, hemoptysis, and fever and AFB semiquantitative sputum scores. The lack of more impressive improvement in fatigue may be a consequence of persistent lung inflammation due to bronchiectasis that is independent of MAC infection and therapy. Testing that assertion would require uniform and aggressive bronchiectasis treatment coadministered with MAC therapy.
Those attempting to evaluate the efficacy of NTM treatment strategies with nonmicrobiologic indices must take into account the inherent limitations imposed by NTM lung disease over and above those associated with TB. The inevitable presence of bronchiectasis in patients with nodular/bronchiectatic MAC lung disease dictates that the correlation between microbiologic response and clinical response to therapy will not be as robust as that seen for patients with TB (3). Even with the limitations and obstacles posed by nodular/bronchiectatic MAC disease, sputum semiquantitative culture analysis was still predictive of symptomatic and radiographic response and ultimately sputum conversion for our patients with MAC lung disease.
The Clinical and Laboratory Standards Institute (CLSI) recommends that broth and solid agar media cultures should be routinely done on all AFB specimens (20). It is evident that some mycobacteriology laboratories do not comply with this recommendation in that (1) they do not use agar plate AFB cultures; (2) they do not report their solid medium culture results; (3) they use only agar slants, which do not allow for quantitation of colonies; or (4) they report agar plate results as positive or negative without quantitation of colonies. The CLSI stated in 2008 that although a recommendation for semiquantitative cultures “should be applied cautiously and in consideration of other factors, including the condition of the host and clinical setting especially when evaluating specimens other than sputum, [semiquantitative results] may help to clarify the degree to which mycobacterial burden can be used to indicate clinical relevance of culture results” (20). Moreover, in 2000, the British Thoracic Society urged “consideration of the degree of bacterial growth and the number of times isolated as an indicator of the relevance of NTM recovery to health” (21).
Our study has some unique aspects that may affect the generalizability of these findings. The data generated here were collected from a single center that has been doing semiquantitative sputum AFB culture analysis for more than two decades. Most sputum specimens submitted for AFB analysis in this study were collected on an outpatient basis and sent by mail or other courier, so that the sample collection method including sputum volume was not uniform. The strong correlation found between semiquantitative culture analysis and both subjective and objective indices suggests that this method of sputum collection is not inadequate and could be applicable to other settings. Because our patient population was limited to those with nodular/bronchiectatic MAC disease, the relevance of these findings, namely the use of semiquantitative scales for predicting disease progression for cavitary MAC disease or lung disease caused by other NTM, remains unproven.
Overall, we found that for patients with nodular/bronchiectatic MAC lung disease treated by antibiotic therapy, semiquantitative sputum culture scores were predictive of long-term sputum conversion, indicative of treatment success; and were correlated with reduced patient symptoms, especially cough, as well as radiographic improvement. We suggest that semiquantitative sputum culture scores can be a useful tool for evaluating new NTM lung disease therapies. Specifically, in place of prolonged and expensive full treatment courses, decreasing quantitative culture scores in the first 2 months of therapy could be used as a surrogate outcome measure for eventual sputum conversion in this disease phenotype. On the basis of these data we believe a recommendation to the CLSI to add Middlebrook 7H10 or 7H11 agar plate cultures, instead of LJ or Middlebrook agar slants, including semiquantitative colony counts would improve the care of patients with nodular/bronchiectatic MAC lung disease. Further study is required to determine the applicability of this approach in the setting of cavitary MAC lung disease or with other NTM respiratory pathogens.
Footnotes
Supported in part by institutional funds from the University of Texas Health Science Center, Tyler, the Amon Carter Foundation (R.J.W.), the Moncrief Foundation (D.E.G.), and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (J.A. and D.R.P.).
Author Contributions: All authors met all four criteria for authorship as suggested by the International Committee of Medical Journal Editors (ICMJE), specifically, substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Originally Published in Press as DOI: 10.1164/rccm.201503-0444OC on June 11, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. ATS Mycobacterial Diseases Subcommittee American Thoracic Society Infectious Diseases Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases Am J Respir Crit Care Med 2007175367–416.[Published erratum appears in Am J Respir Crit Care Med 175:744–745.] [DOI] [PubMed] [Google Scholar]
- 2.Wallace RJ, Jr, Glassroth J, Griffith DE, Olivier KN, Cook JL, Gordin F. American Thoracic Society; Medical Section of the American Lung Association. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997;156(2) Suppl:S1–S25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 3.Hales CM, Heilig CM, Chaisson R, Leung CC, Chang KC, Goldberg SV, Gordin F, Johnson JL, Muzanyi G, Saukkonen J, et al. The association between symptoms and microbiologically defined response to tuberculosis treatment. Ann Am Thorac Soc. 2013;10:18–25. doi: 10.1513/AnnalsATS.201207-038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT, Onyi GO, Steingrube VA, Mazurek GH. Initial clarithromycin monotherapy for Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994;149:1335–1341. doi: 10.1164/ajrccm.149.5.8173775. [DOI] [PubMed] [Google Scholar]
- 5.Wallace RJ, Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: the first 50 patients. Am J Respir Crit Care Med. 1996;153:1766–1772. doi: 10.1164/ajrccm.153.6.8665032. [DOI] [PubMed] [Google Scholar]
- 6.Griffith DE, Brown BA, Girard WM, Griffith BE, Couch LA, Wallace RJ., Jr Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin Infect Dis. 2001;32:1547–1553. doi: 10.1086/320512. [DOI] [PubMed] [Google Scholar]
- 7.Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ., Jr Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus–negative patients. J Infect Dis. 1998;178:121–126. doi: 10.1086/515597. [DOI] [PubMed] [Google Scholar]
- 8.Wallace RJ, Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, York DS, Shepherd S, Griffith DE. Macrolide/azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014;146:276–282. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilber ST, Fu R. Risk ratios and odds ratios for common events in cross-sectional and cohort studies. Acad Emerg Med. 2010;17:649–651. doi: 10.1111/j.1553-2712.2010.00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Marras TK, Prevots DR, Jamieson FB, Winthrop KL Pulmonary MAC Outcomes Group. Variable agreement among experts regarding Mycobacterium avium complex lung disease. Respirology. 2015;20:348–351. doi: 10.1111/resp.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn DL, Catlin BJ, Peterson KL, Judson FN, Sbarbaro JA. A 62-dose, 6-month therapy for pulmonary and extrapulmonary tuberculosis: a twice-weekly, directly observed, and cost-effective regimen. Ann Intern Med. 1990;112:407–415. doi: 10.7326/0003-4819-76-3-112-6-407. [DOI] [PubMed] [Google Scholar]
- 12.Lee AL, Burge A, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. 2013;5:CD008351. doi: 10.1002/14651858.CD008351.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Nicolson CH, Stirling RG, Borg BM, Button BM, Wilson JW, Holland AE. The long term effect of inhaled hypertonic saline 6% in non–cystic fibrosis bronchiectasis. Respir Med. 2012;106:661–667. doi: 10.1016/j.rmed.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med. 2011;105:1831–1835. doi: 10.1016/j.rmed.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Vallilo CC, Terra RM, de Albuquerque AL, Suesada MM, Mariani AW, Salge JM, da Costa PB, Pêgo-Fernandes PM. Lung resection improves the quality of life of patients with symptomatic bronchiectasis. Ann Thorac Surg. 2014;98:1034–1041. doi: 10.1016/j.athoracsur.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Olveira C, Olveira G, Gaspar I, Dorado A, Cruz I, Soriguer F, Quittner AL, Espildora F. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res. 2013;22:597–605. doi: 10.1007/s11136-012-0188-5. [DOI] [PubMed] [Google Scholar]
- 17.Girón Moreno RM, Fernandes Vasconcelos G, Cisneros C, Gómez-Punter RM, Segrelles Calvo G, Ancochea J. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Arch Bronconeumol. 2013;49:415–420. doi: 10.1016/j.arbres.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Wilson CB, Jones PW, O’Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J. 1997;10:1754–1760. doi: 10.1183/09031936.97.10081754. [DOI] [PubMed] [Google Scholar]
- 19.Goyal V, Chang AB. Combination inhaled corticosteroids and long-acting β2-agonists for children and adults with bronchiectasis. Cochrane Database Syst Rev. 2014;6:CD010327. doi: 10.1002/14651858.CD010327.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical Laboratory Standards Institute. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. Laboratory detection and identification of mycobacteria; approved guideline. CLSI document M48-A. [Google Scholar]
- 21.Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Thorax. 2000;55:210–218. doi: 10.1136/thorax.55.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]