Abstract
Rationale: Smoking-related microvascular loss causes end-organ damage in the kidneys, heart, and brain. Basic research suggests a similar process in the lungs, but no large studies have assessed pulmonary microvascular blood flow (PMBF) in early chronic lung disease.
Objectives: To investigate whether PMBF is reduced in mild as well as more severe chronic obstructive pulmonary disease (COPD) and emphysema.
Methods: PMBF was measured using gadolinium-enhanced magnetic resonance imaging (MRI) among smokers with COPD and control subjects age 50 to 79 years without clinical cardiovascular disease. COPD severity was defined by standard criteria. Emphysema on computed tomography (CT) was defined by the percentage of lung regions below −950 Hounsfield units (−950 HU) and by radiologists using a standard protocol. We adjusted for potential confounders, including smoking, oxygenation, and left ventricular cardiac output.
Measurements and Main Results: Among 144 participants, PMBF was reduced by 30% in mild COPD, by 29% in moderate COPD, and by 52% in severe COPD (all P < 0.01 vs. control subjects). PMBF was reduced with greater percentage emphysema−950HU and radiologist-defined emphysema, particularly panlobular and centrilobular emphysema (all P ≤ 0.01). Registration of MRI and CT images revealed that PMBF was reduced in mild COPD in both nonemphysematous and emphysematous lung regions. Associations for PMBF were independent of measures of small airways disease on CT and gas trapping largely because emphysema and small airways disease occurred in different smokers.
Conclusions: PMBF was reduced in mild COPD, including in regions of lung without frank emphysema, and may represent a distinct pathological process from small airways disease. PMBF may provide an imaging biomarker for therapeutic strategies targeting the pulmonary microvasculature.
Keywords: pulmonary microvascular blood flow (PMBF), gadolinium-enhanced MRI, chronic obstructive pulmonary disease (COPD), lung emphysema, small airway disease
At a Glance Commentary
Scientific Knowledge on the Subject
Smoking-related microvascular loss causes end-organ damage in multiple organs. Basic research suggests that a similar process occurs in the lungs; normal smokers with computed tomography (CT) signs of centrilobular emphysema have increased pulmonary perfusion heterogeneity, and blood vessels observable on noncontrast CT are reduced in mostly severe chronic obstructive pulmonary disease (COPD). However, studies demonstrating reduced pulmonary microvascular blood flow (PMBF) in COPD and particularly mild COPD are lacking.
What This Study Adds to the Field
Using noninvasive, gadolinium-enhanced magnetic resonance imaging, we found that PMBF was reduced in participants with mild, in addition to more severe, COPD. PMBF was inversely related to emphysema severity, particularly for centrilobular and panlobular emphysema, and reductions were independent of disease probability measures of small airways disease and gas trapping. This work suggests that pulmonary microvascular changes occur early in COPD and represent a distinct pathological process from small airways disease. PMBF on magnetic resonance imaging may provide an imaging biomarker for therapeutic strategies targeting the pulmonary microvasculature in chronic lung diseases.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally and in the United States (1, 2). Cigarette smoke, the major cause of COPD, has protean effects on the airway epithelium (3) but also causes endothelial damage and loss of the microvasculature in multiple organs, including the brain, kidney, and heart (4). The consequent loss of blood flow causes end-organ damage, and therapies that ameliorate microvascular blood flow, such as angiotensin-receptor blockers, improve function of some of these organs (5). Despite the importance of this preventative and therapeutic target in other organs, there has been little examination of the pulmonary microvasculature early in the course of COPD.
Basic science studies suggest that smoking may have similar effects on the pulmonary and systemic microvasculature (6, 7). A cigarette contains 10 to 500 µg of acrolein (8), which causes apoptosis of human pulmonary microvascular endothelial cells (9) and microvascular damage. Endothelial apoptosis causes emphysema-like changes in mice (10). Hence, smoking-related pulmonary microvascular damage may occur early in COPD; however, there are limited direct data in humans to support this hypothesis.
In 1950, Liebow noted loss of pulmonary capillaries in emphysema and speculated that vascular loss may contribute to emphysema pathogenesis (11). Subsequent studies demonstrated pulmonary vascular endothelial dysfunction, perivascular inflammation, and remodeling of muscular arteries in mild to moderate COPD (12, 13), which challenge the notion that changes in the pulmonary vasculature and perfusion occur only in severe COPD due to hypoxia and parenchymal destruction.
Contrast-enhanced magnetic resonance imaging (MRI) is an established technique to quantify pulmonary perfusion (14). Small, imaging-based studies have found that smokers with normal lung function and a normal quantitative emphysema index but visually defined mild, apical centrilobular emphysema had greater heterogeneity of, although not reduced, pulmonary perfusion (15), and smokers with severe COPD and emphysema had reduced pulmonary perfusion or structural abnormalities (16–18). Other small studies showed perfusion deficits of lung parenchyma on MRI in smokers at risk for COPD and with COPD (19–21). Whether pulmonary microvascular blood flow (PBMF) is reduced in mild COPD and emphysema is, however, unclear.
We therefore assessed PBMF by dynamic contrast-enhanced MRI in a multicenter study sampled from the general population, hypothesizing that PBMF is reduced in mild COPD, as a surrogate for early disease, in addition to more severe COPD and in emphysema.
Some of the results of these studies have been previously reported in the form of abstracts (22–24).
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study enrolled cases of COPD and control subjects in 2009 to 2011 predominantly from two prospective cohort studies, MESA (25) and an emphysema progression study of smokers (26), at four sites. Participants were age 50 to 79 years with ≥10 pack-years smoking history. Exclusion criteria were clinical cardiovascular disease, pulmonary embolism, stage IIIb to V chronic kidney disease, asthma before age 45 years, lung resection, cancer, allergy to gadolinium, claustrophobia, weight >300 lbs, metal in the body, and pregnancy. We selected all eligible participants in the larger MESA Lung Study (27), oversampled participants with obstructive lung function or emphysema on computed tomography (CT) from the remainder of the MESA and from the cohort of smokers, and recruited a small number of participants from outside MESA. Quantitative MRI lung perfusion parameters were obtained at one site; semiquantitative MRI lung perfusion parameters were measured at all four sites. Written informed consent was obtained from all participants, and the protocol was approved by the Institutional Review Boards of all collaborating institutions.
Pulmonary Microvascular Perfusion
Participants underwent cardiac MRI following the protocol of the fifth examination of MESA modified to include dynamic contrast-enhanced pulmonary MRI using 1.5 Tesla MRI (GE and Siemens Healthcare).
Coronal 3D spoiled gradient echo sequences were performed at functional residual volume with a slice thickness of 10 mm at 5-mm intervals between the anterior and the posterior chest wall. A bolus of 0.1 mmol/kg bodyweight gadolinium diethylenetriamine pentaacetate (Magnevist, Berlex, Wayne, NJ) was injected at 5 ml/s, followed by a saline flush of 20 ml at the same rate. First-pass pulmonary perfusion was assessed with an update rate of one image per 1.2 to 2.4 seconds.
Pulmonary microvascular perfusion was assessed on a coronal slice at the level of the trachea in the peripheral 2 cm of the lung as previously described (28). This region was selected to limit assessment to vessels of no more than 500 μm and predominantly less than 200 μm in diameter (29, 30). Quantitative parameters of pulmonary microvascular blood flow (PMBF), volume, and mean transit time were calculated as described in the online supplement. The semiquantitative parameters of signal increase after gadolinium injection and its slope were calculated from signal intensity–time curves; these parameters correlate well with PMBF (28). The coefficient of variation of all measures was 1.5 to 7.6%.
Spirometry
Spirometry was conducted in accordance with American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines (31) following the MESA Lung protocol (32). Post-bronchodilator spirometry was performed after inhalation of two puffs of albuterol.
COPD was defined as a post-bronchodilator FEV1/FVC ratio of <0.70 (33, 34). COPD severity was classified as mild (FEV1 ≥ 80% predicted), moderate (FEV1 50–80% predicted), and severe (FEV1 < 50% predicted) (33). Predicted values were calculated using Hankinson Equations (35) with a 0.88 correction for Asians (32).
Emphysema and Emphysema Subtypes
All participants underwent full-lung CTs at suspended full inspiration on 64-slice helical scanners (GE and Siemens Healthcare) following the MESA-Lung/SPIROMICS protocol (0.984 pitch, 0.5 s, 120 kVp) (36). Expiratory scans were acquired at functional residual volume on a subset. Details are provided in the online supplement.
Emphysematous regions were defined as voxels within the lung field that fell below −950 HU, and the percentage emphysema was defined as the percentage of the lung voxels that were emphysematous (37). Gas trapping on CT was defined as regions on the expiratory scans below −856 HU. The upper limit of normal for percentage emphysema was defined using MESA reference equations (38).
The percentage of the lung with emphysema and each emphysema subtype were assessed qualitatively by experienced thoracic radiologists using a standardized, reproducible protocol as previously described (39).
Registration of PMBF and Emphysematous Regions
To evaluate alterations in PMBF in lung regions that were not emphysematous at an attenuation of −950 HU, we coregistered CT scans to MRI perfusion images for a subset of participants as described in the online supplement.
Disease Probability Measures on CT
Inspiratory and expiratory CT scans were coregistered to yield a continuous metric of small airway disease–like regions, emphysema-like regions, and regions of normal lung. Additional details are provided in the online supplement.
Plethysmography
Body plethysmography was measured with an Autobox 220 Series instrument and with a V6200 Series Autobox (Sensormedics/Viasys Healthcare, Yorba Linda, CA) following ATS/ERS guidelines (40, 41).
Smoking Status and Other Covariates
Age, sex, race/ethnicity, educational attainment, pack-years of smoking, and medical history were self-reported. Height, weight, resting blood pressure, medications, fasting plasma glucose, complete blood count, and oxygen saturation were measured following MESA protocols (42). Current smoking was defined as blood cotinine level >100 ng/ml or urinary cotinine >500 ng/ml on the day of the examination or report of cigarette smoking in the prior 30 days.
Semiautomated contouring of the left ventricle (LV) on MRI (Cardiac Image Modeller [CIM]; Auckland MRI Research Group, Auckland, New Zealand) (43) was used to obtain end-diastolic and end-systolic volumes, the difference in which was multiplied by the heart rate to obtain LV cardiac output. Similar measures were made for the right ventricle as previously described (44).
Statistical Analysis
The sample was stratified by COPD severity for descriptive purposes. Initial tests for COPD status were performed with a t test. Subsequently, linear regression was used to test the association of pulmonary microvascular perfusion with categories of COPD severity. The base model was adjusted for age, sex, race/ethnicity, and cohort of recruitment. This model was then additionally adjusted for smoking status, pack-years, educational attainment, weight, height, oxygen saturation, and LV cardiac output. Because the primary test of interest was the decrement in PMBF in mild COPD, we specified the Holm’s Step-Down test a priori to account for multiple comparisons.
Analyses of percentage emphysema were additionally adjusted for milliamperes. All analyses with continuous dependent variables were weighted according to cohort-specific probabilities of selection and enrollment into the MESA COPD study to account for the sampling approach (45). Robust standard errors were used in the weighted analyses.
Generalized additive models were used to test the linearity of associations and to depict graphically multivariate relationships. Analyses were performed in SAS 9.2 and R 2.14.1.
Results
Participant Characteristics
We enrolled 338 participants, of whom 321 underwent cardiopulmonary MRI, 285 received gadolinium, and 257 had valid pulmonary microvascular perfusion measures assessed by signal increase (see Figure E1 in the online supplement). At one site, 144 participants received a second gadolinium bolus, allowing calculation of PMBF.
The mean age of the 144 participants was 68 ± 7.1 years, 40% were women, 42% were non-white, 35% were current smokers, and the median number of pack-years was 35. Fifty-six percent had COPD (37% with mild, 46% with moderate, and 17% with severe COPD), and the remainder of participants were control subjects. None of the participants had evidence of pulmonary embolism on perfusion MRI or mediastinal tumor or lymphadenopathy on CT.
Patients with more severe COPD were more likely to be white and African American and to have a greater smoking history, lower body mass index, lower oxygen saturation, and obstructive sleep apnea compared with control subjects (Table 1). Nonpulmonary medication use was similar across severities of COPD except calcium-channel blockers, which were more frequently used in severe COPD; pulmonary medications were more commonly used in severe COPD.
Table 1.
Clinical Characteristics of Participants in the MESA COPD Study with Measures of Quantitative Pulmonary Perfusion Stratified by Chronic Obstructive Pulmonary Disease Severity
| No COPD (n = 63) | Mild COPD (n = 30) | Moderate COPD (n = 37) | Severe/Very Severe COPD (n = 14) | |
|---|---|---|---|---|
| Age, yr, mean ± SD | 68.2 ± 6.5 | 68.5 ± 7.0 | 67.1 ± 7.8 | 65.6 ± 8.0 |
| Male sex, n (%) | 35 (55.6) | 21 (70.0) | 21 (56.8) | 9 (64.3) |
| Race/ethnicity, n (%) | ||||
| White | 32 (50.8) | 20 (66.7) | 21 (56.8) | 10 (71.4) |
| African American | 9 (14.3) | 7 (23.3) | 11 (29.7) | 4 (28.6) |
| Hispanic | 13 (30.2) | 3 (10.0) | 4 (10.8) | 0 |
| Chinese | 3 (4.5) | 0 | 1 (2.7) | 0 |
| Education, n (%) | ||||
| ≤High school degree | 17 (27.0) | 6 (20.0) | 10 (27.0) | 2 (14.3) |
| Some college | 15 (23.8) | 5 (16.7) | 8 (21.6) | 7 (50.0) |
| ≥College degree | 31 (49.2) | 19 (63.3) | 19 (51.4) | 5 (35.7) |
| Cigarette smoking status, n (%) | ||||
| Former | 44 (69.8) | 21 (70.0) | 19 (51.4) | 9 (64.3) |
| Current | 19 (30.2) | 9 (30.0) | 18 (48.7) | 5 (35.7) |
| Pack-years of smoking, median (IQR) | 27.0 (18.0–42.6) | 34.3 (23.5–46.0) | 42.5 (37.5–54.7) | 37.5 (17.5–52.5) |
| Height, cm, mean ± SD | 167 ± 9.4 | 172 ± 9.4 | 169 ± 9.2 | 168 ± 8.6 |
| Weight, kg, mean ± SD | 80.3 ± 16.2 | 78.6 ± 13.7 | 75.8 ± 18.9 | 74.3 ± 15.7 |
| Body mass index, kg/m2, mean ± SD | 28.6 ± 5.1 | 26.6 ± 3.6 | 26.2 ± 4.8 | 26.3 ± 4.6 |
| Oxygenation saturation, %, mean ± SD | 97.5 ± 1.5 | 96.7 ± 2.1 | 97.4 ± 1.3 | 96.2 ± 2.1 |
| Percentage <95.7% saturation | 8 (12.9) | 6 (23.1) | 2 (5.9) | 3 (33.3) |
| Hypertension, n (%) | 22 (34.9) | 11 (36.7) | 17 (27.0) | 6 (42.9) |
| Systolic blood pressure, mm Hg, mean ± SD | 119 ± 15.0 | 119 ± 15.2 | 127 ± 14.8 | 125 ± 13.1 |
| Diastolic blood pressure, mm Hg, mean ± SD | 69.3 ± 9.7 | 71.1 ± 10.5 | 73.4 ± 9.4 | 77.2 ± 10.1 |
| Diabetes mellitus, n (%) | 11 (17.5) | 2 (6.7) | 7 (18.9) | 3 (21.4) |
| Fasting plasma glucose, mg/dl, median (IQR) | 100 (94.0–110) | 103 (91.0–108) | 102 (97.0–113) | 94.0 (87.0–108) |
| Obstructive sleep apnea, self-reported, n (%) | 2 (3.2) | 3 (10.0) | 3 (8.1) | 2 (14.3) |
| Medication use | ||||
| Statin, n (%) | 21 (33.3) | 14 (46.7) | 18 (48.7) | 5 (35.7) |
| ACE inhibitors or angiotensin antagonists, n (%) | 11 (17.5) | 11 (36.7) | 13 (35.1) | 3 (21.4) |
| Calcium channel blockers, n (%) | 11 (17.5) | 2 (6.7) | 8 (21.6) | 4 (28.6) |
| β-blockers, n (%) | 7 (11.1) | 4 (13.3) | 9 (24.3) | 1 (7.1) |
| Omega-3, n (%) | 7 (11.1) | 2 (6.7) | 5 (13.5) | 0 |
| Postmenopausal hormones (among women), n (%) | 1 (3.6) | 0 | 0 | 0 |
| Aspirin, n (%) | 28 (44.4) | 18 (60.0) | 19 (51.4) | 4 (28.6) |
| Short-acting bronchodilators, n (%) | 0 | 2 (6.7) | 7 (18.9) | 10 (71.4) |
| Long-acting bronchodilators, n (%) | 1 (1.6) | 1 (3.3) | 2 (5.4) | 3 (21.4) |
| Inhaled or systemic corticosteroids, n (%) | 2 (3.2) | 3 (10.0) | 5 (13.5) | 12 (85.7) |
| Home oxygen therapy, n (%) | 0 | 0 | 0 | 5 (35.7) |
| White blood cell count, billions/L, mean ± SD | 6.5 ± 1.8 | 6.2 ± 1.3 | 7.3 ± 1.9 | 7.6 ± 2.6 |
| Hemoglobin, g/L, mean ± SD | 13.9 ± 1.4 | 14.1 ± 0.9 | 13.8 ± 1.3 | 13.9 ± 0.8 |
| FEV1, % predicted, mean ± SD | 101 ± 16.9 | 92.1 ± 11.4 | 68.6 ± 8.0 | 40.6 ± 6.0 |
| FVC, % predicted, mean ± SD | 98.5 ± 16.2 | 108 ± 13.2 | 92.8 ± 12.4 | 81.1 ± 13.6 |
| FEV1/FVC ratio, mean ± SD | 0.77 ± 0.04 | 0.64 ± 0.05 | 0.57 ± 0.09 | 0.38 ± 0.06 |
| Percentage emphysema−950, median (IQR) | 0.85 (0.43–1.52) | 2.64 (1.04–4.72) | 2.65 (1.05–7.89) | 13.4 (7.11–26.7) |
| Left ventricular cardiac output, l/min, mean ± SD | 5.12 ± 0.95 | 4.80 ± 0.92 | 4.70 ± 0.95 | 4.84 ± 1.32 |
| DlCO, %, mean ± SD (n = 87) | 67.5 ± 9.5 | 64.8 ± 11.6 | 53.4 ± 15.3 | 40.5 ± 14.9 |
| DlCO/Va, %, mean ± SD (n = 87) | 78.5 ± 13.5 | 70.4 ± 15.4 | 67.0 ± 19.2 | 58.1 ± 20.3 |
| RV/TLC ratio, mean ± SD (n = 87) | 0.32 ± 0.06 | 0.32 ± 0.06 | 0.39 ± 0.08 | 0.49 ± 0.08 |
Definition of abbreviations: ACE = angiotensin converting enzyme; COPD = chronic obstructive pulmonary disease; DlCO = diffusing capacity of carbon monoxide; IQR = interquartile range; MESA = Multi-Ethnic Study of Atherosclerosis; RV = residual volume; TLC = total lung capacity.
Pulmonary Microvascular Perfusion in COPD
PMBF was 38% lower in patients with COPD compared with control subjects [mean, 52.1 mlblood/(min · 100 mllung) vs. 82.7 mlblood/(min · 100 mllung); P = 0.004]. The mean difference between subjects with COPD and control subjects was −21.7 mlblood/(min · 100 mllung) (95% confidence interval [CI], −7.3 to −36.0; P = 0.003) after accounting for demographic and anthropological factors, smoking, oxygen saturation, and LV cardiac output.
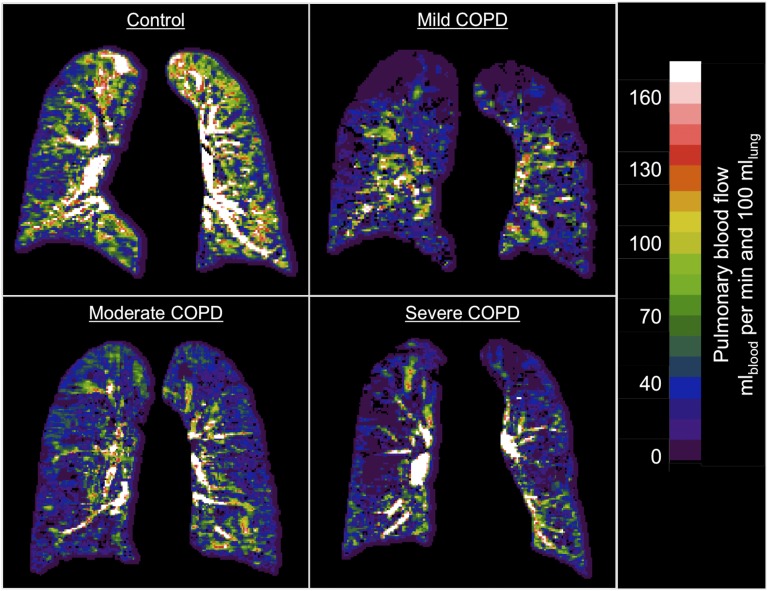
PMBF was significantly reduced in mild COPD in addition to in moderate and severe COPD (P < 0.01 for all comparisons) in both minimally and fully adjusted models (Table 2). The 30% reduction in PMBF in mild COPD was of similar magnitude to that in moderate COPD; in severe COPD, PMBF was reduced by 53%. These differences were clearly visible in the perfusion maps (Figure 1). The relationship of the PMBF to the FEV1/FVC ratio was approximately linear (Figure E2).
Table 2.
Predicted Mean Pulmonary Microvascular Perfusion across Categories of Chronic Obstructive Pulmonary Disease Severity (N = 144)
| None (n = 63) | Mild (n = 30) | Moderate (n = 37) | Severe (n = 14) | |
|---|---|---|---|---|
| Pulmonary microvascular blood flow, mlblood/(min · 100 mllung) | ||||
| Model 1, predicted mean | 81.7 | 56.8* | 58.0* | 38.8* |
| Model 2, predicted mean | 81.4 | 59.7* | 62.7† | 43.1* |
| Pulmonary microvascular blood volume, mlblood/100 mllung | ||||
| Model 1, predicted mean | 5.03 | 3.90† | 4.43 | 3.10† |
| Model 2, predicted mean | 5.05 | 3.96† | 4.04 | 3.23† |
| Mean transit time, s | ||||
| Model 1, predicted mean | 3.84 | 4.36 | 4.51† | 5.14† |
| Model 2, predicted mean | 6.89 | 5.76 | 5.91 | 5.04† |
Model 1 was adjusted for age, sex, race/ethnicity, and cohort. Model 2 was adjusted for variables in model 1 in addition to smoking status, pack-years, education, weight, height, oxygen saturation, and left ventricular cardiac output.
P < 0.01.
P < 0.05.
Figure 1.
Quantitative maps of pulmonary microvascular blood flow (PMBF) of participants with varying chronic obstructive pulmonary disease (COPD) severities and a participant without COPD. Shown are examples of PMBF maps in a participant without COPD and in participants with mild, moderate, and severe COPD. Window width and level are the same for all examples. PMBF is globally reduced in the examples of participants with mild, moderate, and severe COPD, as is blood flow in the region defined as representing the microvasculature (peripheral 2 cm of the lung). PMBF was reduced in emphysematous regions of lung (e.g., superior lung in the case of mild COPD); these regions were excluded in the results provided in Table 5.
In addition, pulmonary microvascular blood volume was reduced in patients with COPD compared with control subjects (mean difference, −1.1 mlblood/100 mllung; 95% CI, −0.2 to −2.1; P = 0.02) and in patients with mild COPD (Table 2). The increase in mean transit time was nonsignificant (multivariate mean difference, 0.3 s; 95% CI, −0.3 to 0.8; P = 0.36).
Pulmonary Microvascular Perfusion and Percentage Emphysema
PMBF was significantly and monotonically related to percentage emphysema on CT (Table 3). Mean PMBF among participants in the highest quintile of percentage emphysema was less than half of that in the lowest. The relationship between PMBF and percentage emphysema was nonlinear (P = 0.015), demonstrating a greater reduction in PMBF in milder disease (Figure E3).
Table 3.
Predicted Mean Values of Pulmonary Microvascular Perfusion Measures by Quintile of Percentage of Emphysematous Lung on Computed Tomography (N = 143)
| CT Percentage Emphysema Predicted Mean Value |
Difference per Unit Increase in log −950 HU (95% CI) | P Value | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 (n = 29) | Quintile 2 (n = 28) | Quintile 3 (n = 29) | Quintile 4 (n = 28) | Quintile 5 (n = 29) | |||
| Pulmonary microvascular blood flow, mlblood/(min · 100 mllung) | |||||||
| Model 1 | 89.8 | 76.2 | 67.7 | 56.3 | 39.2 | −13.8 (−18.6 to −8.89) | <0.001 |
| Model 2 | 91.3 | 77.9 | 69.4 | 58.2 | 41.3 | −13.6 (−18.9 to −8.30) | <0.001 |
| Pulmonary microvascular blood volume, mlblood/100 mllung | |||||||
| Model 1 | 5.88 | 5.21 | 4.79 | 4.23 | 3.39 | −0.68 (−1.04 to −0.31) | <0.001 |
| Model 2 | 5.76 | 4.98 | 4.49 | 3.84 | 2.86 | −0.79 (−1.18 to −0.40) | <0.001 |
| Mean transit time, s | |||||||
| Model 1 | 3.67 | 3.99 | 4.19 | 4.45 | 4.85 | 0.32 (0.13 to 0.52) | 0.01 |
| Model 2 | 3.91 | 3.88 | 4.00 | 4.18 | 4.45 | 0.22 (0.01 to 0.43) | 0.045 |
Definition of abbreviations: CI = confidence interval; CT = computed tomography; HU = Hounsfield units.
Model 1 was adjusted for age, sex, race/ethnicity, and cohort. Model 2 was adjusted for variables in model 1 in addition to smoking status, pack-years, education, weight, height, oxygen saturation, left ventricular cardiac output, and high milliamperes.
Percentage emphysema was also associated with large reductions in pulmonary microvascular blood volume and marginal increases in mean transit time (Table 3).
Pulmonary Microvascular Perfusion and Emphysema Subtypes
Similar to findings for percentage emphysema, PMBF was reduced among participants with radiologist-defined emphysema (adjusted P = 0.01). This reduction was related to the extent of panlobular emphysema and centrilobular emphysema (Table 4), whereas there was no relationship of PMBF to paraseptal emphysema.
Table 4.
The Association of Pulmonary Perfusion Measures with Emphysema Subtypes (N = 142)
| Centrilobular Emphysema (per log Unit Increase) | P Value | Panlobular Emphysema (per log Unit Increase) | P Value | Paraseptal Emphysema (per log Unit Increase) | P Value | |
|---|---|---|---|---|---|---|
| Pulmonary microvascular blood flow, mlblood/(min · 100 mllung) | ||||||
| Model 1, mean difference | −12.3 (−18.9 to −5.58) | <0.001 | −12.3 (−20.9 to −3.59) | 0.006 | −2.07 (−14.7 to 10.6) | 0.75 |
| Model 2, mean difference | −9.65 (−16.3 to −2.97) | 0.005 | −11.2 (−18.7 to −3.66) | 0.004 | 2.91 (−10.0 to 15.8) | 0.66 |
| Pulmonary microvascular blood volume, mlblood/100 mllung | ||||||
| Model 1, mean difference | −0.57 (−1.00 to −0.14) | 0.01 | −0.58 (−1.09 to −0.06) | 0.03 | 0.23 (−0.58 to 1.06) | 0.57 |
| Model 2, mean difference | −0.60 (−1.04 to −0.15) | 0.009 | −0.65 (−0.10 to 0.10) | 0.005 | 0.25 (−0.62 to 1.13) | 0.57 |
| Mean transit time, s | ||||||
| Model 1, mean difference | 0.32 (0.05 to 0.58) | 0.02 | 0.41 (0.06 to 0.76) | 0.02 | 0.45 (−0.02 to 0.91) | 0.06 |
| Model 2, mean difference | 0.15 (−0.11 to 0.42) | 0.25 | 0.24 (−0.04 to 0.53) | 0.10 | 0.06 (−0.40 to 0.52) | 0.81 |
Model 1 was adjusted for age, sex, race/ethnicity, and cohort. Model 2 was adjusted for variables in model 1 in addition to smoking status, pack-years, education, weight, height, oxygen saturation, and left ventricular cardiac output.
Pulmonary Microvascular Perfusion in Nonemphysematous Regions of the Lungs
Results were similar for pulmonary microvascular perfusion and COPD in regions of the lung with attenuation above the standard threshold for emphysema of −950 HU: the mean difference between patients with COPD and control subjects in the fully adjusted model was −28 mlblood/(min · 100 mllung) (95% CI, −53 to −11; P = 0.007). Results by COPD severity (Table 5) demonstrated, if anything, greater decrements in mild COPD compared with the main analysis.
Table 5.
Association of Mean Pulmonary Microvascular Perfusion in Regions of the Lung with Attenuation of More Than −950 Hounsfield Units across Categories of Chronic Obstructive Pulmonary Disease Severity
| No COPD (n = 18) | Mild COPD (n = 12) | Moderate COPD (n = 13) | Severe COPD (n = 5) | |
|---|---|---|---|---|
| Medial pulmonary microvascular blood flow, mlblood/(min · 100 mllung) | ||||
| Model 1, predicted mean | 88.8 | 43.3* | 54.4† | 43.3‡ |
| Model 2, predicted mean | 88.8 | 44.4* | 68.1 | 54.1† |
| Medial pulmonary microvascular blood volume, mlblood/100 mllung | ||||
| Model 1, predicted mean | 5.03 | 3.06* | 4.07 | 3.69 |
| Model 2, predicted mean | 5.03 | 3.01 | 4.15 | 4.03 |
| Medial mean transit time, s | ||||
| Model 1, predicted mean | 3.50 | 4.65† | 3.90 | 5.52* |
| Model 2, predicted mean | 3.51 | 4.43† | 3.58 | 4.96† |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Model 1 was adjusted for age, sex, race/ethnicity, and cohort. Model 2 was adjusted for variables in model 1 in addition to smoking status, pack-years, education, weight, height, oxygen saturation, and cardiac output.
P < 0.001.
P < 0.05.
P < 0.01.
Adjustment for RV Structure and Function
Adjustment for RV end-diastolic volume, mass, or ejection fraction did not attenuate the observed reductions in PMBF in mild or more severe COPD, with emphysema, or with emphysema subtypes (see Table E1 in the online supplement).
Pulmonary Microvascular Perfusion, Disease Probability Measures of Emphysema and Small Airways, and Gas Trapping
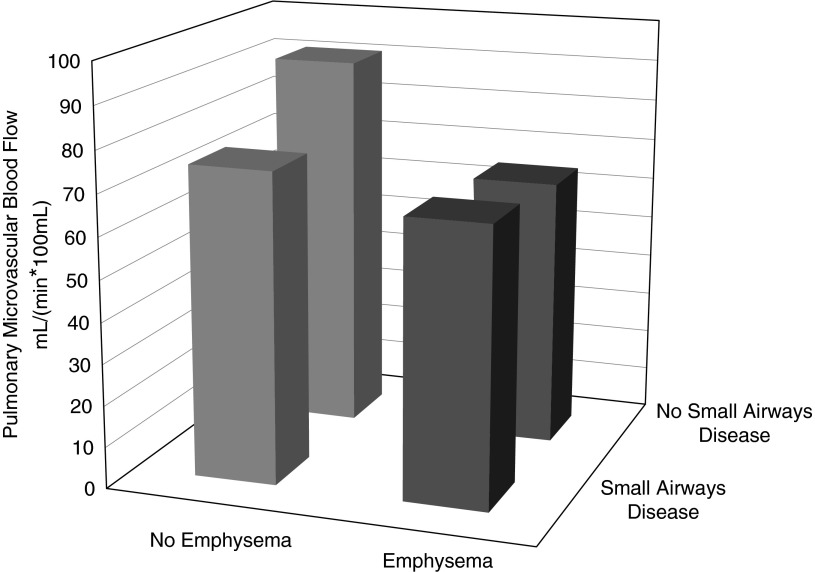
Reductions in PMBF were large and statistically significant in participants with emphysema by disease probability measures regardless of small airway disease measures, whereas PMBF was only modestly and nonsignificantly reduced in those with disease probability measures of small airway disease (Figure 2).
Figure 2.
Mean pulmonary microvascular blood flow (PMBF) among participants with emphysema compared with small airways disease. The presence of emphysema and small airways disease was defined by disease probability measures on co-registered computed tomography results greater than the median value. The mean values of PMBF were adjusted for age, sex, race/ethnicity, cohort, weight, height, and chronic obstructive pulmonary disease. Mean PMBF was reduced in participants with emphysema without (n = 11; P = 0.03) and with (n = 29; P = 0.02) reductions in small airway count but did not differ significantly among participants with reduced small airway count only (n = 13; P = 0.15) compared with those with neither phenotype.
PMBF was inversely associated with the disease probability measures of emphysema and small airways disease in minimally and fully adjusted models, and both were associated with decrements in PMBF independent of the other (Table E2). Results were similar for pulmonary blood volume, but mean transit time was increased in the fully adjusted model only with greater disease probability measures of emphysema.
PMBF was not significantly associated with the plethysmographic measures of gas trapping residual volume or residual volume/total lung capacity (Table E3). Associations of PMBF with mild COPD and percentage emphysema also remained significant with additional adjustment for gas trapping on CT (P = 0.02 and P = 0.006, respectively).
Additional Analyses
The associations of PMBF with any COPD, mild COPD, and percentage emphysema were similar in sensitivity analyses (1) restricted to participants without hypoxemia, participants recruited from MESA and the emphysema progression cohort only, and whites and African Americans only; (2) excluding participants with hypertension, diabetes, or obstructive sleep apnea, using a −910 HU threshold for percentage emphysema; and (3) additionally adjusted for white blood cell count, medication use, and oxygen use (Figures E4A–E4C).
Associations of PMBF with COPD and mild COPD were of somewhat greater magnitude among current smokers than former smokers (Figures E4A and E4B), although the interactions were not statistically significant (e.g., P = 0.58 for COPD). Statistical weighting did not substantially affect the results for percentage emphysema (Figure E4C).
In multivariable analyses that adjusted for both COPD and percentage emphysema, the association of PMBF and percentage emphysema remained significant (P < 0.001), as did that for COPD (P = 0.03).
Signal Increase
A similar pattern of results for pulmonary microvascular perfusion assessed as Signal Increase was observed in the larger sample of 257 patients across categories of COPD severity and with percentage emphysema (Tables E4 and E5).
Discussion
PMBF assessed by dynamic contrast-enhanced MRI was substantially reduced in mild, as well as moderate and severe, COPD and was related to emphysema on CT scan, particularly of the panlobular and centrilobular types. The findings suggest marked microvascular damage in COPD and emphysema, provide evidence for microvascular changes early in the disease process, and suggest a vascular process distinct from small airways disease.
The current results from state-of-the-art MRI scanning are broadly consistent with an earlier meta-analysis of 10 small studies that assessed ventilation and perfusion using the multiple inert gas elimination technique (46). That study demonstrated abnormal ventilation/perfusion heterogeneity in 15 patients with mild COPD predominantly due to increased dispersion of pulmonary blood flow. Similar to the present study, perfusion abnormalities were marked in mild COPD and only slightly worse in severe/very severe COPD. Other smaller studies of pulmonary perfusion in more severe COPD have found consistent associations using, variously, MRI (16, 17), single photon emission tomography (47, 48), and CT perfusion scanning (15, 49). These studies, however, were small, single-center, and generally uncontrolled.
The current results are also consistent with a study of predominantly severe COPD that showed that ratios of small (<5 mm2) to total vessel volumes visible on noncontrast CT were inversely associated with percentage emphysema and reduced in severe COPD (18), although that study assessed neither the pulmonary microvasculature nor blood volume per se. The present study extends the findings to mild COPD and emphysema subtypes with adjustment for multiple cardiac and physiologic factors. Importantly, PMBF was also markedly reduced in regions of the lung that are considered nonemphysematous based on the standard thresholding on CT, suggesting that the observed reductions were not simply due to overt loss of lung tissue.
The present study did not assess the mechanism of the marked decrement in PMBF in mild COPD and emphysema directly; however, cigarette smoking causes endothelial dysfunction in the pulmonary and systemic circulations (6, 50), and a variety of lipid moieties, including ceramide, cause pulmonary endothelial cell apoptosis and emphysema in animal models (10, 51). A growing literature in humans suggests endothelial dysfunction, whether measured ex vivo (13, 52) or in vivo indirectly by flow-mediated dilation of the brachial artery (26, 53) or more directly by endothelial microparticles (54–56), occurs early in COPD and emphysema.
Findings were strongest for emphysema and particularly for panlobular and centrilobular emphysema. The association with panlobular emphysema in humans is consistent with original, but little examined, observations of elastin models of panlobular emphysema in animals (57) and with newer experimental work demonstrating the vascular effects of α1-antitrypsin (58–60) deficiency.
Regional hypoxic pulmonary vasoconstriction undoubtedly contributed to some of the observed decrement in PMBF as mild to moderate hypoxia can contribute to vasoconstriction and remodeling of the subepithelial microvascular bed (61). We did not measure regional hypoxia in the lung or arterial blood gases. However, few patients with mild COPD were hypoxemic, and our results were unchanged among participants without hypoxemia (e.g., oxygen saturation ≥98%). Hence, it is unlikely that hypoxia alone caused the observed marked reduction in pulmonary microvascular perfusion in mild COPD.
An alternative explanation is that gas trapping due to small airways disease reduced PMBF. To address this concern, we used disease probability measures of coregistered scans to define the extent of emphysema and small airways disease and found that PMBF was reduced in patients with emphysema and little to no evidence of small airways disease. Furthermore, gas trapping on plethysmography was not associated with PMBF, and findings were independent of gas trapping on CT. Together, these findings suggest that reduced PMBF is a feature predominantly of emphysematous COPD and less of small airways–related COPD.
Reduced cardiac output is prominent in early COPD and emphysema (62). However, the observed reductions in PMBF were independent of (and probably contributed to) reductions in LV cardiac output. The observed reductions in cardiac output may have contributed to the reduced right ventricular volumes that we recently described in this cohort, particularly in centrilobular emphysema (44). The present findings for PMBF, however, were robust to adjustment for right ventricular structure and function, which implies the primacy of changes in the pulmonary vasculature, particularly in panlobular emphysema.
Sleep apnea is associated with COPD in clinical populations (63), although not in general population samples like the present one (64), and may have a small contribution to pulmonary vascular changes in COPD. Sleep apnea was assessed only by self-report; nonetheless, exclusion of these patients had no appreciable impact on the findings.
We did not validate PMBF against anatomically confirmed microvascular damage in COPD or pulmonary pressures; however, we have previously shown PMBF to be inversely associated with CD31+ endothelial microparticles, markers of endothelial damage (56). Furthermore, gadolinium-enhanced imaging is a well-accepted and validated measure of myocardial microvascular disease in the absence of epicardial coronary (muscular) artery disease (65, 66). Because there is no difference in the lumen diameter of the muscular pulmonary arteries in patients with mild COPD compared with smoking control subjects (12) and because pulmonary perfusion was assessed in the periphery of the lung that is perfused mostly by the microvasculature, it is reasonable to conclude that our measures of pulmonary perfusion represent microvascular perfusion.
We quantified lung perfusion only in a mid-lung coronal slice and did not examine the whole lung. This approach was chosen because we focused on microvascular perfusion rather than redistribution of blood flow.
There remains some disagreement on the optimal definition of COPD. The present one was based upon current guidelines (34), and Figure E2A shows no evidence for an inflection at any threshold of the FEV1/FVC ratio. Mild COPD is not synonymous with early COPD; nonetheless, mild decrements in lung function strongly predict accelerated decline in the FEV1 and progression of COPD (67) such that, it is hypothesized, ongoing prospective follow-up of this cohort will demonstrate the prognostic significance of these measures in early COPD.
Finally, the current study is cross-sectional; hence the direction of the association is not entirely clear and may be subject to selection bias. The latter was minimized by the mostly nested design of the study, and fully nested analyses confirmed the main findings.
In conclusion, PMBF was markedly reduced in mild COPD and emphysema as well as in more severe COPD. These findings, together with prior animal work (10, 68, 69), suggest that reduced PMBF might be implicated in the pathogenesis of emphysematous COPD. PMBF on MRI may be a helpful imaging biomarker for therapies targeting the pulmonary vasculature given that it is reproducible (28), noninvasive, specific, and easily repeatable in the short term. Randomized clinical trials of therapies targeting the microvasculature in early COPD and emphysema are warranted to determine if the observed association underlies a reversible component of COPD pathogenesis.
Acknowledgments
Acknowledgment
The authors thank the other investigators, staff, and participants of the MESA COPD Study for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org. This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments have been incorporated prior to submission for publication.
Footnotes
Supported by National Institutes of Health grants R01-HL093081, R01-HL077612, R01-HL075476, R01-HL-112986, N01-HC95159-HC95169, and UL1 RR024156.
Author Contributions: Conception and design: J.V.-C., D.A.B., E.A.H., S.M.K., J.L., and R.G.B. Data collection: K.H., J.H.M.A., D.A.B., J. Carr, J. Choi, T.A.G., A.S.G., E.A.H., J.L., E.D.M., W.S.P., M.J.P., M.R.P., K.L., J.S., B.M.S., K.W., Y.Y., A.M.Z.-L., and R.G.B. Analysis and interpretation: M.A.P., D.R., and R.G.B. Obtaining of funding: E.A.H., J.L., W.S.P., M.J.P., K.L., K.W., and R.G.B. Drafting the manuscript: K.H. and J.V.-C. Critical revision of the manuscript for important intellectual content: all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201411-2120OC on June 11, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minino AM, Murphy SL. Death in the United States, 2010. NCHS Data Brief. 2012;99:1–8. [PubMed] [Google Scholar]
- 3.Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122:2749–2755. doi: 10.1172/JCI60324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leone A, Landini L. Vascular pathology from smoking: look at the microcirculation! Curr Vasc Pharmacol. 2013;11:524–530. doi: 10.2174/1570161111311040016. [DOI] [PubMed] [Google Scholar]
- 5.Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia. 2012;55:566–578. doi: 10.1007/s00125-011-2398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman MM, Laher I. Structural and functional alteration of blood vessels caused by cigarette smoking: an overview of molecular mechanisms. Curr Vasc Pharmacol. 2007;5:276–292. doi: 10.2174/157016107782023406. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer E, Peinado VI, Castañeda J, Prieto-Lloret J, Olea E, González-Martín MC, Vega-Agapito MV, Díez M, Domínguez-Fandos D, Obeso A, et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur Respir J. 2011;38:617–627. doi: 10.1183/09031936.00105110. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman D, Hecht S. Advances in tobacco carcinogenesis. In: Cooper CS, Grover PL, editors. Chemical carcinogenesis and mutagenesis I. Handbook of experimental pharmacology. Vol. 94, No. I. Heidelberg: Springer; 1990. pp. 63–102. [Google Scholar]
- 9.Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, Cool C, Parr J, Moore MD, Bull TM, et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007;175:676–685. doi: 10.1164/rccm.200605-724OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80:67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 12.Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barberà JA. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 13.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274:L908–L913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 14.Hatabu H, Tadamura E, Levin DL, Chen Q, Li W, Kim D, Prasad PV, Edelman RR. Quantitative assessment of pulmonary perfusion with dynamic contrast-enhanced MRI. Magn Reson Med. 1999;42:1033–1038. doi: 10.1002/(sici)1522-2594(199912)42:6<1033::aid-mrm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Alford SK, van Beek EJ, McLennan G, Hoffman EA. Heterogeneity of pulmonary perfusion as a mechanistic image-based phenotype in emphysema susceptible smokers. Proc Natl Acad Sci USA. 2010;107:7485–7490. doi: 10.1073/pnas.0913880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, Takenaka D, Fujii M, Sugimura K. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353–365. doi: 10.1002/jmri.20137. [DOI] [PubMed] [Google Scholar]
- 17.Jang YM, Oh YM, Seo JB, Kim N, Chae EJ, Lee YK, Lee SD. Quantitatively assessed dynamic contrast-enhanced magnetic resonance imaging in patients with chronic obstructive pulmonary disease: correlation of perfusion parameters with pulmonary function test and quantitative computed tomography. Invest Radiol. 2008;43:403–410. doi: 10.1097/RLI.0b013e31816901ab. [DOI] [PubMed] [Google Scholar]
- 18.Estépar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, Kikinis R, Han MK, Come CE, Diaz AA, et al. COPDGene Study. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan L, Xia Y, Guan Y, Yu H, Zhang TF, Liu SY, Li B. Capability of differentiating smokers with normal pulmonary function from COPD patients: a comparison of CT pulmonary volume analysis and MR perfusion imaging. Eur Radiol. 2013;23:1234–1241. doi: 10.1007/s00330-012-2729-2. [DOI] [PubMed] [Google Scholar]
- 20.Fan L, Xia Y, Guan Y, Zhang TF, Liu SY. Characteristic features of pulmonary function test, CT volume analysis and MR perfusion imaging in COPD patients with different HRCT phenotypes. Clin Respir J. 2014;8:45–54. doi: 10.1111/crj.12033. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y, Guan Y, Fan L, Liu SY, Yu H, Zhao LM, Li B. Dynamic contrast enhanced magnetic resonance perfusion imaging in high-risk smokers and smoking-related COPD: correlations with pulmonary function tests and quantitative computed tomography. COPD. 2014;11:510–520. doi: 10.3109/15412555.2014.948990. [DOI] [PubMed] [Google Scholar]
- 22.Hueper K, Vogel-Claussen J, Parikh M, Austin J, Bluemke D, Carr J, Goldstein T, Gomes A, Hoffman E, Lima J, et al. Veränderungen der lungenperfusion bei patienten mit chronisch obstruktiver Lungenerkrankung (COPD): die MESA COPD Studie [abstract] Fortschr Röntgenstr. 2012;184(S 01):VO318_1. [Google Scholar]
- 23.Hueper K, Vogel-Claussen J, Parikh M, Austin J, Bluemke D, Carr J, Goldstein TA, Gomes AS, Hoffman EA, Lima JAC, et al. Pulmonary parenchymal blood flow in early chronic obstructive pulmonary disease (COPD): the MESA COPD Study [abstract] Proc Intl Soc Mag Reson Med. 2012;0624 [Google Scholar]
- 24.Hueper K, Vogel-Claussen J, Parikh M, Austin J, Bluemke DA, Carr J, Goldstein T, Gomes A, Hoffman EA, Lima J, et al. Pulmonary parenchymal perfusion in early chronic obstructive pulmonary disease (COPD): the MESA COPD Study [abstract] Am J Respir Crit Care Med. 2012;185:A4345. [Google Scholar]
- 25.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 26.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, Shimbo D, Stevenson L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hueper K, Parikh MA, Prince MR, Schoenfeld C, Liu C, Bluemke DA, Dashnaw SM, Goldstein TA, Hoffman EA, Lima JA, et al. Quantitative and semiquantitative measures of regional pulmonary microvascular perfusion by magnetic resonance imaging and their relationships to global lung perfusion and lung diffusing capacity: the multiethnic study of atherosclerosis chronic obstructive pulmonary disease study. Invest Radiol. 2013;48:223–230. doi: 10.1097/RLI.0b013e318281057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Yen RT, McLaurine M, Bledsoe G. Morphometry of the human pulmonary vasculature. J Appl Physiol (1985) 1996;81:2123–2133. doi: 10.1152/jappl.1996.81.5.2123. [DOI] [PubMed] [Google Scholar]
- 30.Weibel ER. A quantitative approach to the morphologic study of the peripheral pulmonary vasculature. Med Thorac. 1962;19:208–214. doi: 10.1159/000192220. [DOI] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) lung study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celli BR, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 34.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 35.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 36.Sieren JP, Hoffman EA, Baumhauer H, Barr RG, Goldin JG, Rennard S. Chicago, IL: Radiological Society of North America; 2011. CT Imaging Protocol Standardization for use in a Multicenter Study: SPIROMICS. [Google Scholar]
- 37.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 38.Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11:898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith BM, Austin JHM, Newell JD, Jr, D'Asousa BM, Rozenshtein A, Hoffman EA.Ahmed F, Barr RG Pulmonary emphysema subtypes on computed tomography: the MESA COPD Study. Am J Med. 2014;127:94, e7–e23. doi: 10.1016/j.amjmed.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 41.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 42.MESA. Field center procedures manual of operations: Multi-Ethnic Study of Atherosclerosis [accessed 2012 Jun 1]. Available from: http://www.mesa-nhlbi.org/PublicDocs/2011/MESAE5_MOPJanuary2011.pdf
- 43.Young AA, Cowan BR, Thrupp SF, Hedley WJ, Dell’Italia LJ. Left ventricular mass and volume: fast calculation with guide-point modeling on MR images. Radiology. 2000;216:597–602. doi: 10.1148/radiology.216.2.r00au14597. [DOI] [PubMed] [Google Scholar]
- 44.Kawut SM, Poor HD, Parikh MA, Hueper K, Smith BM, Bluemke DA, Lima JA, Prince MR, Hoffman EA, Austin JH, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol. 2014;64:2000–2009. doi: 10.1016/j.jacc.2014.07.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith BM, Kawut SM, Bluemke DA, Basner RC, Gomes AS, Hoffman E, Kalhan R, Lima JA, Liu CY, Michos ED, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127:1503–1511, 1511e1-6. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, Roca J, Barberà JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol (1985) 2009;106:1902–1908. doi: 10.1152/japplphysiol.00085.2009. [DOI] [PubMed] [Google Scholar]
- 47.Suga K, Kawakami Y, Iwanaga H, Hayashi N, Seto A, Matsunaga N. Assessment of anatomic relation between pulmonary perfusion and morphology in pulmonary emphysema with breath-hold SPECT-CT fusion images. Ann Nucl Med. 2008;22:339–347. doi: 10.1007/s12149-007-0137-5. [DOI] [PubMed] [Google Scholar]
- 48.Jögi J, Ekberg M, Jonson B, Bozovic G, Bajc M. Ventilation/perfusion SPECT in chronic obstructive pulmonary disease: an evaluation by reference to symptoms, spirometric lung function and emphysema, as assessed with HRCT. Eur J Nucl Med Mol Imaging. 2011;38:1344–1352. doi: 10.1007/s00259-011-1757-5. [DOI] [PubMed] [Google Scholar]
- 49.Pansini V, Remy-Jardin M, Faivre J-B, Schmidt B, Dejardin-Bothelo A, Perez T, Delannoy V, Duhamel A, Remy J. Assessment of lobar perfusion in smokers according to the presence and severity of emphysema: preliminary experience with dual-energy CT angiography. Eur Radiol. 2009;19:2834–2843. doi: 10.1007/s00330-009-1475-6. [DOI] [PubMed] [Google Scholar]
- 50.Talukder MA, Johnson WM, Varadharaj S, Lian J, Kearns PN, El-Mahdy MA, Liu X, Zweier JL. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol. 2011;300:H388–H396. doi: 10.1152/ajpheart.00868.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green LA, Petrusca D, Rajashekhar G, Gianaris T, Schweitzer KS, Wang L, Justice MJ, Petrache I, Clauss M. Cigarette smoke-induced CXCR3 receptor up-regulation mediates endothelial apoptosis. Am J Respir Cell Mol Biol. 2012;47:807–814. doi: 10.1165/rcmb.2012-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barberà JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am J Respir Crit Care Med. 2001;164:709–713. doi: 10.1164/ajrccm.164.4.2101023. [DOI] [PubMed] [Google Scholar]
- 53.Moro L, Pedone C, Scarlata S, Malafarina V, Fimognari F, Antonelli-Incalzi R. Endothelial dysfunction in chronic obstructive pulmonary disease. Angiology. 2008;59:357–364. doi: 10.1177/0003319707306141. [DOI] [PubMed] [Google Scholar]
- 54.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, Mezey JG, Crystal RG. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi T, Kobayashi S, Fujino N, Suzuki T, Ota C, He M, Yamada M, Suzuki S, Yanai M, Kurosawa S, et al. Increased circulating endothelial microparticles in COPD patients: a potential biomarker for COPD exacerbation susceptibility. Thorax. 2012;67:1067–1074. doi: 10.1136/thoraxjnl-2011-201395. [DOI] [PubMed] [Google Scholar]
- 56.Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, Fu J, Liu CY, Bluemke DA, Ventetuolo CE, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema: the Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188:60–68. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayes JA, Korthy A, Snider GL. The pathology of elastase-induced panacinar emphysema in hamsters. J Pathol. 1975;117:1–14. doi: 10.1002/path.1711170102. [DOI] [PubMed] [Google Scholar]
- 58.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel antiapoptotic role for α1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmid ST, Koepke J, Dresel M, Hattesohl A, Frenzel E, Perez J, Lomas DA, Miranda E, Greulich T, Noeske S, et al. The effects of weekly augmentation therapy in patients with PiZZ α1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2012;7:687–696. doi: 10.2147/COPD.S34560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lockett AD, Kimani S, Ddungu G, Wrenger S, Tuder RM, Janciauskiene SM, Petrache I. α₁-Antitrypsin modulates lung endothelial cell inflammatory responses to TNF-α. Am J Respir Cell Mol Biol. 2013;49:143–150. doi: 10.1165/rcmb.2012-0515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polosukhin VV, Lawson WE, Milstone AP, Egunova SM, Kulipanov AG, Tchuvakin SG, Massion PP, Blackwell TS. Association of progressive structural changes in the bronchial epithelium with subepithelial fibrous remodeling: a potential role for hypoxia. Virchows Arch. 2007;451:793–803. doi: 10.1007/s00428-007-0469-5. [DOI] [PubMed] [Google Scholar]
- 62.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 64.Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, O’Connor GT, Punjabi NM, Shahar E Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 65.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Jerosch-Herold M, Jacobs DR, Jr, Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 67.Drummond MB, Hansel NN, Connett JE, Scanlon PD, Tashkin DP, Wise RA. Spirometric predictors of lung function decline and mortality in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1301–1306. doi: 10.1164/rccm.201202-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 69.Weissmann N, Lobo B, Pichl A, Parajuli N, Seimetz M, Puig-Pey R, Ferrer E, Peinado VI, Domínguez-Fandos D, Fysikopoulos A, et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med. 2014;189:1359–1373. doi: 10.1164/rccm.201311-2037OC. [DOI] [PubMed] [Google Scholar]