Abstract
Background
Atrial fibrillation (AF) can be a risk factor for development of significant tricuspid regurgitation (TR). We investigated which clinical and echocardiographic parameters were related to severity of functional TR in patients with lone AF.
Methods
A total of 89 patients with lone AF were enrolled (75 ± 11 years; 48% male): 13 patients with severe TR, 36 patients with moderate TR, and 40 consecutive patients with less than mild TR. Clinical parameters and echocardiographic measurements including right ventricular (RV) remodeling and function were evaluated.
Results
Patients with more severe TR were older and had more frequently persistent AF (each p < 0.001). TR severity was related to right atrial area and tricuspid annular systolic diameter (all p < 0.001). The patients with moderate or severe TR had larger left atrial (LA) volume and increased systolic pulmonary artery pressure (SPAP) than the patients with mild TR (p = 0.04 for LA volume; p < 0.001 for SPAP). RV remodeling represented by enlarged RV area and increased tenting height was more prominent in severe TR than mild or moderate TR (all p < 0.001). Multivariate analysis showed type of AF, LA volume, tricuspid annular diameter and tenting height remained as a significant determinants of severe TR. In addition, tenting height was independently associated with the presence of severe TR (p = 0.04).
Conclusion
In patients with lone AF, TR was related to type of AF, LA volume, tricuspid annular diameter and RV remodeling. Especially, tricuspid valvular tethering seemed to be independently associated with development of severe functional TR.
Keywords: Tricuspid regurgitation, Atrial fibrillation, Tenting height
Introduction
Tricuspid regurgitation (TR) can be caused by pathologic involvement of the tricuspid valve but more often occurs in the structurally normal tricuspid leaflet and chordae which is called functional TR.1),2) Functional TR can occur in several cardiovascular diseases such as left-sided heart disease, myocardial and pulmonary diseases.3) This functional TR was often ignored in the past because it was regarded to be simply related to other pathologic problems such as left sided valvular disease and it can improve after correction of underlying problem. However, multiple recent studies have shown that TR may progess inspite of appropriate repair of the left side valve and that functional TR may be an important factor of adverse clinical outcome. Prior studies have shown that annular dilatation and tethering of the tricuspid valve contribute to the development of functional TR.3),4),5),6) Atrial fibrillation (AF) is suggested as one of the risk factors for development of TR.7),8),9) AF is often associated with left and right atrial enlargement, thus causing annular dilatation.4),7),10) However, not all patients with AF and dilated right atrium (RA) develop significant TR. In this study, we investigated which clinical or echocardiographic parameters were related to severity of functional TR in patients with lone AF. We hypothesized that multifactorial factors such as the geometric change of right ventricle (RV) in addition to tricuspid annular dilatation by RA enlargement would play a role in determining TR severity in the patients with lone AF.
Methods
Study population
A total 89 patients with lone AF were enrolled in this study. By reviewing echocardiographic reports of the patients with lone AF between Jan 2009 and Dec 2014, 36 patients with moderate and 13 patients with severe TR were identified. Then control group was selected from our echocardiographic database during same period and consisted of 40 consecutive patients with lone AF but no more than mild TR. For patients who had multiple echocardiograms, we used the first echocardiogram showing significant TR. The patients with pacemaker, left ventricular (LV) ejection fraction < 50%, rheumatic valvular disease, significant left sided valvular disease, prior valvular surgery, intrinsic tricuspid valvular disease or congenital heart disease were excluded.
Echocardiography
Standard echocardiographic parameters including M-mode, two-dimensional (2D) and Doppler measurements were analyzed in accordance with the American Society of Echocardiography guidelines.11),12) Right atrial area (RAA) was measured by tracing RA endocardium from the lateral aspect of the tricuspid annulus to the septal aspect at the apical 4-chamber view. Right ventricular end-diastolic area (RVEDA) and end-systolic area (RVESA) were obtained from a RV-focused apical 4-chamber view. RV fractional area change (RVFAC) was calculated by (RVEDA - RVESA) / RVEDA × 100.11) RV systolic area was divided by RV long-axis dimension (from the tip of the RV apex to the midpoint of tricuspid annular plane) to derive the RV spherical index.13) Tricuspid annular diameter was measured in the apical 4-chamber view as the distance between the insertion of the septal leaflet and the insertion of the anterior leaflet, and tenting height was also measured as the perpendicular distance between the tip of leaflet coaptation and tricuspid annulus plane at the time of maximal systolic closure.8) Systolic pulmonary artery pressure (SPAP) was estimated from the peak systolic TR velocity using Bernoulli equation, and RA pressure was derived based on the inferior vena cava diameter and its respiratory change. Tricuspid annular plane systolic excursion was acquired by placing M-mode cursor through the tricuspid annulus and measuring the distance of its systolic excursion in the longitudinal plane. Systolic excursion velocity of RV free wall was obtained with a tissue Doppler imaging at the apical 4-chamber view.12) Severity of TR was graded based on the color-flow Doppler using the ratio of TR jet area to the RAA: mild if the ratio < 20%, moderate if 20% to 40% and severe if ≥ 40%.14) Vena contract width more than 7 mm was also defined as having severe TR. All echocardiographic measurements were averaged over 5 cardiac cycles.
Data analysis
Data were expressed as mean ± standard deviation for continuous variables and counts and percentages for categorical variables. We divided the patients into 3 groups according to TR severity and applied one-way analysis of variance with the Scheffe post hoc test for continuous variables or Kruskal-Wallis analysis for categorical variables. The relationship between tenting height and other echocardiographic parameters was examined with Pearson's correlation analysis. We assessed univariate regression analysis with vena contract width and other parameters. In addition, multivariate stepwise linear regression analysis was performed to assess independent determinants among parameters with the variables which had a p value < 0.1 from the univariate regression analysis. Vena contract width was used as a dependent variable in this multivariate linear regression analysis. To avoid collinearity, we constructed 3 different multivariate models using the variables with a variance inflation factor less than 5. Logistic regression was also applied to assess independent parameters in determining severe TR. All statistical analyses were performed using SPSS Statistics Version 19.0 (IBM Corp., Armonk, NY, USA) and STATA 14.0 (STATA Corp., College Station, TX, USA). p value of < 0.05 was considered statistically significant.
Results
Clinical characteristics
A total of 89 patients with lone AF were enrolled (age 75 ± 11 years; 48% male): 13 patients with severe TR, 36 patients with moderate TR, and 40 patients with no more than mild TR. Baseline characteristics of the study population are summarized in Table 1. When the patients were divided into three groups according to the severity of TR, they were similar in gender and underlying diseases such as diabetes and hypertension. However, the patients who have more severe TR were older (p < 0.001). All patients with moderate or severe TR had persistent AF, whereas 38% of patients with no more than mild TR had paroxysmal AF (Table 2). Duration of AF was related to the severity of TR (p = 0.008).
Table 1. Baseline characteristics of overall patients.
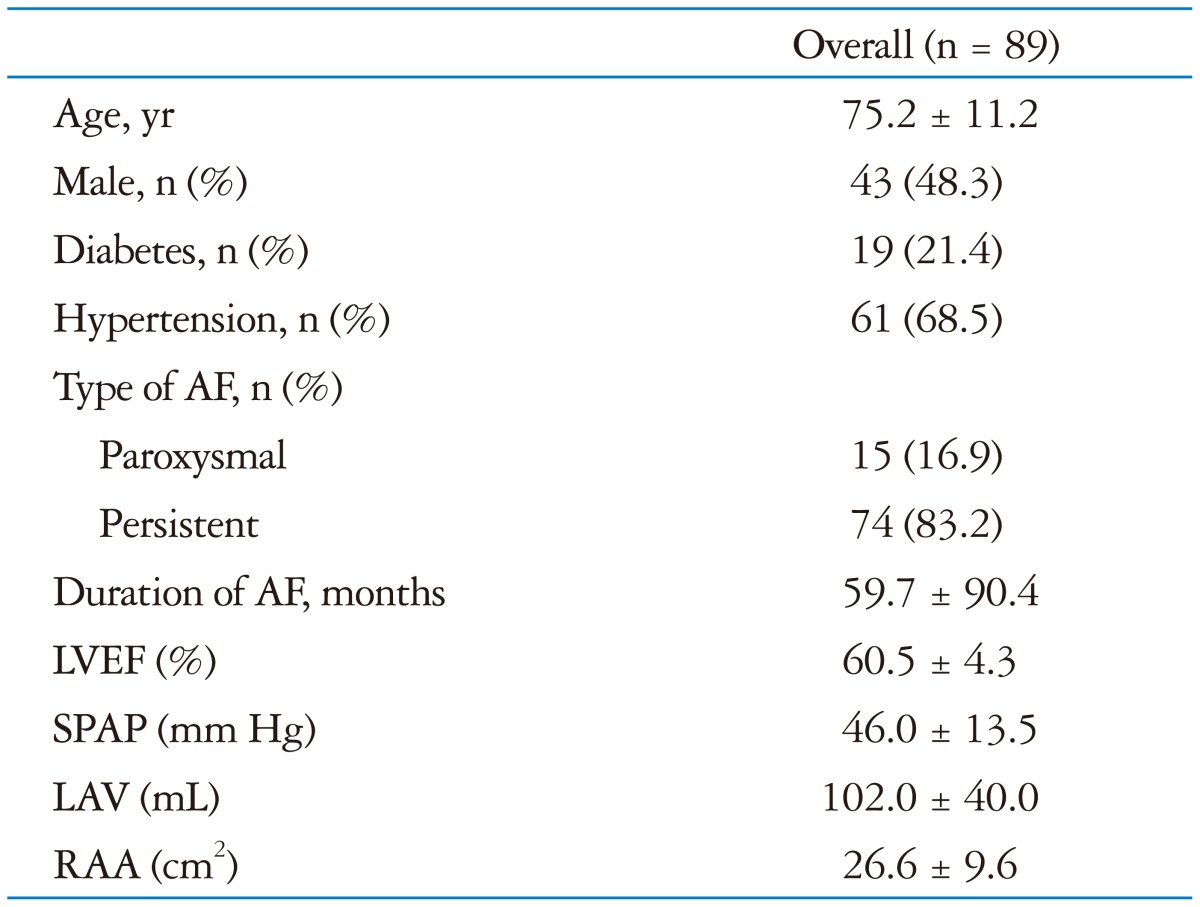
AF: atrial fibrillation, LAV: left atrial volume, LVEF: left ventricular ejection fraction, RAA: right atrial area, SPAP: systolic pulmonary artery pressure
Table 2. Clinical characteristics in patients with AF according to TR severity.
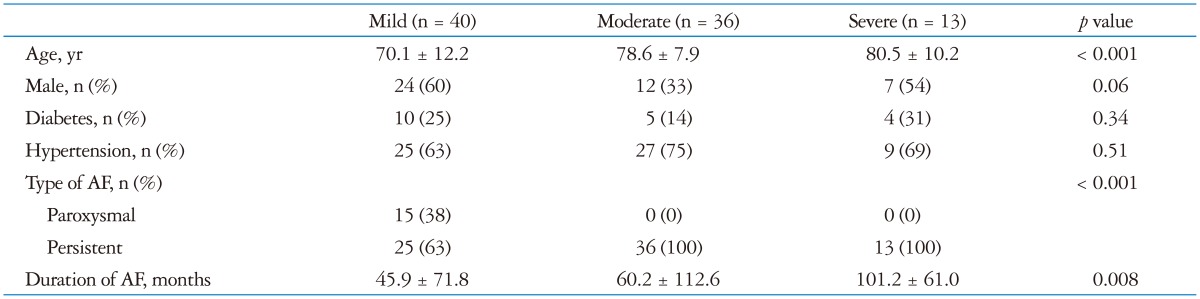
AF: atrial fibrillation, TR: tricuspid regurgitation
Echocardiographic parameters according to TR severity
There was no significant difference between the groups regarding LV size, LV mass, and LV systolic function (Table 3). Left atrial (LA) volume was larger in the patients with moderate or severe TR as compared to the patients with mild TR (p = 0.04). In addition, RA area as well as tricuspid annular diameter were larger in the patients with more severe TR than in the patients with milder TR (p < 0.001). RV systolic and diastolic area, RV spherical index and tenting height were larger in the patients with severe TR than with mild or moderate TR (p < 0.001), whereas they were comparable between mild and moderate group. SPAP was higher in the patients with moderate or severe group than with mild group (p < 0.001). RV function, reflected by several parameters such as RVFAC, systolic excursion velocity, and tricuspid annular plane systolic excursion, was similar between the groups.
Table 3. Echocardiographic parameters in patients with AF according to TR severity.
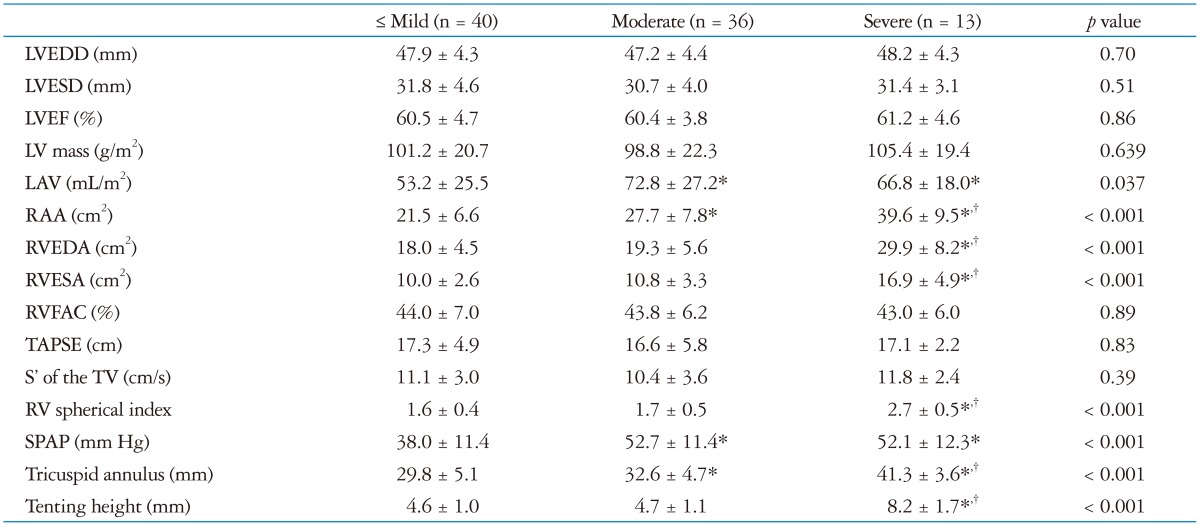
*Indicates p ≤ 0.05 as compared to group with less than mild TR, †Indicates p ≤ 0.05 as compared to group with moderate TR. AF: atrial fibrillation, LAV: left atrial volume, LVEDD: left ventricular end-diastolic dimension, LVEF: left ventricular ejection fraction, LVESD: left ventricular end-systolic dimension, RAA: right atrial area, RVEDA: right ventricular end-diastolic area, RVESA: right ventricular end-systolic area, RVFAC: right ventricular fractional area change, S': systolic myocardial velocity, SPAP: systolic pulmonary artery pressure, TAPSE: tricuspid annular plane systolic excursion, TR: tricuspid regurgitation, TV: tricuspid valve
Relationship between tenting height and right heart remodeling
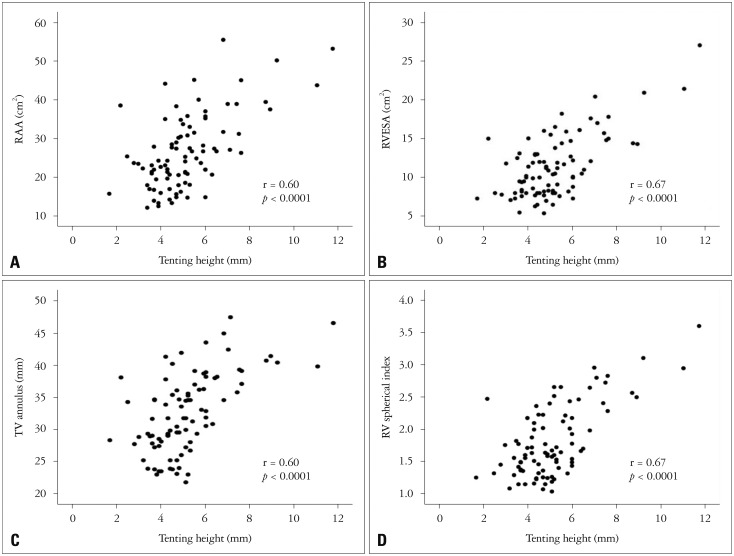
Tenting height was associated with RA size, RV size and tricuspid annular diameter (r = 0.60, p < 0.0001 for RAA; r = 0.70, p < 0.0001 for RVEDA; r = 0.67, p < 0.0001 for RVESA; r = 0.60, p < 0.0001 for tricuspid annular diameter). In addition, the correlation between the tenting height and RV spherical index was significant (r = 0.67, p < 0.0001) (Fig. 1).
Fig. 1. Relationship between tenting height and RAA (A), RVESA (B), TV annulus (C), and RV spherical index (D). RAA: right atrial area, RV: right ventricle, RVESA: right ventricular end-systolic area, TV: tricuspid valve.
Determinants of severe TR
Univariate analysis showed TR severity was related to age, type of AF, LA volume, RA area, RV area, RV spherical index, SPAP, tricuspid annulus and tenting height (Table 4, Fig. 2). Multivariate regression demonstrated type of AF, LA volume, tricuspid annular diameter and tenting height were independently associated with TR severity (Table 4). By multivariate logistic analysis with tenting height, tricuspid annular diameter and LA volume, tenting height was independently associated with the presence of severe TR (p = 0.04).
Table 4. Univariate and multivariate regression analyses of factors associated with tricuspid regurgitation severity.
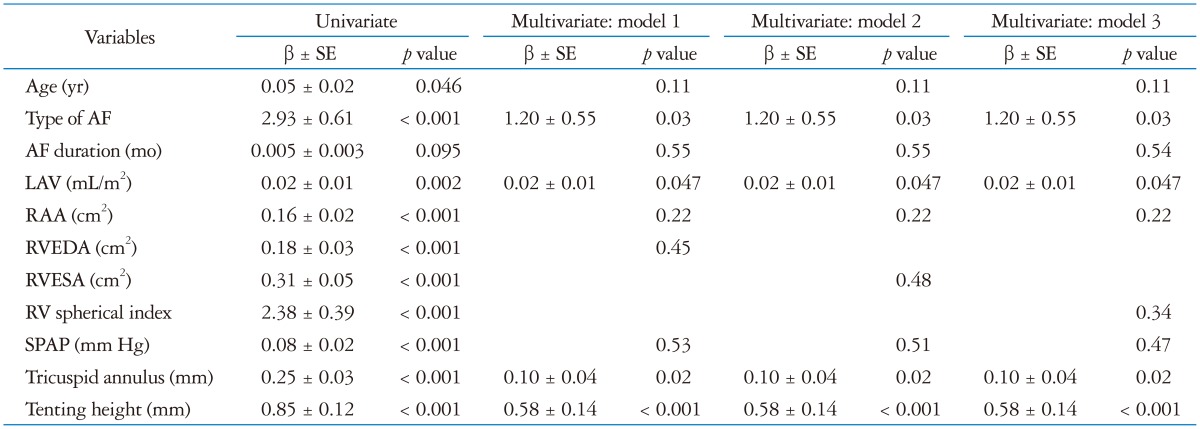
AF: atrial fibrillation, LAV: left atrial volume, RAA: right atrial area, RV: right ventricle, RVEDA: right ventricular end-diastolic area, RVESA: right ventricular end-systolic area, SPAP: systolic pulmonary artery pressure
Fig. 2. Relationship between vena contracta and age (A), LAV (B), TV annulus (C), and tenting height (D). LAV: left atrial volume, TV: tricuspid valve.
Discussion
TR is a common echocardiographic finding. Although functional TR has somewhat been ignored in the past, it has attracted attention following clinical observations that functional TR itself can be an important predictor of clinical outcomes.2),15) Functional TR is known to be related to tricuspid annular dilatation, which can be caused by RV remodeling in various conditions.3),4),5) Also, tethering of the tricuspid leaflets is often observed. While AF is known to contribute to the development of significant TR, the mechanism of TR development in AF is not clear. Most previous studies about functional TR included the patients with a variety of diseases and enrolled the patients with AF as only a part of study population.3),4),16),17) In our study, we assessed which clinical and echocardiographic parameters would contribute to severe TR in AF, and type of AF, LA volume, tricuspid annular diameter and tenting height were independently related to more severe TR in lone AF. The patients with more severe TR had larger RA and tricuspid annular size, greater RV size, and more globular RV than milder TR group. Tenting height was strongly related to RA and RV enlargement as well as RV spherical deformation.
AF can cause significant LA and RA enlargement, thus potentially leading to dilatation of mitral and tricuspid annuli located at the inferior edge of the atrium.10) But annular dilatation and valvular regurgitation are reported to be greater in the tricuspid valve than in the mitral valve because the fibrous skeleton is less developed in the tricuspid valve.7) Previous data have reported aging and right heart enlargement as important pathophysiologic mechanism for developing severe TR in the patents with AF.8),9) In our study, the patients with moderate or severe TR had larger annular dilatation with more RA enlargement than the patients with no more than mild TR while the patients with severe TR showed significantly greater RV size with globular shape and more tethering of the tricuspid valve, reflected by increased tenting height. Isolated tricuspid annular dilatation relating to RA enlargement might not be enough to cause severe functional TR in the patients with lone AF. Further tricuspid annular enlargement with RV dilatation and remodeling might be required to develop severe TR, leading to an altered ventricular force balance to close the leaflets and incomplete closure of the tricuspid leaflets. However, we could not elucidate causal relationship in our cross-sectional study design and RV enlargement can be caused by significant TR. Significant TR can dilate right heart chamber more and dilated right heart would deteriorate TR. So, TR can beget TR.
Prior studies revealed that the annular plane became larger, more planar, and circular in patients with functional TR as opposed to saddle shape in control subjects.18) Spinner et al.19) showed that RV dilatation can result in displacement of all of the papillary muscles using three-dimensional echocardiography. They demonstrated that apical and lateral displacement of all papillary muscles was related to TR severity, with the exception of lateral displacement of anterior papillary muscle, along with leaflet tethering. Displacement of the papillary muscles and annular dilatation would make the papillary muscle to move away from the annulus, thus leading to leaflet tethering. Our study showed that leaflet tethering can contribute to the presence of severe TR in AF. This finding is compatible with other previous studies, which revealed tricuspid valve tethering as an important geometric cause of functional TR.5),20),21),22) Relative positioning of the papillary muscles may also be a significant contributor to the tethering of the leaflets.
Study limitation
Several limitations of our analysis should be noted. First, our study could not elucidate the causal relationship because of cross-sectional design as pointed out previously. Second, we graded TR severity based on the ratio of TR jet area to the RAA and vena contracta width. While TR jet area and vena contracta are commonly used in assessing TR grade and most TR jet was a central jet in our population, more quantitative assessment of TR such as proximal isovelocity surface area can be recommended.1),23),24) Also, we used 2D echocardiography in measuring several parameters including tricuspid annular diameter. Although 2D echocardiography is widely used in clinical practice and also for research currently especially for the patients with AF, 2D images suffer from inherent limitations such as incomplete comprehension of the three-dimensional tricuspid annulus. Three-dimensional echocardiography with one beat acquisition might provide more accurate measurements with more reproducible landmarks, but it is necessary to take account of its relative lower temporal and spatial resolution compared to 2D echocardiography. Finally, the study population with severe TR was relatively small, thus decreasing statistical power.
Conclusions
Tenting height, together with RA and RV enlargement as well as RV spherical deformation, tricuspid annular diameter and LA volume were independently associated with TR severity in the patients with persistent lone AF. While the causal relationship is not elucidative in this study, tricuspid valvular tethering may predominantly contribute to development of severe TR in this population. Further large, prospective cohort studies with more quantitative methods will be required to elucidate the precise mechanisms and a determinant for developing severe TR in the patients with lone AF.
Acknowledgements
This work was supported by Inha University Hospital and Inha University Research Grants (INHA-40899).
References
- 1.Badano LP, Muraru D, Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013;34:1875–1885. doi: 10.1093/eurheartj/ehs474. [DOI] [PubMed] [Google Scholar]
- 2.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 3.Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, Mahoney DW, Enriquez-Sarano M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:314–323. doi: 10.1161/CIRCIMAGING.111.967919. [DOI] [PubMed] [Google Scholar]
- 4.Mutlak D, Lessick J, Reisner SA, Aronson D, Dabbah S, Agmon Y. Echocardiography-based spectrum of severe tricuspid regurgitation: the frequency of apparently idiopathic tricuspid regurgitation. J Am Soc Echocardiogr. 2007;20:405–408. doi: 10.1016/j.echo.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Sagie A, Schwammenthal E, Padial LR, Vazquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol. 1994;24:446–453. doi: 10.1016/0735-1097(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim HK, Lee SP, Kim YJ, Sohn DW. Tricuspid regurgitation: clinical importance and its optimal surgical timing. J Cardiovasc Ultrasound. 2013;21:1–9. doi: 10.4250/jcu.2013.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Otsuji Y, Yoshifuku S, Yuasa T, Zhang H, Takasaki K, Matsukida K, Kisanuki A, Minagoe S, Tei C. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J. 2002;66:913–916. doi: 10.1253/circj.66.913. [DOI] [PubMed] [Google Scholar]
- 8.Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. 2012;29:140–146. doi: 10.1111/j.1540-8175.2011.01565.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki N, Kondo F, Kubo T, Okawa M, Matsumura Y, Kitaoka H, Yabe T, Furuno T, Doi Y. Severe tricuspid regurgitation in the aged: atrial remodeling associated with long-standing atrial fibrillation. J Cardiol. 2006;48:315–323. [PubMed] [Google Scholar]
- 10.Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, Weyman AE. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990;82:792–797. doi: 10.1161/01.cir.82.3.792. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. quiz 786-8. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda S, Gillinov AM, Song JM, Daimon M, Kongsaerepong V, Thomas JD, Shiota T. Echocardiographic insights into atrial and ventricular mechanisms of functional tricuspid regurgitation. Am Heart J. 2006;152:1208–1214. doi: 10.1016/j.ahj.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 15.Topilsky Y, Nkomo VT, Vatury O, Michelena HI, Letourneau T, Suri RM, Pislaru S, Park S, Mahoney DW, Biner S, Enriquez-Sarano M. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185–1194. doi: 10.1016/j.jcmg.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB, Choi YS. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006;98:236–242. doi: 10.1016/j.amjcard.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 17.Seo HS, Ha JW, Moon JY, Choi EY, Rim SJ, Jang Y, Chung N, Shim WH, Cho SY, Kim SS. Right ventricular remodeling and dysfunction with subsequent annular dilatation and tethering as a mechanism of isolated tricuspid regurgitation. Circ J. 2008;72:1645–1649. doi: 10.1253/circj.cj-08-0237. [DOI] [PubMed] [Google Scholar]
- 18.Ton-Nu TT, Levine RA, Handschumacher MD, Dorer DJ, Yosefy C, Fan D, Hua L, Jiang L, Hung J. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006;114:143–149. doi: 10.1161/CIRCULATIONAHA.106.611889. [DOI] [PubMed] [Google Scholar]
- 19.Spinner EM, Lerakis S, Higginson J, Pernetz M, Howell S, Veledar E, Yoganathan AP. Correlates of tricuspid regurgitation as determined by 3D echocardiography: pulmonary arterial pressure, ventricle geometry, annular dilatation, and papillary muscle displacement. Circ Cardiovasc Imaging. 2012;5:43–50. doi: 10.1161/CIRCIMAGING.111.965707. [DOI] [PubMed] [Google Scholar]
- 20.Hung J. The pathogenesis of functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg. 2010;22:76–78. doi: 10.1053/j.semtcvs.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, Thomas JD, Shiota T. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975–979. doi: 10.1161/01.CIR.0000156449.49998.51. [DOI] [PubMed] [Google Scholar]
- 22.Mikami T, Kudo T, Sakurai N, Sakamoto S, Tanabe Y, Yasuda H. Mechanisms for development of functional tricuspid regurgitation determined by pulsed Doppler and two-dimensional echocardiography. Am J Cardiol. 1984;53:160–163. doi: 10.1016/0002-9149(84)90702-1. [DOI] [PubMed] [Google Scholar]
- 23.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 24.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]