The field of pulmonary vascular disease remains a rapidly growing area of research. In 2014, investigators discovered new genetic determinants of pulmonary hypertension (PH) risk and described novel molecular mechanisms of disease pathogenesis. New studies have provided significant insights into our understanding of disease complexity, highlighting critical roles of cellular metabolism, inflammation, and regulatory function of microRNAs that could lead to development of novel targeted therapeutics (1). Studies of the prediction of outcomes in pulmonary vascular disease and venous thromboembolism could lead to more efficient and effective management strategies. This review provides a brief summary of the many scientific accomplishments in the field of pulmonary vascular disease during the past year.
American Thoracic Society Statements and Guidelines
An American Thoracic Society (ATS) Statement (2) focused on current and future approaches to phenotyping patients with PH. Although the current clinical classification has been useful for clinical trials and regulatory approval (3), the World Health Organization (WHO) categories may miss more subtle variability and endotypes that could not only point to potential new therapeutics but also result in the use of advanced therapies in a “personalized” or “precision” medicine approach. This Statement highlighted the importance of a more granular approach to phenotyping patients, particularly in studies of genetics and genomics, which provided some justification for an NHLBI-sponsored multicenter study of patients with pulmonary hypertension using ’omics technology (PVDOMICS [Pulmonary Vascular Disease Phenomics]).
A comprehensive, evidence-based ATS Clinical Practice Guideline focused on the diagnosis, risk stratification, and management of PH in sickle cell disease (SCD) (4). The Guidelines outlined the complex underlying pathobiology of SCD characterized by both pulmonary vascular remodeling and left ventricular diastolic dysfunction and the attributable risk of hemolytic anemia in the pathogenesis. The roles of echocardiography, N-terminal pro-brain natriuretic peptide, and right heart catheterization in screening, diagnosis, and mortality risk assessment, and treatment approaches were discussed in depth. Specifically, up to 10% of unselected adult patients with SCD have PH defined by right heart catheterization and are at increased risk of death. Screening to identify this population of patients with SCD at high risk of having PH and of death was recommended, but the diagnosis of PH requires right heart catheterization. Therapies should target the underlying hematological condition, including hydroxyurea and iron chelation, oxygen, and transfusion as indicated. Pulmonary arterial hypertension (PAH)-specific therapies were not recommended for most patients with PH in the setting of SCD (strong recommendation, moderate-quality evidence). For selected patients meeting certain hemodynamic criteria, a trial of a prostacyclin analog or endothelin receptor antagonist was suggested (weak recommendation, very-low-quality evidence). The guidelines clearly summarized the knowledge gaps to be addressed with further studies.
Epidemiology and Outcomes
The outcome in acute pulmonary embolism (PE) is heavily dependent on whether the patient is hypotensive or shows evidence of shock or not. Risk stratification within a “low-risk” group is more difficult; being able to decide which patients could be treated as outpatients and which are at risk of adverse outcomes could facilitate safe, cost-effective triage. Jiménez and colleagues (5) created a prediction rule derived from 848 individuals in the PROTECT (Prognostic Value of CT) study, which recruited adult patients with acute PE who were hemodynamically stable and not treated with thrombolytics at PE diagnosis from 12 hospitals in Spain between 2009 and 2011. This rule was validated in 529 normotensive patients with acute PE in the PREP (Prognostic Factors for Pulmonary Embolism) study (6). The outcome of interest was a “complicated course” within 30 days, defined as death, hemodynamic collapse, or recurrent PE.
The overall pretest probability of a complicated course was 7.4% in the derivation and 4.5% in the validation cohorts. Using recursive partitioning, a Simplified Pulmonary Embolism Severity Score (sPESI) of 0 points (i.e., age ≤ 80 yr, no history of cancer or chronic cardiopulmonary disease, heart rate < 110 bpm, systolic blood pressure ≥ 100 mm Hg, and oxygen saturation ≥ 90%) and brain natriuretic peptide levels less than 100 pg/ml were found in 25% of the derivation population and demonstrated a very low risk of death (0.9%, 95% confidence interval [CI], 0–2.2%; or a negative predictive value of 99%, 95% CI, 96–100%). This combination was found in 36% of the validation cohort with a 30-day complicated course risk of 0% (or negative predictive value of 100%; one-sided 97.5% CI, 98.1%). An sPESI score greater than 0 (with the presence of any factor listed above), brain natriuretic peptide greater than 100 pg/ml, troponin greater than 0.05 ng/ml, and a proximal deep venous thrombosis on lower limb compression ultrasound was present in only 3.6% of patients in the derivation cohort and predicted a 25.8% (95% CI, 10.4–41.2%) chance of having a complicated course. In the validation cohort, 4.1% had this high-risk profile, with a positive predictive value of 21.2% (95% CI, 9.0–38.9%).
The identification of those at low risk for complications applied to more patients and reduced the probability of a complicated course from 4 to 7% (already quite small) to 1% or less. The prediction of “high risk” only applied to a small portion of this patient population, and even among those with this designation, most (75–85%) did not have complications. The majority of patients in the derivation and validation cohorts remained unclassified by the above two categories. Randomized clinical trials (RCTs) using this rule in the treatment and triage of patients with PE (most likely the low-risk group) should be the next step.
Autoimmunity and inflammation have been studied as important mechanisms in PAH. Specifically, the high risk of PAH in patients with connective tissue disease has been attributed to autoimmunity. Becker and colleagues (7) found that patients with connective tissue disease–related PAH, systemic sclerosis–related PAH (SSc-PAH), or SSc alone had higher levels of functional autoantibodies against the Ang II type-1 receptor (AT1R) and the endothelin receptor Type A (ETAR) (which up-regulate ERK1/2 and increase endothelial cell [EC]-dependent cytokine secretion) than patients with idiopathic PAH, chronic thromboembolic pulmonary hypertension, and congenital heart disease PAH.
Certain levels of anti-ETAR and AT1R antibodies were associated with a diagnosis of PAH in a prospective cohort of 253 patients with SSc without PAH at baseline; this cutoff was derived from receiver operating characteristic curves with areas of approximately 0.64 (0.50 indicates chance), indicating weak discrimination. In a multivariate model, however, only systemic pulmonary artery (PA) pressure by echocardiography at baseline predicted the onset of PAH. In a smaller group of patients with SSc-PAH, higher antibody levels were associated with a greater risk of mortality; again, these cutoffs were derived from receiver operating characteristic curves with areas of approximately 0.67. At this point, such antibody measurements are more likely to have mechanistic importance than clinical usefulness, considering the low discriminatory power for prediction of disease or survival.
Although pediatric pulmonary vascular disease is commonly subsumed under the WHO categories most recently revised in Nice (3), these conditions differ from those seen in adults. del Cerro Marin and colleagues (8) initiated the Spanish Registry for Pediatric Pulmonary Hypertension that collected data retrospectively at 21 pediatric cardiology centers throughout the country from 1998 and prospectively from January 2009. Patients were classified according to the WHO and the pediatric Panama Classifications (3, 9). Only two (<1%) of the children were lost to follow-up. Almost one-third had multifactorial disease showing the complexity of the phenotypes of pediatric PH. Predictors of better outcome in the entire cohort included (1) a diagnosis of PAH, (2) older age at diagnosis, (3) better functional class at diagnosis, and (4) lower right atrial pressure. In children with PAH, age at diagnosis younger than 2 years, worse functional class at diagnosis, lower cardiac index, and higher right atrial pressure were associated with an increased risk of death. Continued follow-up of this pediatric cohort will provide important information about the long-term outcomes of pediatric pulmonary vascular disease.
Yorke and colleagues across the UK (10) published the emPHasis-10, a short questionnaire to assess health-related quality of life in PH. A simple scoring system and nonproprietary status make it a very attractive patient-reported outcome for observational research and clinical trials. Although further studies are needed, the emPHasis-10 is an important advance in measuring how PH affects our patients and whether we are succeeding at making them feel better with our therapies.
Pulmonary Hypertension in the Setting of Parenchymal Lung Disease
Despite the progress in the understanding of the mechanisms and treatment of PAH and chronic thromboembolic pulmonary hypertension, more common forms of PH remain understudied and without specific effective medical treatment. PH in the setting of diffuse parenchymal lung disease, especially idiopathic pulmonary fibrosis (IPF), is associated with reduced exercise capacity and an increased risk of death (11, 12), but treatment attempts have been disappointing (13). Even more concerning, some studies of therapies for PH in IPF have gone unpublished (ACTIVE [A Clinical Trial in IPF to Improve Ventilation and Exercise], NCT00109681 and ARTEMIS-PH [Study of Ambrisentan in Subjects with Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis], NCT00879229). Corte and colleagues (14) showed the challenges of studying treatments for PH in interstitial lung disease in their double-blind RCT, which allocated patients with either IPF or fibrotic nonspecific interstitial pneumonia to bosentan (n = 40) or to placebo (n = 20). The authors found no difference in a reduction in pulmonary vascular resistance (PVR) index of 20% over 16 weeks (the primary end point; ∼30% in both groups), 6-minute-walk distance, health-related quality of life, or oxygen saturation. This study demonstrated the challenges of studying a small subset of a population with a rare disease with a particularly high morbidity and mortality, but important lessons may be gleaned. First, the use of something other than an intermediate or ultimate end point of a disease as a primary end point should be supported by data suggesting surrogacy. Even in PAH, drug-induced changes in PVR only explain a small amount of the therapeutic impact on outcomes in the short term (15). The lack of any signal in multiple other clinical end points, however, supports the findings. Second, the high risk of drop-out, death, and other reasons for not measuring the primary end point show the importance of using end points that will be measurable in all (or virtually all) subjects at the end of a study. Third, the publication of this study is itself important. Patients enroll in clinical trials with the altruistic notion that their participation will result in an impact on the field and a betterment of the human condition; studies that are completed but are never published in peer-reviewed journals leave this motivation unfulfilled and are questionable from several vantage points (16). Finally, this study shows the importance of performing RCTs in types of PH other than PAH and not just using PAH therapies indiscriminately.
Although PH is well known to accompany IPF, the prevalence and importance of PH in cystic fibrosis (CF) is less clear. Hayes and colleagues (17) used the Organ Procurement and Transplant Network Standard Transplant Analysis and Research Database to study patients with CF who were listed for lung transplantation between 1987 and 2013, did not undergo lung transplantation, and had complete data. There was close to a doubling in the risk of death for those with mean pulmonary artery pressure (mPA) greater than 25 mm Hg and a two to four times increased risk of death for those with mPA greater than 35 mm Hg. The generalizability of this study was greatly limited by the exclusion of the vast majority (75% or more) of the patients with CF actually listed for transplant (including those surviving to transplant) and the exclusion (by design) of patients who were too well to undergo evaluation (or who were evaluated and not listed). Lung function, likely a strong confounder of the association between PH and survival, was not included in the multivariate analyses, and differences in spirometry could explain the results. Missing data and the potential for selection bias (by excluding those who underwent transplant) were other important limitations. The authors propose a change in the “standard of care” to screen and focus on early diagnosis of PH in CF, which may be premature based on current knowledge.
Blood, Body Mass Index, and Bad Endothelium
The complexity of the interplay between blood flow and organ perfusion, baseline endothelial function, and vascular integrity and response after the transfusion of aged stored red blood cells (RBCs) has come into clearer focus. It is hypothesized that aged stored blood hemolyzes during storage and after transfusion with the released cell-free hemoglobin and red cell microparticles inhibiting NO signaling and impairing vascular function. Berra and colleagues (18) performed a crossover study of the effect of duration of blood storage in overweight patients with documented systemic endothelial dysfunction. With 2-week intervals between interventions, each volunteer received autologous leukoreduced blood stored for 2 to 3 days (3d challenge) or blood stored for 38 to 42 days with (40d challenge) or without NO inhalation (40d + NO challenge). The study was powered to detect a difference in the reactive hyperemia index (the primary end point), a measure of vasodilation, in the 5 minutes after transitory ischemia of one arm. Echocardiographic measurements were added to the assessment after the first four patients were enrolled, and the report focuses on pulmonary artery acceleration time (PAAT), which can be used to estimate mPA. There were no changes in the reactive hyperemia index based on duration of storage. However, the 40d challenge reduced PAAT and increased estimated mPA compared with the 3d challenge. 40d blood also increased plasma hemoglobin levels, NO consumption by plasma hemoglobin, serum iron, and indirect bilirubin levels. NO inhalation increased PAAT (reduced estimated mPA) and reduced NO consumption, which appeared to depend on the change in plasma hemoglobin and account for the increase in mPA, albeit in cross-sectional analyses. Supernatant-free hemoglobin was strongly associated with NO consumption, which was directly related to the change in estimated mPA.
This study showed that transfusion of RBCs after short- and long-term storage did not affect systemic vascular function in overweight and obese patients with preexisting endothelial dysfunction. The PAAT decrease after transfusion of blood stored for a longer duration may be related to changes in systemic NO processing. The clinical implications of subclinical increases in mPA (and possibly PVR) are currently unknown, but NO consumption may be a key mechanism.
Interestingly, a large multicenter RCT of fresher versus older stored RBC has been published. The RECESS (Red Cell Storage Duration) study of 1,098 cardiac surgery patients in the United States (19) randomized patients to RBCs stored less than 10 days or more than 21 days. The primary outcome was the change in multiorgan dysfunction score through Day 7. The median time of storage was 7 days in the fresh stored blood group and 28 days on the older stored blood group. There were no differences in the primary outcome of multiple organ dysfunction score or 7-day or 28-day mortality rates. How do we interpret these clinical trial results in the context of the preclinical and human physiological studies? One possible explanation is that the animal and physiological human studies have evaluated blood at the limits of storage, which for human blood banking approaches 42 days, whereas the clinical studies have explored median storage times of 28 days. More studies looking at the safety of blood storage and transfusion after longer storage times are required to address these concerns.
Sex, Endogenous Estrogen, and Aromatase Inhibitors
The best-established risk factor for idiopathic and heritable PAH is female sex. Even so, multiple studies have shown that women with PAH have better outcomes than men, potentially explained by better right ventricular (RV) function and response to therapy (20–24). Extensive evidence supports both protective and pathological roles of estrogen in PH, a phenomenon known as the “estrogen paradox” (25). By studying lungs from rodent PH models and human pulmonary artery vascular smooth muscle cells (PAVSMC), Mair and colleagues (26) found that, compared with male PAVSMC, female PAVSMC had higher levels of aromatase, an enzyme responsible for estrogen synthesis, and reduced levels of bone morphogenic protein receptor type II (BMPRII), which may explain the increased incidence of PAH in women. The aromatase inhibitor anastrozole (approved by the U.S. Food and Drug Administration for the treatment of breast cancer) and inhibition of estrogen receptor-α increased BMPRII and attenuated PH exclusively in the female animals, suggesting a sex-specific adverse effect of estrogenic signaling. Aromatase inhibitors are currently being studied in a small RCT of patients with PAH (NCT01545336).
Novel Gene Modifiers in PH
Although the role of mutations in the gene that codes for BMPRII (BMPR2) in PAH pathogenesis is well known, there is significant unexplained variation in phenotypic expression (27, 28). A cohort study by Girerd and colleagues (29) revealed that patients with mutations in the BMPR2 cytoplasmic tail, which do not affect the kinase domain and downstream Smad signaling, were older at diagnosis, had less severe hemodynamic abnormalities, and were more likely to have a long-term response to calcium channel blockers than affected carriers with mutations in other BMPR2 regions, suggesting that mutation location may impact disease severity. Another group (30) identified noncoding promoter variants of prostacyclin synthase associated with increased gene expression that were overrepresented in nonaffected BMPR2 carriers, implying a protective effect. de Jesus Perez and colleagues (31) used whole-exome sequencing of patients with idiopathic PAH without BMPR2 mutations to identify TopBP1, encoding topoisomerase DNA II–binding protein 1, as a new genetic cause of PAH and showed that the deficiency of the protein product in PA endothelial cells (PAEC) led to increased susceptibility to DNA damage and apoptosis.
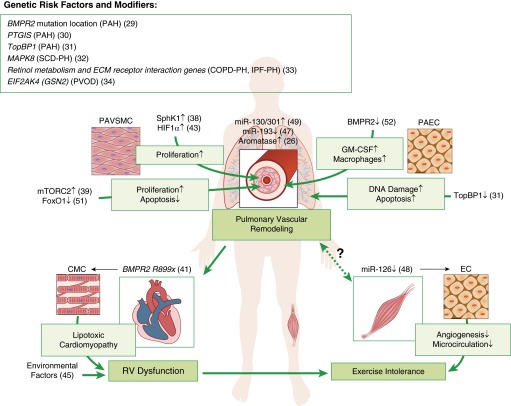
Although PAH has been the focus of most genetic and genomic studies, other forms of PH may have genomic risk modifiers. Zhang and colleagues (32) found that patients with SCD with precapillary PH had up-regulation of hypoxic response genes and reduced expression of MAPK8, which encodes a proapoptotic mitogen-activated protein kinase. Homozygosity for a MAPK8 polymorphism was present in all of the patients with precapillary SCD PH, supporting its pathogenic role. Hoffmann and colleagues (33) uncovered distinct gene expression patterns in remodeled PAs from patients with chronic obstructive pulmonary disease (COPD)- and IPF-associated PH, with the most significant differences in retinol metabolism and extracellular matrix receptor interaction pathways. Eyries and colleagues (34) used whole-exome sequencing to detect recessive functional mutations in EIF2AK4 (GCN2), which encodes protein kinase-negative regulator of translation initiation factor-2 and protein synthesis in individuals with pulmonary venoocclusive disease. These studies highlight the complex genetic architecture of PH and could lead to new diagnostic tools and/or molecular targets for therapy (Figure 1).
Figure 1.
Novel genetic risk factors, gene modifiers, and new mechanisms underlying the pathogenesis of pulmonary hypertension. Up arrow indicates up-regulation; down arrow indicates down-regulation. A number in parentheses corresponds to a source article in the References. BMPR = bone morphogenic protein receptor; CMC = cardiomyocytes; COPD = chronic obstructive pulmonary disease; EC = endothelial cells; FOXO1 = forkhead box O transcription factor 1; GM-CSF = granulocyte macrophage colony-stimulating factor; HIF = hypoxia-inducible factor; IPF = idiopathic pulmonary fibrosis; miR = microRNA; mTORC2 = mammalian target of rapamycin complex 2; PAEC = pulmonary artery endothelial cells; PAH = pulmonary arterial hypertension; PAVSMC = pulmonary artery vascular smooth muscle cells; PH = pulmonary hypertension; PVOD = pulmonary venoocclusive disease; RV = right ventricular; SCD = sickle cell disease; SphK = sphingosine kinase; TopBP = topoisomerase DNA II–binding protein.
Cell Fate in PH
Pulmonary vascular remodeling is integral to many forms of PH, and a number of cellular sources of neomuscularization of precapillary PAs and neointima formation have been implicated (35). Two groups used PAVSMC and EC tracing in transgenic mice to determine their fate in PH development. Sheikh and colleagues (36) found that hypoxia caused existing distal PAVSMC to recapitulate an earlier developmental program. PAVSMC dedifferentiated, migrated distally, proliferated, and then redifferentiated, giving rise to new distal PAVSMC. Qiao and colleagues (37) found that microvascular PAEC in the monocrotaline model turned on smooth muscle protein expression and contributed to neointima formation, strongly supporting the role of endothelial–mesenchymal transition in pulmonary vascular remodeling.
Pulmonary Vascular Remodeling, Metabolism, and RV Dysfunction
Metabolic abnormalities, a pathological component of PAH, have attracted significant interest from the research community, extending observations from cancer biology. Chen and colleagues (38) demonstrated that increased PAVSMC-specific expression of sphingosine kinase 1, and elevated levels of its product, sphingosine-1-phosphate, caused PAVSMC proliferation in human PAH and experimental hypoxia-induced PH, and inhibition prevented the development of PH. Goncharov and colleagues (39) found that mammalian target of rapamycin (mTOR) complex 2 (mTORC2), a master regulator of cell metabolism and survival (40), supported a proliferative, apoptosis-resistance human PAH PAVSMC phenotype via inhibiting the metabolic sensor AMP-activated protein kinase (AMPK). They provided evidence that the mTORC2-AMPK is a potential target pathway for therapeutic intervention.
Hemnes and colleagues (41) showed that global overexpression of dominant-negative BMPR2 R899X induced RV dysfunction with an impaired hypertrophic response and intracardiomyocyte accumulation of triglycerides and ceramide, known mediators of lipotoxicity and apoptosis, respectively. Metformin decreased RV lipid deposition. RV samples from patients with heritable PAH also showed lipid deposition and impaired fatty acid oxidation, suggesting the potential for AMPK activators to reduce lipotoxic cardiomyopathy and improve RV function (42). Interestingly, Ball and colleagues (43) found that vascular smooth muscle cell–specific deletion of HIF1α, a key pathologic player in hypoxia-induced PH (44), attenuated pulmonary vascular remodeling and RV systolic pressure increase but not RV hypertrophy. Together with a study showing that exposure to higher levels of air pollutants was linked to greater RV mass in adults without clinical cardiovascular disease (45), these data show that the determinants of RV morphology may be varied and go beyond simple measures of afterload (Figure 1).
MicroRNAs in PH
Growing evidence supports an important role of microRNAs, short noncoding RNAs that regulate protein-coding RNA transcription and translation (46), in PH pathogenesis. Sharma and colleagues (47) reported that miR-193 deficiency in human PAH and experimental PH increased plasma levels of oxidized lipids and promoted PH progression. Potus and colleagues (48) showed that a deficiency of endothelial-specific proangiogenic miR-126 in patients with PAH attenuated angiogenesis via overexpression of its downstream target Sprouty-related EVH1 domain-containing protein 1, leading to impaired skeletal muscle microcirculation and exercise intolerance. Bertero and colleagues (49) used an innovative network-based bioinformatic approach to identify miR-130/301 family as a top-ranked master regulator of subordinate miRNAs. The miR-130/301 family was up-regulated in the pulmonary vasculature in PAH and experimental PH. It was induced by multiple PH triggers, including hypoxia, inflammatory cytokines IL-1β and IL-6, and BMPR2 or CAV1 knockdown. Importantly, miR-130/301 controlled the PPARγ-STAT3-miR-204 pathway in PAVSMC, the apelin-miR-424/503-FGF2 pathway in PAEC, cell proliferation, and PH development. Although more work should be done, these findings suggest the potential attractiveness of microRNA-based therapies (Figure 1).
Inflammation and PH
Genetic, metabolic, and signaling abnormalities underlying PAH pathogenesis are closely linked to altered inflammation and immune responses (50). Savai and colleagues (51) found that inflammatory signals induced PAVSM-specific down-regulation of Forkhead box O transcription factor 1 (FoxO1), a key negative regulator of cell proliferation, in human and experimental PH. This induced PAVSMC proliferation, reduced apoptosis, and promoted PH, whereas up-regulation of FoxO1 had the opposite effect. Sawada and colleagues (52) found that the combination of BMPRII deficiency and increased levels of tumor necrosis factor led to PAEC-specific production of granulocyte macrophage colony-stimulating factor and demonstrated a critical role of this potent proinflammatory chemokine in macrophage recruitment and worsening of PH (Figure 1).
Conclusions
This review highlights the richness of investigation into pulmonary vascular disease, spanning basic mechanisms of vascular physiology and cellular metabolism, proliferation, and senescence to genetics and the regulation of protein signaling by microRNAs. Clinical studies have been enlightened by better understanding of genetics and phenotypes and by studying the determinants of outcome. As the best studies generate more questions than answers, there is much work to do to better understand pulmonary vascular disease in the years to come.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 2.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Klings ES, Machado RF, Barst RJ, Morris CR, Mubarak KK, Gordeuk VR, Kato GJ, Ataga KI, Gibbs JS, Castro O, et al. American Thoracic Society Ad Hoc Committee on Pulmonary Hypertension of Sickle Cell Disease. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189:727–740. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiménez D, Kopecna D, Tapson V, Briese B, Schreiber D, Lobo JL, Monreal M, Aujesky D, Sanchez O, Meyer G, et al. On Behalf Of The Protect Investigators. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189:718–726. doi: 10.1164/rccm.201311-2040OC. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez O, Trinquart L, Caille V, Couturaud F, Pacouret G, Meneveau N, Verschuren F, Roy P-M, Parent F, Righini M, et al. Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study. Am J Respir Crit Care Med. 2010;181:168–173. doi: 10.1164/rccm.200906-0970OC. [DOI] [PubMed] [Google Scholar]
- 7.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. 2014;190:808–817. doi: 10.1164/rccm.201403-0442OC. [DOI] [PubMed] [Google Scholar]
- 8.del Cerro Marín MJ, Sabaté Rotés A, Rodriguez Ogando A, Mendoza Soto A, Quero Jiménez M, Gavilán Camacho JL, Raposo Sonnenfeld I, Moya Bonora A, Albert Brotons DC, Moreno Galdó A REHIPED Investigators. Assessing pulmonary hypertensive vascular disease in childhood: data from the Spanish registry. Am J Respir Crit Care Med. 2014;190:1421–1429. doi: 10.1164/rccm.201406-1052OC. [DOI] [PubMed] [Google Scholar]
- 9.Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, Haworth SG, Ivy D, Lopes AA, Raj JU, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1:286–298. doi: 10.4103/2045-8932.83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yorke J, Corris P, Gaine S, Gibbs JSR, Kiely DG, Harries C, Pollock V, Armstrong I. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J. 2014;43:1106–1113. doi: 10.1183/09031936.00127113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132:998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 12.Rivera-Lebron BN, Forfia PR, Kreider M, Lee JC, Holmes JH, Kawut SM. Echocardiographic and hemodynamic predictors of mortality in idiopathic pulmonary fibrosis. Chest. 2013;144:564–570. doi: 10.1378/chest.12-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW Idiopathic Pulmonary Fibrosis Clinical Research Network. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corte TJ, Keir GJ, Dimopoulos K, Howard L, Corris PA, Parfitt L, Foley C, Yanez-Lopez M, Babalis D, Marino P, et al. BPHIT Study Group. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190:208–217. doi: 10.1164/rccm.201403-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventetuolo CE, Gabler NB, Fritz JS, Smith KA, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Are hemodynamics surrogate end points in pulmonary arterial hypertension? Circulation. 2014;130:768–775. doi: 10.1161/CIRCULATIONAHA.114.009690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan SD, Carbone RG. Pulmonary hypertension due to fibrotic lung disease: hidden value in a neutral trial. Am J Respir Crit Care Med. 2014;190:131–132. doi: 10.1164/rccm.201406-1094ED. [DOI] [PubMed] [Google Scholar]
- 17.Hayes D, Jr, Tobias JD, Mansour HM, Kirkby S, McCoy KS, Daniels CJ, Whitson BA. Pulmonary hypertension in cystic fibrosis with advanced lung disease. Am J Respir Crit Care Med. 2014;190:898–905. doi: 10.1164/rccm.201407-1382OC. [DOI] [PubMed] [Google Scholar]
- 18.Berra L, Pinciroli R, Stowell CP, Wang L, Yu B, Fernandez BO, Feelisch M, Mietto C, Hod EA, Chipman D, et al. Autologous transfusion of stored red blood cells increases pulmonary artery pressure. Am J Respir Crit Care Med. 2014;190:800–807. doi: 10.1164/rccm.201405-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, Granger S, Bennett-Guerrero E, Blajchman MA, Scavo V, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–1429. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, et al. Sex hormones are associated with right ventricular structure and function: the MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183:659–667. doi: 10.1164/rccm.201007-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;43:523–530. doi: 10.1183/09031936.00027613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard H-J, Boonstra A, Vonk Noordegraaf A. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145:1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umar S, Rabinovitch M, Eghbali M. Estrogen paradox in pulmonary hypertension: current controversies and future perspectives. Am J Respir Crit Care Med. 2012;186:125–131. doi: 10.1164/rccm.201201-0058PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115:189–202. doi: 10.1161/CIRCRESAHA.115.303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajkumar R, Ahmad F. The genomic complexity underlying pulmonary arterial hypertension: from Mendel to networks. Am J Respir Crit Care Med. 2014;189:1152–1154. doi: 10.1164/rccm.201403-0556ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girerd B, Coulet F, Jaïs X, Eyries M, Van Der Bruggen C, De Man F, Houweling A, Dorfmüller P, Savale L, Sitbon O, et al. Characteristics of pulmonary arterial hypertension in affected carriers of a mutation located in the cytoplasmic tail of bone morphogenetic protein receptor type 2. Chest. 2015;147:1385–1394. doi: 10.1378/chest.14-0880. [DOI] [PubMed] [Google Scholar]
- 30.Stearman RS, Cornelius AR, Lu X, Conklin DS, Del Rosario MJ, Lowe AM, Elos MT, Fettig LM, Wong RE, Hara N, et al. Functional prostacyclin synthase promoter polymorphisms: impact in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:1110–1120. doi: 10.1164/rccm.201309-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jesus Perez VA, Yuan K, Lyuksyutova MA, Dewey F, Orcholski ME, Shuffle EM, Mathur M, Yancy L, Jr, Rojas V, Li CG, et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:1260–1272. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Zhang W, Ma S-F, Desai AA, Saraf S, Miasniakova G, Sergueeva A, Ammosova T, Xu M, Nekhai S, et al. Hypoxic response contributes to altered gene expression and precapillary pulmonary hypertension in patients with sickle cell disease. Circulation. 2014;129:1650–1658. doi: 10.1161/CIRCULATIONAHA.113.005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann J, Wilhelm J, Marsh LM, Ghanim B, Klepetko W, Kovacs G, Olschewski H, Olschewski A, Kwapiszewska G. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:98–111. doi: 10.1164/rccm.201401-0037OC. [DOI] [PubMed] [Google Scholar]
- 34.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, Chelghoum N, Coulet F, Bonnet D, Dorfmüller P, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 35.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Reports. 2014;6:809–817. doi: 10.1016/j.celrep.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR, Berry GJ, Pearl RG, Kao PN. Endothelial fate mapping in mice with pulmonary hypertension. Circulation. 2014;129:692–703. doi: 10.1161/CIRCULATIONAHA.113.003734. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 41.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuder RM, Robinson JC, Graham BB. Fat and cardiotoxicity in hereditary pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:247–249. doi: 10.1164/rccm.201312-2240ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med. 2014;189:314–324. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JSK, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, Lima JA, Szpiro AA, Van Hee VC, Kawut SM The Multi-ethnic Study of Atherosclerosis. Traffic-related air pollution and the right ventricle: the multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med. 2014;189:1093–1100. doi: 10.1164/rccm.201312-2298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2015;52:139–151. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130:776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay È, Breuils-Bonnet S, Ribeiro F, Porlier A, Maltais F, Bonnet S, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:318–328. doi: 10.1164/rccm.201402-0383OC. [DOI] [PubMed] [Google Scholar]
- 49.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 52.Sawada H, Saito T, Nickel NP, Alastalo T-P, Glotzbach JP, Chan R, Haghighat L, Fuchs G, Januszyk M, Cao A, et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med. 2014;211:263–280. doi: 10.1084/jem.20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]