Abstract
Wiwaxiids are a problematic group of scale-covered lophotrochozoans known from Cambrian Stages 3–5. Their imbricating dorsal scleritome of leaf-like scales has prompted comparison with various annelids and molluscs, and has been used as a template to reconstruct the articulation pattern of isolated Small Shelly Fossils. The first articulated specimens of Wiwaxia from the Cambrian Stage 3 Chengjiang Konservat-Lagerstätte show that the Wiwaxia scleritome comprised nine equivalent transverse rows associated with outgrowths of soft tissue, but did not possess a separate zone of anterior sclerites. This serial construction is fundamentally incompatible with the circumferential disposition of sclerites in early molluscs, but does closely resemble the armature of certain annelids. A deep homology with the annelid scleritome must be reconciled with Wiwaxia’s mollusc-like mouthparts and foot; together these point to a deep phylogenetic position, close to the common ancestor of annelids and molluscs.
The distinctive mid-Cambrian organism Wiwaxia is best known for its stalked carbonaceous sclerites, which together comprise an imbricated dorsal scleritome. Articulated scleritomes have previously been reported from five localities spanning 15 million years1,2,3,4,5,6. The constitution of the Wiwaxia scleritome is remarkably conserved, notwithstanding species-level variety in sclerite proportions and orientation. An anterior zone of sclerites is followed by eight transverse rows across the body, with morphologies varying consistently according to location: ventrolateral sclerites are sickle-shaped; lower-lateral sclerites are oval; upper-lateral sclerites are rounded and symmetrical, and dorsal sclerites are asymmetric. In addition, mature specimens – those longer than a centimetre – exhibit twin series of erratically arranged dorsal spines. The anterior body region seemingly corresponds to a distinct zone of rounded sclerites4, although the exact relationship of these anterior sclerites to the transverse rows is unclear.
The construction of the sclerites and scleritome represents important, if ambiguous, evidence with respect to the phylogenetic affiliation of Wiwaxia. The sclerites bear the distinctive signature of microvillar secretion – an internal microstructural fabric of long, narrow chambers – which assigns Wiwaxia to the lophotrochozoan total group7. (The persistent occurrence of these striations through the full length of intact sclerites distinguishes them from the parallel striations reported in certain ecdysozoan sclerites8,9). Beyond this, the interpretation of the scleritome is more ambiguous. Some authors10,11 have favoured an annelid analogue, but others12,13 have emphasized similarities with aculiferan molluscs – accommodating the conspicuously mollusc-like nature of the Wiwaxia foot (as observed in a small number of specimens) and feeding apparatus12,14.
Here we report a new species of Wiwaxia based on articulated specimens from the Cambrian Stage 3 Chengjiang Lagerstätte. Post-mortem enrolment and soft tissue preservation in these fossils, in combination with critical W. corrugata material from the Burgess Shale, allows a timely re-evaluation of the Wiwaxia scleritome, and expounds the scleritome’s implications for the affinity of this confounding taxon.
Material and Methods
Five new Wiwaxia specimens, each comprising part and counterpart, have been collected from Chengjiang by the Early Life Institute working team, and deposited in the Early Life Institute and Department of Geology, Northwest University, Xi’an, China (Prefix: ELI). These complete, articulated specimens represent organisms in various states of enrolment, preserved at various orientations to the plane of splitting. Sclerites and mouthparts are represented by regions with a deep purple to black colouration, and in the best cases correspond to an intact layer of carbon. Regions corresponding to soft tissue are coated with rust-coloured framboids, apparently arising through the oxidation of pyrite.
Burgess Shale specimens of W. corrugata are deposited in the Smithsonian Institution National Museum of Natural History (NMNH), Washington DC, and the Royal Ontario Museum (ROM), Toronto, and represent unweathered carbonaceous compression fossils associated with diagenetic aluminosilicate films15.
Systematic Palaeontology
This published work and the nomenclatural acts it contains have been registered in Zoobank: http://zoobank.org/Referencesurn:lsid:zoobank.org:pub:1B5E0AE5-2FB2-4EFF-B35B-8293D919DEE8
Family Wiwaxiidae Walcott, 1911 (nom. corr. Howell, 1962)
Genus Wiwaxia Walcott, 1911
Emended diagnosis
Ovoid body bearing nine transverse rows of ribbed carbonaceous sclerites, arranged in bundles and directed towards the posterior. Anteriormost sclerite row terminal. Sclerites comprising narrow root and wide blade, and incorporating narrow internal longitudinal chambers. Sclerite morphology varying consistently across each transverse row; medial sclerites rounded, ventro-lateral sclerites elongate and curved, usually with pointed tip. Dorsal surface of adults often with elongate spinose sclerites. Ventral surface comprising unarmoured ‘foot’. Toothed feeding apparatus comprising two to three rows of curved carbonaceous teeth arranged on tongue-like supporting apparatus.
Wiwaxia papilio sp. nov.
LSID. urn:lsid:zoobank.org:act:E5A1A18C-D087-4FA1-AC60-A0CE65603E9B
Derivation of name
Papilio (Latin), butterfly, reflecting the butterfly-like arrangement of the fans of sclerites.
Holotype
ELI-W001 (Fig. 1a–b), an almost complete dorsoventral specimen preserving mouthparts and soft tissue.
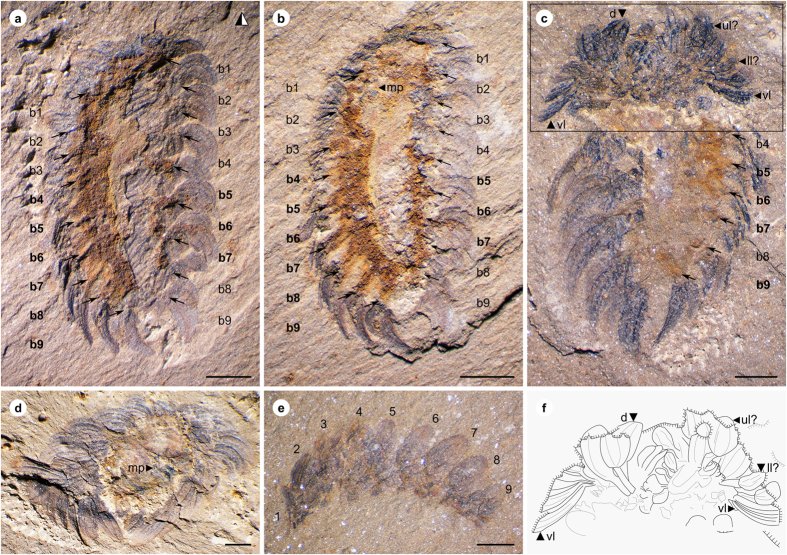
Figure 1. Wiwaxia papilio sp. nov. from Chengjiang.
(a,b), ELI-W001, Holotype, part (horizontally mirrored) and counterpart, ventral view showing bundles of sclerites (b1–b9) associated with rust-coloured outgrowths of soft tissue (bold-face lettering), showing mouthparts (mp); (c), ELI-W004, ventral view, with anterior body curved up to display anterior sclerite zone; sclerites in anterior row express morphologies of ventrolateral (vl), lower lateral (ll), upper lateral (ul) and dorsal (d) sclerites; interpretative sketch in (f); (d), ELI-W003, anterior view showing mouthparts (mp); (e), ELI-W005, lateral view, illustrating anterior sclerite row and eight subsequent dorsal sclerites (1–9). Scale bars: 1 mm. Z. Zhang and M. Smith created the images.
Paratypes
ELI-W002–ELI-W005 (Figs 1c–e, 2a,b).
Figure 2. Mouthparts of Wiwaxia.
(a,b), ELI-W003, Wiwaxia papilio sp. nov., optical (a) and backscatter electron (b) images; (c,d) Wiwaxia corrugata from the Burgess Shale; (c), NMNH 277890; (d), NMNH 271947. Scale bars = 250 μm. Z. Zhang and M. Smith created the images.
Stratigraphic setting
Specimens were collected from the yellowish-green to greyish-green mudstones of the Chengjiang Lagerstätte at the Jianshan Section in Haikou, Kunming. Other taxa recovered from this site include the early agnathan Haikouichthys16 and the echinoderm-like vetulocystids17.
Diagnosis
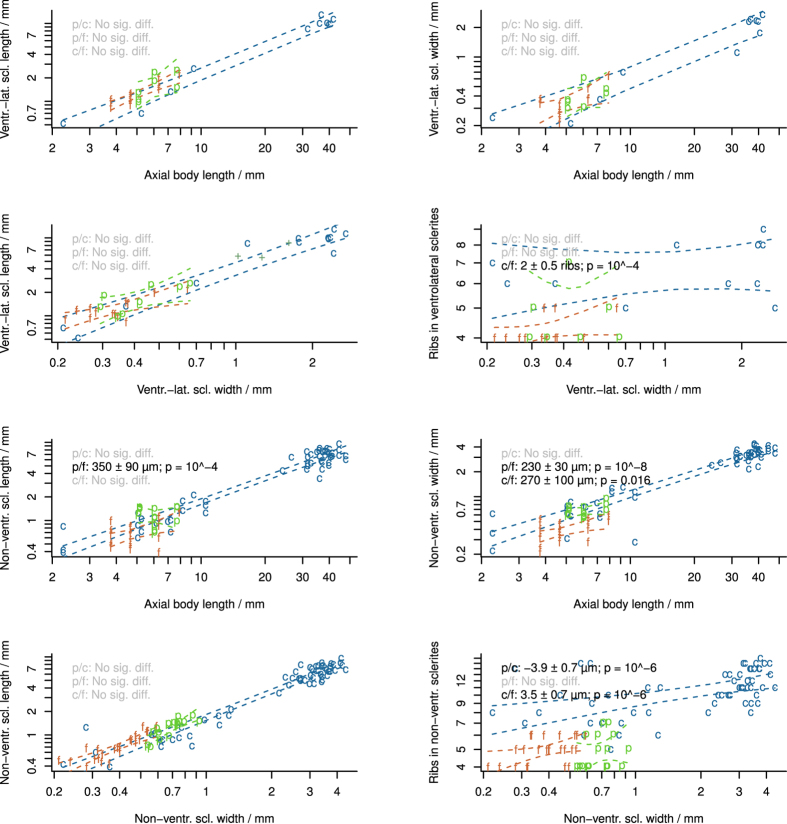
Single order of widely spaced sclerite ribs (4–6 ribs on sclerites 500–1000 μm in length). Non-ventrolateral sclerites long and wide relative to body length (Fig. 3).
Figure 3. Summary of sclerite measurements in Wiwaxia species.
c W. corrugata; f W. foliosa; p, W. papilio sp. nov.; + , isolated Chengjiang sclerites. Dashed lines denote 95% the confidence envelope of regression lines. Within each panel, regression line gradients are not significantly different. Panel legends report significant pairwise differences in the location of the y-intercept. M. Smith created the images. For source data and statistical code, see Supplementary Datasets.
Remarks
The examined material resembles juvenile specimens of Wiwaxia corrugata in terms of its overall body size, the form of its mouthparts, the relatively large size of dorsal sclerites, the broad yet short ventrolateral sclerites, and the absence of dorsal spines. Adult specimens are conceivably represented by the larger isolated sclerites that have also been reported from Chengjiang18 (Fig. 3), though this material is difficult to exclude from other Wiwaxia species. W. papilio sp. nov. is distinguished from W. corrugata based on the low number ribs on its sclerites; detailed comparison with other species is hampered by the shortage of comparative material1,5.
Description
The articulated specimens of W. papilio sp. nov. (Figs 1 and 4) are 5–8 mm long, and exhibit the arrangement of sclerites typical of juvenile Wiwaxia specimens: an anterior region of sclerites followed by eight further transverse rows, with no dorsal spines. Ventrolateral sclerites are siculate, whereas other sclerites are rounded and about twice as long as broad. (The limited preservation of the dorsal surface precludes a detailed description of the dorsal most sclerites.) Each ventro-lateral sclerite fully overlaps its posterior neighbour (per ref. 2, but contra ref. 12). Two specimens preserve mouthparts with two to three rows of carbonaceous teeth (Fig. 2a,b). As the morphology of individual teeth is indistinct, it is not clear whether or not small lateral teeth are present; otherwise, no substantive differences from W. corrugata (Fig. 2c,d) are evident.
Figure 4. Reconstruction of Wiwaxia papilio sp. nov.
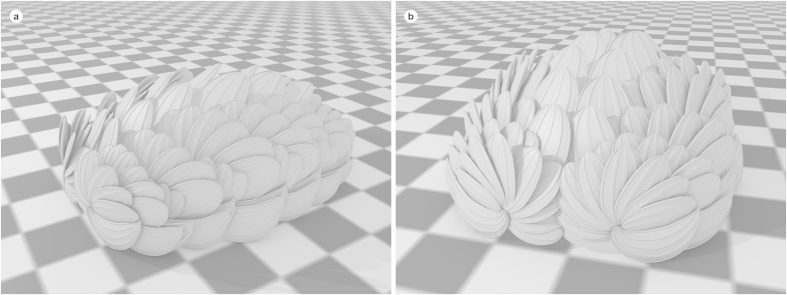
(a) lateral view, showing arrangement of transverse rows; (b), frontal view, showing fan-like arrangement of anterior sclerites. Checkers = 1 mm. M. Smith and Z. Zhang created the images.
The anterior row of sclerites is made up of two bilaterally-paired rosettes, across which the morphology of sclerites varies in the same fashion as it does elsewhere on the body: the most ventral sclerites are siculate, whereas the more dorsal sclerites are rounded and occasionally asymmetric (Figs 1c and 4b). Siculate sclerites also form part of the anterior row of Burgess Shale specimens of Wiwaxia corrugata (e.g. Fig. 5b,d), occurring at the lateral edges of the scleritome but not skirting the front of the animal (contra ref. 4). As such, the anterior sclerites do not form a distinct zone of the scleritome, but represent a (ninth) transverse row of sclerites.
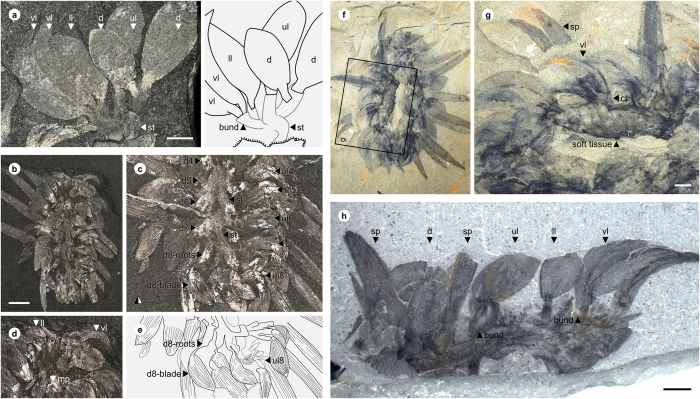
Figure 5. Wiwaxia corrugata from the Burgess Shale.
(a) NMNH 199948, partial row of sclerites and associated connective tissue; (b–e), ROM 57707, connective tissue associated with bundles of dorsal and upper lateral sclerites; images courtesy Jean-Bernard Caron; c is horizontally mirrored counterpart of specimen in b (f–g), ROM 61511, outgrowth of connective tissue associated with bundle of ventrolateral sclerites; (h), NMNH 199953; transverse row of sclerites articulated by connective tissue. M. Smith created the images. Abbreviations: bund, bundling of sclerites; ct, connective tissue; d, dorsal sclerite; d4–8, bundles of dorsal sclerites; ll, lower lateral sclerite; mp, mouthpart; sp, spine; ul, upper lateral sclerite; ul4–8, bundles of upper lateral sclerites; vl, ventrolateral sclerite. Scale bars = 2 mm.
The ventral surface of the fossils is represented by an iron-rich region that we interpret as soft tissue in a position dorsal to the foot. Bundles of sclerites insert into lateral projections of this iron-rich region (Fig. 1a–c). Sclerite bundles are anchored in equivalent projections in W. corrugata (Fig. 5a–g; see ref. 12), where equivalent projections are connected by transverse bands of connective tissue (Fig. 5h); these themselves are embedded in the soft tissue of the organism (Fig. 5h).
Discussion
Distribution of Wiwaxia
This report represents the first occurrence of articulated Wiwaxia in the shallow water communities represented by the Chengjiang fauna. The five new specimens are all less than a centimetre long and lack spines; by analogy with W. corrugata, they represent juveniles. The only evidence of adult Wiwaxia individuals at Chengjiang comes from a single assemblage of ventro-lateral sclerites, corresponding in size to those of adult W. corrugata18; in contrast, there are a hundred adult Wiwaxia in the Burgess Shale for every five juveniles12. This reflects a more general scarcity of adult Wiwaxia specimens in shallow-water settings. Bedding-surface fossils from the Buchava, Hongjingshao and Kaili formations exclusively correspond to juvenile size ranges and morphologies2,3,19. (These localities, like Chengjiang, preserve shallow-water communities; in the case of Kaili, shallow-water taxa were washed into deeper waters before burial19,20,21,22,23).
In contrast, deep-water settings are replete with adult Wiwaxia. The deep water Tsinghsutung (=Qingxudong) Formation contains disarticulated sclerites that correspond to the size range of sclerites in adult Wiwaxia corrugata, and includes elongate sclerites that conceivably represent spines3,24. The Spence Shale and Sinsk Biota, which were deposited below storm wave base25,26, contains articulated and disarticulated sclerites belonging to Wiwaxia adults1,6,27. And in the Burgess Shale, adult Wiwaxia are present in great abundance at the deeper water localities on Fossil Ridge and Mount Stephen4,12,28,29 but have not yet been found in the shallow-water Marble Canyon locality30.
Wiwaxia juveniles occur in almost all geographic and ecological settings31,32, perhaps reflecting planktonic larval dispersal33. The rarity of adult specimens in shallow waters may therefore represent failure to reach maturity in these environments – whether through active migration to deeper water, or through accentuated predation pressure on adult organisms.
Phylogenetic implications of scleritome constitution
Since the discovery of the first articulated specimens10, sclerite disposition has played a central role in determining Wiwaxia’s biological affinity.
One obvious analogue to Wiwaxia sclerites are the conspicuous dorsal scales (elytra) of aphroditid and polynoid annelids10,34 – but these fleshy outgrowths are not secreted by microvilli, so cannot be equivalent to Wiwaxia sclerites4,11. The modified paleal chaetae of chrysopetalid annelids represent a more promising analogue11; as with Wiwaxia sclerites, chrysopetalid paleae occur in a series of bundles or fans across a transverse rows35,36, and indeed sclerite morphology even varies from siculate lateral sclerites to more symmetrical dorsal sclerites37. This correspondence also rings true on the level of sclerite construction: chrysopetalid paleae, like Wiwaxia sclerites, comprise a proximal root and a broad distal blade, and on a more superficial level may exhibit ribs, a granular ornament, and a distal prong (cf. ref 32).
Despite this compelling similarity, there is a fundamental objection to a chrysopetalid affinity: chrysopetalids are fundamentally derived crown-group annelids38,39,40,41,42, whereas Wiwaxia lacks key synapomorphies such as biramous parapodia, palps and aciculae and thus belongs outside the annelid crown group7,42,43,44. Equally problematic is the location of the ventral mouthparts in Wiwaxia beneath the second or third sclerite row: this is difficult to reconcile with the anterior position of the annelid prostomium. As such, the detailed similarity between Wiwaxia sclerites and those of chrysopetalids must be attributed to convergent evolution.
Could the molluscs provide a more reasonable analogue for the Wiwaxia scleritome? Of the extant molluscs, only aculiferans (=Polyplacophora + Aplacophora) bear comparable sclerites. Polyplacophoran sclerites exhibit crystalline cores that are surrounded by a thin cuticular layer with a microvillar texture45,46, which is conceivably homologous (at a deep level) to the sclerites of Wiwaxia12 – but polyplacophoran sclerites are arranged in concentric zones rather than transverse rows, and exhibit a broadly quincuncial disposition47 rather than occurring in bundles. Aplacophoran molluscs do exhibit transverse rows of dorsal sclerites at early developmental stages13,48 – but stem-group aplacophorans resemble polyplacophorans49,50, meaning that Wiwaxia would have to represent a surprisingly early and extremely derived aplacophoran that retained larval features to adulthood and developed a precise sclerite organization unseen in modern representatives12. As such, no living mollusc provides a convincing analogue for Wiwaxia’s scleritome.
Although the detailed construction of the Wiwaxia scleritome has no precise equivalent in modern or fossil groups, this is not to say that it does not share homologies at a deeper level. Carbonaceous sclerites are secreted by microvilli in bryozoans, brachiopods, molluscs and annelids46,51,52, and are likely homologous across Lophotrochozoa53. The iterated arrangement of Wiwaxia sclerites is paralleled by basal annelids, and possibly brachiopods54,55,56. Finally, the bundling of sclerites in Wiwaxia could conceivably foreshadow the neuropodial and notopodial bundles observed in crown-group annelids. Whilst it is conceivable that the iterated nature of the Wiwaxia scleritome arose convergently, we prefer to assume homology in the absence of clear evidence to the contrary.
The broadly annelid-like serial construction of the Wiwaxia scleritome must be balanced against the conspicuously mollusc-like nature of its mouthparts and foot12,14,57,58. An equivalent paradox is represented in Hallucigenia, which bears onychophoran-like claws alongside cycloneuralian-like mouthparts; this is resolved if cycloneuralian-like mouthparts occurred in the common ancestor of onychophorans and cycloneuralians59. As the affinity of Wiwaxia is less well established, there is more than one way to reconcile its molluscan and annelidan features. Either annelid-like sclerite rows or molluscan mouthparts may have been present in the common ancestor of annelids and molluscs and been retained for some time in the stem lineages of each phylum.
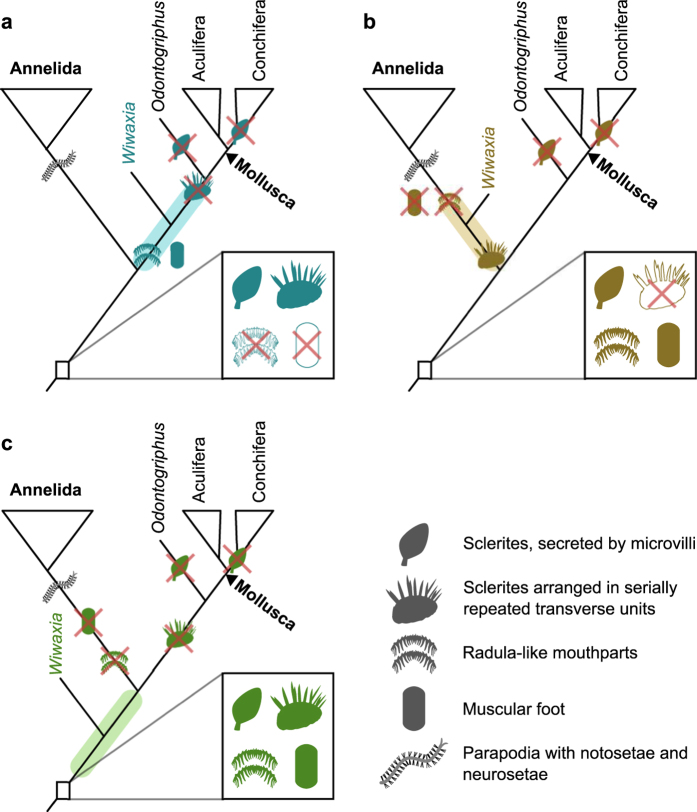
If Wiwaxia is a stem-group mollusc (Fig. 6a), a scleritome of iterated rows was conceivably ancestral to annelids and molluscs, and was later rearranged into the circumferential format of aculiferans. If Wiwaxia is a stem-annelid (Fig. 6b), a muscular foot and radula-like mouthparts are ancestral to molluscs and annelids, with a serially arranged scleritome unique to the annelid stem and ultimately leading to metamerism and full segmentation in the crown group. Under this arrangement, annelids replaced their ancestrally mollusc-like mouthparts with an independently-derived and non-homologous14 jaw, just as onychophorans replaced their cycloneuralian-like mouthparts with independently-derived jaws59. The third possibility is that Wiwaxia falls in the stem lineage of Mollusca + Annelida. Under this scenario, both phyla exhibit a loss or overprinting of primitive features: the foot and mouthparts in annelids, the transverse sclerite arrangement in molluscs.
Figure 6. Possible affinities of Wiwaxia.
(a), in molluscan stem group; (b), in annelid stem group; (c), in stem group to Mollusca + Annelida. Boxes denote implied morphology of deep ancestors of Annelida + Mollusca; branches are illustrated with implied gains or losses of characters. M. Smith and Z. Zhang created the images.
Ultimately, a more complete record of early Lophotrochozoan evolution is needed before the polarity of these distinctive characters can be resolved. But whatever the exact phylogenetic position of Wiwaxia, it clearly diverged before the modern phyla had attained their distinctive body plans, and thus represents a valuable proxy for the common ancestor of molluscs and annelids.
Additional Information
How to cite this article: Zhang, Z. et al. New reconstruction of the Wiwaxia scleritome, with data from Chengjiang juveniles. Sci. Rep. 5, 14810; doi: 10.1038/srep14810 (2015).
Supplementary Material
Acknowledgments
Jean-Bernard Caron and Nicholas Butterfield kindly provided images of Burgess Shale material, collected with by permission of Parks Canada. We thank Jean-Bernard Caron, Peter Fenton, Mark Florence and Gene Hunt for access to Burgess Shale material, and Lars Holmer for hospitality and access to facilities. J.-P Zhai and M.-R Cheng (in Xi’an) assisted with fossil preparation, and X.-L Zhang, J Han and J.-N Liu kindly assisted with field work. We acknowledge funding from the National Natural Science Foundation of China (NSFC 41425008, to ZZF), the Fok Ying Tung Education Foundation (G121016, to ZZF), the Ministry of Education of China (NCET-11-1046, to ZZF), the Geological Society of America (MRS) and Clare College, Cambridge (MRS). Fossil collection was partly financed by the National 973 Program (2013CB835002) and 111 Projects of China (P201102007).
Footnotes
Author Contributions Z.Z. designed and conceived the study and prepared the fossils. All authors participated in the discussion and analysis of these fossils. Fossil collection from Chengjiang was partly organized by D.S. M.S. prepared the manuscript with input from Z.Z. All authors reviewed the manuscript. M.S. studied Burgess Shale comparative material and produced the fossil reconstructions.
References
- Conway Morris S., Selden P. A., Gunther G., Jamison P. M. & Robison R. A. New records of Burgess Shale-type taxa from the middle Cambrian of Utah. J. Paleo. in press (2015). [Google Scholar]
- Yang J., Smith M. R., Lan T., Hou J. & Zhang X. Articulated Wiwaxia from the Cambrian Stage 3 Xiaoshiba Lagerstätte. Sci. Rep. 4, 4643 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.-J., Zhao Y.-L., Peng J. & Yang Y.-N. New Wiwaxia material from the Tsinghsutung Formation (Cambrian Series 2) of Eastern Guizhou, China. Geol. Mag. 151, 339–348 (2014). [Google Scholar]
- Conway Morris S. The Middle Cambrian metazoan Wiwaxia corrugata (Matthew) from the Burgess Shale and Ogygopsis Shale, British Columbia, Canada. Phil. Trans. R. Soc. Lond. B 307, 507–582 (1985). [Google Scholar]
- Zhao Y.-L., Qian Y. & Li X. S. Wiwaxia from Early-Middle Cambrian Kaili Formation in Taijiang, Guizhou. Acta Pal. Sin. 33, 359–366 (1994). [Google Scholar]
- Ivantsov A. Yu. et al. Unique Sinsk localities of early Cambrian organisms (Siberian platform) [in Russian]. Paleontol. Institut, Akad. Nauk SSSR, Tr. 284, 1–143 (2005). [Google Scholar]
- Butterfield N. J. Hooking some stem-group ‘worms’: fossil lophotrochozoans in the Burgess Shale. BioEssays 28, 1161–1166 (2006). [DOI] [PubMed] [Google Scholar]
- Caron J.-B., Smith M. R. & Harvey T. H. P. Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians. Proc. R. Soc. B 280, 20131613 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Harvey T. H. P. & Butterfield N. J. Data from: The macro- and microfossil record of the Cambrian priapulid Ottoia. Dryad Digit. Repos. 10.5061/dryad.km109 (2015). [DOI] [Google Scholar]
- Walcott C. D. Cambrian Geology and Paleontology II, no. 5. Middle Cambrian annelids. Smithson. Misc. Collect. 57, 109–144 (1911). [Google Scholar]
- Butterfield N. J. A reassessment of the enigmatic Burgess Shale fossil Wiwaxia corrugata (Matthew) and its relationship to the polychaete Canadia spinosa Walcott. Paleobiology 16, 287–303 (1990). [Google Scholar]
- Smith M. R. Ontogeny, morphology and taxonomy of the soft-bodied Cambrian ‘mollusc’ Wiwaxia. Palaeontology 57, 215–229 (2014). [Google Scholar]
- Scheltema A. H. & Ivanov D. L. An aplacophoran postlarva with iterated dorsal groups of spicules and skeletal similarities to Paleozoic fossils. Invertebr. Biol. 121, 1–10 (2002). [Google Scholar]
- Smith M. R. Mouthparts of the Burgess Shale fossils Odontogriphus and Wiwaxia: implications for the ancestral molluscan radula. Proc. R. Soc. B 279, 4287–4295 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield N. J., Balthasar U. & Wilson L. A. Fossil diagenesis in the Burgess Shale. Palaeontology 50, 537–543 (2007). [Google Scholar]
- Shu D.-G. et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature 421, 526–529 (2003). [DOI] [PubMed] [Google Scholar]
- Shu D.-G., Conway Morris S., Han J., Zhang Z.-F. & Liu J.-N. Ancestral echinoderms from the Chengjiang deposits of China. Nature 430, 422–428 (2002). [DOI] [PubMed] [Google Scholar]
- Zhao F. et al. First report of Wiwaxia from the Cambrian Chengjiang Lagerstätte. Geol. Mag. 152, 378–382 (2015). [Google Scholar]
- Fatka O., Kraft P. & Szabad M. Shallow-water occurrence of Wiwaxia in the Middle Cambrian of the Barrandian area (Czech Republic). Acta Pal. Pol. 56, 871–875 (2011). [Google Scholar]
- Lin J.-P. Review of the Depositional Environment of the Kaili Formation (Cambrian Series 2-3 Boundary Interval: China). Mem. Assoc. Australas. Palaeontol. 37, 131–149 (2009). [Google Scholar]
- Hu S. Taphonomy and palaeoecology of the early Cambrian Chengjiang Biota from eastern Yunnan, China. Berliner Paläobiologische Abhandlungen 7, 1–197 (2005). [Google Scholar]
- Zeng H., Zhao F., Yin Z., Li G. & Zhu M. A Chengjiang-type fossil assemblage from the Hongjingshao Formation (Cambrian Stage 3) at Chenggong, Kunming, Yunnan. Chin. Sci. Bull. 59, 3169–3175 (2014). [Google Scholar]
- Gaines R. R., Mering J. a., Zhao Y. & Peng J. Stratigraphic and microfacies analysis of the Kaili Formation, a candidate GSSP for the Cambrian Series 2-Series 3 boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 311, 171–183 (2011). [Google Scholar]
- Kuang W.-L., Yang S.-X., Yu P.-R. & Lao K.-T. Sedimentary characteristics and geological significance of turbidites in the Lower Cambrian Qingxudong Formation at Huayuan county, northwestern Hunan. Chinese J. Geol. (Scientia Geol. Sin.) 2, 347–358 (2008). [Google Scholar]
- Robison R. A. in The Early Evolution of Metazoa and the Significance of Problematic Taxa (eds. Simonetta A. M. & Conway Morris S.) 77–98 (Cambridge University Press, 1991). [Google Scholar]
- Ivantsov A. Yu. et al. Palaeoecology of the Early Cambrian Sinsk biota from the Siberian Platform. Palaeogeogr. Palaeoclimatol. Palaeoecol. 220, 69–88 (2005). [Google Scholar]
- Conway Morris S. & Robison R. A. More soft-bodied animals and algae from the Middle Cambrian of Utah and British Columbia. Univ. Kansas Paleontol. Contrib. 122, 23–84 (1988). [Google Scholar]
- Aitken J. D. & McIlreath I. A. The Cathedral Reef Escarpment: a Cambrian great wall with humble origins. Geos 13, 17–19 (1984). [Google Scholar]
- O’Brien L. J., Caron J.-B. & Gaines R. R. Taphonomy and depositional setting of the Burgess Shale Tulip Beds, Mount Stephen, British Columbia. Palaios 29, 309–324 (2014). [Google Scholar]
- Caron J.-B., Gaines R. R., Aria C., Mángano M. G. & Streng M. A new phyllopod bed-like assemblage from the Burgess Shale of the Canadian Rockies. Nat. Commum. 5, 3210 (2014). [DOI] [PubMed] [Google Scholar]
- Smith M. R., Hughes G. M. G., de la Parra F. & Vargas M. C. Sclerites and possible mouthparts of Wiwaxia from the temperate palaeolatitudes of Colombia, South America. Lethaia (in press). 10.1111/let.12154 [DOI] [Google Scholar]
- Butterfield N. J. & Harvey T. H. P. Small Carbonaceous Fossils (SCFs): a new measure of early Paleozoic paleobiology. Geology 40, 71–74 (2012). [Google Scholar]
- Han J., Zhang Z.-F. & Liu J.-N. A preliminary note on the dispersal of the Cambrian Burgess Shale-type faunas. Gondwana Res. 14, 269–276 (2008). [Google Scholar]
- Piotrowski C. N. in The Coral Triangle: The 2011 Hearst Philippine Biodiversity Expedition (eds. Williams G. C. & Gosliner T. M.) 155–164 (California Academy of Sciences, 2014). [Google Scholar]
- Westheide W. & Watson Russell C. Ultrastructure of Chrysopetalid paleal chaetae (Annelida, Polychaeta). Acta Zool. 73, 197–202 (1992). [Google Scholar]
- Watson C., Chivers A. J., Narayanaswamy B. E., Lamont P. & Turnewitsch R. Chrysopetalidae (Annelida: Phyllodocida) from the Senghor Seamount, north-east Atlantic: taxa with deep-sea affinities and morphological adaptations. Mem. Museum Victoria 71, 311–325 (2014). [Google Scholar]
- Watson Russell C. Paleaequor, a new genus of polychaete worm (Chrysopetalidae). Rec. Aust. Museum 38, 153–174 (1986). [Google Scholar]
- Dahlgren T. G., Lundberg J., Pleijel F. & Sundberg P. Morphological and molecular evidence of the phylogeny of Nereidiform polychaetes (Annelida). J. Zool. Syst. Evol. Res. 38, 249–253 (2000). [Google Scholar]
- Rouse G. W. & Fauchald K. Cladistics and polychaetes. Zool. Scr. 26, 139–204 (1997). [Google Scholar]
- Zrzavý J., Říha P., Piálek L. & Janouškovec J. Phylogeny of Annelida (Lophotrochozoa): total-evidence analysis of morphology and six genes. BMC Evol. Biol. 9, 189 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck T. H. et al. Phylogenomic analyses unravel annelid evolution. Nature 471, 95–98 (2011). [DOI] [PubMed] [Google Scholar]
- Weigert A. et al. Illuminating the base of the annelid tree using transcriptomics. Mol. Biol. Evol. 31, 1391–1401 (2014). [DOI] [PubMed] [Google Scholar]
- Eibye-Jacobsen D. A reevaluation of Wiwaxia and the polychaetes of the Burgess Shale. Lethaia 37, 317–335 (2004). [Google Scholar]
- Butterfield N. J. Exceptional fossil preservation and the Cambrian Explosion. Integr. Comp. Biol. 43, 166–177 (2003). [DOI] [PubMed] [Google Scholar]
- Blumrich J. Das Integument der Chitonen. Zeitschrift für Wissenschaftliche Zool. 52, 404–476 plates 23–30 (1891). [Google Scholar]
- Fischer F. P., Maile W. & Renner M. Die Mantelpapillen und Stacheln von Acanthochiton fascicularis L. (Mollusca, Polyplacophora). Zoomorphologie 94, 121–131 (1980). [Google Scholar]
- Vinther J. & Nielsen C. The Early Cambrian Halkieria is a mollusc. Zool. Scr. 34, 81–89 (2005). [Google Scholar]
- Nielsen C., Haszprunar G., Ruthensteiner B. & Wanninger A. Early development of the aplacophoran mollusc Chaetoderma. Acta Zool. 88, 231–247 (2007). [Google Scholar]
- Sutton M. D., Briggs D. E. G., Siveter D. J., Siveter D. J. & Sigwart J. D. A Silurian armoured aplacophoran and implications for molluscan phylogeny. Nature 490, 94–97 (2012). [DOI] [PubMed] [Google Scholar]
- Scherholz M., Redl E., Wollesen T., Todt C. & Wanninger A. Aplacophoran mollusks evolved from ancestors with polyplacophoran-like features. Curr. Biol. 23, 2130–2134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. P. The resemblance of bryozoan gizzard teeth to ‘annelid-like’ setae. Acta Zool. 56, 283–289 (1975). [Google Scholar]
- Gustus R. M. & Cloney R. A. Ultrastructural similarities between setae of brachiopods and polychaetes. Acta Zool. 53, 229–233 (1972). [Google Scholar]
- Giribet G. et al. in Animal Evolution—Genomes, Fossils, and Trees (eds. Telford M. J. & Littlewood D. T. J.) 52–64 (Oxford University Press, 2009) 10.1093/acprof:oso/9780199549429.003.0006. [DOI] [Google Scholar]
- Temereva E. & Malakhov V. The evidence of metamery in adult brachiopods and phoronids. Invertebr. Zool. 8, 87–101 (2011). [Google Scholar]
- Parry L., Tanner A. & Vinther J. The origin of annelids. Palaeontology 57, 1091–1103 (2014). [Google Scholar]
- Zhang Z.-F et al. An Early Cambrian agglutinated tubular lophophorate with brachiopod characters. Sci. Rep. 4, 4682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J.-B., Scheltema A. H., Schander C. & Rudkin D. A soft-bodied mollusc with radula from the Middle Cambrian Burgess Shale. Nature 442, 159–163 (2006). [DOI] [PubMed] [Google Scholar]
- Caron J.-B., Scheltema A. H., Schander C. & Rudkin D. M. Reply to Butterfield on stem-group worms: fossil lophotrochozoans in the Burgess Shale. BioEssays 29, 200–202 (2007). [DOI] [PubMed] [Google Scholar]
- Smith M. R. & Caron J.-B. Hallucigenia’s head and the pharyngeal armature of early ecdysozoans. Nature 523, 75–78 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.