Lymphoid neogenesis, the formation of tertiary lymphoid structures (1), within distal lung parenchyma indisputably characterizes advanced chronic obstructive pulmonary disease (COPD) (2, 3). Many such lung lymphoid follicles (LLFs) contain germinal centers, indicating immunoglobulin class-switching. Some LLFs in advanced COPD produce auto-antibodies that could fuel lung destruction (4, 5). These findings make lung B cells highly suspicious, especially when congregating in LLF.
However, proximity does not prove culpability. It is challenging to know conclusively when LLF are detrimental and when they are responding appropriately to threatening microbial signals. The stakes on this question are substantial. COPD is on track to become the world’s leading killer within this century. Therapies proven to arrest its progression in individual patients are lacking. B cells could be eliminated therapeutically in COPD using rituximab, if proven to be prudent. Are LLF B cells in advanced COPD bad, or beneficial? Or might they have begun good but become subverted by the processes they sought to control?
In this issue of the Journal, new clues come from two groups (6, 7) with long-term interest in this question. Polverino and colleagues (pp. 695–705) primarily used human lung tissue resected for clinical indications supplemented by in vitro experiments (6), whereas Seys and colleagues (pp. 706–718) also employed their established murine cigarette smoke-exposure model (7). Both studies convincingly associate LLF accumulation in COPD with dysregulation of B-cell activating factor (BAFF), and the latter found that blocking BAFF partially reduced lung pathology in mice (7).
BAFF is a 285-amino acid glycoprotein essential for the survival of conventional B cells beyond the first (T1) post–bone marrow stage. Known formally as tumor necrosis factor ligand superfamily member 13B (TNFSF-13B) (plus multiple aliases reflecting discovery by several groups), BAFF exists as a transmembrane protein (CD257) and as active cleaved and secreted soluble molecules. Its predominant sources are monocytes, macrophages, conventional and follicular dendritic cells, and some T cells. Human B cells do not normally produce BAFF, but can during Epstein Barr virus infection or after in vitro stimulation via their B-cell antigen receptor plus CD40 (8). BAFF deficiencies are associated with immunoglobulin deficiency, whereas excesses are found in autoimmune diseases including systemic lupus. BAFF binds to three receptors, including most specifically and avidly to BAFFR.
These interesting papers contain both congruent and complementary findings. Each elegantly demonstrated BAFF expression within human LLF. Seys and colleagues found increased lung concentrations of both BAFF messenger RNA and protein in Global Initiative for Obstructive Lung Disease (GOLD) stage II COPD, relative to never-smokers, but importantly, neither differed significantly from smokers without obstruction (7). Focusing on LLF and examining a wider COPD severity range, Polverino and colleagues demonstrated that BAFF expression by LLF B cells correlated directly with LLF size and COPD spirometric severity, and inversely with apoptotic B-cell detection (6). They further showed that cigarette smoke extract induced concentration-dependent effects on murine B cells in vitro: low levels induced BAFF, whereas high levels caused apoptosis that could be blocked by exogenous BAFF, unexpectedly in a nuclear factor-κB-dependent manner (6). Closing the loop mechanistically, Seys and colleagues tested BAFF blockade in their murine model, using a decoy receptor (BAFFR-Fc) both prophylactically and therapeutically. BAFFR-Fc administration reduced LLF extent and lung inflammation and reduced emphysema, but only when used prophylactically, and then only as assessed by destructive index, but not by mean linear intercept.
Both studies meticulously employed cutting-edge techniques and a robust number of human subjects (146 [6] and 70 [7], respectively). Limitations are minor (e.g., immunolocalization was complicated by the likelihood that not all BAFF was cell-associated), leading to the prudent characterization of staining, as “in the vicinity of” B cells, CD4+ (but not CD8+) T cells, and fibroblastic reticular cells (7). The latter are one of three stromal cell types essential for lymphoid neogenesis (9), a point adding to mechanistic understanding of BAFF in COPD.
So, do these novel discoveries constitute another “smoking gun” (10) establishing adaptive immunity as central to COPD progression? Before reaching that verdict, we urge caution, based on the timing of LLF expansion, confirmed by both studies to be primarily late during COPD progression, and the dominant role in immune system development of interactions with microbes, especially bacteria.
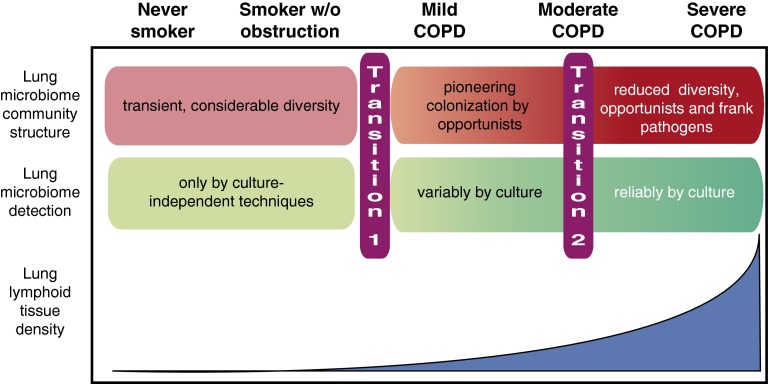
In multiple longitudinal studies, FEV1 declined more greatly in spirometrically mild COPD than in advanced disease. Yet lung B-cell (and T-cell) abundance is low in GOLD stage I COPD (2), unlike the expansion of lung mononuclear phagocytes that begins soon after initiating smoking (11). This disparity between histology and spirometry suggests that, despite the intriguing effects of BAFF blockade in mice (7), whatever processes drive the initial transition from preserved to impaired lung function in some human smokers (Figure 1, transition 1), they are unlikely to be caused by lung B cells, as neither they nor LLF are common at that stage.
Figure 1.
Relationship between lung microbiome community structure and intrapulmonary lymphoid tissue abundance in cigarette smoking–induced chronic obstructive pulmonary disease (COPD). In never-smokers and smokers without airflow obstruction, lung bacterial communities are diverse and shaped by repeated elimination of bacteria derived from the upper airway. Lung bacteria are detectable only by sequencing, and some may be nonviable. In mild to moderate COPD, although sequencing indicates that microbe numbers are not necessarily greater, they become variably detectable by culture (indicating viability). Whether this change (transition 1) causes obstruction or is a marker of shared underlying causes is undefined. A further change occurs in moderate to severe COPD (transition 2), with reduced diversity and predominance of opportunistic and even pathogenic species. The relationship of this transition to spirometric severity differs between COPD phenotypes, and whether it drives progression outside exacerbations is unproven. Importantly, expansion of lung lymphoid follicles occurs relatively late during pathogenesis and parallels altered microbial community structure.
Similarly, although the lung microbiome community structure of smokers without airflow obstruction does not differ from that of never-smokers (12), what data exist (13) indicate it changes progressively in the fraction of smokers who progress to COPD (Figure 1, transition 2). No publications have yet directly related reduced lung microbiome diversity to LLF extent. Nevertheless, reasonable doubt should exist that early lung lymphoid neogenesis develops independent from lung microbiome alteration, which neither study claims. Conversely, autonomous BAFF elaboration could plausibly contribute to dysregulated inflammation or even frank autoimmunity in advanced COPD, especially combined with the known defective function of both T-regulatory and T-helper cells (14, 15).
Hence, important new insights have been gained from these papers (6, 7), but even more must be learned before novel therapies, including the several BAFF-inhibitory drugs under evaluation in multiple myeloma, can be contemplated for use in COPD. The markedly abnormal lung microbiome of some patients with advanced COPD likely drives symptoms and contributes to exacerbation severity, and thereby mortality. Specific IgA may be important for these patients, and is certainly essential to maintain homeostasis with our gut microbiome. Moreover, B cells also inhibit inflammation via IL-10 or IL-35 (16), which merit greater study in COPD. Before testing BAFF elimination, it will be crucial to identify and validate biomarkers that define in exactly which COPD phenotypes lung B cells are misbehaving versus beneficial. Intervening earlier in COPD progression will require determining whether lung dysbiosis, perhaps in association with specific COPD endotypes, causes the initial transition to airflow obstruction (Figure 1, transition 1) or is simply a biomarker for the processes that do. Both goals can be accomplished most expediently by longitudinally analyzing the interplay of pulmonary immunity, lung microbiome, and clinical outcomes in cohorts of patients with COPD of distinct phenotypes and a range of severities.
Acknowledgments
Acknowledgment
The authors thank Dr. Robert P. Dickson, Dr. Peter M. Henson, Dr. Carlos H. Martinez, and all the members of the SPIROMICS Steering Committee for helpful discussions.
Footnotes
The authors are supported by Merit Review Awards I01 CX000911 from the Clinical Science Research and Development Service and I01 BX001389 from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs; and U01 HL098961 and R01 HL114447 and Contract No. HHSN26820090016C from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. 2014;124:953–959. doi: 10.1172/JCI71611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.van der Strate BW, Postma DS, Brandsma CA, Melgert BN, Luinge MA, Geerlings M, Hylkema MN, van den Berg A, Timens W, Kerstjens HA. Cigarette smoke-induced emphysema: A role for the B cell? Am J Respir Crit Care Med. 2006;173:751–758. doi: 10.1164/rccm.200504-594OC. [DOI] [PubMed] [Google Scholar]
- 4.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 5.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:156–163. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, Rios A, Jahn A, Sauleda J, Divo M, et al. B cell–activating factor: an orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seys LJM, Verhamme FM, Schinwald A, Hammad H, Cunoosamy DM, Bantsimba-Malanda C, Sabirsh A, McCall E, Flavell L, Herbst R, et al. Role of B cell–activating factor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:706–718. doi: 10.1164/rccm.201501-0103OC. [DOI] [PubMed] [Google Scholar]
- 8.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–317. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Aguzzi A, Kranich J, Krautler NJ. Follicular dendritic cells: origin, phenotype, and function in health and disease. Trends Immunol. 2014;35:105–113. doi: 10.1016/j.it.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Kheradmand F, Shan M, Corry DB. Smoking gun: mature dendritic cells in human lung provide clues to chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1166–1167. doi: 10.1164/rccm.200909-1391ED. [DOI] [PubMed] [Google Scholar]
- 11.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291:755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- 12.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, et al. Lung HIV Microbiome Project. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sze MA, Dimitriu PA, Suzuki M, McDonough JE, Campbell JD, Brothers JF, Erb-Downward JR, Huffnagle GB, Hayashi S, Elliott WM, et al. The host response to the lung microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:438–445. doi: 10.1164/rccm.201502-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barceló B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agustí AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31:555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 15.Freeman CM, McCubbrey AL, Crudgington S, Nelson J, Martinez FJ, Han MK, Washko GR, Jr, Chensue SW, Arenberg DA, Meldrum CA, et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS One. 2014;9:e96421. doi: 10.1371/journal.pone.0096421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]