Abstract
Background/Purpose
Plantar fasciitis (PF), a common condition affecting physically active individuals, is typically treated with orthotics, two to four months of stretching programs, and/or surgery. Primal Reflex Release Technique™ (PRRT) is thought to reduce over‐arousal of the nervous system through down‐regulation of the primal reflexes. The technique has been suggested as a novel treatment method for patients suffering from PF. The purpose of this case series was to examine the effects of PRRT on patients with PF.
Description of Cases
The PRRT technique was applied in eight consecutive cases of PF in physically active subjects. The Numeric Pain Rating Scale, the Disability in the Physically Active (DPA) Scale, and the Patient Specific Functional Scale (PSFS) were administered to identify patient‐reported pain and dysfunction.
Outcomes
Primal Reflex Release Technique (PRRT) was an effective treatment for subjects with either acute or chronic PF. The use of the PRRT treatment resulted in an average reduction in plantar fascia pain across all subjects that was both statistically significant and clinically following a single treatment. Statistically and clinically significant improvements on averaged measures of function, such as the DPA Scale and PSFS, were also found over the course of treatment.
Discussion
In this case series, the use of PRRT produced positive changes in terms of improvements in reported pain and dysfunction and a shorter time to resolution, when compared to traditional treatment methods for PF reported in the literature. Subjects who undergo PRRT treatment for both acute and chronic PF may experience reduction in pain and improvement of function that exceeds what is experienced in traditional conservative therapy programs found in the available literature. Clinicians should consider the regional interdependence model in order to identify underlying related factors when evaluating and treating PF. The autonomic nervous system may play a role in the perception of pain and should be addressed during treatment.
Level of Evidence
Level 4 – case series
Keywords: Autonomic nervous system, primal reflex, regional interdependence, up‐regulation.
Background and Purpose
Plantar fasciitis (PF) is a condition that results in one million medical visits each year, with primary care physicians seeing 62% of all patients who seek medical care for PF.1 Factors that have been linked to PF include age, increased body mass index, decreased ankle dorsiflexion, prolonged periods of weight bearing, pes planus foot type, and increased metatarsal pressure and forefoot pronation during gait.2–4 Runners commonly experience PF, with incidence rates ranging from 4.5%‐10%.5,6
Traditionally, diagnosis of PF is made following a detailed history combined with clinical evaluation.7,8 Primary symptoms include: descriptions of throbbing, piercing, or stabbing pain; inferior heel pain when bearing weight, especially in the morning or after inactivity; pain that improves after brief activity but worsens with prolonged activity.7,8 The pain is often insidious in nature, occurring without report of a direct mechanism of injury.9 The primary physical exam finding is tenderness to palpation at the medial calcaneal tubercle,7,8 while some researchers suggest limitations in dorsiflexion10 and strength (i.e., plantarflexion, toe flexion)10,11 are also present in individuals with PF. Neurological findings during an exam point towards a differential diagnosis of tarsal tunnel syndrome or abductor digiti quinti nerve entrapment.7,8 The transition from an acute PF diagnosis to a chronic PF is traditionally made when the symptoms have been present for a minimum of ten months.12,13 When diagnosing PF, it is important to consider both the insidious onset and the possibility of spontaneous recovery with the condition. Cases of PF have recovered without surgical intervention within 10‐11 months of onset.14
Regional interdependence relates to the concept that dysfunction in one area or system of the body may result in perceived pain or deficiency in another region of the body.15,16 One system of the body that may contribute to plantar fascia pain is the nervous system and its associated network of reflexes. Overstimulation of the nervous system can result in pain and dysfunction.17,18 Primal reflexes control unlearned movement patterns and are triggered as protective defense mechanisms for the body.19 The withdrawal reflex and the startle reflex are two examples of primal reflexes.20 When an individual goes through the fight, flight or freeze response, the muscles reflexively tense in preparation for the response.21 Following activation of primal reflexes, pain may be produced through “up‐regulation”, a sustained period of heightened arousal of the nervous system.22
Primal Reflex Release Technique™ (PRRT) is a treatment paradigm that falls under the regional interdependent approach to patient care and involves down‐regulating an overstimulated autonomic nervous system in order to reduce patterns of pain.18 The paradigm is designed to address the neural system by resetting (recalibrating) hyper‐aroused primal reflexes within the body.22 Sensitized areas are located using bilateral palpation during a one‐minute nociceptive evaluation.23 If, however, a specific condition is the problem of interest, the clinician can follow a specific PRRT procedure as treatment. The treatment involves providing 12 seconds of light, swift sensation in the form of repetitive deep tendon reflexes (DTR) that tap or stimulate the skin to inhibit painful areas.23,24 These reflex stimulations are generally performed lightly (as to not initiate a pain response) with several repetitions. A potential explanatory theory is that these repetitive reflex stimulations send many impulses to the spinal cord, which may cause the spinal cord and brain to temporarily “overload” and “reset”. When this happens, the brain may evaluate the situation and determine the current circumstances. If there was no actual current pathology or illness, rather only a faulty neurological circuit, the brain will clear the faulty pattern. This mechanism is similar to what may be happening in the gate theory of pain control.
Designed for use with both acute and chronic conditions, the developers of PRRT created a protocol for patients with neuromuscular dysfunction presenting as PF. The PRRT treatment for PF is based on the previously discussed foundational theory and requires the clinician to address neurological adaptations that may cause pain or may arise as a result of repetitive stress to the nervous system. A five‐step process for evaluating and treating plantar fasciitis has been identified. The steps involve the neuromuscular “resetting” of five areas, including the: sacroiliac joint (through the hip adductors), peroneal tendons, triceps surae complex, hamstring musculature, and the toe flexors.25
The purpose of this case series was to examine the effects of PRRT on PF in active individuals. Research questions included: (a) Does a single bout of PRRT reduce pain on the Numerical Pain Rating Scale (NPRS) in subjects with PF? (b) Does PRRT improve scores on the NPRS, Patient‐Specific Functional Scale (PSFS) and the Disablement in the Physically Active (DPA) Scale from initial visit to discharge? (c) Do subjects report continued resolution of the symptoms at 2 week, 1 month, and 2 month follow‐ups?
Description of Cases: Subject History and Systems Review
A total of eight physically active subjects (4 cross country athletes, 2 track athletes, 1 lacrosse athlete, and 1 university employee) ranging in age from 18‐40 years (mean=22.22 ± 6.76 years) presented to the athletic training clinic with complaints of plantar fascia pain (Table 1). The current study of subjects presenting with plantar fascia pain features an a priori design. All subjects were evaluated in the same manner to determine eligibility for inclusion. Outcome measures were collected for all subjects enrolled in the study. The PRRT treatment protocol was identical for all subjects. No other intervention (e.g., stretching, change in footwear) was applied and no activity modifications were imposed. Each subject gave informed consent to the use of data concerning his/her case for publication. Subject confidentiality was protected according to the United States’ Health Insurance Portability and Accountability Act (HIPAA).
Table 1.
Demographic information for patients with plantar pain. Onset is listed as acute or chronic with duration of symptoms prior to initiation of treatment listed in parentheses
| ID | Age | Gender | Sport | Unilateral or Bilateral | Onset (duration of symptoms) |
|---|---|---|---|---|---|
| 1 | 19 | Female | Cross Country | Unilateral | Chronic (1 year) |
| 2 | 19 | Female | Cross Country | Unilateral | Chronic (2 years) |
| 3 | 21 | Female | Lacrosse | Unilateral | Acute (2 months) |
| 4 | 21 | Female | Track and Field | Bilateral | Acute (2.5 months – L; 2 days – R) |
| 5 | 40 | Male | Recreational | Unilateral | Chronic (1 year) |
| 6 | 20 | Female | Track and Field | Unilateral | Acute (1 week) |
| 7 | 21 | Female | Cross Country | Unilateral | Acute (2 days) |
| 8* | 18 | Female | Cross Country | Unilateral | Acute (1 day) |
Patient presented with lateral plantar pain and was excluded from the study. No patient‐outcomes measures were collected for this patient and she was not included in statistical analysis beyond basic demographics.
Clinical Impression #1
Subjects were included in the study if they presented with: pain or tenderness in the medial arch and at the insertion of the plantar fascia at the medial tubercle of the calcaneus; pain with walking or dorsiflexion of the toes, especially in the morning. Subjects were excluded if they had known neurological impingement, cancer, a history of recent fracture, or acute lower extremity surgery. One subject presenting with plantar foot pain was excluded due to pain located along the lateral border of the foot, leaving seven subjects to be included in the study.
Examination
Evaluation included an extensive history relating to pain location, intensity, frequency/duration, and prior ankle sprains (Table 2). All subjects (N = 7) presented with plantar fascia pain that was either acute (N=5) or chronic (N=3) in nature (subject #4 had bilateral pain). For the purposes of this study, chronic PF was defined as having minimum symptom duration of 10 months,8 with acute PF symptoms lasting less than 10 months.
Table 2.
Patient evaluation information.
| ID | Location of Pain | Description of Pain | MOI | Onset | Prior PF History | Activities that Worsen Pain | Activities that Improve pain | Medications |
|---|---|---|---|---|---|---|---|---|
| 1 | Medial Arch | Aching | Running | Chronic | Yes | Pushing off | Rest | None |
| 2 | Heel | Sharp | Overuse | Chronic | Yes | Balancing; single leg activities | Massage | None |
| 3 | Medial Arch | Sharp | Overuse | Acute | No | Pounding activities | Ice, stretching | Medrol; Ibuprofen |
| 4a (left) | Medial Arch | Stinging | Cast Removal | Acute | No | Walking in flats; pushing off; weight shift | Nothing helps | None |
| 4b (right) | Medial Arch | Sharp | Running | Acute | No | Running | Rest | None |
| 5 | Heel | Aching | Changing Shoes | Chronic | No | Running; standing | Stretching | OTC NSAIDs |
| 6 | Medial Arch | Aching | Changing Shoes | Acute | Yes | Stairs; walking | Rest | None |
| 7 | Medial Arch | Sharp | Running | Acute | Yes | Walking; running; sprinting | Rest, ice | None |
MOI = mechanism of injury; PF = plantar fascitits; OTC NSAIDs = over‐the‐counter non‐steroidal anti‐inflammatories.
Clinical Impression #2
The NPRS was administered pre‐ and post‐treatment (all sessions) and at discharge. The DPA Scale and the PSFS were also administered at initial evaluation and discharge. Following evaluation and collection of initial outcomes measures, the subject was treated with the PRRT treatment for PF described below. Subjects were discharged after reporting being symptom‐free for two weeks. Total number of treatments and days to resolution were tracked for each subject. The primary investigator followed‐up with subjects at two weeks, one month, and two months after discharge.
Intervention
Outcome Measures
Outcome measures are necessary to determine treatment efficacy. The outcome measures chosen should allow clinicians to calculate a minimal clinically important difference (MCID) for their results. The Numeric Pain Rating Scale (NPRS) is a method for patients to rate pain on a scale from “no pain” (score of zero) to “worst possible pain” (score of 10). Scores on the NPRS can be collected for “current” pain, “best” pain, “worst” pain, or a cumulative score where these three values are averaged to get a 24 hour general pain rating.26 The Disablement in the Physically Active (DPA) Scale, geared towards the physically active, includes questions relating to health‐related quality of life, impairment, functional limitations, and disabilities. The DPA Scale is completed by the patient and ranges in scores from 0 to 64.27 On the Patient Specific Functional Scale (PSFS), patients are asked to identify up to five difficult activities that are associated with their condition and then rate the activities on a scale of difficulty from “unable to perform activity” (score of zero) to “able to perform activity at the same level as before injury” (score of 10). Scores for the PSFS may be analyzed for a single activity or as an average score for all of the activities recorded.28 For the NPRS, an MCID has been established as a two‐point change.29,30 An MCID of six points on the DPA Scale has been established for patients with chronic conditions and nine points for patients with acute conditions.27 The MCID of the PSFS is condition dependent and ranges from two points for an average score to three points for a single activity score.28
Treatment Procedure
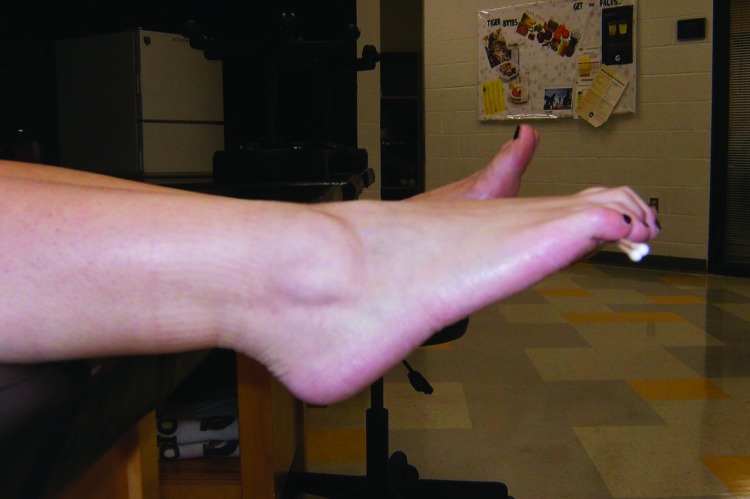
The first four steps of the PRRT treatment required the subject to maintain a specific position while a 12‐second application of DTR stimulation was performed in each of the four areas. The first location involved applying the stimulation above and below the medial knee (Figure 1), while the second location was at the peroneal tendons with the subject holding the foot in eversion (Figure 2). The third and fourth sites involved releasing tension in the gastrocnemius and hamstring muscles, respectively (Figures 3, 4). For the gastrocnemius, the stimulation was applied simultaneously over the patellar tendon and anterior tibialis tendon while the hamstring release involved stimulation to the patella tendon and hamstring muscle belly. The final step of the treatment required the subject to grip two cotton‐tipped applicators with the toes while performing sustained maximal plantarflexion of the ankle (Figure 5). The subject was instructed to maintain the position of toe flexion and ankle plantarflexion even if any cramping sensations occurred. Once the cramp was eliminated, the subject was instructed to keep the toes curled but bring the ankle out of plantarflexion and into a neutral position. If no cramping sensation occurred, the “toe curl” was discontinued after one minute. The “toe curl” portion was performed twice (up to two minutes), while the other four segments were performed once for 12‐seconds each. The entire treatment lasted approximately one minute for the first four steps and two to four minutes for the fifth step (no more than five minutes overall). Following the full PRRT treatment for PF, subjects were asked to get up from the plinth and walk around. The NPRS was repeated at this point in time.
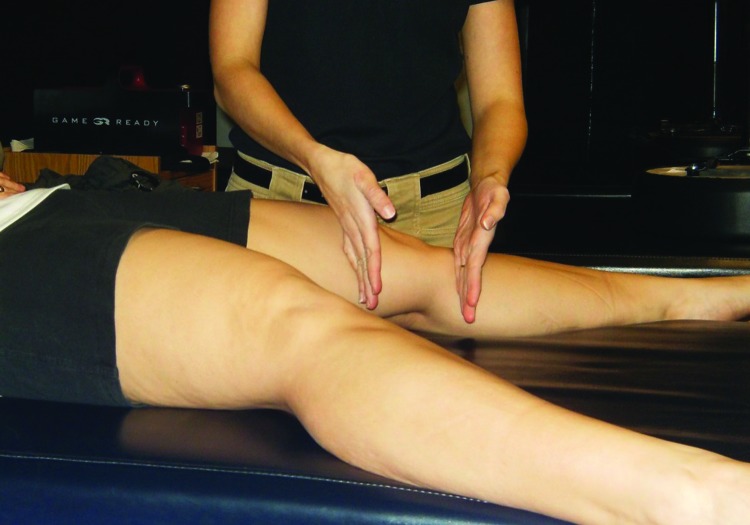
Figure 1.
Medial knee/SI joint reset. Stimulation is applied above and below the medial knee.
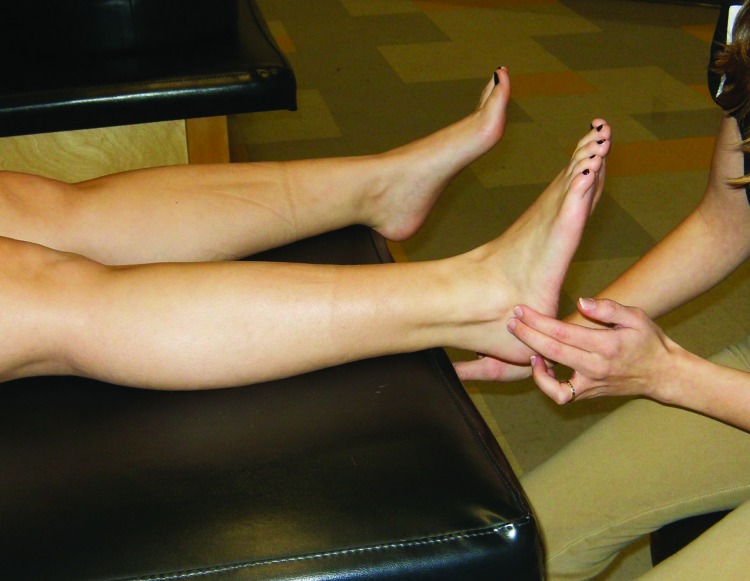
Figure 2.
Peroneal tendon reset. Patient actively holds ankle in eversion while stimulation is applied along the distal peroneal tendons.
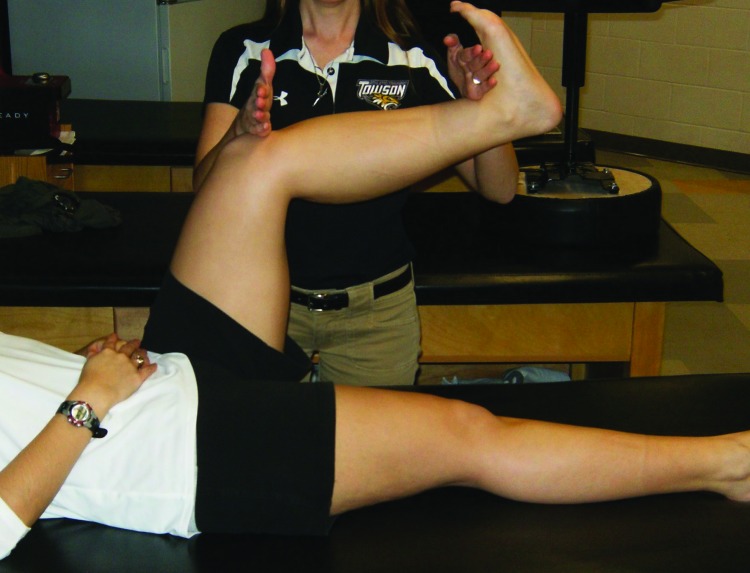
Figure 3.
Gastrocnemius reset. Patient maintains hip and knee flexion and ankle dorsiflexion while the clinician applies stimulation to the patella tendon and ankle dorsiflexors.
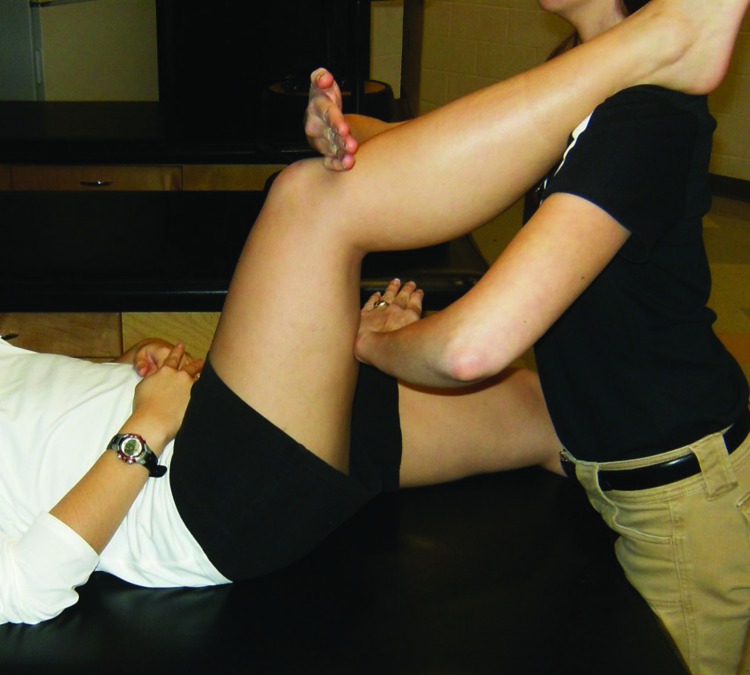
Figure 4.
Hamstring reset. Patient rests foot on clinician's shoulder while stimulation is applied to the patella tendon and hamstring muscle belly.
Figure 5.
Toe curl. Patient holds two cotton‐tipped applicators in the toes while plantarflexing the ankle.
Outcomes
Following initial assessment that included seven subjects (total of 8 feet), two subjects were unable to meet follow‐up assessment expectations due to scheduling conflicts and were removed from study. A total of five subjects (6 feet) were willing to participate in the study beyond the initial assessment and treatment session (Table 3). For the five subjects (6 feet) who continued the study beyond initial assessment, the average number of treatments was 3.33 ± 1.97 with 14.83 ± 17.7 days to resolution.
Table 3.
Patient discharge and follow‐up information.
| ID | Total Treatments | Days to Resolution | 2 Week Follow‐up | 1 Month Follow‐up | 2 Month Follow‐up |
|---|---|---|---|---|---|
| 1 | 4 | 17 | Pain‐free | Episode(s) | Episode(s)* |
| 2 | 4 | 6 | Pain‐free | Pain‐free | Pain‐free |
| 4a | 6 | 48 | Pain‐free | Pain‐free | Episode(s) |
| 4b | 4 | 16 | Pain‐free | Pain‐free | Pain‐free |
| 6 | 1 | 1 | Pain‐free | Pain‐free | Pain‐free |
| 7 | 1 | 1 | Pain‐free | Pain‐free | Pain‐free |
Patient was discharged at two weeks pain‐free. Two‐week follow‐up was conducted two weeks post‐discharge (four weeks after last treatment session). The asterisk indicates that this patient's episode listed at 2‐month follow‐up was the same episode listed during the 1‐month follow‐up (i.e. – patient was pain‐free at two month follow‐up, but had had a recurrence during the first month of that two month period).
Numerical Pain Rating Scale
Immediate Effects
A paired t‐test was used to analyze the immediate pre‐post treatment effect of PRRT on that subject's current pain rating on the NPRS. A statistically significant difference between the current NPRS score pre‐treatment (mean=3.25 ± 1.39 points) for the 8 evaluated feet and the current NPRS score post‐treatment (mean=1.19 ± 0.998 points) was found (p≤0.002; 95% CI: 1.00, 3.12) (Table 4). The Cohen's d value of 1.48 suggests a large effect size from the treatment,31 while the mean change (2.06 ± 1.27) suggests that the treatment was effective enough to produce a MCID on the NPRS in one visit.29,30
Table 4.
Change in NPRS scores at initial evaluation and from initial evaluation to discharge.
| Mean difference | p‐value | Confidence Interval | Cohen's D | |
|---|---|---|---|---|
| Initial Current Pre to Current Post | 2.06 (1.27) | 0.002* | 1.00, 3.12 | 1.48 |
| Initial Average to Discharge Average | 3.00 (1.4) | 0.003∞ | 1.53, 4.47 | 2.14 |
Results of paired t‐tests for NPRS scores.
Significant differences between NPRS current score pre‐treatment and NPRS current score post‐treatment.
Significant differences between NPRS average score at initial evaluation and NPRS average score at discharge. Results presented as mean (standard deviation).
Discharge and Follow‐up
To assess the effectiveness of the PRRT treatment to address pain from initial exam to discharge for the five remaining subjects (for a total of six feet), a paired t‐test was performed on the average NPRS scores reported during initial and discharge exam. A statistically significant difference was found between initial exam average NPRS scores (mean=3.00 ± 1.4 points) and discharge exam average NPRS scores (mean=0 ± 0 points) (p≤0.003; 95% CI: 1.53, 4.47). The Cohen's d value of 2.14 suggests a large effect size, while the mean change (3.00 ± 1.40) suggests that the treatment was effective enough to produce a MCID on the NPRS for the average reported pain (Table 4). Additionally, all subjects who were included beyond the initial evaluation (for a total of six feet) were discharged pain free and continued to have a full resolution of pain at two‐week follow‐up (Table 3).
Disablement in the Physically Active (DPA) Scale
A paired t‐test was used to analyze the change in score of the DPA Scale from initial exam to discharge. The DPA Scale score at discharge (mean=1.20 ± 2.68 points) was significantly lower than initial DPA Scale score (mean=23.0 ± 12.02 points) (p≤0.012; CI 8.10, 35.5), as shown in Table 5. The mean 21.8 point reduction in DPAS score surpassed the established MCID in the literature27 and each subject experienced a reduction that satisfied the MCID. The Cohen's d effect size (d = 1.8) suggested a high level of practical significance for this change. Additionally, all subjects reported a DPA Scale score at discharge that was within the range expected for healthy, asymptomatic individuals.27
Table 5.
Changes in functional outcomes measures from initial visit to discharge.
| Initial | Discharge | Mean Difference | Significance | Confidence Interval | Cohen's D | |
|---|---|---|---|---|---|---|
| PSFS* | 4.95 (1.67) | 9.71 (0.59) | −4.76 (1.45)* | 0.002 | −6.56, −2.97 | −2.85 |
| DPAS | 23.0 (12.02) | 1.20 (2.68) | 21.8 (11.03) | 0.012 | 8.10, 35.5 | 1.81 |
Results presented as mean (standard deviation).
Note that for the PSFS, a negative number reflects a positive change (i.e., patient improvement). Outcomes measures utilized were the Patient Specific Functional Scale (PSFS) and the Disability in the Physically Active Scale (DPAS).
Patient Specific Functional Scale
A paired t‐test was used to analyze the change in the PSFS score during initial exam and discharge exam. The analysis revealed the PSFS score at discharge (mean=9.71 ± 0.59 points) was significantly better than the initial PSFS score (mean=4.95 ± 1.67 points) (p≤0.002; CI: ‐6.56, ‐2.97), as shown in Table 5. The Cohen's d effect size (d=2.9) suggested a high level of practical significance and the mean change value exceeded the required MCID value for the PSFS.28 Additionally, each subject reported a change large enough to indicate an MCID was experienced.
Discussion
Among subjects with PF (N = 8 feet), a single, initial treatment of PRRT resulted in immediate improvement on the NPRS. The NPRS improvement was both statistically significant and clinically meaningful, with a large effect size. The results indicate that performing a single treatment of PRRT led to a meaningful, immediate reduction in pain. Of note, the subjects in this study had lower initial pain scores compared to the available literature (average of 3.0 on the NPRS versus 6.2‐6.6 on the Visual Analog Scale,32 respectively). One possible reason is that many of the available studies4,32,33 contain middle‐aged, often sedentary subjects while the majority (6/7) of the subjects in this study were otherwise healthy, active, Division I athletes. In addition to improvements in pain, the PRRT technique also improved scores on the PSFS and the DPA Scale from initial visit to discharge (N=6 feet). The subjects (N=6 feet) who completed multiple sessions of PRRT experienced complete resolution of pain on the NPRS at discharge.
The technique also appeared to have long‐lasting results for a majority of subjects without any continued intervention. At the two‐week follow‐up, 100% of the subjects remained pain‐free and did not report any return of their symptoms. At the one month follow‐up, 83% of the subjects were pain‐free, as one subject (ID #1) reported minor episodic pain with running. At the two‐month follow‐up, 83% of the subjects were pain‐free; one subject (ID #4a) experienced a return of minor episodic pain while running, while the other subject (ID #1) who reported pain at the 1 month follow‐up had not experienced any new pain episodes from the previous follow‐up.
The effectiveness of traditional treatments to reduce pain and improve function in PF patients is conflicting and depends on the specific intervention chosen (e.g., stretching or night splints). Other researchers using the pain sub‐scale of the Foot Function Index (FFI) identified that both plantar fascia specific stretching (PFSS) and Achilles tendon stretching reduced overall pain after eight weeks.12 While there was no difference between groups in overall pain reduction, the PFSS reduced pain “at its worst” and with “first steps in the morning” to a significantly greater degree than the Achilles tendon stretching program.12 At two year follow‐up, the subjects in both groups reported a continued reduction in pain on the FFI pain sub‐scale.33 Both calcaneal taping and stretching programs reduced pain according to a Visual Analog Scale (VAS), with calcaneal taping providing a significantly higher pain reduction than a stretching program after one week.34 While the reported studies improved pain scores, pain was not reported to be eliminated completely. Likewise, studies examining function have shown mixed results after conservative treatment. Achilles stretching and PFSS stretching together did not reduce scores on the PSFS at one week follow‐up.34 Lee, McKeon, & Hertel35 revealed through meta‐analysis that orthoses improve foot function in as little as six weeks and that improvements are maintained after 12 weeks. The use of night splints is not as effective as orthoses after 12 week follow‐up.35
Although no peer‐reviewed articles specific to plantar fasciitis were found, PRRT has been reported to positively affect pain and function in other areas of the body. Carnahan reported using PRRT to eliminate shoulder pain and improve strength in five physical therapy visits.36 In this unpublished case study, a sedentary, middle‐aged male with chronic shoulder pain was treated using PRRT in conjunction with shoulder range of motion and strengthening exercises.36 The PRRT system has also been used to successfully treat chronic conditions. McKeon's unpublished dissertation focused on a subject suffering from nine months of gastrointestinal and body pain. For this subject, five treatments of PRRT eliminated all pain and the subject returned to full function after nine visits.24 The subject was able to maintain her pain‐free, full function condition at 22‐month follow‐up.24
Currently, the established stretching programs for treatment of PF are commonly prescribed for eight weeks to four months in duration.12,37‐39 The subjects in the current study needed only 3.33 visits (average 14.83 days) to discharge. Likewise, in the current study, subjects reported both a statistically significant and clinically meaningful improvement on the NPRS immediately after the initial treatment of PRRT.29,30 Additionally, all subjects reported elimination of pain at discharge as well as improved function. Both the DPAS and PSFS revealed statistically significant and clinically meaningful improvements from baseline to discharge.27,28
Limitations and Future Research
Limitations of this study include lack of a control group or comparison group. The lack of a control group in combination with the potential for spontaneous resolution of PF may be a contributing factor in the favorable outcomes demonstrated in this group of active, generally healthy subjects. Also, this study included a small sample size and relatively specific patient population. Post‐hoc power analysis revealed a power of 0.95 for the immediate changes and a power of 0.98 at discharge, suggesting that the sample size may have been appropriate given the large effect sizes. Moreover, as with any study examining humans, non‐compliance from subjects can be problematic. The immediate effectiveness of the PRRT intervention in these subjects led a few to miss scheduled treatment sessions and these cases took longer to resolve. Thus, the authors hypothesize the number of days until resolution could have been reduced for the subjects (Patient ID #4) who missed treatment sessions (Table 3). Despite these subject issues, the results indicate an effective treatment across all subjects in this study.
Future studies should include more large scale multi‐site study of this PRRT technique to treat apparent PF. Also, cohort studies comparing PRRT versus other treatment interventions in both acute PF and chronic PF subjects would be helpful in determining the effectiveness of the technique. Additional research is also necessary to determine the effectiveness of PRRT on treating other conditions and determining the longevity of a single treatment, as well as the cumulative effects of multiple PRRT treatments. Future research specific to PRRT should address the components of the PRRT treatment in further detail (e.g., must all components of the treatment be applied, does the order of application matter).
Conclusions
The present case series is the first to consider the use of PRRT for the treatment of PF. In this case series, the use of PRRT produced both immediate and long‐term positive changes on patient reported outcome measures including the NPRS, PSFS, and DPA Scale after an average of 3.3 treatments. Available literature dictates that traditional treatment for plantar fascia pain takes weeks to months to achieve an effect. Traditional treatments involve focusing on the local area (e.g. – plantar fascia stretching) or looking up the kinetic chain (e.g. – calf stretching). The PRRT technique for PF may be effective because it includes treatments for apparent gastrocnemius and hamstring tightness, as well as toe flexor endurance and neurological system sensitization. The results of this case series support a comprehensive evaluation of patients with plantar fasciitis to include a regionally interdependent approach by considering the role of the nervous system in the regulation and arousal of primal reflexes. Although the current results lend credence to the effectiveness of PRRT for PF, more research is needed to establish the effectiveness of the technique at treating other conditions.
REFERENCES
- 1.Riddle DL Pulisic M Pidcoe P Johnson RE. Risk factors for plantar fasciitis: A matched case‐control study. J Bone Joint Surg Am. 2003;85(5):872‐877. [DOI] [PubMed] [Google Scholar]
- 2.Beeson P. Plantar fasciopathy: Revisiting the risk factors. Foot and Ankle Surgery. 2014;20:160‐165. [DOI] [PubMed] [Google Scholar]
- 3.Riddle DL Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: A national study of medical doctors. Foot and Ankle International. 2004;25(5):303‐310. [DOI] [PubMed] [Google Scholar]
- 4.Werner RA Gell N Hartigan A Wiggerman N Keyserling WM. Risk factors for plantar fasciitis among assembly plant workers. PM&R. 2010;2(2):110‐116. [DOI] [PubMed] [Google Scholar]
- 5.Chandler TJ Kibler WB. A biomechanical approach to the prevention, treatment, and rehabilitation of plantar fasciitis. Sports Medicine. 1993;15(5):344‐352. [DOI] [PubMed] [Google Scholar]
- 6.Lopes AD Junior LCH Yeung SS Costa LOP. What are the main running‐related musculoskeletal injuries? Sports Med. 2012;42(10):891‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole C Seto C Gazewood J. Plantar fasciitis: Evidence‐based review of diagnosis and therapy. American Family Physician. 2005;72:2237‐2242. [PubMed] [Google Scholar]
- 8.Roxas M. Plantar fasciitis: Diagnosis and therapeutic considerations. Alternative Medicine Review. 2005;10(2):83‐93. [PubMed] [Google Scholar]
- 9.League AC. Current concepts review: Plantar fasciitis. Foot & Ankle International. 2008;29(3):358‐366. [DOI] [PubMed] [Google Scholar]
- 10.Kibler WB Goldberg C Chandler TJ. Functional biomechanical deficits in running athletes with plantar fasciitis. American Journal of Sports Medicine. 1991;19(1):66‐71. [DOI] [PubMed] [Google Scholar]
- 11.Allen RH Gross MT. Toe flexors strength and passive extension range of motion of the first metatarsophalangeal joint in individuals with plantar fasciitis. J Orthop Sports Phys Ther. 2003;33(8):468‐478. [DOI] [PubMed] [Google Scholar]
- 12.DiGiovanni BF Nawoczenski DA Lintal ME Moore EA Murray JC Wilding GE Baumhauer JF. Tissue‐specific plantar fascia stretching exercise enhances outcomes in patients with chronic heel pain: A prospective, randomized study. Journal of Bone and Joint Surgery. 2003;85A(7):1270‐1277. [DOI] [PubMed] [Google Scholar]
- 13.DiGiovanni BF Moore AM Zlotnicki JP Pinney SJ. Preferred management of recalcitrant plantar fasciitis among orthopaedic foot and ankle surgeons. Foot and Ankle International. 2012;33(6):507‐512. [DOI] [PubMed] [Google Scholar]
- 14.Davis PF Severud E Baxter DE. Painful heel syndrome: Results of nonoperative treatment. Foot & Ankle International. 1994;15(10):531.535. [DOI] [PubMed] [Google Scholar]
- 15.Sueki DG Cleland JA Wainner RS. A regional interdependence model of musculoskeletal dysfunction: Research, mechanisms, and clinical implications. Journal of Manual and Manipulative Therapy. 2013;21(2):90‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainner RS Whitman JM Cleland JA Flynn TW. Regional interdependence: A musculoskeletal examination model whose time has come. Journal of Orthopaedic and Sports Physical Therapy. 2007;37(11):658‐660. [DOI] [PubMed] [Google Scholar]
- 17.Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. St. Louis, MO: Elsevier Saunders; 2013. [Google Scholar]
- 18.Fantazzi F Snyder A Snyder M. Cyber PT. Primal reflex release technique: Welcome to a paradigm shift. 7 May 2008. Available at http://www.cyberpt.com/prrt.asp. Accessed November 27 2014. [Google Scholar]
- 19.Kasprowicz DE. Understanding the autonomic nervous system–A missing piece in the treatment of chronic pain. ND. Retrieved from http://www.boernepti.com/media/file/340330/Understanding%20the%20ANS.pdf. Accessed January 17 2015. [Google Scholar]
- 20.Slaughter V. Primitive reflexes. Salem Press Encyclopedia Of Science [serial online]. September 2013; Available from: Research Starters, Ipswich, MA: Accessed January 17, 2015. [Google Scholar]
- 21.Rothschild B. The Body Remembers. The Psychophysiology of Trauma and Trauma Treatment. New York, NY: W.W. Norton & Company, Inc; 2000. [Google Scholar]
- 22.Iams J. Primal Reflex Release Technique. What is the primal reflex release technique for pain relief? 2012. Available at http://www.theprrt.com/what-is-the-primal-reflex-release-technique-for-pain-relief.php. Accessed November 27 2014. [Google Scholar]
- 23.Iams J. When reflexes rule: A new paradigm in understanding why some patients don't get well. Advance for Physical Therapy and Rehab Medicine. 2005;16(3):41. [Google Scholar]
- 24.McKeon N. Use of primal reflex techniques in the treatment of chronic pain: A case study. [dissertation] St. Augustine, FL: University of St. Augustine; 2009. [Google Scholar]
- 25.Iams J. Plantar fasciitis [Video]. Primal Reflex Release Technique. http://www.theprrt.com/etc/video/index.php?file=plantar-fascitis55. Accessed August 1 2014. [Google Scholar]
- 26.Mintken PE Glynn P Cleland JA. Psychometric properties of the shortened disabilities of the arm, shoulder, and hand questionnaire (QuickDASH) and numeric pain rating scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18:920‐926. [DOI] [PubMed] [Google Scholar]
- 27.Vela LI Denegar C. The disablement in the physically active scale, part II: The psychometric properties of an outcomes scale for musculoskeletal injuries. Journal of Athletic Training. 2010;45(6):630‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn KK Jennings S Richardson G Vliet DV Hefford C Abbott JH. The patient‐specific functional scale: Psychometrics, clinimetrics, and application as a clinical outcome measure. Journal of Orthopaedic and Sports Physical Therapy. 2012;42(1):30‐42. [DOI] [PubMed] [Google Scholar]
- 29.Farrar JT Young JP LaMoreaux L Werth JL Poole M. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain. 2001;94:149‐158. [DOI] [PubMed] [Google Scholar]
- 30.Pool JJ Ostelo RW Hoving JL Bouter LM de Vet HC. Minimal clinically important change of the neck disability index and the numerical rating scale for patients with neck pain. Spine. 2007;32(26):3047‐3051. [DOI] [PubMed] [Google Scholar]
- 31.Hurley WL Denegar CR Hertel J. Research Methods: A Framework for Evidence‐Based Clinical Practice. Baltimore, MD: Lippincott, Williams, & Wilkins; 2011. [Google Scholar]
- 32.Klein SE Dale AM Hayes MH Johnson JE McCormick JJ Racette BA. Clinical presentation and self‐reported patterns of pain and function in patients with plantar heel pain. Foot & Ankle International. 2012;33(9):693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiGiovanni BF Nawoczenski DA Malay DP Graci PA Williams TT Wilding GE Baumhauer JF. Tissue‐specific plantar fascia stretching exercise improves outcomes in patients with chronic plantar fasciitis: A prospective clinical trial with 2 year follow‐up. Journal of Bone and Joint Surgery. 2006;88A(8):1775‐1781. [DOI] [PubMed] [Google Scholar]
- 34.Hyland MR Webber‐Gaffney A Cohen L Lichtman SW. Randomized controlled trial of calcaneal taping, sham taping, and plantar fascia stretching for the short‐term management of plantar heel pain. Journal of Orthopaedic and Sports Physical Therapy. 2006;36(6):364‐371. [DOI] [PubMed] [Google Scholar]
- 35.Lee SY McKeon P Hertel J. Does the use of orthoses improve self‐reported pain and function measures in patients with plantar fasciitisϿ. A meta‐analysis. Physical Therapy in Sport. 2009;10:12‐18. [DOI] [PubMed] [Google Scholar]
- 36.Carnahan K. Inclusion of primal reflex release technique (PRRT) plan of care for shoulder pain: A case study. ND. Retrieved from http://theprrt.com/files/prrt-patient-case-study.pdf. Accessed November 27 2014. [Google Scholar]
- 37.Pfeffer G, et al. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int. 1999;20(4):214–221. [DOI] [PubMed] [Google Scholar]
- 38.Porter D Barrill E Oneacre K May BD. The effects of duration and frequency of Achilles tendon stretching on dorsiflexion and outcome in painful heel syndrome: A randomized, blinded, control study. 2002;23(7):619‐624. [DOI] [PubMed] [Google Scholar]
- 39.Probe RA Baca M Adams R Preece C. Night splint treatment for plantar fasciitis: A prospective randomized study. Clinical Orthopaedics and Related Research. 1999;368:190‐195. [PubMed] [Google Scholar]