Abstract
Clostridium perfringens is estimated to be the second most common bacterial cause of foodborne illness in the United States, causing one million illnesses each year. Local, state, and territorial health departments voluntarily report C. perfringens outbreaks to the U.S. Centers for Disease Control and Prevention through the Foodborne Disease Outbreak Surveillance System. Our analysis included outbreaks confirmed by laboratory evidence during 1998–2010. A food item was implicated if C. perfringens was isolated from food or based on epidemiologic evidence. Implicated foods were classified into one of 17 standard food commodities when possible. From 1998 to 2010, 289 confirmed outbreaks of C. perfringens illness were reported with 15,208 illnesses, 83 hospitalizations, and eight deaths. The number of outbreaks reported each year ranged from 16 to 31 with no apparent trend over time. The annual number of outbreak-associated illnesses ranged from 359 to 2,173, and the median outbreak size was 24 illnesses. Outbreaks occurred year round, with the largest number in November and December. Restaurants (43%) were the most common setting of food preparation. Other settings included catering facility (19%), private home (16%), prison or jail (11%), and other (10%). Among the 144 (50%) outbreaks attributed to a single food commodity, beef was the most common commodity (66 outbreaks, 46%), followed by poultry (43 outbreaks, 30%), and pork (23 outbreaks, 16%). Meat and poultry outbreaks accounted for 92% of outbreaks with an identified single food commodity. Outbreaks caused by C. perfringens occur regularly, are often large, and can cause substantial morbidity yet are preventable if contamination of raw meat and poultry products is prevented at the farm or slaughterhouse or, after contamination, if these products are properly handled and prepared, particularly in restaurants and catering facilities.
Introduction
Clostridium perfringens is an anaerobic, Gram-positive, spore-forming bacillus (McClane, 2007) and a natural inhabitant of soil and the intestinal tracts of humans and other warm-blooded mammals (Brynestad and Granum, 2002). It causes an estimated one million illnesses each year, making it the second most common bacterial cause of food-borne illness in the United States (Scallan et al., 2011). C. perfringens is also ranked among the leading causes of bacterial foodborne disease outbreaks in other countries, including Australia, Japan, England, and Wales (Dalton et al., 2004; Komatsu et al., 2012; Gormley et al., 2011). Outbreaks of C. perfringens illness are commonly associated with consuming contaminated meat and poultry products (Wen and McClane, 2004; Brynestad and Granum, 2002).
Typically, C. perfringens spores germinate in raw or cooked foods under anaerobic conditions; when ingested, these vegetative cells sporulate, which allows elaboration of the C. perfringens enterotoxin [CPE] that causes human disease (Juneja et al., 2010). Most isolates causing human disease have a chromosomally encoded cpe gene; isolates carrying this gene on a plasmid are not typically associated with foodborne infections (McClane, 2007). C. perfringens can proliferate rapidly in food because the vegetative cells can double in as little as 10 minutes (Labbe, 1989). The vegetative cells can grow at 15–50°C; however, they are most likely to grow at 45°C (Brynestad and Granum, 2002). The vegetative cells and spores of C. perfringens carrying the cpe gene on the chromosome are far more heat resistant than those carrying this gene on a plasmid (McClane, 2007; Sarker et al., 2000), favoring their survival even in cooked foods.
We summarized epidemiologic data on foodborne disease outbreaks caused by C. perfringens reported to the U.S. Centers for Disease Control and Prevention’s (CDC’s) Foodborne Disease Outbreak Surveillance System from 1998 through 2010 and used these data to determine how C. perfringens outbreaks differ from reported foodborne outbreaks as a whole. This is the first summary of U.S. outbreaks caused by C. perfringens illness in 30 years.
Materials and Methods
A foodborne disease outbreak is defined as the occurrence of two or more cases of a similar illness resulting from ingestion of a common food (CDC, 2011). Local, state, and territorial health departments report outbreaks of illness caused by C. perfringens and other foodborne pathogens by submitting a web-based standard form to CDC’s Foodborne Disease Outbreak Surveillance System (CDC, 2011). Information collected for each outbreak includes the number of illnesses, hospitalizations, and deaths; age groups and gender categories of affected persons; reported food vehicles; symptoms of affected persons; contributing factors; and settings of food preparation and consumption.
Foodborne outbreak reports having first illness onset dates from 1998 through 2010 were included. The etiology was classified as confirmed if ≥ 106 C. perfringens was isolated from the stool of two or more ill persons, if C. perfringens enterotoxin was detected in the stool of two or more ill persons, or if ≥ 105 organisms per gram were isolated from the implicated food vehicle (CDC, 2000). Outbreaks that did not meet these criteria were considered suspect (CDC, 2000) and were not included in the analysis.
Reported foods that contained a single contaminated ingredient or in which all ingredients belonged to a single commodity were classified into one of 17 single food commodities (e.g., beef, dairy, leafy vegetables) (Painter et al., 2009). Foods that contained ingredients from more than one commodity or that could not be assigned to one of the 17 commodities were not assigned to any commodity (CDC, 2011).
The annual rate of outbreaks during 1998–2010 was calculated for each state by using population data from the U.S. Census Bureau; the 2004 population was used as the denominator because it was the midpoint of the years included in the analysis (Census, 2010). We also compared selected characteristics of confirmed C. perfringens outbreaks with foodborne outbreaks of other confirmed etiologies reported during 1998–2010.
Results
From 1998 to 2010, 14,918 foodborne disease outbreaks were reported, which resulted in 298,197 illnesses, 9,691 hospitalizations, and 232 deaths. Among these outbreaks, 823 (6%), which caused 28,543 (10%) illnesses, were confirmed or suspected to be caused by C. perfringens. On average, confirmed C. perfringens outbreaks comprised 5% of the total confirmed foodborne disease outbreaks reported each year. In all years, C. perfringens was the second most common cause of both confirmed and suspected bacterial foodborne disease outbreaks, after Salmonella. Of the 823 confirmed and suspected C. perfringens outbreaks, 289 (35%) were confirmed, which resulted in 15,208 illnesses, 83 hospitalizations, and eight deaths. These 289 confirmed C. perfringens outbreaks were included in further analyses.
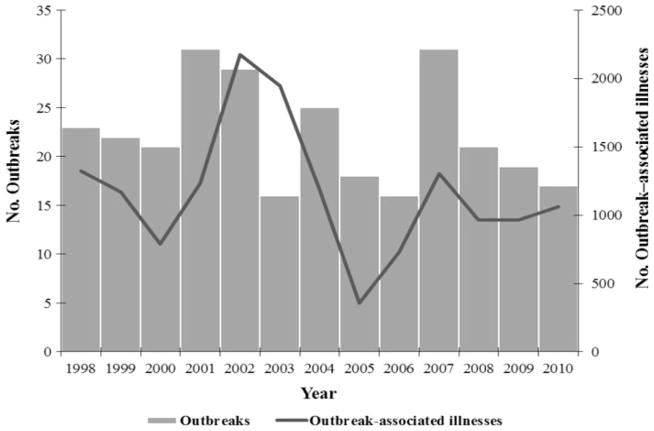
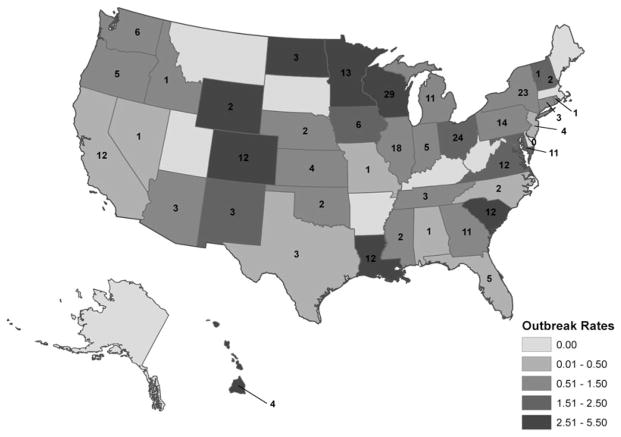
A median of 21 confirmed outbreaks was reported each year (range, 16–31), with no apparent pattern in the number of outbreaks reported over time (Fig. 1). Outbreaks were reported by 40 states; no geographic patterns were discernible (Fig. 2). The median rate of confirmed C. perfringens outbreaks reported by states reporting at least one outbreak was 1.17 per 1,000,000 persons (range, 0.13–5.26) (Fig. 2). No multistate outbreaks (e.g., outbreaks where exposure to the implicated food occurred in more than one state) were reported.
FIG. 1.
Number of Clostridium perfringens outbreaks and outbreak-associated illnesses, United States, 1998–2010.
FIG. 2.
Number and rate of reported Clostridium perfringens outbreaks per 1,000,000 population by state—United States, 1998–2010.
A median of 1,166 outbreak-associated illnesses was reported each year (range, 359–2,173) (Fig. 1), and the median outbreak size was 24 illnesses (range, 2–950). The median incubation period was 11 h (range, 4–19) (Table 1). Among outbreak-associated illnesses for which symptoms were reported, the most common were diarrhea (8,980 illnesses, 91%) and abdominal cramps (6,989 illnesses, 73%). Vomiting (1,328 illnesses, 14%) was much less common. A higher proportion of illnesses in C. perfringens outbreaks was in males when compared with the 5,765 other foodborne outbreaks with a confirmed etiology (64% vs. 45%, Relative Risk [RR] = 2.08, 95% Confidence Interval [CI] = 2.00–2.15). If outbreaks occurring in prison or jail settings were excluded, 44% of illnesses in C. perfringens outbreaks were in males, compared with 43% in other foodborne outbreaks with a confirmed etiology. For both C. perfringens and other outbreaks with a confirmed etiology, the 20–49-year-old age group (55% and 45%, respectively) had the highest number of illnesses reported; however, this proportion was higher in C. perfringens outbreaks than in the combined others.
Table 1.
Characteristics of Outbreak-Associated Illnesses Caused by Clostridium perfringens and Other Confirmed Etiologies, United States, 1998–2010
| Characteristic | C. perfingens, n (%) | Other etiologies, n (%) |
|---|---|---|
| Incubation period | ||
| Hours | 11 | 28 |
| Range | 4–19 | 8–41 |
| Symptoms | ||
| Diarrhea | 8,980 (91) | 94,031 (84) |
| Abdominal cramps | 6,989 (73) | 70,308 (69) |
| Vomiting | 1,328 (14) | 62,849 (58) |
| Gendera | 12,565 (100) | 127,017 (100) |
| Male | 8,092 (64) | 56,911 (45) |
| Age group (years) | 11,343 (100) | 120,654 (100) |
| < 1 | 3 (0) | 636 (1) |
| 1–4 | 76 (1) | 4,394 (4) |
| 5–19 | 1,622 (14) | 21,334 (18) |
| 20–49 | 6,280 (55) | 54,005 (45) |
| ≥ 50 | 2,681 (24) | 33,904 (28) |
| Unknown | 681 (6) | 6,381 (5) |
If outbreaks occurring in a prison or jail setting are omitted, most outbreak-associated illnesses occurred in females for both C. perfringens and other outbreaks with a confirmed etiology (56% vs. 57%, respectively).
A food vehicle was reported for 254 (88%) C. perfringens outbreaks, 144 (50%) of which were attributed to a single food commodity. Beef was the most common single commodity reported (66 outbreaks, 46%), followed by poultry (43 outbreaks, 30%), pork (23 outbreaks, 16%), and other foods (12 outbreaks, 8%). Combined, meat and poultry commodities accounted for 92% of single food commodity outbreaks. Among outbreaks associated with the beef commodity, the most common food vehicle reported was roast beef (25 outbreaks, 38%). Among the 110 outbreaks that could not be classified into a single commodity (i.e., the food contained ingredients from >1 commodity), 78 (71%) reported a food containing ingredients from the meat and poultry commodities.
Outbreaks of C. perfringens illness occurred in all months, with a peak in November (37 outbreaks, 13%) and December (33 outbreaks, 11%), and a smaller peak in April (31 outbreaks, 11%) and May (28 outbreaks, 10%) (Fig. 3). By commodity, December accounted for the largest number of beef (10 outbreaks; 15% of beef-associated outbreaks) and poultry outbreaks (9 outbreaks; 21% of poultry-associated outbreaks) (Fig. 3).
FIG. 3.
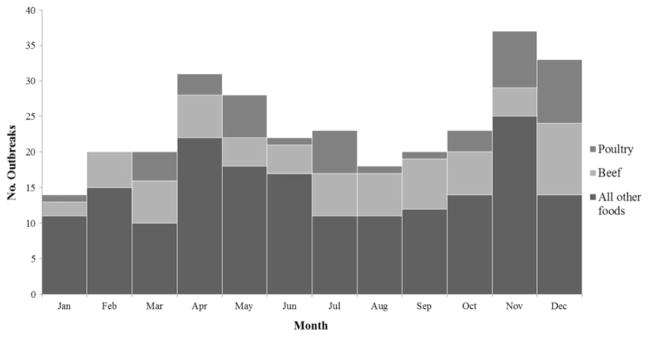
Food commodities associated with Clostridium perfringens outbreaks, by month, United States, 1998–2010.
Restaurants were the most common setting of food preparation and consumption (Table 2). The contaminated food item was prepared in a restaurant in 43% of outbreaks, followed by a catering facility (19%), private home (16%), prison or jail (11%), and other setting (10%). The food was most often eaten in a restaurant (26%), followed by private home (15%), prison or jail (11%), workplace (11%), and other setting (37%). Of the 121 outbreaks in which the food was prepared in a restaurant, it was eaten in another setting in almost half (40%). When compared with other foodborne outbreaks, a higher proportion of C. perfringens outbreaks involved food prepared in catering facilities (19% vs. 8%) and prisons (11% vs. 1%). The two largest reported outbreaks occurred in prisons. In 2002, 950 persons became ill, and one person was hospitalized after eating contaminated roast beef gravy at a prison in Illinois. In 2003, 880 illnesses and 35 hospitalizations occurred at a prison in Louisiana after consuming corn contaminated with C. perfringens; this outbreak accounted for more hospitalizations than any other C. perfringens outbreak during the study period.
Table 2.
Characteristics of Outbreaks Caused by Clostridium perfringens and Other Confirmed Etiologies, United States, 1998–2010a
| Characteristics | C. perfringens, n (%) | Other etiologies, n (%) |
|---|---|---|
| Total outbreaks | 289 (100) | 5,765 (100) |
| January | 14 (5) | 419 (7) |
| February | 20 (7) | 367 (6) |
| March | 20 (7) | 434 (8) |
| April | 31 (11) | 527 (9) |
| May | 28 (10) | 562 (10) |
| June | 22 (8) | 621 (11) |
| July | 23 (8) | 540 (9) |
| August | 18 (6) | 556 (10) |
| September | 20 (7) | 427 (7) |
| October | 23 (8) | 408 (7) |
| November | 37 (13) | 429 (7) |
| December | 33 (11) | 475 (8) |
| Setting of food preperation | 280 (100) | 5,222 (100) |
| Restaurant | 121 (43) | 2,814 (54) |
| Catered event | 53 (19) | 414 (8) |
| Private home | 46 (16) | 1,041 (20) |
| Prison or jail | 32 (11) | 74 (1) |
| Other | 28 (10) | 879 (17) |
| Setting of food consumption | 274 (100) | 5,223 (100) |
| Restaurant | 70 (26) | 2,389 (46) |
| Private home | 41 (15) | 1,336 (26) |
| Prison or jail | 31 (11) | 85 (2) |
| Workplace | 30 (11) | 310 (6) |
| Other | 102 (37) | 1,103 (21) |
1998–2008 data downloaded on June 14, 2010 and 2009–2010 data downloaded on August 2, 2012.
At least one contributing factor was reported for 232 (80%) outbreaks. The most common factor reported (114 outbreaks, 39%) was “allowing foods to remain at room or warm outdoor temperature for several hours,” followed by “slow cooling” (111 outbreaks, 38%), and “insufficient time and/or temperature during hot holding” (93 outbreaks, 32%). At least one of these three factors was reported for 188 (65%) outbreaks. The most common contributing factor reported during November–December was “slow cooling” (33 outbreaks, 11%), whereas “allowing foods to remain at room or warm outdoor temperature for several hours” (85 outbreaks, 29%) was the most common contributing factor reported during the rest of the year.
Discussion
Outbreaks caused by C. perfringens were the second most common cause of bacterial foodborne disease outbreaks in the United States during 1998–2010. Outbreaks occurred regularly, were often large, and caused substantial morbidity. Although the number of outbreaks fluctuated from year to year, no trends were discernible. At an estimated medical cost of $539 per case (Scharff et al., 2009), confirmed C. perfringens outbreaks alone cost an estimated $8,197,112 during this 13-year period. The true human and economic burden of C. perfringens is likely much higher, as many outbreaks are never investigated or confirmed, and many illnesses occur sporadically.
More than 90% of C. perfringens outbreaks attributed to a single food commodity were due to meat and poultry products. This is consistent with an earlier study on the epidemiology of C. perfringens (Shandera et al., 1983) and is not surprising because the organism is common in animals and ubiquitous in the environment (Labbe and Juneja, 2006) and because C. perfringens grows readily under pH conditions between 6.0 and 7.0, a range similar to that of most meat and poultry products (Labbe and Juneja, 2006). C. perfringens type A, including isolates producing the cpe enterotoxin associated with human disease, has also been isolated from raw meat and poultry at retail (Wen and McClane, 2004; Limbago et al., 2012) and in food service establishments (Bryan and McKinley, 1979). Contamination of meats and other foods has been documented to occur through contact of carcasses with feces as well as via cross-contamination by other foods or contaminated surfaces during food processing or preparation (Juneja et al., 2010). However, the most important animal or environmental reservoirs for CPE-producing C. perfringens and points at which intervention would be most effective to reduce C. perfringens contamination remain unknown (McClane, 2007). Most C. perfringens contamination appears to be on the surface of meats; however, low levels of C. perfringens spores have been isolated from whole muscle tissues (Taormina et al., 2003).
C. perfringens outbreaks were most common during November and December, months in which many holiday gatherings and events occur. During the holidays, people tend to gather in large groups to eat foods such as roasts, gravies, and poultry that are often cooked in large batches or prepared ahead of serving. November and December were also the months during which most C. perfringens outbreaks attributable to beef and poultry occurred. In contrast, foodborne disease outbreaks caused by most other etiologies occurred more frequently during the summer months. Education about properly preparing and handling foods that are commonly prepared ahead of time or allowed to sit at room temperature before serving could help reduce C. perfringens outbreaks, especially if directed broadly at the many persons who prepare and handle such foods, including restaurant workers, caterers, and food workers in institutional settings, as well as home cooks. Similarly, the finding that food was commonly prepared in a restaurant but consumed in another setting illustrates a need for prevention activities focused on proper handling and storage of prepared foods.
C. perfringens vegetative cells will be inactivated at temperatures around 60°C (Juneja et al., 2001), but even foods cooked to proper temperatures can harbor C. perfringens spores. Ideal conditions for complete spore inactivation are less well defined (Akhtar et al., 2009), and include a combination of temperature and pressure. Because spores that survive cooking can actually germinate and produce vegetative cells at a higher rate following heat treatment (Juneja et al., 2010), adherence to recommendations for food cooling and hot holding are particularly important to prevent C. perfringens outbreaks. Refrigeration of foods at temperatures less than 10°C will inhibit bacterial growth (Traci and Duncan, 1974; Strong et al., 1966).
The large proportion of C. perfringens outbreaks for which the etiology was not confirmed and the wide variation among states in the rate of reported outbreaks strongly suggest that the number of reported outbreaks is only a small percentage of those that occur. C. perfringens illness is typically mild and self-limiting; therefore, most infected persons do not seek medical care, and among those who do, only a small portion is tested for C. perfringens (Labbe and Juneja, 2006). Because few public health laboratories have the resources to characterize strains of C. perfringens isolated in outbreaks, these data were not available. Also, not all outbreaks are detected, and even among those that are detected, not all are investigated or reported. The dynamics of outbreak detection combined with the fact that medical care seeking is unlikely for this pathogen suggests that small outbreaks may be less likely to be detected. This analysis included only confirmed outbreaks; if suspected outbreaks were included, the number of outbreaks would have increased nearly threefold.
Conclusion
Information gathered from outbreak reports provide information that can contribute to prevention. C. perfringens outbreaks are preventable with proper food handling and preparation, particularly in restaurants and catering facilities (Shandera et al., 1983). Proper cooking, cooling, and hot holding can decrease the growth of C. perfringens and the formation of vegetative cells in food. To ensure that vegetative cells are eliminated, foods should be cooked at or reheated to a temperature of ≥ 75°C (Bryan, 1969). Restaurant inspections, consumer education, and enhanced efforts in food production environments provide an opportunity to improve food handling and minimize contamination of foods. Limited information is available about environmental reservoirs for CPE–producing C. perfringens; identifying these reservoirs and factors associated with contamination of meat products might lead to control measures at the farm or slaughterhouse level that could decrease food contamination (McClane, 2007).
Acknowledgments
The findings in this report are based, in part, on contribution by state, territorial, tribal, and local health departments.
Footnotes
Disclosure Statement
No competing financial interests exist.
References
- Akhtar S, Paredes-Sabja D, Torres JA, Sarker MR. Strategy to inactivate Clostridium perfringens spores in meat products. Food Microbiol. 2009;26:272–277. doi: 10.1016/j.fm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Bryan FL. What the sanitarian should know about Clostridium perfringens foodborne illness. J Milk Food Technol. 1969;32:381–389. [Google Scholar]
- Bryan FL, McKinley TW. Hazard analysis and control of roast beef preparation in foodservice establishments. J Food Prot. 1979;42:4–18. doi: 10.4315/0362-028X-42.1.4. [DOI] [PubMed] [Google Scholar]
- Brynestad S, Granum PE. Clostridium perfringens and foodborne infections. Int J Food Microbiol. 2002;74:195–202. doi: 10.1016/s0168-1605(01)00680-8. [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Surveillance for foodborne disease outbreaks—United States, 1993–1997. MMWR Morb Mortal Wkly Rep. 2000;49:1–62. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Surveillance for foodborne disease outbreaks—United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:1197–1202. [PubMed] [Google Scholar]
- [Census] United States Census Bureau. [accessed September 14, 2012];Population estimates. 2010 Available at: http://www.census.gov.
- Dalton CB, Gregory J, Kirk MD, Stafford RJ, Givney R, Kraa E, Gould D. Foodborne disease outbreaks in Australia, 1995 to 2000. Commun Dis Intell. 2004;28:211–224. doi: 10.33321/cdi.2004.28.19. [DOI] [PubMed] [Google Scholar]
- Gormley FJ, Little CL, Rawal N, Gillespie IA, Lebaigue S, Adak GK. A 17-year review of foodborne outbreaks: Describing the continuing decline in England and Wales (1992–2008) Epidemiol Infect. 2011;139:688–699. doi: 10.1017/S0950268810001858. [DOI] [PubMed] [Google Scholar]
- Juneja VK, Novak JS, Eblen BS, McClane BA. Heat resistance of Clostridium perfringens vegetative cells affected by prior heat shock. J Food Saf. 2001;21:127–139. [Google Scholar]
- Juneja VK, Novak JS, Labbe RL. Clostridium perfringens. In: Juneja VK, Sofos JN, editors. Pathogens and Toxins in Foods: Challenges and Interventions. Washington, DC: ASM Press; 2010. pp. 53–70. [Google Scholar]
- Komatsu H, Inui A, Sogo T, Fujisawa T. Clostridium perfringens. Nihon Rinsho. 2012;70:1357–1361. [PubMed] [Google Scholar]
- Labbe RG. Clostridium perfringens. In: Doyle MP, editor. Foodborne Bacterial Pathogens. New York: Marcel Dekker; 1989. pp. 192–234. [Google Scholar]
- Labbe RG, Juneja VK. Clostridium perfringens gastroenteritis. In: Riemann HP, Cliver DO, editors. Foodborne Infections and Intoxications. San Diego: Academic Press; 2006. pp. 137–163. [Google Scholar]
- Limbago B, Thompson AD, Greene SA, Maccannell D, Macgowan CE, Jolbitado B, Hardin HD, Estes SR, Weese JS, Songer JG, Gould LH. Development of a consensus method for culture of Clostridium difficile from meat and its use in a survey of U.S. retail meats. Food Microbiol. 2012;32:448–451. doi: 10.1016/j.fm.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClane BA. Clostridium perfringens. In: Doyle MP, Beuchat LR, editors. Food Microbiology: Fundamentals and Frontiers. Washington, DC: ASM Press; 2007. pp. 423–444. [Google Scholar]
- Painter JA, Ayers T, Woodruff R, Blanton E, Perez N, Hoekstra RM, Griffin PM, Braden C. Recipes for foodborne outbreaks: A scheme for categorizing and grouping implicated foods. Foodborne Pathog Dis. 2009;6:1259–1264. doi: 10.1089/fpd.2009.0350. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Shivers RP, Sparks SG, Juneja VK, McClane BA. Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid genes versus chromosomal enterotoxin genes. Appl Environ Microbiol. 2000;66:3234–3240. doi: 10.1128/aem.66.8.3234-3240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—Major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff R, McDowell J, Medeiros L. Economic cost of foodborne illness in Ohio. J Food Prot. 2009;72:128–136. doi: 10.4315/0362-028x-72.1.128. [DOI] [PubMed] [Google Scholar]
- Shandera WX, Tacket CO, Blake PA. Food poisoning due to Clostridium perfringens in the United States. J Infect Dis. 1983;147:167–170. doi: 10.1093/infdis/147.1.167. [DOI] [PubMed] [Google Scholar]
- Strong DH, Weiss KF, Higgins LW. Survival of Clostridium perfringens in starch pastes. J Am Diet Assoc. 1966;49:191–195. [PubMed] [Google Scholar]
- Taormina PJ, Bartholomew GW, Dorsa WJ. Incidence of Clostridium perfringens in commercially produced cured raw meat product mixtures and behavior in cooked products during chilling and refrigerated storage. J Food Prot. 2003;66:72–81. doi: 10.4315/0362-028x-66.1.72. [DOI] [PubMed] [Google Scholar]
- Traci PA, Duncan CL. Cold shock lethality and injury in Clostridium perfringens. Appl Microbiol. 1974;28:815–821. doi: 10.1128/am.28.5.815-821.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, McClane BA. Detection of enterotoxigenic Clostridium perfringens type A isolates in American retail foods. Appl Environ Microbiol. 2004;70:2685–2691. doi: 10.1128/AEM.70.5.2685-2691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]