Abstract
We conducted a phase 1 trial evaluating the oral nucleoside analogue clofarabine in patients with relapsed/refractory non-Hodgkin lymphoma. Patients were treated once daily on days 1 through 21 of a 28 day cycle for a maximum of 6 cycles. The study was conducted with a 3+3 design with ten additional patients treated at the recommended phase 2 dose. Thirty patients were enrolled including indolent B-cell lymphomas (21), mantle cell (6), and diffuse large B-cell lymphoma (3). The primary toxicities were hematologic including grade 3–4 neutropenia (53%) and thrombocytopenia (27%). Three mg was determined to be the recommended phase 2 dose. Tumor volume was reduced in 70% of patients, and the overall response rate was 47% including 27% complete remissions. Responses were seen in indolent B-cell lymphomas and mantle cell lymphoma. At a median follow-up of 17 months, 68% of responding patients remain in ongoing remission. Oral clofarabine was well tolerated with encouraging efficacy in indolent B-cell lymphomas and mantle cell lymphomas, warranting further investigation.
Keywords: nucleoside analogue, non-Hodgkin’s lymphoma, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma
INTRODUCTION
Non-Hodgkin lymphomas (NHL) are a heterogeneous collection of diseases accounting for approximately 70,000 new cases diagnosed each year in the United States, and close to 20,000 deaths.[1] Though often highly treatable diseases, many lymphomas will not be cured with initial therapy and will require treatment at the time of recurrence. The indolent NHLs, including follicular lymphoma (FL), marginal zone lymphoma (MZL), small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), and lymphoplasmacytic lymphoma (LPL), generally present at advanced stage and are incurable with conventional therapies. Though incurable, these diseases tend to be chemotherapy responsive with high rates of initial response, but they exhibit progressively lower rates of response and remission duration with subsequent lines of therapy. Available systemic therapies for relapsed indolent NHLs include rituximab, chemotherapy, chemoimmunotherapy, or radioimmunotherapy. There is no standard therapy at the time of relapse for indolent lymphomas, with selection of treatment guided by each patient’s prior therapy, comorbidities, and personal preferences. Additional effective treatment options are needed in relapse, particularly those that preserve quality of life.
Nucleoside analogues have demonstrated clinical efficacy in the treatment of non-Hodgkin lymphomas and are used in predominantly intravenous formulations for the treatment of relapsed or refractory disease.[2,3] Clofarabine is a novel nucleoside analogue that is FDA approved in an intravenous formulation for the treatment of children with relapsed acute lymphoblastic leukemia. Like the other nucleoside analogues, clofarabine must be converted to the 5′-triphosphate form by deoxycytidine kinase (dCK) to be active within cells. The triphosphate form of clofarabine is an inhibitor of both DNA polymerase α and ribonucleotide reductase.[4] These effects lead to depletion of intracellular deoxynucleotide triphosphate pools, and inhibition of elongation of DNA strands during synthesis.[5] Clofarabine has potential pharmacologic advantages over existing nucleoside analogues, including being superior to fludarabine in inhibiting ribonucleotide reductase, and being more efficient than cladribine as a substrate for dCK.[6,7] Unlike most traditional chemotherapeutics, anti-cancer activity is observed in vitro in non-dividing cells and in cells with a low proliferation rate, making it an appealing therapy for low-grade lymphomas.[8,9] Activity of the intravenous formulation of clofarabine in relapsed NHL has been established, but with significant hematologic toxicities.[10,11] Preclinical evaluation of clofarabine in a xenograft model suggested improved efficacy when administered at low dose on a prolonged administration schedule. We therefore performed a phase 1 study of a novel oral formulation of clofarabine administered in an extended dosing schedule. This is the first study of the oral formulation in non-Hodgkin lymphomas.
PATIENTS AND METHODS
Patients
Patients were eligible if they had non-Hodgkin lymphoma that had relapsed or been refractory to prior therapy. There was no limit on the number of prior therapies. Patients were ≥18 years old with histologically confirmed NHL, measurable disease, ECOG performance status ≤2, absolute neutrophil count (ANC) ≥1,500/mcL, platelets ≥100,000/mcL, creatinine ≤1.5mg/dL or creatinine clearance ≥60 mL/min/1.73 m2 for patients with creatinine levels above institutional upper limit of normal (ULN). Total bilirubin was ≤2.0 X institutional ULN, and AST (SGOT)/ALT (SGPT) ≤2.5 X institutional ULN.
Patients with known leptomeningeal or brain metastases were excluded. Prior autologous stem cell transplantation was not allowed during the dose escalation phase, but was allowed in the 10 patient dose-expansion at the MTD as long as six months or more had elapsed since high-dose chemotherapy. Allogeneic stem cell transplantation was excluded. Due to the pattern of responses manifest during dose-escalation, eligible histologies in the dose expansion were limited to indolent B-cell lymphomas and mantle cell lymphoma. No prior allogeneic stem cell transplantation was allowed.
This study was conducted in accordance with the Declaration of Helsinki, approved by the institutional review board of participating centers, and registered with clinicaltrials.gov (NCT00644189).
Study Design
This was an open label phase 1 study of oral clofarabine taken once daily on days 1–21 of a 28 day cycle. Patients could receive up to 6 total cycles in the absence of disease progression or unacceptable toxicity. The dose-escalation phase was conducted with a traditional 3+3 design, followed by a 10 patient dose expansion at the recommended phase 2 dose. During dose escalation, patients were treated at 1mg, 2mg, 4mg, and then de-escalated to 3mg due to late cytopenias observed in the 4mg cohort.
Escalation of clofarabine to the next dose level proceeded if 3 of 3 subjects in a given cohort completed cycle 1 without experiencing a dose-limiting toxicity (DLT). If one subject within any dose level experienced a DLT, a total of six subjects were enrolled at that dose level. If less than 2 subjects of 6 experienced a DLT, accrual proceeded to the next dose level. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3). DLTs were defined as: ANC of Grade 3 or 4 plus fever; grade 4 ANC for ≥5 days; any platelet count of <25,000/μL, grade 3 or 4 nausea, vomiting, diarrhea if persistent despite optimal antiemetic or anti-diarrheal therapy, grade 3 elevation of transaminases if persistent for > 7 days, any other grade 3 or 4 toxicity, or any grade 2 or greater toxicity (other than alopecia, fatigue, insomnia, and dry skin) that did not recover before the next cycle of therapy. After the 28 day DLT assessment period, dose reductions were allowed.
The maximum tolerated dose (MTD) was defined as the greatest dose at which ≤1 of 6 subjects experienced a DLT during cycle ≤1. If DLTs were observed in 1 subjects of a 6 subject cohort, but dose reductions in cycles 2–6 were required in ≥3 subjects at that dose level, then the dose was de-escalated by 1mg. If ≤1 subject of that 6 subject cohort experiences a DLT with cycle 1 and ≤2 subjects in that cohort require dose reductions with cycles 2–6, then this was declared the recommended phase 2 dose (RP2D).
Statistical Analysis
All patients receiving at least one dose of study drug were considered evaluable for safety and efficacy. Radiographic restaging with CT scans was performed following cycles 2 and 6, and earlier if clinically indicated. Response to treatment was defined according to the revised International Response Criteria for non-Hodgkin lymphomas, including FDG-PET scans for high-grade histologies and mantle cell lymphoma; PET was not used in the staging of low-grade histologies.[12]
The primary objective of this phase I study was determination of the MTD. Descriptive statistics were used to characterize patients at baseline. Best overall response rate (ORR) is reported and its confidence interval was computed by the method of exact binomial confidence interval. Overall survival (OS) was defined as the time from registration to death, or censored at last known date of survival. Progression-free survival (PFS) was defined as the time from registration to progression or death without evidence of progression. For cases without documentation of progression, follow-up was censored at the date of last disease assessment without progression. Duration of Response (DOR) is defined as the time from initial achievement of response to documented progression. OS, PFS and DOR curves were generated using the Kaplan-Meier method, with 95% confidence intervals calculated using Greenwood’s formula. During dose-escalation, if the true rates of DLTs were 0.1, 0.2, 0.3, 0.4 and 0.5, there would be a probability of dose escalation of 91%, 71%, 49%, 31%, and 17%, respectively.
RESULTS
Thirty patients were enrolled and received at least one dose of study drug and are included in the analysis. The median age was 62. The most common histologies were follicular lymphoma (7), marginal zone lymphoma (7), mantle cell lymphoma (6) and small lymphocytic lymphoma (6). Three patients had diffuse large B-cell lymphoma. The median number of prior regimens was 1, with a maximum of 7 prior therapies. All but one patient had previously received rituximab. Eight patients’ disease was considered rituximab-refractory, defined as progressing during or within 6 months of prior rituximab. Six patients’ disease was chemotherapy refractory, defined as progressing during or within 6 months of prior cytotoxic chemotherapy. One patient had previously undergone autologous stem cell transplantation. No patients had received radioimmunotherapy. Patient characteristics are summarized in table I.
Table I.
Baseline Characteristics
| n | 30 |
|
| |
| Median Age, range | 62 (47–88) |
|
| |
| Gender | |
| Male | 15 (50%) |
| Female | 15 (50%) |
|
| |
| ECOG PS | |
| 0 | 20 (67%) |
| 1 | 8 (27%) |
| 2 | 2 (7%) |
|
| |
| Histology | |
| Follicular lymphoma | 7 (23%) |
| Marginal Zone lymphoma | 7 (23%) |
| Mantle Cell lymphoma | 6 (20%) |
| Small Lymphocytic lymphoma | 6 (20%) |
| Diffuse Large B-cell lymphoma | 3 (10%) |
| Lymphoplasmacytic lymphoma | 1 (3%) |
|
| |
| Median number of prior therapies | 1 (range 1–7) |
|
| |
| Rituximab-refractory | 8 (27%) |
|
| |
| Refractory to prior cytotoxic chemotherapy | 6 (20%) |
|
| |
| Prior rituximab | 29 (97%) |
|
| |
| Prior R-CHOP-like | 15 (50%) |
|
| |
| Prior R-CVP | 9 (30%) |
|
| |
| Prior fludarabine | 4 (13%) |
|
| |
| Prior autologous SCT | 1 (3%) |
R-CHOP=rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R-CVP=rituximab, cyclophosphamide, vincristine and prednisone; SCT=stem cell transplant
Safety
The median number of cycles received was 6 (range 1–6). The most common grade 2–4 adverse events were anemia (63%), neutropenia (60%), thrombocytopenia (30%), and fatigue (27%). Neutropenic fever occurred in only 1 patient, and grade 3–4 infections occurred in 10% of patients. Sixty-seven percent of patients experienced grade 3–4 toxicity during the course of the study. Toxicity is detailed in table II.
Table II.
Toxicity
| Toxicity Type | Total (n=30) | ||
|---|---|---|---|
| Grade | |||
| 2 | 3 | 4 | |
| Percent | Percent | Percent | |
| Anemia | 50 | 10 | 3 |
| Neutropenia | 7 | 23 | 30 |
| Thrombocytopenia | 3 | 13 | 13 |
| Fatigue | 27 | . | . |
| Infection | 7 | 10 | . |
| Rash | 3 | . | . |
| Constipation | 3 | . | . |
| Diarrhea | 3 | . | . |
| Dyspepsia | 3 | . | . |
| Nausea | 3 | . | . |
| Vomiting | 3 | . | . |
| Abdominal discomfort | 3 | . | . |
| Febrile neutropenia | . | 3 | . |
| Elevated Bilirubin | 7 | . | . |
| Hypophosphatemia | 7 | 3 | . |
| Generalized weakness | 3 | . | . |
| Neuropathy | 3 | . | . |
| Headache | 13 | . | . |
| Dyspnea | . | 3 | . |
There were no DLTs at the 1mg and 2mg dose levels. At the 4mg dose level, 1 of 6 patients had a DLT (thrombocytopenia), but the 5 remaining patients all required pre-specified dose reductions with ongoing therapy due to progressive cytopenias. The dose was therefore de-escalated to 3mg, and 6 additional subjects were accrued without a DLT and with only 1 patient requiring a subsequent dose reduction. This was declared the recommended phase 2 dose and 10 additional patients were accrued.
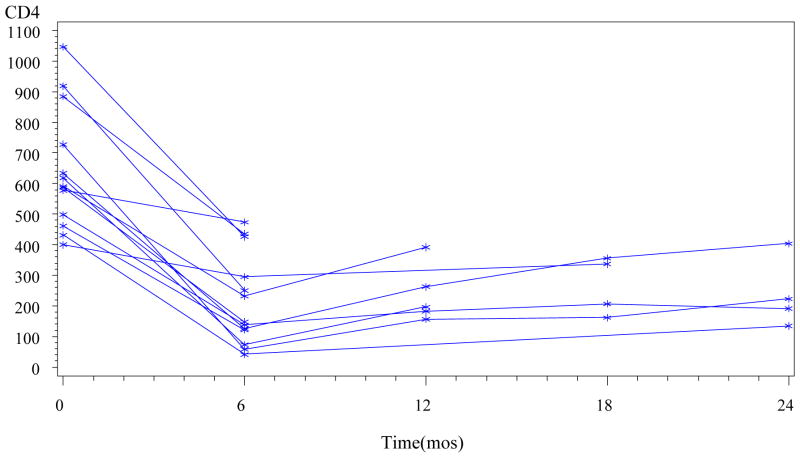
Effect of therapy on T-cell populations was analyzed in 13 patients who completed 6 cycles of therapy for whom T-cell subsets were assessed prior to and at completion of therapy. The CD4 count significantly declined to an average of 34% of baseline after 6 cycles of treatment (p=0.002, Wilcoxon signed-rank test) (figure 1). The CD8 count significantly declined to an average of 55% of baseline (p=0.003, Wilcoxon signed-rank test). Following completion of therapy, serial measurements in patients not undergoing further therapy showed very gradual improvement over time without any patients CD4 counts returning to their baseline value, but at limited follow-up. At 6 months after completion of therapy, T-cell subsets available in 5 patients showed mean CD4 and CD8 counts at 40% and 93% of their baseline values, respectively (figure 1).
Fig. 1.
CD4 counts are graphed in 13 patients who completed 6 cycles of therapy. The 6 month time point denotes end of treatment. Each line denotes one patient.
Efficacy
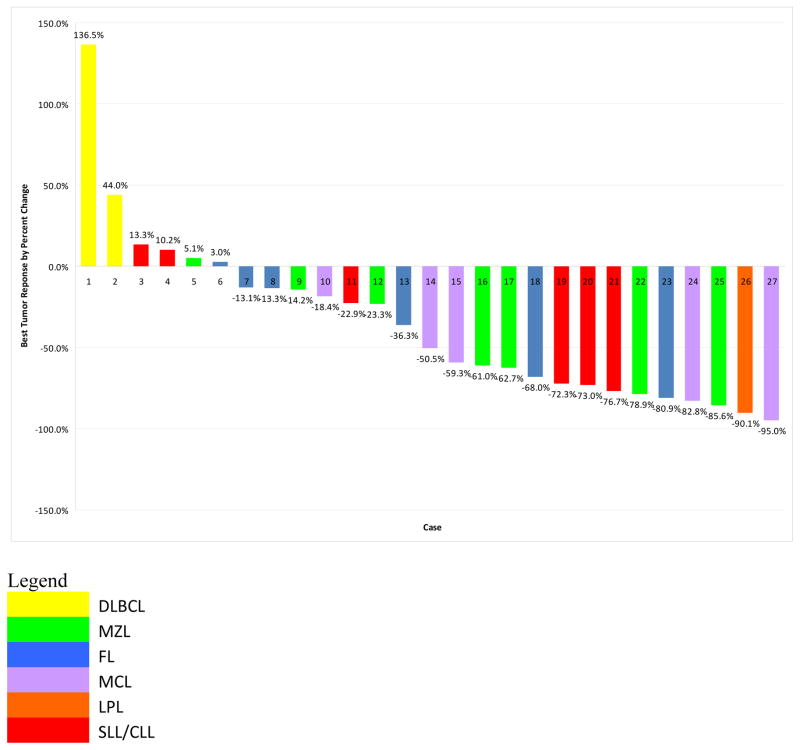
Thirty patients received at least one dose of study drug and are included in the efficacy analysis. Responses are summarized in table III and displayed in figure 2. Seventy percent of patients had a reduction in tumor volume. The overall response rate was 47% (90% CI [31%, 63%]). There were 8 complete responses (27%) and 6 partial responses (20%). Ten patients (33%) had stable disease (SD), and 3 (10%) had progressive disease (PD). Among 16 patients treated at the RP2D, the response rate was 56% with 5 CRs and 4 PRs. Among 21 patients with indolent histologies (FL, MZL, SLL, and LPL) there were 10 responses (48%) including 6 complete responses (29%). Among 6 patients with MCL, there were 4 responses (67%), 2 of which were CRs. Responses by dose level and histology are detailed in tables IV and V. Among the 6 patients with chemorefractory disease, there were 2 PRs. Four patients had previously received prior fludarabine, 2 of whom responded (1 CR and 1 PR). Among 8 patients with rituximab-refractory disease, there were 3 PRs and 1 CR.
Table III.
Response
| Best Response | Frequency | Percent [95% CI] |
|---|---|---|
| OR | 14 | 47 [28–66] |
| CR | 8 | 27 [12–46 |
| PR | 6 | 20 [8–39 |
| SD | 10 | 33 [17–53 |
| PD | 3 | 10 [2–27] |
| Not evaluable for response* | 3 | 10 [2–27] |
Patients not evaluable for response came off study prior to restaging for reasons other than clinical progression
CR=complete response; OR=overall response; PD=progressive disease; PR=partial response; SD=stable disease
Fig. 2.
Waterfall plot depicting best response of evaluable patients. Histology is coded by color. 70% of patients had a reduction in tumor size and the overall response rate was 47%
Table IV.
Response by Dose Level
| Dose level | n | Responses | Response detail |
|---|---|---|---|
| 1mg | 3 | 0 | 0 |
| 2mg | 3 | 1 | 1 PR (MZL) |
| 3mg | 16 | 9 | 5 CR (1 SLL, 2 MZL, 2 MCL) 4 PR (2 SLL, 1 FL, 1 MCL) |
| 4mg | 6 | 4 | 3 CR (LPL, FL, MZL) 1 PR (MCL) |
CR=complete response; FL=follicular lymphoma; LPL=lymphoplasmacytic lymphoma; MCL=mantle cell lymphoma; MZL=marginal zone lymphoma; OR=overall response; PR=partial response; SLL=small lymphocytic lymphoma
Table V.
Response by Histology
| Histology | n | Responses |
|---|---|---|
| Follicular | 7 | 2 |
| Marginal Zone | 7 | 4 |
| Mantle Cell | 6 | 4 |
| Small Lymphocytic | 6 | 3 |
| Diffuse Large B-cell | 3 | 0 |
| Lymphoplasmacytic | 1 | 1 |
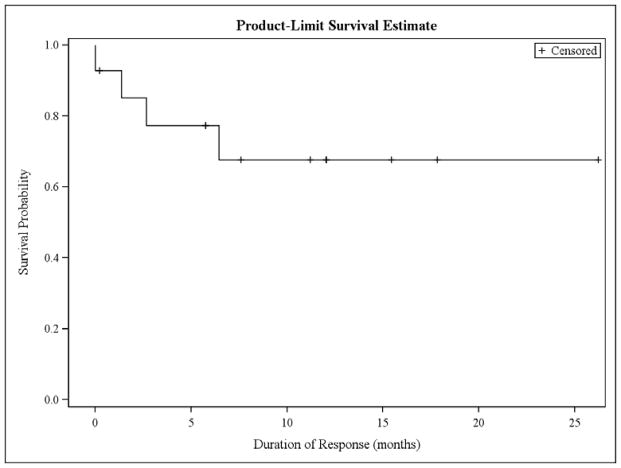
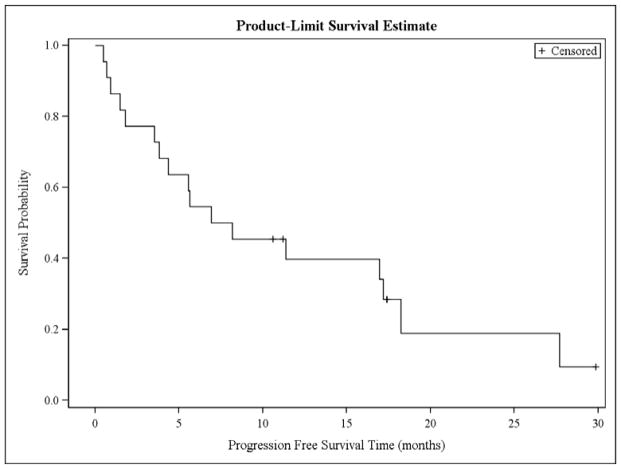
Among responders, the median DOR has not been reached with 4 of 14 patients having progressed (figure 3). At 17 months, 68% of responders remain without progression (90% CI [45%, 90%]) (figure 4). Among responding patients with indolent histologies, the median DOR has not been reached at 17 months with 86% of responders remaining free from progression (90%CI [64%, 91%]). Among 4 responders with MCL, the median DOR was 6.2 months with 1 of 4 patients remaining free from progression (90%CI [0%, 61%]). The median PFS for all patients was 8 months (90% CI [4, 17]). At 17 months, the PFS was 34% (90% CI [17%, 51%]). Among 30 treated patients, 12 have died. The median OS was 31 months (90% CI [19-NA]), and the 17 month OS was 75% (90% CI [62%, 89%]). Causes of death were lymphoma in 9 patients, infection in 2 patients (both occurring after patients were removed from protocol and received additional therapies), and unknown in 1 patient.
Fig. 3.
Duration of response for patients achieving a complete or partial response. The median DOR has not been reached
Fig. 4.
Progression-free survival for all patients. The median PFS is 8 months and 34% of patients remain progression-free at 17 months
DISCUSSION
We report results from a phase 1 study of oral clofarabine and find the dose of 3mg daily on days 1–21 of a 28 day cycle to be well tolerated and associated with an encouraging response rate of 56% in relapsed/refractory indolent B-cell NHL and MCL patients treated at the RP2D. The median PFS is 8 months, but among responders, the DOR has not yet been reached. The primary toxicities were hematologic, with delayed cytopenias making the higher dose level of 4mg untenable.
This is the first report of oral clofarabine in non-Hodgkin lymphomas. Two prior studies have assessed the intravenous formulation. The dose of 4mg/m2 IV for 5 days every 28 days was found to be the MTD, producing an ORR of 47% and median DOR of 7 months.[11] Responders included patients with DLBCL, FL, SLL, MZL and peripheral T-cell lymphoma. The primary toxicities were hematologic. A second study of IV clofarabine in NHL evaluated a tenfold higher dose of 40mg/m2, but this led to prolonged myelosuppression in the first 6 patients, prompting early closure of the study.[10]
Nucleoside analogues have been widely used in relapsed/refractory lymphoma. Intravenous fludarabine monotherapy produces response rates of 30–60% in relapsed indolent lymphomas, with increased response rates observed when combined with rituximab.[14–16] Intravenous cladribine produces response rates of 30–45%, also increased when combined with rituximab.[17–19] While the majority of chemotherapy agents employed for relapsed/refractory NHL are administered intravenously, patients may prefer oral agents provided they offer similar therapeutic efficacy.[20] An oral formulation of fludarabine was reported to show an overall response rate of 65% in a phase 2 trial in relapsed indolent B-cell NHL, though 70% of patients experienced grade 3 or 4 neutropenia.[21]
Most of the patients in our study had indolent histologies and MCL. The DOR was encouraging in low-grade diseases with the median having not yet been reached, but was shorter in MCL patients with a median of 6 months, consistent with results of other novel agents in this setting,[22–24] though the number of MCL patients in this phase 1 study is small. Only 3 patients in this study had DLBCL, and none were treated at the RP2D, so we cannot draw inferences as to efficacy in this population; however, the low-dose prolonged dosing strategy and activity of this agent in non-dividing cells are likely to be more applicable to diseases with low-grade kinetics. Of note, though patients in our study received up to 7 prior lines of therapy, the median number of prior therapies was 1, and only 1 patient had a prior autologous stem cell transplant, so the majority of patients were not heavily pre-treated, and are therefore likelier to demonstrate chemosensitive disease. Despite this, we did see responses in rituximab-refractory and chemotherapy-refractory lymphomas, though less so in the latter group. Cytopenias were the most common toxicity. We also observed a significant decline in CD4 counts in patients on study with only gradual improvement that did not normalize to baseline values during the course of the study. Though no opportunistic infections were observed, this warrants attention in future studies.
In summary, in this phase 1 study, oral clofarabine at 3mg daily on days 1–21 of a 28 day cycle was well-tolerated and exhibited preliminary evidence of efficacy in relapsed/refractory indolent B-cell lymphomas. A phase 2 study of oral clofarabine in relapsed/refractory indolent lymphomas and mantle cell lymphoma is ongoing. Potential future directions include incorporating oral clofarabine in combination chemoimmunotherapy strategies as well as evaluation as a component of upfront therapy, particularly in elderly or frail patients who are not good candidates for intravenous treatment approaches.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Lenz G, Hiddemann W, Dreyling M. The role of fludarabine in the treatment of follicular and mantle cell lymphoma. Cancer. 2004;101:883–893. doi: 10.1002/cncr.20483. [DOI] [PubMed] [Google Scholar]
- 3.Sigal DS, Miller HJ, Schram ED, Saven A. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010;116:2884–2896. doi: 10.1182/blood-2010-02-246140. [DOI] [PubMed] [Google Scholar]
- 4.Parker WB, Shaddix SC, Chang CH, et al. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5′-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- 5.Xie KC, Plunkett W. Deoxynucleotide pool depletion and sustained inhibition of ribonucleotide reductase and DNA synthesis after treatment of human lymphoblastoid cells with 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl) adenine. Cancer Res. 1996;56:3030–3037. [PubMed] [Google Scholar]
- 6.Lotfi K, Mansson E, Spasokoukotskaja T, et al. Biochemical pharmacology and resistance to 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine, a novel analogue of cladribine in human leukemic cells. Clin Cancer Res. 1999;5:2438–2444. [PubMed] [Google Scholar]
- 7.Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 8.Carson DA, Wasson DB, Esparza LM, Carrera CJ, Kipps TJ, Cottam HB. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine. Proc Natl Acad Sci U S A. 1992;89:2970–2974. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genini D, Adachi S, Chao Q, et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96:3537–3543. [PubMed] [Google Scholar]
- 10.Blum KA, Hamadani M, Phillips GS, et al. Prolonged myelosuppression with clofarabine in the treatment of patients with relapsed or refractory, aggressive non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50:349–356. doi: 10.1080/10428190902730227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabhan C, Davis N, Bitran JD, et al. Efficacy and safety of clofarabine in relapsed and/or refractory non-Hodgkin lymphoma, including rituximab-refractory patients. Cancer. 2011;117:1490–1497. doi: 10.1002/cncr.25603. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 14.Redman JR, Cabanillas F, Velasquez WS, et al. Phase II trial of fludarabine phosphate in lymphoma: an effective new agent in low-grade lymphoma. J Clin Oncol. 1992;10:790–794. doi: 10.1200/JCO.1992.10.5.790. [DOI] [PubMed] [Google Scholar]
- 15.Hochster HS, Kim KM, Green MD, et al. Activity of fludarabine in previously treated non-Hodgkin’s low-grade lymphoma: results of an Eastern Cooperative Oncology Group study. J Clin Oncol. 1992;10:28–32. doi: 10.1200/JCO.1992.10.1.28. [DOI] [PubMed] [Google Scholar]
- 16.Czuczman MS, Koryzna A, Mohr A, et al. Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol. 2005;23:694–704. doi: 10.1200/JCO.2005.02.172. [DOI] [PubMed] [Google Scholar]
- 17.Tulpule A, Schiller G, Harvey-Buchanan LA, et al. Cladribine in the treatment of advanced relapsed or refractory low and intermediate grade non-Hodgkin’s lymphoma. Cancer. 1998;83:2370–2376. doi: 10.1002/(sici)1097-0142(19981201)83:11<2370::aid-cncr17>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Kong LR, Huang CF, Hakimian D, et al. Long term follow-up and late complications of 2-chlorodeoxyadenosine in previously treated, advanced, indolent non-Hodgkin’s lymphoma. Cancer. 1998;82:957–964. [PubMed] [Google Scholar]
- 19.Nagai H, Ogura M, Kusumoto S, et al. Cladribine combined with rituximab (R-2-CdA) therapy is an effective salvage therapy in relapsed or refractory indolent B-cell non-Hodgkin lymphoma. Eur J Haematol. 2011;86:117–123. doi: 10.1111/j.1600-0609.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15:110–115. doi: 10.1200/JCO.1997.15.1.110. [DOI] [PubMed] [Google Scholar]
- 21.Tobinai K, Watanabe T, Ogura M, et al. Phase II study of oral fludarabine phosphate in relapsed indolent B-Cell non-Hodgkin’s lymphoma. J Clin Oncol. 2006;24:174–180. doi: 10.1200/JCO.2005.03.9313. [DOI] [PubMed] [Google Scholar]
- 22.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 24.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]