Abstract
The use of multiple origins for chromosome replication has been demonstrated in archaea. Similar to the dormant origins in eukaryotes, some potential origins in archaea appear to be inactive during genome replication. We have comprehensively explored the origin utilization in Haloferax mediterranei. Here we report three active chromosomal origins by genome-wide replication profiling, and demonstrate that when these three origins are deleted, a dormant origin becomes activated. Notably, this dormant origin cannot be further deleted when the other origins are already absent and vice versa. Interestingly, a potential origin that appears to stay dormant in its native host H. volcanii lacking the main active origins becomes activated and competent for replication of the entire chromosome when integrated into the chromosome of origin-deleted H. mediterranei. These results indicate that origin-dependent replication is strictly required for H. mediterranei and that dormant replication origins in archaea can be activated if needed.
 Archaea use multiple origins for chromosome replication, but some potential origins appear to be inactive during the genome replication. Here, Yang et al. show that when active origins are deleted from Haloferax mediterranei, a dormant origin is activated and essential, suggesting an origin-dependent replication.
Archaea use multiple origins for chromosome replication, but some potential origins appear to be inactive during the genome replication. Here, Yang et al. show that when active origins are deleted from Haloferax mediterranei, a dormant origin is activated and essential, suggesting an origin-dependent replication.
Chromosome replication starts at specific sites known as origins, where initiator proteins bind and recruit replication machinery components1,2. Bacteria use a single origin, which is usually adjacent to the initiator gene dnaA, to replicate their chromosome, whereas eukaryotes perform chromosome replication with multiple replication origins that are recognized by origin recognition complexes2,3. Dormant origins are replication origins that are not used in a normal cell cycle4. If DNA encounters replication stress or if adjacent origins fail to fire, dormant origins can be activated to help complete chromosome replication4,5,6, providing an important mechanism for cells to deal with replication stress and to maintain genomic stability7. For organisms with multiple replication origins on a chromosome, the coordination of origin utilization is vital to ensure complete and accurate genome duplication.
Chromosome replication using multiple replication origins has been widely recognized in archaea. The combination of an autonomously replicating sequence (ARS) assay or two-dimensional gel electrophoresis with marker frequency analysis (MFA) has led to the identification of multiple replication origins in Sulfolobus species8,9,10, haloarchaea11,12,13,14, Aeropyrum pernix15 and Pyrobaculum calidifontis16, thus greatly expanding our knowledge of archaeal replication origins. Archaeal replication origins generally contain an AT-rich region flanked by conserved repetitive DNA sequences designated as origin recognition boxes (ORBs)17. The initiator Cdc6 shows homology to both Orc1 and Cdc6 in eukaryotes and is responsible for ORB recognition and minichromosome maintenance (MCM) helicase recruitment18,19. As in bacteria, an archaeal replication origin usually lies upstream of the initiator gene orc1/cdc6 (ref. 20). Studies in Sulfolobus islandicus and Haloarcula hispanica have demonstrated that the firing of specific origins is dependent on their adjacent initiators10,21, which facilitate the direct regulation of each origin. Based on the origin features described above, multiple cdc6-associated replication origins in 15 fully sequenced haloarchaeal genomes have been predicted12. However, not all of these origins were shown to be active in the genome-wide analysis of replication profiles14,22, similar to the presence of dormant origins in eukaryotes. However, no dormant origin has been experimentally identified in archaea.
The origin usage in one cell cycle was investigated in Sulfolobus acidocaldarius, which possesses three origins on its chromosome. The three replication origins fire once per cell cycle, with oriC1 and oriC3 firing slightly earlier than oriC2 (ref. 23). For the three origins in S. islandicus and the two origins on the chromosome of H. hispanica, gene knockout experiments demonstrated that no single origin was essential, but at least one orc1/cdc6 gene (for S. islandicus) or one origin (for H. hispanica) was required for chromosome replication10,21. Intriguingly, all active replication origins on the chromosome in H. volcanii H26 can be knocked out, and the resultant strain grows even faster than the parent strain22. Owing to the requirement for RadA recombinase, which is essential for homologous recombination, homologous recombination was proposed to account for replication initiation in this origin deletion strain of H. volcanii22.
In this study, the utilization of multiple replication origins and the consequences of origin deletion were investigated in Haloferax mediterranei, which belongs to the same genus as H. volcanii. We identified the replication origins on the chromosome and examined origin utilization throughout the growth phase. Surprisingly, knocking out all active origins on the chromosome led to the activation of a dormant replication origin, oriC4-cdc6H. The oriC4 is essential for chromosome replication in the absence of all other active origins, and the three active origins cannot be deleted simultaneously if oriC4 has been knocked out. Interestingly, when oriC4-cdc6H was replaced with oriP2-orc13, a putative dormant origin from H. volcanii, oriP2-orc13 could also efficiently initiate the replication of the entire chromosome of H. mediterranei in the absence of all other active origins. These results demonstrate that dormant origins can be activated in archaea if needed, and that chromosome replication in H. mediterranei, unlike that in H. volcanii, strictly requires origin-dependent initiation.
Results
Coordinated utilization of three chromosomal origins
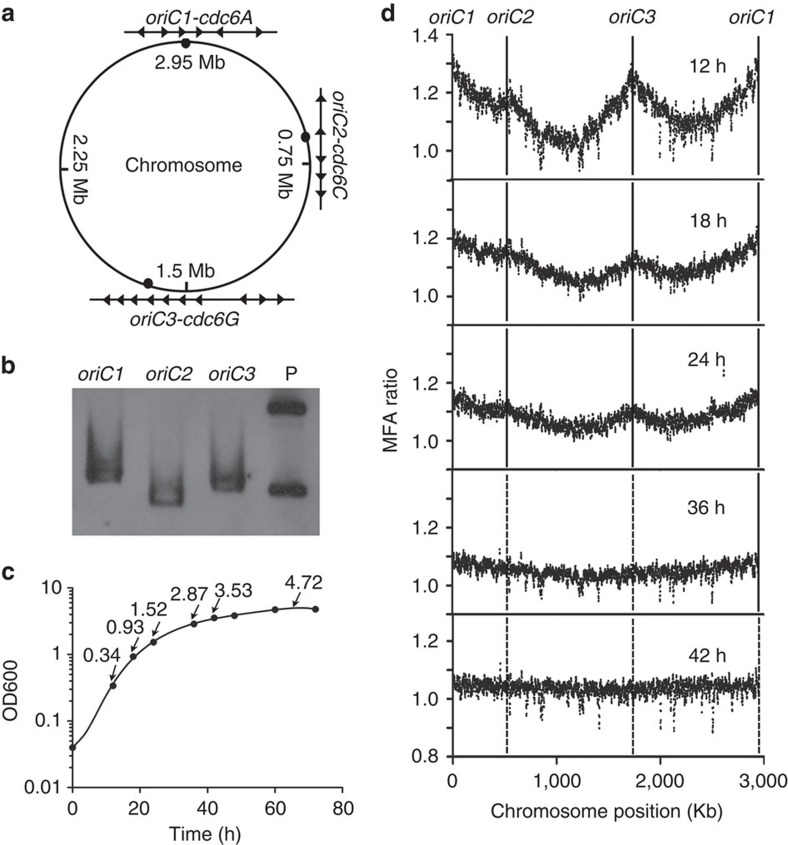
Genome sequencing has revealed that multiple initiator genes (cdc6) are encoded in the H. mediterranei genome, with eight in the main chromosome and five in the three megaplasmids24. Because replication origins are dependent on adjacent initiator proteins (Cdc6) for firing10,21, the replication origins of the H. mediterranei chromosome were predicted using Cdc6 and potential ORBs associated with these initiator genes12. In addition, as the genomes of H. mediterranei and H. volcanii share high homology over the entire chromosome, except for a 1-Mb fragment inversion between the two rRNA operons, the predicted origins and cdc6 genes were also compared with those of H. volcanii. Of the eight cdc6 genes in the H. mediterranei chromosome, cdc6A, cdc6C and cdc6G share high identity (>90%) with the three main replication initiator genes orc1, orc2 and orc5, respectively, in H. volcanii. These three cdc6 genes were also adjacent to inverted ORB repeats, representing a conserved characteristic of archaeal replication origins (Fig. 1a and Supplementary Fig. 1). Therefore, the three predicted cdc6 genes with adjacent ORB-containing regions were designated as oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G (Fig. 1a).
Figure 1. Three replication origins are utilized for chromosome replication in coordination with cell growth.
(a) Prediction of the replication origins in the H. mediterranei chromosome. The three replication origins are indicated with filled ovals, and the corresponding ORB motifs are represented by triangles. (b) The ARS assay for the predicted origins. The corresponding fragments oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G were cloned into the non-replicating plasmid pBI501 and the resulting plasmids pBI501-oriC1-cdc6A, pBI501-oriC2-cdc6C and pBI501-oriC3-cdc6G (Supplementary Table 1) were transferred into strains with the corresponding fragments deleted. Southern blot analysis was performed, using the bla gene as the probe. Lane P contains the plasmid pBI501-oriC3-cdc6G isolated from E. coli, and the other lanes contain the crude DNA extracted from the corresponding H. mediterranei transformants. (c) The growth curve of H. mediterranei DF50. The time points at which the cultures were collected for MFA are indicated with arrows, and the corresponding OD600 values are labelled. (d) The utilization of replication origins throughout the growth phase. The chromosome replication profiles of H. mediterranei at 12, 18, 24, 36 and 42 h were generated by MFA. Ratios of the marker hybridization signals from the indicated time-point samples versus the stationary phase sample (66 h) are plotted against the chromosome position. The three replication origins oriC1, oriC2 and oriC3 are indicated at the top. The utilized origins are marked with solid lines, while the non-utilized origins are marked with dotted lines.
Genetic assays were performed to investigate the ARS activities of the three predicted origins. Three fragments, oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G, were cloned into the non-replicating plasmid pBI501 (Supplementary Table 1), which cannot be replicated in any haloarchaea if no haloarchaeal ARS fragment is inserted into it. The resulting plasmids were transferred into H. mediterranei strains (Table 1) with the corresponding chromosome fragments deleted to avoid integration of the plasmids into the chromosome. As expected, the transformants were obtained. The presence of autonomously replicating plasmids in the corresponding transformants was determined by Southern blot analysis (Fig. 1b), which was further confirmed by retransferring the plasmids from H. mediterranei back into Escherichia coli (Supplementary Fig. 2). These results clearly showed that the three predicted origins were all capable of autonomous replication.
Table 1. Strains used in this study.
| H. mediterranei strains | Relevant characteristics | Source or reference |
|---|---|---|
| DF50 | ΔpyrF strain of H. mediterranei ATCC 33500 | Liu et al.26 |
| DFA50 | trpA deletion mutant of DF50 | This study |
| DF50ΔoriC1-cdc6A | oriC1-cdc6A deletion mutant of DF50 | This study |
| DF50ΔoriC2-cdc6C | oriC2-cdc6C deletion mutant of DF50 | This study |
| DF50ΔoriC3-cdc6G | oriC3-cdc6G deletion mutant of DF50 | This study |
| DF50ΔoriC4-cdc6H | oriC4-cdc6H deletion mutant of DF50 | This study |
| DF50ΔoriP1-cdc6L | oriP1-cdc6L deletion mutant of DF50 | This study |
| DF50ΔoriP2-cdc6M | oriP2-cdc6M deletion mutant of DF50 | This study |
| DF50ΔoriC1 | oriC1 deletion mutant of DF50 | This study |
| DF50ΔoriC2 | oriC2 deletion mutant of DF50 | This study |
| DF50ΔoriC3 | oriC3 deletion mutant of DF50 | This study |
| DF50ΔoriC4 | oriC4 deletion mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC2 | oriC1 and oriC2 deletion mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC3 | oriC1 and oriC3 deletion mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC2ΔoriC3 | oriC1, oriC2 and oriC3 deletion mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC2ΔoriC4 | oriC1, oriC2 and oriC4 deletion mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC3ΔoriC4 | oriC1, oriC3 and oriC4 deletion mutant of DF50 | This study |
| DFA50ΔoriC1ΔoriC2ΔoriC3 | oriC1, oriC2, oriC3 and trpA deletion mutant of DF50 | This study |
| DFA50ΔoriC4::trpA | ΔoriC4::trpA mutant of DFA50 | This study |
| DF50R | p.tna-radA mutant of DF50 | This study |
| DF50ΔoriC1ΔoriC2ΔoriC3R | p.tna-radA mutant of DF50ΔoriC1ΔoriC2ΔoriC3 | This study |
| H13 | ΔoriC4-cdc6H::trpA-oriP2-orc13 mutant of DFA50ΔoriC1ΔoriC2ΔoriC3 | This study |
Whole-genome DNA microarray-based MFA was then used to examine the replication profile of H. mediterranei and the utilization of the three predicted origins throughout the entire growth phase. According to the growth curve, samples for DNA extraction were collected in the early exponential phase (12 h), middle exponential phase (18, 24 h), late exponential phase (36, 42 h) and stationary phase (66 h) (Fig. 1c). The MFA results for the 12-h sample revealed three peaks, suggesting that three origins were used to initiate chromosome replication. The three peaks, located proximal to cdc6A, cdc6C and cdc6G, represented oriC1, oriC2 and oriC3, respectively (Fig. 1d). The variable heights of the three peaks could reflect different initiation efficiencies of these origins, indicating that oriC1 is likely more efficient at all of the time points (Fig. 1d). The MFA peak heights of the origins decreased gradually from 12 to 24 h, but the pattern of the differential initiation efficiencies of the origins was maintained (Fig. 1d). At 36 h, only the peak corresponding to oriC1 was slightly visible, probably because only a small percentage of cells were cycling at this time point. The profile of the 42-h sample showed no prominent peaks (Fig. 1d), which was consistent with strain growth near the stationary phase. Therefore, despite the different initiating efficiencies, the three replication origins were used in coordination with cell growth.
Activation of a dormant origin
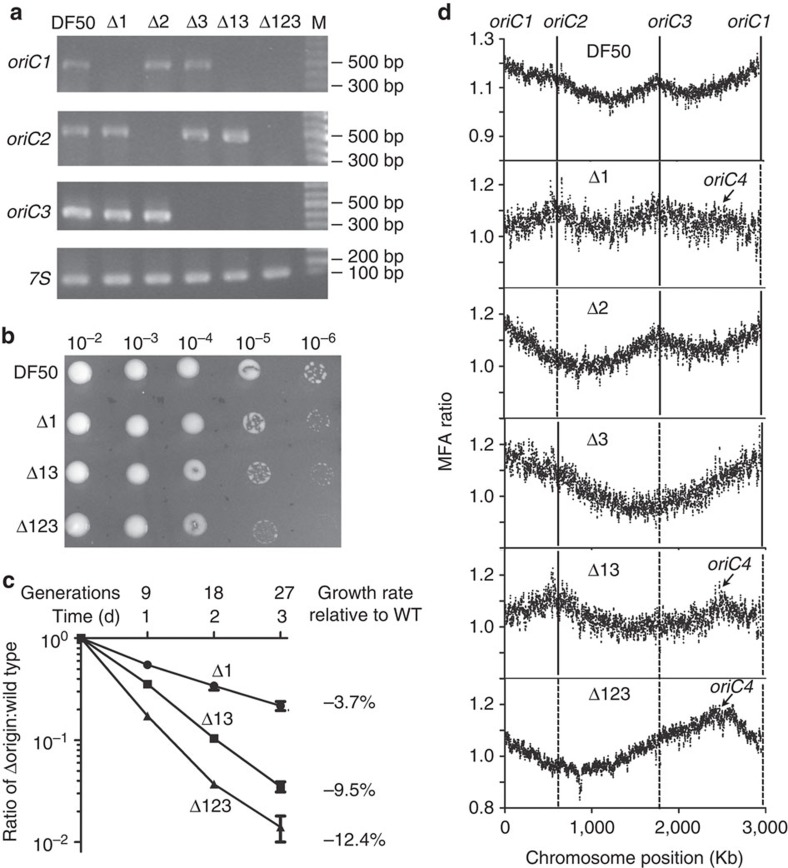
The above results demonstrated that three origins were used to replicate the chromosome, but with different initiating efficiencies. To investigate origin utilization when specific origins were deleted and to test the viability of the strain lacking all of these active origins on the chromosome, the three origins were targeted for knockout. Interestingly, knockout of each origin, double knockout of the oriC1 and oriC3 and simultaneous knockout of all three origins could be successfully obtained (Fig. 2a). These origin-deleted strains grew more slowly than the wild-type strain (Fig. 2b), with growth defect of ∼3.7% (for ΔoriC1) to 12.4% (for ΔoriC1ΔoriC2ΔoriC3) (Fig. 2c). However, the observed survival and growth of the DF50ΔoriC1ΔoriC2ΔoriC3 strain indicated that efficient chromosome replication could still occur in the absence of the three main origins.
Figure 2. Activation of the dormant origin oriC4 for chromosome replication in the origin deletion strains.
(a) The origin deletion strains DF50ΔoriC1 (Δ1), DF50ΔoriC2 (Δ2), DF50ΔoriC3 (Δ3), DF50ΔoriC1ΔoriC3 (Δ13) and DF50ΔoriC1ΔoriC2ΔoriC3 (Δ123) were confirmed by PCR analyses. The primers were specific for oriC1, oriC2, oriC3 and 7S; 7S was used as an internal control. (b) The growth phenotypes of DF50ΔoriC1, DF50ΔoriC1ΔoriC3 and DF50ΔoriC1ΔoriC2ΔoriC3 compared with that of DF50. Serial dilutions of stationary phase cells (in equal amounts) were spotted onto AS-168 plates supplemented with uracil. (c) Pairwise growth competition assays comparing DF50 (wild-type) and origin deletants. The average with the s.e. of three parallel experiments is plotted. (d) Comparison of the chromosome replication profiles of DF50, DF50ΔoriC1 (Δ1), DF50ΔoriC2 (Δ2), DF50ΔoriC3 (Δ3), DF50ΔoriC1ΔoriC3 (Δ13) and DF50ΔoriC1ΔoriC2ΔoriC3 (Δ123). The peaks corresponding to the activation of oriC4 are indicated with arrows.
Microarray-based MFA was then used to examine the replication profile of strains with deletions of one, two or all three of the origins. In the single origin deletion strains, the peaks corresponding to the deleted origins disappeared (Fig. 2d), supporting the observation that the three origins on the chromosome were active. Notably, a new prominent peak of replication initiation emerged in the strain that had all three origins deleted, whereas the three peaks corresponding to the original origins disappeared. This new peak, located near the cdc6H gene, represented a new replication origin and was designated oriC4 (Fig. 2d). Remarkably, the new peaks corresponding to oriC4 were also observed in DF50ΔoriC1ΔoriC3 and perhaps in DF50ΔoriC1, but not in DF50ΔoriC2 or DF50ΔoriC3 (Fig. 2d). Therefore, when the active origins on the chromosome were knocked out, a dormant replication origin was activated to replicate the entire chromosome either alone or in combination with other active origins. Interestingly, we noticed that the degree of activation or the initiation efficiency of oriC4 was variable, as the peaks corresponding to oriC4 in the replication profiles of the origin deletion strains had different heights (Fig. 2d). In the replication profile of DF50ΔoriC1, the oriC4 peak was only slightly visible, whereas in that of DF50ΔoriC1ΔoriC3 the oriC4 peak was nearly the same height as the oriC2 peak. Finally, in DF50ΔoriC1ΔoriC2ΔoriC3, the oriC4 peak was the only peak and had a prominent height. Therefore, the activation of oriC4 was positively correlated with the number of replication origins knocked out. To the best of our knowledge, this is the first report of the activation of a dormant origin in the domain of Archaea.
The dormant oriC4 depends on adjacent cdc6H for firing
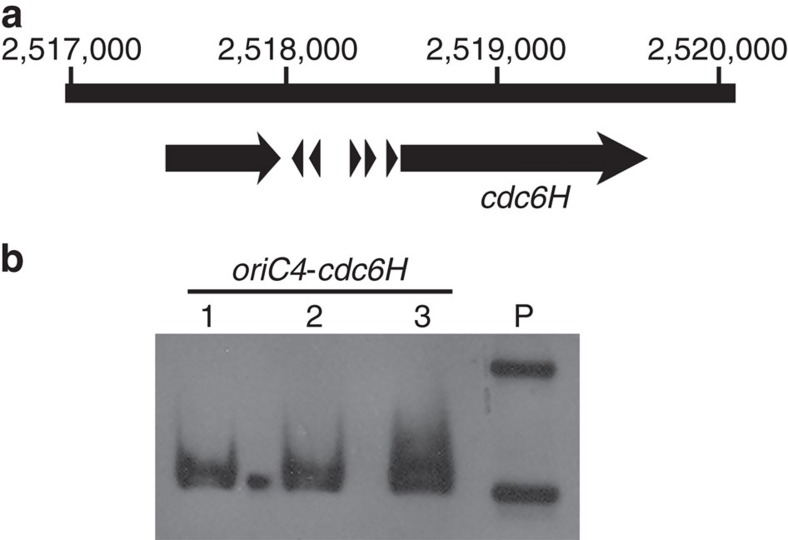
The new peak, corresponding to oriC4, observed in the replication profile of DF50ΔoriC1ΔoriC2ΔoriC3 was proximal to cdc6H; however, its precise location and the crucial elements responsible for its replication initiation remained undetermined. Interestingly, cdc6H was adjacent to inverted ORB repeats (within the predicted oriC4), as was observed for cdc6A, cdc6C and cdc6G, among the eight cdc6 genes in the H. mediterranei chromosome (Fig. 3a and Supplementary Fig. 1). Therefore, oriC4 was proposed to be dependent on cdc6H for firing, and an ARS assay was used to test this proposal. The fragments oriC4, cdc6H and oriC4-cdc6H were individually cloned into pBI501, and their autonomous replication abilities were examined via transformation of the DF50ΔoriC4-cdc6H strain. Although no transformants were observed for the single oriC4 or cdc6H fragment, transformants corresponding to the oriC4-cdc6H fragment were obtained, and Southern blot analysis confirmed the presence of autonomous replicating plasmids in the transformants (Fig. 3b). Therefore, oriC4-cdc6H is a novel replication origin. Although oriC4-cdc6H is dormant in the chromosome of wild-type H. mediterranei, it is capable of autonomous plasmid replication and can be activated for chromosome replication in H. mediterranei in the absence of other active origins.
Figure 3. oriC4 is dependent on cdc6H for firing.
(a) Sequence features of the predicted location of oriC4. ORB motifs adjacent to the cdc6H gene are indicated with triangles. The numbering refers to the chromosomal position. (b) The ARS assay for oriC4-cdc6H. The fragments oriC4, cdc6H and oriC4-cdc6H were examined for autonomous replicating ability, using DF50ΔoriC4-cdc6H as the transformation host. Transformants were only obtained from the transformation corresponding to oriC4-cdc6H. Southern blot analysis was performed, using the bla gene as the probe. Lane P contains the plasmid pBI501-oriC4-cdc6H (Supplementary Table 1) isolated from E. coli, whereas the other lanes (lane 1–3) contain crude DNA extracted from the H. mediterranei transformants.
Recent acquisition of oriC4 may account for its dormancy
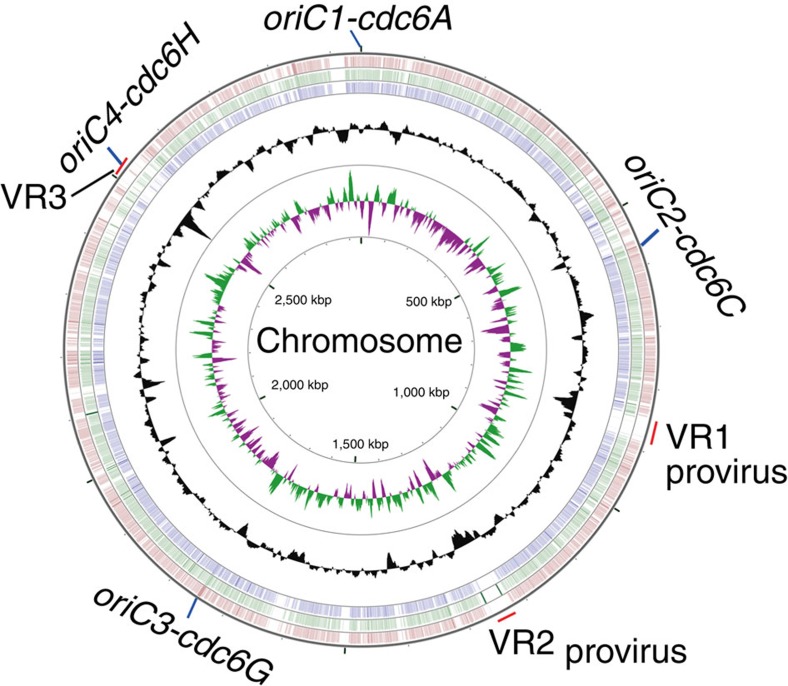
To determine the evolutionary difference between the dormant origin and the active origins, we analysed the distribution of H. mediterranei-type origins in the Haloferax genus by comparing Cdc6 from H. mediterranei against the other 14 species via BLAST. The three active origins oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G were found to be conserved in these Haloferax species (Table 2). However, the dormant origin oriC4-cdc6H was much less conserved and was present only in H. mediterranei and two other species, namely H. larsenii and H. elongans (Table 2). Interestingly, sequence context analysis revealed that oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G were located in the conserved chromosomal context, whereas oriC4-cdc6H was located in the variable chromosomal context (Fig. 4). The conservation of these three active replication origins indicated that these origins were present in the initial Haloferax in the evolutionary process. However, in contrast to these original active replication origins, the dormant origin oriC4-cdc6H was likely acquired more recently. We propose that the recently acquired oriC4-cdc6H may be less competitive in the recruitment of inherent replication initiation factors (such as MCM), which may contribute to its dormant state in the wild-type strain.
Table 2. Distribution of H. mediterranei-type replication origins in Haloferax species.
| 12 Haloferax species* | Haloferax larsenii | Haloferax elongans | |
|---|---|---|---|
| oriC1-cdc6A | + | + | + |
| oriC2-cdc6C | + | + | + |
| oriC3-cdc6G | + | + | + |
| oriC4-cdc6H | — | + | + |
*12 Haloferax species: H. volcanii, H. sulfurifontis, H. mucosum, H. denitrificans, H. prahovense, H. gibbonsii, H. alexandrinus, H. lucentense, Haloferax sp. ATCC BAA-644, Haloferax sp. ATCC BAA-645, Haloferax sp. ATCC BAA-646 and Haloferax sp. BAB-2207.
Figure 4. Comparative genomics of the replication origins of Haloferax species.
Starting from the outside, the first, second and third circles represent the DNA sequence BLASTN hits of the H. mediterranei chromosome against H. volcanii, Haloferax elongans ATCC BAA-1513 and H. alexandrinus JCM 10717, respectively. The fourth and fifth circles represent the G+C content and GC skew of the H. mediterranei chromosome, respectively. The four replication origins and three large variable G+C content regions (VR1, VR2 and VR3) on the H. mediterranei chromosome are indicated. The origins oriC1-cdc6A, oriC2-cdc6C and oriC3-cdc6G are located in the conserved chromosomal region, whereas oriC4-cdc6H is located in a variable chromosomal region (VR3).
The oriC4 is essential in absence of other active origins
The dormant origin oriC4 was activated for chromosome replication in the absence of the other three active origins. To verify the necessity and importance of oriC4 in strains lacking the primary active origins, we attempted to knock out oriC4 in the DF50ΔoriC1ΔoriC2ΔoriC3 strain and in the DF50 and DF50ΔoriC1ΔoriC3 strains. Although knocking out oriC4 in DF50 and DF50ΔoriC1ΔoriC3 was easy, we failed to knock out oriC4 in DF50ΔoriC1ΔoriC2ΔoriC3 after screening more than 600 FOAr (5-fluoroorotic acid resistant) colonies from two independent experiments (Table 3). This result indicated that oriC4 is essential for chromosome replication in the absence of the other three origins. The necessity of oriC4 was also tested using gene disruption experiments. Because the archaeal replication origin depends on an adjacent initiator protein for firing, oriC4 inactivation was performed by disrupting the cdc6H gene. Notably, the disruption of cdc6H in DF50 and DF50ΔoriC1ΔoriC3 resulted in a large number of large colonies, whereas the disruption of cdc6H in DF50ΔoriC1ΔoriC2ΔoriC3 only resulted in sporadic tiny transformants (Supplementary Fig. 3a). PCR analysis of the tiny transformants revealed both disrupted and intact cdc6H genes (Supplementary Fig. 3b). As H. mediterranei has multiple copies of its chromosome25, the heterozygosity of cdc6H genes in this disruption approach further supports the requirement for oriC4 in the DF50ΔoriC1ΔoriC2ΔoriC3 strain.
Table 3. Isolation of oriC4 knockouts in different H. mediterranei strains.
| Starting strain | Knockouts of oriC4/colonies analysed |
|---|---|
| DF50 | 8/14 |
| DF50ΔoriC1ΔoriC3 | 3/42 |
| DF50ΔoriC1ΔoriC2ΔoriC3 | 0/(>600) |
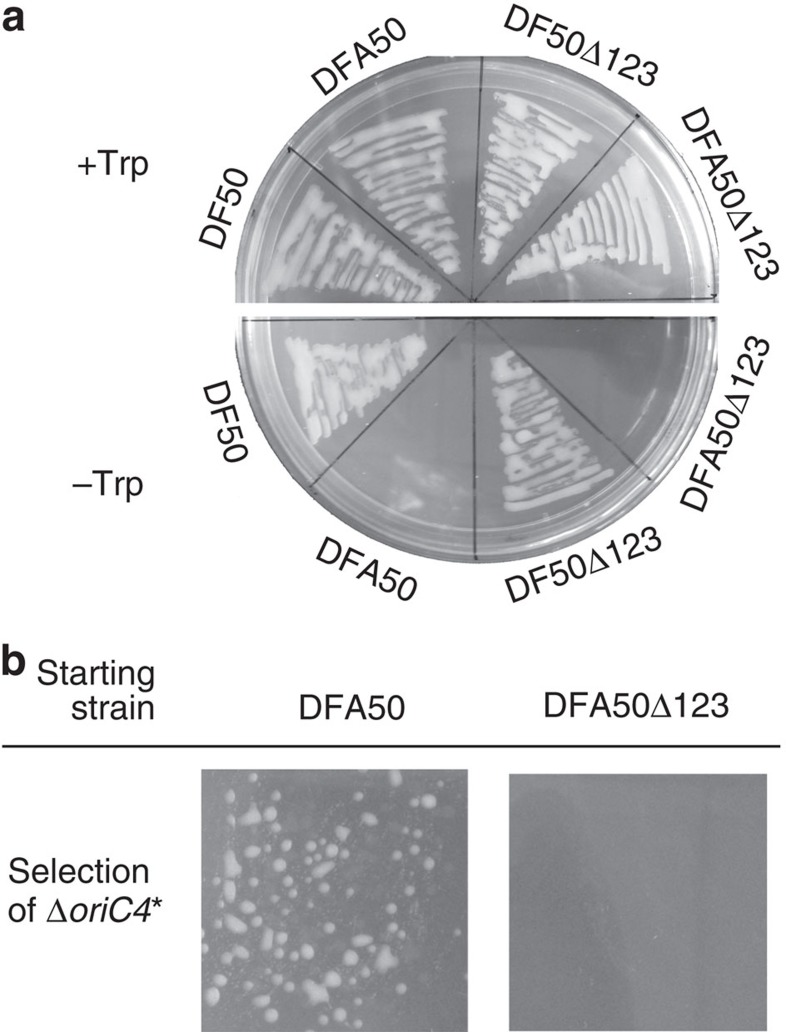
To confirm the requirement for oriC4 in DF50ΔoriC1ΔoriC2ΔoriC3, we also developed a trpA-marked positive selection system in H. mediterranei based on the pyrF-based gene knockout system26. First, trpA was knocked out to construct the tryptophan auxotrophic strains DFA50 and DFA50ΔoriC1ΔoriC2ΔoriC3 (Fig. 5a). Then, oriC4 was replaced with the trpA marker. Although we successfully established the trpA-marked knockout of oriC4 in DFA50, we did not obtain any isolates for the oriC4 knockout in DFA50ΔoriC1ΔoriC2ΔoriC3 (Fig. 5b), reconfirming the requirement for oriC4 in the absence of the other three origins. Remarkably, knocking out oriC3 in DF50ΔoriC1ΔoriC2ΔoriC4 and knocking out oriC2 in DF50ΔoriC1ΔoriC3ΔoriC4 were also unsuccessful. All of these results clearly indicate that origin-dependent initiation is strictly required for chromosome replication in H. mediterranei and that oriC4 is essential for chromosome replication in the H. mediterranei DF50ΔoriC1ΔoriC2ΔoriC3 strain.
Figure 5. oriC4 is essential for chromosome replication in the absence of the other three origins.
(a) Cells of the DF50 and DF50ΔoriC1ΔoriC2ΔoriC3 (DF50Δ123) strains and of the corresponding trpA knockout mutant strains, named DFA50 and DFA50ΔoriC1ΔoriC2ΔoriC3 (DFA50Δ123), respectively, were streaked onto AS-168SY plates supplemented with uracil and with or without tryptophan. (b) For the positive selection of trpA-marked knockouts of oriC4 in DFA50 and DFA50ΔoriC1ΔoriC2ΔoriC3, the corresponding propagated culture of one pop-in colony in the AS-168 broth was plated on AS-168SY plates supplemented with uracil and 5-FOA and without tryptophan. *The cell density of the corresponding culture of DFA50ΔoriC1ΔoriC2ΔoriC3 for plating was 10 times that of DFA50.
Interestingly, radA expression was significantly higher in DF50ΔoriC1ΔoriC2ΔoriC3 than in DF50 (Supplementary Fig. 4a). To test whether radA is essential in DF50ΔoriC1ΔoriC2ΔoriC3, as was observed in H. volcanii lacking all active origins22, radA was placed under the control of the inducible promoter p.tna, as described by Hawkins et al.22 Although the growth of DF50ΔoriC1ΔoriC2ΔoriC3 was slower in the absence of RadA (when radA expression was not induced), unlike H. volcanii, RadA was not strictly required for its viability (Supplementary Fig. 4b). This finding further confirms that the chromosome replication of this origin-less H. mediterranei is origin dependent, which differs from the recombination-dependent replication recently proposed for H. volcanii22.
Identification of the dormant origin oriP1-cdc6L on pHM500
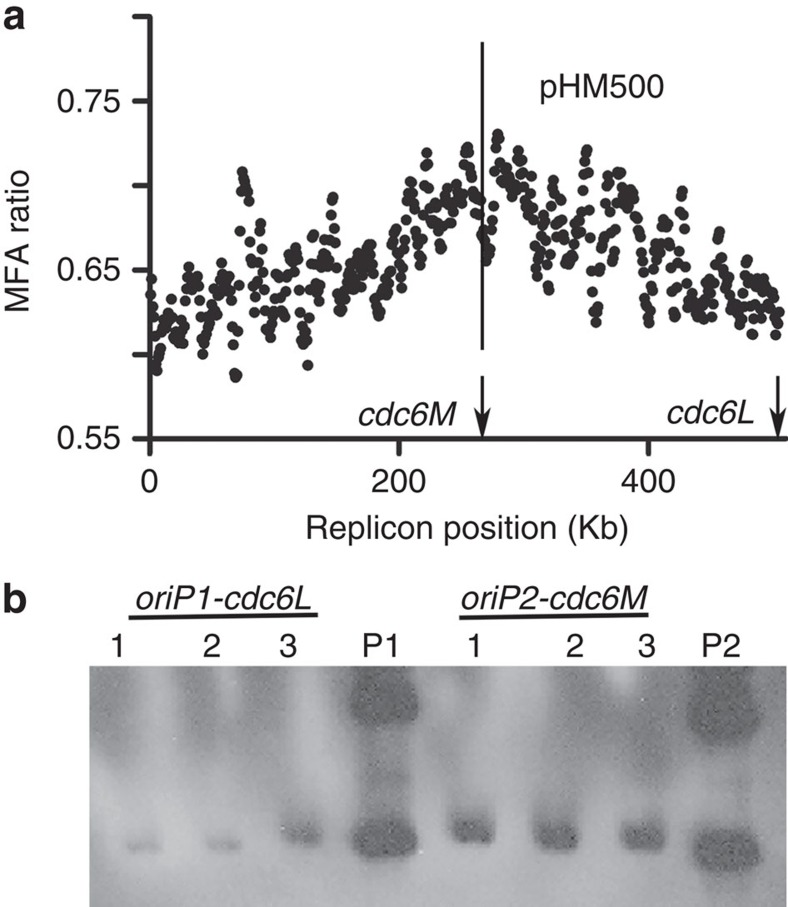
We also assessed extrachromosomal replicons in H. mediterranei to determine whether other dormant origins exist. Two cdc6 genes encoded on pHM500, cdc6L and cdc6M, are adjacent to inverted ORB repeats, indicating that both genes are associated with replication origins (Supplementary Fig. 1). However, the pHM500 replication profile in the wild-type strain displayed only one peak located adjacent to cdc6M (Fig. 6a), indicating that oriP2-cdc6M is the active origin for pHM500 replication in the wild-type strain. We hypothesized that oriP1-cdc6L, acting as a dormant origin in the wild-type strain, would be activated for replication in the absence of oriP2-cdc6M. To test this hypothesis, we generated mutants lacking oriP1-cdc6L or oriP2-cdc6M; the autonomous replication abilities of the two origins were confirmed using an ARS assay (Fig. 6b and Supplementary Fig. 5a), indicating that either origin could be used to replicate the replicon in the absence of the other origin. In addition, our attempt to knock out oriP1-cdc6L in ΔoriP2-cdc6M was unsuccessful, and the disruption of cdc6L in ΔoriP2-cdc6M resulted in only a few tiny colonies (Supplementary Fig. 5b, 5c), indicating that oriP1-cdc6L is essential in the absence of oriP2-cdc6M. Therefore, we proposed that the dormant origin oriP1-cdc6L would be activated for pHM500 replication in the absence of oriP2-cdc6M.
Figure 6. The prediction and identification of a dormant origin on pHM500.
(a) The replication profile of pHM500. The active replication origin is indicated by a vertical line, and the positions of cdc6L and cdc6M are indicated by vertical arrows. (b) ARS assays for oriP1-cdc6L and oriP2-cdc6M. The plasmids pBI501-oriP1-cdc6L and pBI501-oriP2-cdc6M (Supplementary Table 1) were transferred into strains with the corresponding fragments deleted. Southern blot analysis was performed, using the bla gene as the probe. Lanes P1 and P2 contain plasmids isolated from E. coli with pBI501-oriP1-cdc6L for P1 and pBI501-oriP2-cdc6M for P2, whereas the other lanes contain crude DNA extracted from the corresponding H. mediterranei transformants.
Heterogeneous oriP2-orc13 initiates chromosome replication
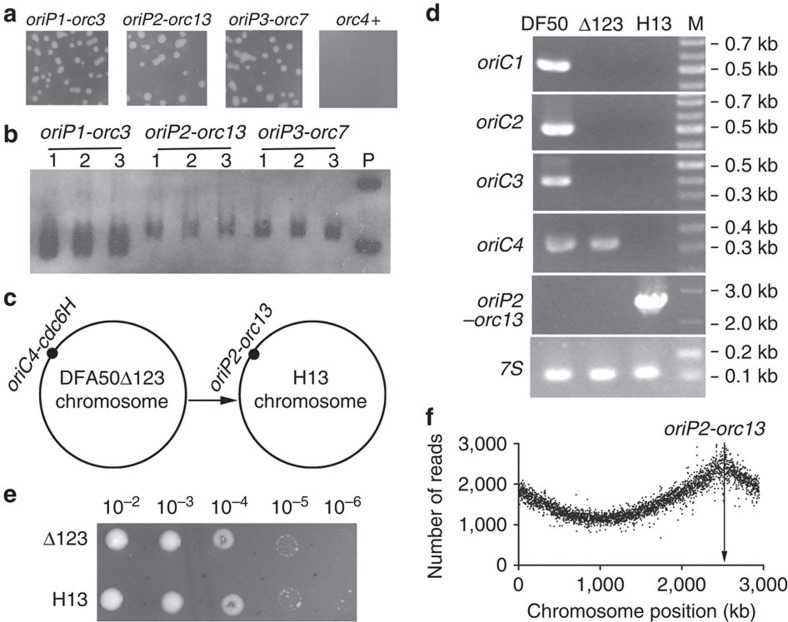
The replication origins of H. volcanii have been extensively characterized11,22, but no dormant origins have been reported in previous investigations. In the case of H. mediterranei, the two identified dormant origins oriC4-cdc6H and oriP1-cdc6L displayed the common features that both initiator genes were adjacent to the inverted ORB motifs and that no peaks of replication initiation emerged at the corresponding locations in the replication profiles of the wild-type strain. When these features were used to evaluate the potential origins in the H. volcanii main chromosome and in pHV4, which is integrated into the main chromosome of H. volcanii H26 (ref. 22), three orc1/cdc6 genes associated with adjacent intergenic regions were found to fulfil these criteria: orc4 on the main chromosome, and orc13 and orc7 on pHV4 (refs 12, 22) (Supplementary Table 2). Although no initiating activity was detected for these three fragments (putative origins) in the replication profiles of the origin-deleted H. volcanii22, we wondered whether they could be activated to replicate the H. mediterranei chromosome, given that dormant origins can be activated in H. mediterranei if needed. First, we tested the autonomous replicating abilities of the three fragments with an ARS assay in H. mediterranei and used the active origin oriP1-orc3 as a positive control. In this assay, the DF50ΔoriP2-cdc6M strain was selected as the transformation host because orc3 and cdc6M share high identities (98%), whereas no cdc6 gene in H. mediterranei shares high identity with orc4, orc13 or orc7 (<55%). Consistent with the results obtained for H. volcanii11, no transformants were observed for the corresponding orc4 fragment transformation. However, the other three fragments, oriP1-orc3, oriP2-orc13 and oriP3-orc7, had autonomous replicating ability in H. mediterranei (Fig. 7a,b).
Figure 7. The dormant origin oriP2-orc13 from H. volcanii can initiate the replication of the entire H. mediterranei chromosome.
(a) ARS assays for H. volcanii oriP1-orc3, orc4+ and the potential dormant origins oriP2-orc13 and oriP3-orc7. Plasmids pBI501-oriP1-orc3, pBI501-oriP2-orc13, pBI501-oriP3-orc7and pBI501-orc4+ (Supplementary Table 1) were transferred into DF50ΔoriP2-cdc6M. Fragment orc4+ refers to the orc4 gene plus the adjacent upstream intergenic region. Colonies were observed after 3 days at 37 °C. (b) Southern blot analysis using the bla gene as the probe. Lane P contains the plasmid pBI501-oriP3-orc7 isolated from E. coli, whereas the other lanes contain crude DNA extracted from the corresponding H. mediterranei transformants. (c) Schematic diagram of the replication origin of the H. mediterranei H13 chromosome. H13 was constructed by replacing the oriC4-cdc6H region in DFA50ΔoriC1ΔoriC2ΔoriC3 (DFA50Δ123) with the oriP2-orc13 region from H. volcanii. (d) The origin-replaced mutant H13 was confirmed by PCR analyses, with DF50 and DF50ΔoriC1ΔoriC2ΔoriC3 (Δ123) as controls. The primers were specific for oriC1, oriC2, oriC3, oriC4, oriP2-orc13 and 7S; 7S was used as an internal control. (e) The growth phenotype of H13 compared with DF50ΔoriC1ΔoriC2ΔoriC3 (Δ123). Serial dilutions of stationary phase cells (equal amounts) were spotted onto AS-168 plates supplemented with uracil. (f) Marker frequency distribution for the H13 chromosome. High-throughput sequencing was performed for the total DNA from the exponential phase of H13, and the number of reads was calculated for every 1-kb window and plotted against the chromosome position. The position of the replication origin oriP2-orc13 is marked with an arrow.
To test the initiating activity of the putative dormant origins from H. volcanii for chromosome replication in H. mediterranei, we attempted to replace the only origin (oriC4-cdc6H) on the chromosome of H. mediterranei DFA50ΔoriC1ΔoriC2ΔoriC3 with the H. volcanii oriP2-orc13 origin. Considering the autonomous replicating ability of oriP2-orc13, we expected that knocking in the oriP2-orc13 fragment into the chromosome via a plasmid vector would be difficult. Thus, a linear DNA fragment containing oriP2-orc13 and a trpA marker (for positive selection of the replacement) was used for gene replacement. Replacement of the oriP2-orc13 origin at the oriC4-cdc6H position (Fig. 7c) was successfully conducted, as confirmed by both PCR (Fig. 7d) and Southern blot analysis (Supplementary Fig. 6), and the mutant strain was designated H. mediterranei H13 (Fig. 7c). Growth of the H13 strain containing only the oriP2-orc13 replication origin was comparable to that of DF50ΔoriC1ΔoriC2ΔoriC3 (Fig. 7e). Notably, the genome-wide replication profile of H13 revealed that chromosome replication was initiated only from oriP2-orc13 (Fig. 7f). Thus, the putative dormant origin oriP2-orc13 from H. volcanii can be used to efficiently replicate the entire chromosome of H. mediterranei in the absence of the other active origins.
Discussion
The initiation of chromosome replication from multiple origins is very common in archaea. Based on the conserved characteristics of archaeal origins, multiple cdc6-associated replication origins have been predicted in 15 fully sequenced haloarchaeal genomes12. However, not all of these predicted origins are active, as revealed by genome-wide MFA14,22. Therefore, it is likely that dormant replication origins are widely distributed in haloarchaea. Because dormant origins can be activated for replicon replication in the absence of other active origins, they can act as backup sites for replication initiation when surrounding origins are inactivated or when replication forks stall, as has been demonstrated for dormant origins in eukaryotes27,28. In contrast to active origins, dormant origins in haloarchaea were proposed to have been recently acquired (Fig. 4), possibly from environmental plasmids, viruses or other haloarchaea. Interestingly, similar to our results that the recently acquired dormant origin is competent to replicate the entire chromosome of the origin-deleted H. mediterranei, suppression of the initiation defect of chromosome replication (for example, in the dnaA or oriC-deleted mutants) by plasmid or prophage integration has also been reported in bacteria29,30. As horizontal gene transfer among haloarchaea is common and the acquisition of replication origins is favourable for the transfer of new genetic content31,32, we propose that the acquired origins that accompanied foreign genetic contents are important for haloarchaea to shape the chromosomal structure and to adapt to harsh and variable environments. In addition, these recently acquired origins can be active or dormant as a result of different intracellular and extracellular conditions; this may act as an additional adaptive feature for these haloarchaea.
Interestingly, oriC4 is dormant in wild-type H. mediterranei but is significantly activated in DF50ΔoriC1ΔoriC2ΔoriC3. In eukaryotes, competition among origins for limiting replication factors such as CDC45, Sld2 and Sld3 determines their initiation time and efficiency33,34. Because the H. mediterranei chromosome is polyploid25, as observed in other haloarchaea35, and all origins share the common helicase MCM, MCM may be one of the limiting factors, as previously proposed for H. volcanii22. Therefore, we can speculate that the ability of oriC4-cdc6H to recruit MCM may be weaker than that of other active origins, resulting in the dormant state of oriC4 in the wild-type strain. The deletion of active origins would liberate more MCM molecules, allowing Cdc6H to recruit MCM and initiate replication at oriC4. The gradually increased activation of oriC4-cdc6H in the origin deletion strains supports this hypothesis (Fig. 2d). In particular, the firing ability of oriC4 appears to be suppressed by oriC1, the most efficient origin on the chromosome, as oriC4 could be slightly activated in the absence of oriC1. Interestingly, according to several phylogenetic analyses of Cdc6, Cdc6A is the most conserved Cdc6 and is distributed among all haloarchaeal genomes as well as other archaeal genomes12,36. Comparative genomic analysis has indicated that oriC4-cdc6H was recently acquired by H. mediterranei (Fig. 4). Therefore, the recent acquisition of oriC4-cdc6H may impair its ability to recruit MCM compared with the other active origins, which are products of a longer period of evolution.
Although the activity of each origin was different, the three active origins of H. mediterranei displayed a similar dynamic pattern in the chromosomal replication profiles throughout the growth phase (Fig. 1d). This finding indicates that mechanisms exist for regulating utilization of the multiple origins. For bacteria with a single origin on the chromosome, intiation regulation depends on the availability and activity of DnaA at the origin2. For archaea with multiple origins on the chromosome, the corresponding initiators may have similar mechanisms for the regulation of initiation. In S. acidocaldarius, transcription of the three initiator genes associated with the origins showed the strongest cell cycle-specific induction around the G1 phase10,37, which is important for initiator binding at the origins and for replication initiation. Gene expression analysis of H. mediterranei revealed that the transcription of all origin-associated cdc6 genes on the chromosome was decreased in the stationary phase compared with the exponential phase38; this finding is consistent with the active utilization of origins during the exponential phase. Apart from the usage of distinct initiator proteins, archaeal replication origins (including the dormant origins) in one cell share common MCM and GINS39, and other unknown factors40. These factors may interact with the distinct oriCs and Cdc6s with different efficiencies, thereby coordinating the firing or inactivation of distinct origins, as discussed above. More importantly, multiple origins acting as a group may also be regulated by these factors via the same mechanisms to ensure the coordination of DNA replication with cell growth.
Notably, although both H. mediterranei and H. volcanii belong to the Haloferax genus, share high homology over the entire chromosome and grow well after the deletion of all known active origins, we demonstrated that the growth of H. mediterranei lacking known active origins is dependent on the activation of a dormant origin. It has been proposed that the H. volcanii H26 lacking known active origins may initiate replication using a recombination-dependent mechanism22. Importantly, our results demonstrated that activation of the dormant origin is essential for chromosome replication in the absence of the other active origins. This is consistent with results observed for other archaea such as S. islandicus and H. hispanica, in which at least one origin is required for chromosome replication10,21,41. For H. volcanii H26 (ref. 22), Michel and Bernander41 have proposed an alternative explanation, suggesting that dormant or newly integrated origins may be responsible for the viability of H. volcanii strains lacking the main replication origins; however, no experimental data are available yet to support this speculation. In this study, we have provided the first evidence that a dormant replication origin can be activated and become essential for chromosome replication on the deletion of all other primary origins. Interestingly, although oriP2-orc13 and oriP3-orc7 in H. volcanii were previously shown to stay dormant in the absence of other active origins22, these origins exhibited ARS activities in H. mediterranei (Fig. 7a,b), and at least oriP2-orc13 (oriP3-orc7 has not yet been tested) was able to initiate replication of the entire H. mediterranei chromosome without other active origins (Fig. 7f). It is possible that H. mediterranei but not H. volcanii may encode factors that are important for the activities of oriP2-orc13 and oriP3-orc7 or that H. volcanii may encode suppressive factors for these putative origins. Difference also exists for the essentiality of RadA for the origin-deleted H. mediterranei and H. volcanii. RadA is strictly required for H. volcanii in the absence of all active origins22. In contrast, although the growth of DF50ΔoriC1ΔoriC2ΔoriC3 was slowed in the absence of RadA and radA gene expression in DF50ΔoriC1ΔoriC2ΔoriC3 was significantly increased, RadA was not strictly required for the viability of H. mediterranei in which the main origins had been deleted (Supplementary Fig. 4). This further supports the notion that activation of the dormant origin but not recombination-dependent initiation is responsible for genome replication in H. mediterranei DF50ΔoriC1ΔoriC2ΔoriC3. Surprisingly, H. volcanii H26 lacking the known active origins exhibits accelerated growth22, whereas H. mediterranei lacking the main origins exhibits slightly slower growth (Fig. 2b,c and Fig. 7e). The reason for this growth discrepancy is unknown, but all of these results indicate that there might be substantial differences between H. volcanii and H. mediterranei, even though they belong to the same genus.
In summary, our characterization of origin utilization in H. mediterranei reveals for the first time that a dormant replication origin can be activated and becomes essential for chromosome replication in the absence of the other active origins in archaea. This further supports the notion that chromosome replication may generally require at least one replication origin. In addition, our results also suggest that replication initiation from multiple origins (including dormant ones) could be extensively regulated, thus providing a model system for investigating the regulatory mechanisms of Cdc6-dependent replication initiation. The observed difference between origin-less H. mediterranei and H. volcanii in the dependence on dormant origin activation may permit the identification of factors that are important for origin activity in haloarchaea.
Methods
Strains and plasmids
The H. mediterranei strains used in this study are listed in Table 1, and the constructed plasmids and corresponding oligonucleotides are listed in Supplementary Table 1. The plasmids used for gene knockout and gene disruption were derived from pHFX26, and the plasmids used for the ARS assay were derived from pBI501 (Supplementary Table 1). E. coli JM109 and JM110 cells were grown in Luria–Bertani medium containing 100 μg ml−1 ampicillin at 37 °C. The former strain was used for constructing plasmids, and the latter strain was used for preparing demethylated plasmids for the transformation of H. mediterranei42. H. mediterranei strains were cultivated at 37 °C in the nutrient-rich medium AS-168 (ref. 12) or in AS-168SY (AS-168 medium without yeast extract). When required, uracil, 5-FOA and tryptophan were added to the medium at final concentrations of 50 μg ml−1, 250 μg ml−1 and 50 μg ml−1, respectively.
DNA preparation
The genomic DNA used for microarray analysis or high-throughput sequencing was prepared using the phenol–chloroform method21. The DNA was resuspended in Tris-EDTA buffer and stored at −20 °C. The crude genomic DNA used for Southern blot analysis was extracted by directly lysing H. mediterranei transformants with 200 μl double-distilled H2O and 100 μl phenol–chloroform, and the liquid supernatant was collected for the following analysis.
Whole-genome DNA microarray-based MFA
Whole-genome DNA microarrays (12 × 135 K) were designed and manufactured by CapitalBio (Beijing, China) and Roche NimbleGen (Madison, USA), respectively, according to the whole genomic sequence of H. mediterranei24. Approximately 34 50-mer probes per kb, encompassing the entire genomic sequence, were designed.
Genomic DNA was extracted from the H. mediterranei cultures at specific time points, as indicated in Fig. 1c, or during the exponential phase (OD600≈0.5) and the stationary phase (OD600≈4.0), for the origin deletion strains. The exponential phase and stationary phase genomic DNA was then labelled with Cy3 and Cy5 (NimbleGen Dual-Colour Labelling Kit), respectively, according to the NimbleGen protocol. Array hybridization, washing and scanning and data normalization were performed as described in the study by Forsberg et al.43 The ratios were calculated as exponential phase values versus stationary phase values for each probe, and outliers (ratio >2 or <0.5) were excluded from the analyses. For each analysis, the excluded outliers accounted for <0.4% of the total probes; thus, ∼34 valid probes per kb remained. Owing to the high density of the probes, the ratios of the 30 probes were averaged as 1 point located at the first probe. The MFA graph was plotted with a 5-point window and a 1-point slide.
High-throughput DNA sequencing and data analysis
Owing to the lack of probes specific for oriP2-orc13, the replication profile of H. mediterranei H13 was measured by high-throughput DNA sequencing. The genomic DNA was extracted from H. mediterranei H13 culture during the exponential phase (OD600≈0.35). Library preparation and sequencing with Illumina HiSeqTM 2500 were performed by Biomarker Technologies (Beijing, China), following Illumina protocols (San Diego, CA). Clean reads recovered after filtering low quality reads and artificially redundant reads were mapped to the H. mediterranei genome with the Burrows–Wheeler Alignment tool44. The number of reads was calculated for every 1-kb window and plotted against the chromosome position.
pyrF marker-based genetic manipulation
The gene knockout experiments were conducted according to previously described procedures26. Briefly, two fragments (500–800 bp) located upstream and downstream of the target gene or fragment were amplified from H. mediterranei genomic DNA and ligated onto the pHFX plasmid. The constructed plasmids were transferred into the H. mediterranei strains. The polyethylene glycol-mediated transformation method45 was used, and the knockouts were achieved via double-crossover homologous recombination. When the knockout strains were screened, 5-FOAr colonies (FOAr indicates that the second homologous recombination has occurred) were selected on AS-168SY plates supplemented with uracil and 5-FOA. PCR analysis was then used to screen the knockout mutants.
Gene disruption was achieved via the single-crossover method. Briefly, a ∼500-bp fragment in the middle of the target gene was amplified and inserted into the pHFX plasmid. The constructed plasmids were transferred into H. mediterranei to disrupt the target gene through single-crossover homologous recombination.
Replacement of the radA promoter with the inducible promoter p.tna was conducted similar to the gene knockout, with minor modifications. Briefly, the upstream and downstream fragments of the radA promoter were placed flanking the p.tna fragment. The resultant plasmid pBI501-ptnaradA (Supplementary Table 1) was transferred into H. mediterranei strains to obtain the recombinant strain.
trpA marker-based genetic manipulation
The trpA-marked positive selection system was constructed based on the pyrF-based gene knockout system26. First, the tryptophan auxotrophic strain DFA50 was constructed by knocking out the trpA gene with the plasmid pHFX-ΔtrpA (Supplementary Table 1). Then, the trpA open reading frame under the hsp5 promoter46 was inserted into pBI501 to obtain pHFA101.
For the knockout of oriC4, the upstream and downstream fragments of oriC4 were placed flanking Phsp5-trpA. The resulting plasmid pHFA101ΔoriC4 was transferred into DFA50, and a pop-in event was verified by PCR analysis. For positive selection of the pop-out event, the propagated culture of one pop-in colony in AS-168 broth was plated on AS-168SY plates supplemented with uracil and 5-FOA but without tryptophan. The oriC4 knockouts could be selected on these plates because of the replacement of oriC4 with Phsp5-trpA.
For the replacement of oriC4-cdc6H on the DFA50ΔoriC1ΔoriC2ΔoriC3 chromosome with oriP2-orc13, the upstream and downstream fragments of oriC4-cdc6H were placed flanking a trpA-oriP2-orc13 fragment (Supplementary Table 1). The ΔoriC4-cdc6H::trpA-oriP2-orc13 cassette was then cut from the resultant plasmid pHFA101-ΔoriC4-cdc6H::oriP2-orc13 and transferred into DFA50ΔoriC1ΔoriC2ΔoriC3. Transformants were selected on AS-168SY plates supplemented with uracil but without tryptophan to ensure the replacement of oriC4-cdc6H with trpA-oriP2-orc13.
ARS assay
The ARS assay experiments were performed as previously described, with some modifications12. The target fragment was amplified and cloned into the non-replicating plasmid pBI501 (Supplementary Table 1), which could not be replicated in H. mediterranei unless an ARS was inserted. To avoid integration of the plasmids into the chromosome, the resulting plasmids were transferred into H. mediterranei strains (pyrF-) with the corresponding fragments deleted. If the cloned fragments could confer autonomous replication to the plasmid pBI501 with a pyrF selection marker gene, the transformants could be obtained on an AS-168SY plate without uracil. Plasmid recovery in the transformants, indicating the ARS ability of the cloned fragment, was verified using Southern blot analysis, as previously described12. This was further confirmed by retransferring the plasmids from H. mediterranei back to E. coli. As the plasmids for the ARS assay contained a replication origin of an E. coli plasmid, the corresponding E. coli transformants will and can only be obtained in this retransformation if the plasmids can autonomously replicate in H. mediterranei and can thus be isolated from the H. mediterranei transformants.
Full-length, uncropped Southern blot images are provided in Supplementary Fig. 7.
Cell growth comparison and pairwise growth competition
To compare growth of the H. mediterranei DF50 (wild type) and the origin-mutant strains on solid plates, a serial dilution spotting assay was performed. Briefly, the wild-type and mutant strains were grown in AS-168 broth supplemented with uracil to the stationary phase (OD600≈4.0). The cell densities of the strains were adjusted to be equal before 5 10-fold serial dilutions were made with AS-168 broth (10−2 to 10−6). Then, 5 μl of each dilution was spotted on an AS-168 plate supplemented with uracil. Photographs were taken 2 to 3 days after spotting.
To compare growth in liquid culture, pairwise growth competition assays were performed as previously described47, with minor modifications. Briefly, the H. mediterranei DF50 and the origin-mutant strains (1:1 ratio, and ∼4 × 106 for each) were mixed and inoculated in 10 ml AS-168 broth supplemented with uracil. The mixed culture was cultivated for ∼1 day to a density of ∼108 cells per ml, and 5 × 106 cells were then inoculated in 10 ml of the same fresh medium and cultured for an additional day; this process was repeated once. To count the cells, the culture from each time point was diluted, and 150 μl aliquots were plated on AS-168 plates supplemented with uracil. The wild-type and origin-mutant cells were identified by PCR, and the ratio was calculated based on >120 random colonies.
Prediction and distribution of replication origins
The intergenic region immediately upstream of cdc6 was assessed for the presence of consensus repeats with MEME software48 (motif size: 18-35; OMOPS model). The consensus repeats were manually evaluated, and those with a ‘G-string'11 were selected as the ORB elements. Only the intergenic regions with inverted ORB elements were considered as replication origins. To analyse the distribution of H. mediterranei-type replication origins in the Haloferax species, the BlastP programme (BLOSUM62 matrix) was used, comparing origin-associated Cdc6 proteins from H. mediterranei with those from certain Haloferax genomes (http://blast.ncbi.nlm.nih.gov/)49. Only those Cdc6 proteins with identities >80% (ref. 12) were recognized.
Genome resources and comparative genomics
Two completed genomes, H. mediterranei ATCC 33500 and H. volcanii DS2 (ref. 50), and the contigs of 13 draft genomes, H. sulfurifonti ATCC BAA 897 (ref. 51), H. mucosum ATCC BAA 1512 (ref. 51), H. denitrificans ATCC 35960 (ref. 51), H. prahovense DSM 18310, H. larsenii JCM 13917, H. gibbonsii ATCC 33959, H. elongans ATCC BAA-1513, H. alexandrinus JCM 10717, H. lucentense DSM 14919, Haloferax sp. ATCC BAA-644, Haloferax sp. ATCC BAA-645, Haloferax sp. ATCC BAA-646 and Haloferax sp. BAB-2207, were available through NCBI (http://www.ncbi.nlm.nih.gov/genome/). All comparative genomic analyses were performed with the CGView Server52 using the default parameters (http://stothard.afns.ualberta.ca/cgview_server/).
Additional information
Accession codes: The DNA microarray and high-throughput sequencing data have been deposited in the NCBI GEO library under accession numbers GSE70597 and GSE70592.
How to cite this article: Yang, H. et al. Activation of a dormant replication origin is essential for Haloferax mediterranei lacking the primary origins. Nat. Commun. 6:8321 doi: 10.1038/ncomms9321 (2015).
Supplementary Material
Supplementary Figures 1-7, Supplementary Tables 1-2 and Supplementary References
Acknowledgments
This work was partially supported by grants from the National Natural Science Foundation of China (31100893, 31271334 and 31330001) and the Hundred Talents Program of the Chinese Academy of Sciences (to H. X.).
Footnotes
Author contributions H.X. conceived the study; H.Y., Z.W. and H.X. designed the experiments; H.Y., J.L., X.L. and S.C. performed the experiments; H.Y., Z.W., J.L., L.W. and H.X. analysed the data; and H.Y. and H.X. wrote the paper.
References
- Sclafani R. A. & Holzen T. M. Cell cycle regulation of DNA replication. Annu. Rev. Genet. 41, 237–280 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A., Hood I. V. & Berger J. M. Mechanisms for initiating cellular DNA replication. Annu. Rev. Biochem. 82, 25–54 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Ozaki S., Keyamura K. & Fujimitsu K. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat. Rev. Microbiol. 8, 163–170 (2010). [DOI] [PubMed] [Google Scholar]
- Mechali M. Eukaryotic DNA replication origins: many choices for appropriate answers. Nat. Rev. Mol. Cell Biol. 11, 728–738 (2010). [DOI] [PubMed] [Google Scholar]
- Santocanale C., Sharma K. & Diffley J. F. Activation of dormant origins of DNA replication in budding yeast. Genes Dev. 13, 2360–2364 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X. Q. & Blow J. J. Chk1 inhibits replication factory activation but allows dormant origin firing in existing factories. J. Cell Biol. 191, 1285–1297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X. Q., Jackson D. A. & Blow J. J. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 21, 3331–3341 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. P. et al. Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell 116, 25–38 (2004). [DOI] [PubMed] [Google Scholar]
- Lundgren M., Andersson A., Chen L., Nilsson P. & Bernander R. Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc. Natl Acad. Sci. USA 101, 7046–7051 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson R. Y. et al. Specificity and function of archaeal DNA replication initiator proteins. Cell Rep. 3, 485–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norais C. et al. Genetic and physical mapping of DNA replication origins in Haloferax volcanii. PLoS Genet. 3, e77 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Liu H., Liu J., Liu X. & Xiang H. Diversity and evolution of multiple orc/cdc6-adjacent replication origins in haloarchaea. BMC Genomics 13, 478 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker J. A. et al. Multiple replication origins of Halobacterium sp. strain NRC-1: properties of the conserved orc7-dependent oriC1. J. Bacteriol. 191, 5253–5261 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelve E. A., Martens-Habbena W., Stahl D. A. & Bernander R. Mapping of active replication origins in vivo in thaum- and euryarchaeal replicons. Mol. Microbiol. 90, 538–550 (2013). [DOI] [PubMed] [Google Scholar]
- Robinson N. P. & Bell S. D. Extrachromosomal element capture and the evolution of multiple replication origins in archaeal chromosomes. Proc. Natl Acad. Sci. USA 104, 5806–5811 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelve E. A., Lindas A. C., Knoppel A., Mira A. & Bernander R. Four chromosome replication origins in the archaeon Pyrobaculum calidifontis. Mol. Microbiol. 85, 986–995 (2012). [DOI] [PubMed] [Google Scholar]
- Wu Z., Liu J., Yang H. & Xiang H. DNA replication origins in archaea. Front. Microbiol. 5, 179 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. D. Archaeal orc1/cdc6 proteins. Subcell. Biochem. 62, 59–69 (2012). [DOI] [PubMed] [Google Scholar]
- Akita M. et al. Cdc6/Orc1 from Pyrococcus furiosus may act as the origin recognition protein and Mcm helicase recruiter. Genes Cells 15, 537–552 (2010). [DOI] [PubMed] [Google Scholar]
- Lindas A. C. & Bernander R. The cell cycle of archaea. Nat. Rev. Microbiol. 11, 627–638 (2013). [DOI] [PubMed] [Google Scholar]
- Wu Z., Liu J., Yang H., Liu H. & Xiang H. Multiple replication origins with diverse control mechanisms in Haloarcula hispanica. Nucleic Acids Res. 42, 2282–2294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M., Malla S., Blythe M. J., Nieduszynski C. A. & Allers T. Accelerated growth in the absence of DNA replication origins. Nature 503, 544–547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggin I. G., McCallum S. A. & Bell S. D. Chromosome replication dynamics in the archaeon Sulfolobus acidocaldarius. Proc. Natl Acad. Sci. USA 105, 16737–16742 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. et al. Complete genome sequence of the metabolically versatile halophilic archaeon Haloferax mediterranei, a poly(3-hydroxybutyrate-co-3-hydroxyvalerate) producer. J. Bacteriol. 194, 4463–4464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Characterization of the minimal replicon of pHM300 and independent copy number control of major and minor chromosomes of Haloferax mediterranei. FEMS Microbiol. Lett. 339, 66–74 (2013). [DOI] [PubMed] [Google Scholar]
- Liu H., Han J., Liu X., Zhou J. & Xiang H. Development of pyrF-based gene knockout systems for genome-wide manipulation of the archaea Haloferax mediterranei and Haloarcula hispanica. J. Genet. Genomics 38, 261–269 (2011). [DOI] [PubMed] [Google Scholar]
- Vujcic M., Miller C. A. & Kowalski D. Activation of silent replication origins at autonomously replicating sequence elements near the HML locus in budding yeast. Mol. Cell. Biol. 19, 6098–6109 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata T. et al. Stalled fork rescue via dormant replication origins in unchallenged S phase promotes proper chromosome segregation and tumor suppression. Mol. Cell 41, 543–553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Hirota Y. & Jacob F. On the process of cellular division in Escherichia coli: replication of the bacterial chromosome under control of prophage P2. Proc. Natl Acad. Sci. USA 68, 2407–2411 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Caro L., Berg C. M. & Hirota Y. Chromosome replication in Escherichia coli. IV. Control of chromosome replication and cell division by an integrated episome. J. Mol. Biol. 55, 441–456 (1971). [DOI] [PubMed] [Google Scholar]
- Allers T. & Mevarech M. Archaeal genetics—the third way. Nat. Rev. Genet. 6, 58–73 (2005). [DOI] [PubMed] [Google Scholar]
- DeMaere M. Z. et al. High level of intergenera gene exchange shapes the evolution of haloarchaea in an isolated Antarctic lake. Proc. Natl Acad. Sci. USA 110, 16939–16944 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Y. & Nurse P. Establishing the program of origin firing during S phase in fission Yeast. Cell 136, 852–864 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantiero D., Mackenzie A., Donaldson A. & Zegerman P. Limiting replication initiation factors execute the temporal programme of origin firing in budding yeast. EMBO J. 30, 4805–4814 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuert S., Allers T., Spohn G. & Soppa J. Regulated polyploidy in halophilic archaea. PLoS ONE 1, e92 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann K., Forterre P., Brochier-Armanet C. & Gribaldo S. Global phylogenomic analysis disentangles the complex evolutionary history of DNA replication in archaea. Genome Biol. Evol. 6, 192–212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M. & Bernander R. Genome-wide transcription map of an archaeal cell cycle. Proc. Natl Acad. Sci. USA 104, 2939–2944 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang L., Liu J., Cai L. & Xiang H. Genome-wide analysis of gene expression in stationary phase and genetic characterization of stationary-phase-dependent halocin gene expression in the haloarchaeon Haloferax mediterranei. J. Genet. Genomics 40, 441–444 (2013). [DOI] [PubMed] [Google Scholar]
- Ishino Y. & Ishino S. Rapid progress of DNA replication studies in Archaea, the third domain of life. Sci. China Life Sci. 55, 386–403 (2012). [DOI] [PubMed] [Google Scholar]
- Matsunaga F., Forterre P., Ishino Y. & Myllykallio H. In vivo interactions of archaeal Cdc6/Orc1 and minichromosome maintenance proteins with the replication origin. Proc. Natl Acad. Sci. USA 98, 11152–11157 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. & Bernander R. Chromosome replication origins: do we really need them? Bioessays 36, 585–590 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao D. et al. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl. Microbiol. Biotechnol. 97, 3027–3036 (2013). [DOI] [PubMed] [Google Scholar]
- Forsberg L. A. et al. Age-related somatic structural changes in the nuclear genome of human blood cells. Am. J. Hum. Genet. 90, 217–228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline S. W., Lam W. L., Charlebois R. L., Schalkwyk L. C. & Doolittle W. F. Transformation methods for halophilic archaebacteria. Can. J. Microbiol. 35, 148–152 (1989). [DOI] [PubMed] [Google Scholar]
- Lu Q. et al. Dissection of the regulatory mechanism of a heat-shock responsive promoter in Haloarchaea: a new paradigm for general transcription factor directed archaeal gene regulation. Nucleic Acids Res. 36, 3031–3042 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas S., Shunburne L., Ngo H. P. & Allers T. Mre11-Rad50 promotes rapid repair of DNA damage in the polyploid archaeon Haloferax volcanii by restraining homologous recombination. PLoS Genet. 5, e1000552 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L., Williams N., Misleh C. & Li W. W. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34, W369–W373 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- Hartman A. L. et al. The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS ONE 5, e9605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E. A. et al. Sequencing of seven haloarchaeal genomes reveals patterns of genomic flux. PLoS ONE 7, e41389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. R. & Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 36, W181–W184 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7, Supplementary Tables 1-2 and Supplementary References