Abstract
Complex interactions between the immune system and the brain might have important aetiological and therapeutic implications for neuropsychiatric brain disorders. A possible association between schizophrenia and the immune system was postulated over a century ago, and is supported by epidemiological and genetic studies pointing to links with infection and inflammation. Contrary to the traditional view that the brain is an immunologically privileged site shielded behind the blood–brain barrier, studies in the past 20 years have noted complex interactions between the immune system, systemic inflammation, and the brain, which can lead to changes in mood, cognition, and behaviour. In this Review, we describe some of the important areas of research regarding innate and adaptive immune response in schizophrenia and related psychotic disorders that, we think, will be of interest to psychiatric clinicians and researchers. We discuss potential mechanisms and therapeutic implications of these findings, including studies of anti-inflammatory drugs in schizophrenia, describe areas for development, and offer testable hypotheses for future investigations.
Introduction
Complex immune–brain interactions that affect neural development, survival, and function might have causal and therapeutic implications for many disorders of the CNS1–5 including psychiatric illness.2 Multiple sclerosis, previously thought to be solely neurological, is increasingly recognised as secondary to immune dysfunction.3 High concentrations of the circulating proinflammatory cytokine interleukin 6 in childhood have been reported to be associated with increased risk of subsequent psychosis and depression in young adult life,2 and elimination of autoantibodies against neuronal cell surface proteins by immunotherapy has led to symptomatic improvement in some cases of first episode psychosis.6 In this Review, we discuss whether research is entering a new era of immunopsychiatry that will change the understanding of the brain’s disorders, in which manifestations include, but are rarely restricted to, mental symptoms. Substantial evidence supports a role for the immune system in the pathogenesis of depression and schizophrenia, which is consistent with the well known clinical and aetiological (including genetic) overlap between these disorders. Here, we describe some of the important areas of research that implicate the innate and adaptive immune response in the pathogenesis of schizophrenia and related psychotic disorders through effects on neurotransmitters, neurodevelopment, and degeneration. We assess potential therapeutic implications of these findings and existing treatment studies of anti-inflammatory agents in schizophrenia.
The aim of this Review is not only to summarise key evidence about the link between immune system and schizophrenia, but also to identify gaps in knowledge and provide suggestions for improvement, including testable hypotheses for future investigations. The aim is also to give a holistic view, rather than an exhaustive review, of a landscape of increasing relevance to people with schizophrenia and those who treat them.
The immune system and the brain share some fundamental characteristics. Both are highly integrated, complex systems with memory, which develop through interactions with the external environment, are able to distinguish between self and non-self, and respond adaptively.7,8 Historically, the brain has been thought of as an immunologically privileged site, shielded behind the blood–brain barrier,9 but immune components of the brain, such as microglia that constitute about 10% of the brain cell mass (equal to neurons), derive from the haemopoietic system beyond the CNS.10 In response to systemic inflammation, microglia release cytokines that bind to specific receptors on neurons8 and affect neurotransmitters, synaptic plasticity, and cortisol concentrations, leading to changes in mood, cognition, and behaviour.1,5
The immune and infection link to psychosis
The immune system consists of a complex organisation of cells and mediators that has evolved largely to protect human beings from infection and malignancy.8 It can be broadly thought about as consisting of an innate response, acting as a rapid, non-specific first line of defence, and an adaptive response that is slower and antigen specific. The innate response is mediated by neutrophils and macrophages that recognise and clear invading organisms. Inflammatory cytokines, secreted by macrophages and other cells, help this process. The adaptive response involves immunological memory, and consists of T (thymic) lymphocytes that recognise antigens and cause lysis of infected cells, and B lymphocytes that secrete antibodies as part of the humoral response.8
Schizophrenia is a disabling disorder characterised by positive (delusions and hallucinations), negative (social withdrawal and apathy), and cognitive symptoms (poor executive function and memory). It affects around 1% of the population at some point in their lives, with onset characteristically during the period of brain development that follows puberty, and lasts until the end of the third decade.11 Schizophrenia is multifactorial; it is associated with multiple genetic loci that confer risk, in addition to developmental and postnatal risk factors.12 A possible association between schizophrenia and the immune system was postulated more than a century ago (panel 1), and is supported by epidemiological studies that suggest links with infection and systemic inflammation.13–16
Serologically confirmed prenatal maternal infection with any of several pathogens (including influenza, herpes simplex virus type 2, cytomegalovirus, and the intracellular parasite Toxoplasma gondii), clinically diagnosed non-specific viral and bacterial infections, and increased maternal C-reactive protein concentrations during pregnancy have all been associated with schizophrenia in the adult offspring.15,17 Reduced concentrations of acute-phase proteins in neonates might increase the risk of adult psychosis by increasing susceptibility to infections in early life.18 Acute-phase proteins are released as part of the innate immune response and consist of several mediators with different physiological functions.8 Exposure to neurotropic virus in early childhood is associated with increased risk of subclinical psychotic experiences in adolescence.19 The finding that the risk of schizophrenia is almost doubled in adult survivors of childhood CNS viral infection shows that the phase during which infection can increase the risk of future neuropsychiatric disorders is not confined to the prenatal period.14 Adult schizophrenia is also associated with increased rates of various infections, including those caused by T gondii.20
Childhood autoimmune conditions are associated with subclinical psychotic experiences in adolescents21 and schizophrenia in adults.22 The prevalence of autoimmune conditions is increased in people with schizophrenia and their unaffected first-degree relatives.23 Furthermore, risk of schizophrenia increases in a linear fashion with the number of severe infections in individuals with a previous history of autoimmune disease.22 Thus, the links between schizophrenia and a range of infections and autoimmune conditions suggest a common underlying pathway, probably involving the inflammatory immune response. In addition to its own effects on the brain, inflammation is thought to increase the permeability of the blood–brain barrier and to help with penetration of immune components into the brain.24
Support for an immune-mediated cause in schizophrenia comes from genome-wide association studies that report significant associations between schizophrenia and markers close to the major histocompatibility complex (MHC) region on chromosome 6.25,26 This region contains many immune-related genes, including those involved in antigen presentation and inflammatory mediators. A 2014 genome-wide association study27 identified 108 genetic loci (83 previously undetected) associated with schizophrenia. Broadly, these represent genes expressed in the brain and immune cells involved in adaptive immunity (CD19 and CD20B lymphocytes), in addition to the MHC. Moreover, associations with the immune-related genes remained significant after the MHC region was excluded, suggesting that these findings were not driven by the strong association at the MHC. Another genome-wide association study28 reported substantial genetic overlap involving the MHC region between schizophrenia and multiple sclerosis, a condition characterised by immune dysfunction.
Immune system in the pathogenesis of schizophrenia and related psychosis
The abnormalities of the immune system seen in schizophrenia and related psychosis are diverse and overlapping, and involve many immune components. Here, we discuss components of the innate immune response (cytokines and microglia), and components of the adaptive immune response (lymphocyte subsets and anti-neuronal cell surface antibodies). We do not cover autoimmunity involving various brain regions, thyroid, thymus, and antibodies to dietary antigens such as gliadin and casein. Key questions pertaining to the entire field have been summarised in panel 2.
Heterogeneity between studies might point towards uncertainty or suggest heterogeneity in the causes and pathogenesis of the schizophrenia syndrome. Changing of the focus of research from syndrome to symptom, or constellation of symptoms, would be helpful to fully understand the role of inflammation and immunity in neuropsychiatric disorders. Many current studies have inadequately accounted for confounding factors such as body mass and smoking. More studies have focused on blood rather than CNS immune markers, and few have examined associations between immune markers and either symptoms or cognition in schizophrenia. Rarer still are longitudinal studies of immune markers and schizophrenia that comment on cause and effect between immunity and psychiatric syndrome. Associations between immune markers, stress, and cortisol in schizophrenia are also poorly understood. Despite these gaps in the literature, present knowledge is consistent with a role for the immune system in the pathophysiology of schizophrenia and related psychotic disorders.
Inflammatory cytokines
Meta-analyses of many cross-sectional studies show that schizophrenia is associated with disruption of the cytokine milieu and the propensity for the production of proinflammatory cytokines.16,29,30 Longitudinal studies of inflammatory markers and subsequent psychotic disorders are scarce. Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort2 suggest that increased serum concentration of the proinflammatory cytokine interleukin 6 at age 9 years is associated with twofold increased risk of development of a psychotic disorder at age 18 years. The study also reports a robust dose-response association between increased interleukin 6 concentrations in childhood and subsequent risk of subclinical psychotic experiences in young adulthood, which persists after several potential confounders are taken into account, including sex, body mass, and psychological and behavioural problems preceding the measurement of childhood interleukin 6.2 No associations between serum C-reactive protein concentrations at baseline and future psychiatric disorders were seen, but another longitudinal study31 reported increased risk of late, or very late, onset schizophrenia for increased serum C-reactive protein concentrations at baseline. Further longitudinal studies are needed to confirm whether the increase in serum concentrations of proinflammatory cytokines in schizophrenia and related psychosis is the cause or consequence of illness, although these findings suggest causal mechanisms.
Antipsychotic-naive first-episode psychosis30 and acute psychotic relapse16 are also associated with increased serum concentrations of interleukin 6 and other proinflammatory cytokines, such as tumour necrosis factor α (TNFα), interleukin 1β, interferon γ, and decreased serum concentrations of anti-inflammatory cytokine interleukin 10, which are normalised after remission of symptoms with antipsychotic treatment.16 Reduced interleukin 2 (involved in immune regulation) production in vitro by T cells collected from patients with schizophrenia was thought to be indicative of autoimmune causes of psychosis.32 However, acute psychosis is associated with no substantial changes in serum interleukin 2 concentrations.16 The concentration of soluble interleukin 2 receptor increases in schizophrenia,16,30 which is likely to be a compensatory mechanism that inhibits interleukin 2 production. Thus, the data are consistent with an increase in proinflammatory cytokines in acute psychosis. However, few studies have adjusted for important immune-modulatory factors such as body mass or smoking,16 or examined cytokines in cerebrospinal fluid, where an increase in interleukin 6 concentration33,34 has been reported in schizophrenia. One study35 reported increased serum interleukin 6 concentrations in people with an at-risk mental state for psychosis compared with healthy controls. Some data suggest that serum cytokine concentrations, including interleukin 6, are associated with illness severity, duration, and antipsychotic therapy,16,36–39 but little is known regarding the associations between stress, cortisol and cytokine concentrations in different stages of schizophrenia. Therefore, more studies are needed to understand the associations between cytokine concentrations, disease prodrome, progression, and treatment response. Longitudinal studies of first-episode psychosis, individuals at clinical high risk for development of psychosis, and those with treatment refractory illness would be useful to examine these issues. Rather than merely reporting group differences in cytokine serum or cerebrospinal fluid concentrations, future studies should examine associations between cytokines, cognitive and social functioning, comorbid physical illness, and structural and functional brain indices in people with psychosis and healthy controls.
In rodents, studies have noted physiological roles for cytokines in memory and learning, including long-term potentiation, synaptic plasticity, and neurogenesis.40 Mild systemic inflammation has been reported to produce impairments in spatial memory in human beings via its action on glucose metabolism in the medial temporal lobe.41 Whether cytokine-mediated inflammatory processes underlie cognitive dysfunction in schizophrenia, an integral part of the syndrome, is an important hypothesis that needs investigation.
Longitudinal associations between interleukin 6 and both psychosis and depression might indicate a transdiagnostic effect.2 A longitudinal association between serum C-reactive protein, a marker of systemic inflammation, and subsequent symptoms of posttraumatic stress disorder has been reported.42 Thus, understanding the early-life biopsychosocial determinants of cytokine serum concentrations would be crucial to elucidate whether increased concentrations of cytokines could explain the association between early-life adversity and the risk of various psychiatric illnesses in adulthood.43
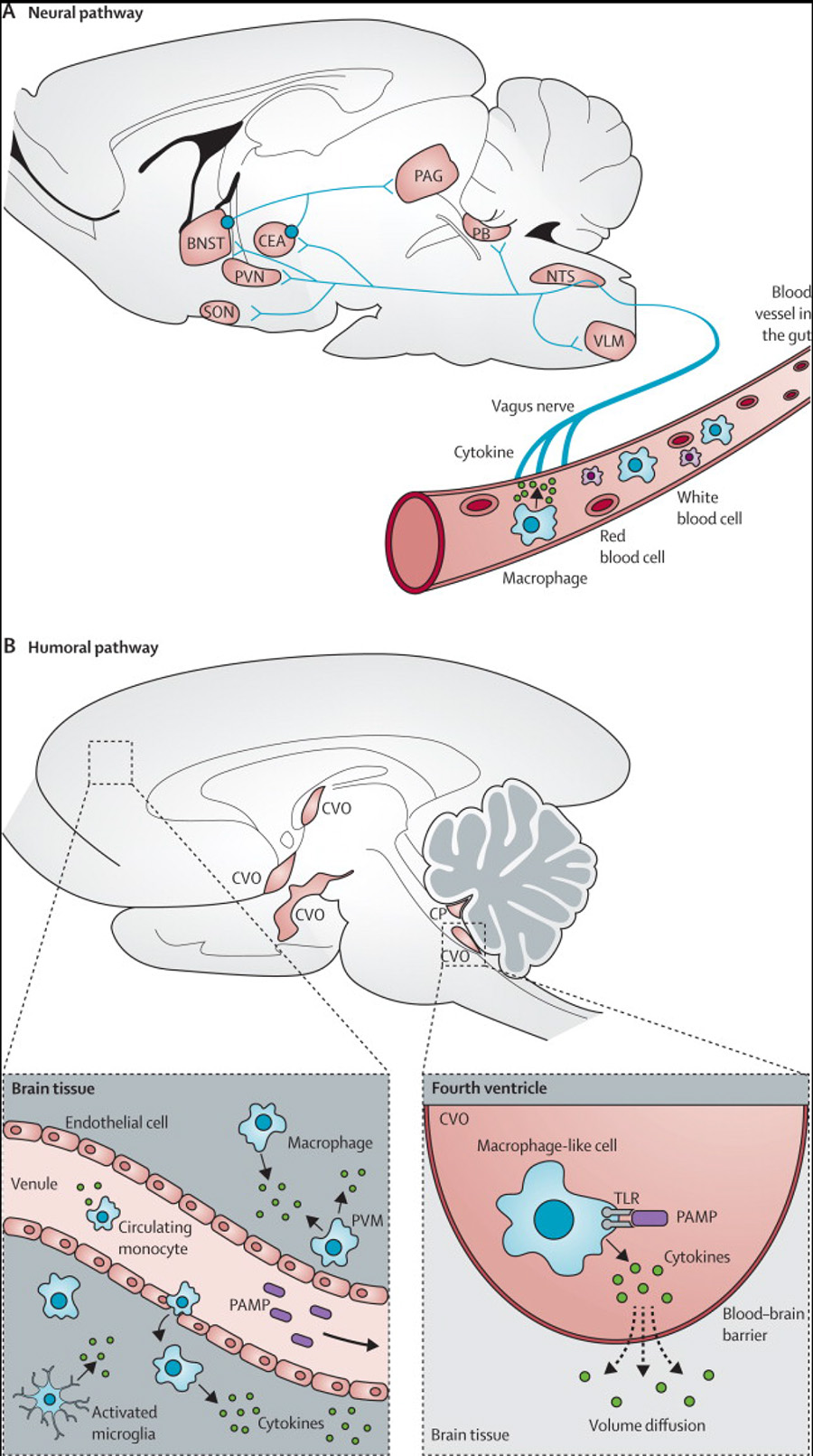
Studies in mice have shown how peripheral cytokines, such as interleukin 6, can affect the brain (figure 1).1,4 In the neural pathway, circulating interleukin 6 binds to receptors on the vagus nerve, and the signal reaches hypothalamic brain nuclei via the brainstem by retrograde axonal transport. Once within the CNS, the cytokine signal is amplified, which activates microglia, leading to the secretion of proinflammatory cytokines, chemokines, and proteases within the brain.1 These messengers activate IDO1, an enzyme that metabolises tryptophan along the kynurenine pathway, leading to increased concentrations of kynurenic acid and its metabolite quinolonic acid, both of which are involved in glutamatergic neurotransmission.44 Cytokines also increase oxidative stress by raising the concentration of toxic nitric oxide, and activate the hypothalamic–pituitary–adrenal axis, leading to the release of cortisol.1,5 These effects could contribute to the negative, cognitive, and positive symptoms of schizophrenia, and also to the impaired mood, cognition, and perception that are important parts of other psychiatric disorders. Indeed, non-specific peripheral immune activation caused by injection of lipopolysaccharide in healthy volunteers increases serum interleukin 6 concentrations, in addition to inducing low mood, anxiety, and reduced cognitive performance.45 Furthermore, cytokines have substantial effects on microglia that, in turn, are crucial for the maintenance of effective neuronal and synaptic health.10
Figure 1. How peripheral immune signals reach the brain to contribute to neuropsychiatric symptoms.
(A) Neural pathway: peripheral cytokines activate the vagus nerve, and the signal reaches brain nuclei by retrograde axonal transport. (B) Humoral pathway: panel on right shows macrophage-like cells residing in the CVOs and the CP responding to circulating PAMPs by producing cytokines which enter the brain by volume diffusion. Panel on left shows that inflammation activates cerebral vascular endothelial cells. This leads to increased transmigration of monocytes into the brain, and activation of microglia and perivascular macrophages. All of these cells release proinflammatory cytokines in the brain. Adapted with permission from Dantzer and colleagues.1 NTS=nucleus tractus solitarius. VLM=ventrolateral medulla. CEA=central amygdala. PVN=periventricular nucleus. SON=supraoptic nucleus. PB=parabrachial nucleus. PAG=periaqueductal grey. BNST=bed nucleus for stria terminalis. CP=choroid plexus. TLR=Toll-like receptor. PAMP=pathogen-associated molecular patterns. CVO=circumventricular organ. PVM=peri-vascular macrophage.
Microglia
Microglia, the resident immune cells of the brain, constitute 10% of all non-neuronal or glial cells, which in turn constitute 90% of the adult human brain.10 Similar to macrophages, these cells originate from myeloid precursor cells and are thought to migrate into the CNS during the early neonatal period.46 In the healthy brain, microglia retain a downregulated phenotype (resting state),47 yet continue to survey and respond to the surrounding brain microenvironment. If the brain is subject to injury, inflammation, or in response to systemic inflammation, microglia develop an activated phenotype characterised by morphological changes, upregulation of surface receptors, the potential to activate T cells, and the release of various inflammatory mediators, including cytokines.48
Neuroinflammation is characterised by the activation of microglia cells, which show an increase in the expression of the translocator protein (TSPO). Neuroimaging studies using PET and a TSPO ligand provide evidence for neuroinflammation in recent-onset schizophrenia,49 and in acute exacerbations of schizophrenia.50 These studies49,50 report increased binding of this ligand in the entire grey matter and hippocampus, suggesting that neuroinflammation might contribute to grey matter volume loss and cognitive deterioration in schizophrenia. However, such studies are few in number and the existing studies have included small numbers of people with schizophrenia and healthy controls. An alternative explanation for increased binding of the TSPO ligand could be its affinity for activated astrocytes, which are seen in schizophrenia but are unrelated to neuroinflammation.50 Many patients with schizophrenia are treated with benzodiazepines, which can also affect the binding of this ligand. Thus, identification of reliable markers of microglial activation is needed so they can be used for in-vivo imaging or measured in blood to investigate whether microglial activation corresponds with clinical severity and treatment response.
Previously activated microglia can respond more strongly to a new stimulus.51 Microglia are likely to retain an immune memory of the neuropathology, which in turn is associated with heightened responsiveness to new systemic inflammation.48 Thus, early developmental insults such as childhood CNS or severe systemic infection might have a priming effect on microglia,51 increasing microglial activation and psychosis risk after subsequent infections. Whether the association between early-life CNS infection and adult schizophrenia14 could be explained by changes in microglia can be tested using longitudinal birth cohorts. Induced pluripotent stem cell technology has been successfully used to create neurons by reprogramming human fibroblast cells; neurons derived from patients with schizophrenia have diminished neuronal connectivity and decreased neurite number and glutamate receptor expression compared with neurons derived from healthy controls.52 Whether this technology can be used to study non-neuronal brain cells such as microglia remains to be seen.
Antineuronal cell surface autoantibodies
A possible role of brain-reactive autoantibodies in the causation of schizophrenia has been discussed since the early 20th century.53 Autoantibodies against components of various brain regions, cellular proteins, and dietary antigens, such as gliadin and casein, in serum and cerebrospinal fluid have been seen in schizophrenia and related psychosis.54,55 Antibodies against neuronal cell-surface targets, N-methyl-D-aspartate (NMDA) receptor, and components of the voltage-gated potassium channel complex have been reported in some patients with first episode psychosis and schizophrenia defined according to the diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV).6,56–59 Some of these antibodies have been typically associated with anti-NMDA receptor encephalitis, a progressive illness that often starts with psychotic symptoms or seizures, and subsequently manifests other neurological and autonomic features.60 When present in patients with encephalitis, the antibodies are deemed to be pathogenic, and removal of the antibodies is associated with clinical improvement. Early identification of the antibodies and treatment with immunotherapy has been reported to predict good clinical outcome.61 Whether this finding applies for patients with antibodies and a purely psychiatric presentation remains to be tested, although a few case descriptions exist to support this.6 Randomised controlled trials of immunotherapy as an adjunct of standard antipsychotic treatment for antibody-associated cases of psychosis are needed to examine this question.
Could NMDA receptor antibody seropositivity in some people with psychosis be, in fact, undiagnosed anti-NMDA receptor encephalitis? Diagnostic misclassification is unlikely to be the sole explanation for this finding because almost none of the NMDA receptor antibody seropositive cases of psychosis have IgG class NMDA receptor antibodies against the NR1 subunit alone (one of the subunits of this antibody).59,62 An association between NMDA receptor antibody and schizophrenia is biologically plausible; in healthy volunteers, blockade of this receptor with ketamine produced psychotic symptoms.63 Furthermore, a 2014 study of schizoprenia64 reported that de-novo mutations, in the form of chromosomal copy number changes, affect glutamatergic post-synaptic proteins that form part of the receptor.
NMDA receptor antibody seropositivity is not restricted to patients with schizophrenia alone. Patients with psychiatric disorders, such as schizophrenia, depression, and bipolar disorder, are collectively about three times more likely to have elevated NMDA receptor antibody titres than controls, based on high-specificity (but not low-specificity) seropositivity thresholds.62 This underscores the need for further cross-sectional and longitudinal studies of psychiatric cases and healthy controls, which employ standardised assay methods and seropositivity threshold definitions. Preclinical studies are also needed to elucidate pathogenic mechanisms of these antibodies in psychosis and other mental illnesses.
T lymphocytes
T cells are thymus-derived lymphocytes which, very simply, can be thought of as either CD8-expressing cytotoxic cells or CD4-expressing helper cells, and can have both proinflammatory and anti-inflammatory roles. Evidence suggests a role for T cells in the causes of schizophrenia. Acute psychosis is associated with the activated phenotype of lymphocytes within the CNS compared with controls.65 In post-mortem studies, immunohistology has allowed direct visualisation of both increased T cell and B cell numbers within the hippocampus in patients with schizophrenia.66 These changes were especially evident in those patients with predominantly negative, rather than positive symptoms.67 However, inconsistencies exist between studies as to whether schizophrenia is associated with increased or decreased numbers of lymphocytes in the peripheral circulation. A 2013 meta-analysis68 reported increased numbers of cells positive for CD56 (a marker of natural killer cells and activated T cells), and an increased CD4/CD8 T cell ratio in schizophrenia. The study also underscored important limitations of the existing data. Many studies do not control for immune-mediating variables, such as smoking, body mass, stress-associated cortisol concentrations, and medication. Findings from individual studies are difficult to compare because they report percentages rather than cell numbers.
Well controlled studies reporting absolute cell numbers are needed to fully understand any associations between T cells and schizophrenia. Since schizophrenia is a heterogeneous condition, different T-cell subtypes might be associated with differing symptoms. Thus studies of T cells might be more informative after stratification of cases by symptom profile. To fully understand the role of T cells in schizophrenia, an understanding of the function of these cells is also needed. Future studies should assess the cytokine profile of these cells, their activation status, and gene expression profiles.
How might immune dysregulation lead to the manifestation of schizophrenia?
Effects on neurotransmitters
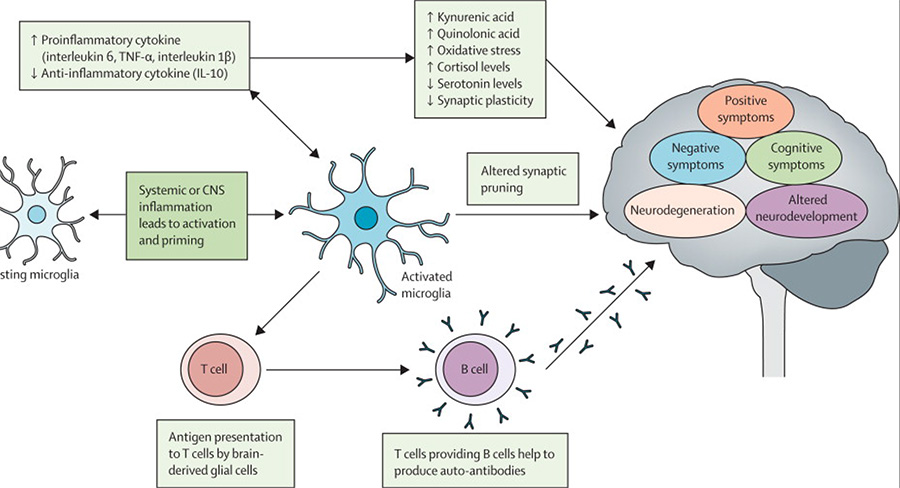
Studies in mice suggest an association between prenatal infection and inflammation and disturbance of neurotransmitter systems (glutamate, dopamine, and γ-aminobutyric acid [GABA]) in the offspring.69 However, little is known about the direct effects of the inflammatory immune response on neurotransmitter systems in human beings. NMDA receptor antagonism and glutamatergic hypofunction have long been proposed as underlying mechanisms for psychotic symptoms and cognitive dysfunction in schizophrenia.63,70 Evidence suggests that proinflammatory cytokines increase the concentration of kynurenic acid, which is a metabolite of tryptophan and the only naturally occurring NMDA receptor antagonist in the human CNS (figure 2).71,72
Figure 2. Possible mechanisms of immune-mediated causation of psychosis.
TNFα=tumour necrosis factor α. CNS=central nervous system.
Studies of the cyclooxygenase (COX) pathway might lead to a better understanding of the role of kynurenic acid in psychosis. COX1 inhibition increases the concentration of kynurenic acid, while COX2 inhibition decreases this concentration.73 This finding might explain some of the side-effects of COX1 inhibitors, such as psychotic symptoms and cognitive dysfunction. Furthermore, celecoxib (a selective COX2 inhibitor) has been reported to improve clinical symptoms in schizophrenia.74,75 Therefore, systematic evaluation of the neuropsychiatric side-effects of COX1 and COX2 inhibitors from existing randomised controlled trial data would be useful.
The association between neurotransmitters and immune mediators can be reciprocal and a few publications have reported immunoregulatory functions for dopamine.76 T cells express dopamine receptors, the stimulation of which promotes upregulation of adhesion molecules and cytokine production.77,78 Increased expression of the dopamine D3 receptor and increased synthesis of the proinflammatory cytokine interferon γ by lymphocytes have been reported in unmedicated patients with schizophrenia.79 The opposite has been reported in Parkinson’s disease,80 a condition characterised by CNS dopamine depletion. Further studies are needed to understand the effects of inflammation on neurotransmission and vice versa in both healthy people and people with psychiatric disorders.
Effects on neurodegeneration
That a neurodegenerative process is active in schizophrenia beyond that seen in healthy people is suggested by progressive clinical deterioration, cognitive decline, and loss of cortical grey matter in combination with histopathological evidence of neuronal atrophy and reductions in the number of neuronal synapses and dendrites.81–83 Activated microglia are increasingly recognised as an important component in the pathogenesis of other degenerative brain conditions, such as Alzheimer’s disease, in which they seem to have wide-ranging effects.48 Could microglial activation also contribute to neurodegeneration in schizophrenia? Microglial activation interferes with neuronal survival by increasing oxidative stress and decreasing neurotrophic support.5 Schizophrenia is associated with changes in serum, plasma, and red blood cell markers of oxidative stress.84 A population-based longitudinal study85 reported a strong association between delirium and subsequent dementia and cognitive decline in older adults, which is not mediated by classical neuropathologies associated with dementia. Systemic inflammation, a common cause of delirium, might underlie this association. Indeed severe systemic illness in the elderly has been reported to be associated with subsequent cognitive decline and functional deterioration.86,87 In the future, studies should examine whether inflammatory processes could explain progressive cognitive decline and brain volume loss in some cases of schizophrenia.
Effects on neurodevelopment
Interference with brain development from early-life infection/inflammation is consistent with a neurodevelopmental view of schizophrenia.88,89 The association of adult schizophrenia with a variety of early-life infections14,15 suggests that a common pathway is probably involved: the proinflammatory immune response. This notion is supported by studies in mice. Simulated viral or bacterial infection, or direct injection with interleukin 6, in pregnant mice has been reported to produce intermediate phenotypes related to schizophrenia in the adult offspring.90 Some of these phenotypes, such as deficits in sensory gating and abnormal latent inhibition, are reversible by treatment with clozapine.91 Since infections are widespread in the general population, interactions with genetic or other factors are likely. Indeed, an additive effect of family history of psychosis and prenatal infection in the causation of schizophrenia has been reported in a Finnish cohort.92 In future, studies should examine the gene–infection interaction, and whether a sensitive period exists during development when exposure to infection is more harmful.
Genome-wide association studies and epidemiological studies indicate some overlap of genetic susceptibility between schizophrenia and serious infection.26,93 Childhood infection might have a priming effect on microglia (discussed previously). Thus, early-life infection, by affecting gene expression or in the presence of preexisting genetic liability, might lead to a distinct or pathological immune response. This might, in turn, lead to CNS alterations that make these individuals susceptible to developing psychotic illness subsequently in life. Studies with detailed phenotypic characterisation of early development and immune and genetic data are necessary to test this hypothesis.
The human microbiome and the gut–brain axis: an emerging area of interest
The intestinal microbiota consists of a vast bacterial community that resides primarily in the lower gut and lives in a symbiotic association with the host. A bidirectional neurohumoral communication system, known as the gut–brain axis, integrates the host gut and brain activities. The intestinal microbiota is thought to affect brain development and function via this axis, and thus might be relevant for neuropsychiatric disorders.94,95 Bacterial colonisation of germ-free mice increases metabolism of tryptophan, leading to more than doubling of concentrations of 5-hydroxy tryptophan and its metabolites, including kynurenic acid (which is relevant for psychosis as previously described). A study in mice96 suggests that manipulation of the intestinal microbiota can alter host cognitive function and behaviour.
The vagus nerve—which plays a central part in relaying of the systemic cytokine signal to the brain—might also be important for gut–brain communication. In mice, the anxiolytic and antidepressant effects of ingesting a specific strain of Lactobacillus can no longer be seen after vagotomy.97 Evidence shows increased intestinal inflammation in individuals with schizophrenia compared with controls, and similarly, in people with first episode psychosis who have not taken antipsychotics compared with those receiving antipsychotics.98 Studies in rats99 and human beings100 suggest that manipulation of the gut microbial composition affects systemic cytokine concentrations. Thus, intestinal microbiota might affect the brain and behaviour by changing systemic cytokine concentrations in schizophrenia. This hypothesis is important and warrants examination. In the future, studies should examine the associations between intestinal microbiota and behavioural, cognitive, and neurochemical phenotypes in psychiatric disorders and healthy controls.
Therapeutic implications of an immunological understanding of schizophrenia
The present understanding of the association between the immune system and risk of psychotic disorders holds promise for novel approaches for detection, treatment, and prevention. These approaches might include development or repurposing of drugs to target inflammatory pathways, immunotherapy for antibody-associated cases of psychosis and other mental illnesses, and stratification of patients by their immune phenotype to inform treatment decisions and measure treatment response. Indeed, a 2014 molecular study101 reported two distinct subgroups of patients with schizophrenia characterised by predominant abnormalities in either immune molecules or growth factors and hormones.
Randomised controlled trials of anti-inflammatory drugs as adjuncts to standard therapy have shown promising results in schizophrenia (table),74,75,102–118 although such trials are few and have often involved small samples.119 Celecoxib has been reported to improve cognitive function in the early stages of schizophrenia,106,108 but the use of COX2 inhibitors is problematic because they can increase risk of heart disease.120 Minocycline, a centrally acting tetracyclic anti-inflammatory drug, has been reported to improve negative symptoms and cognitive function in schizophrenia.111,112 Large multicentre trials are needed, with stratification of patients by their immune phenotype. This approach proved useful in a randomised controlled trial of infliximab (a TNFα antagonist) in treatment-resistant depression, in which no overall efficacy was noted but infliximab improved depressive symptoms in patients with high concentrations of inflammatory markers at the start of the trial.121 The absence of clinical benefit from conventional antidepressants has been suggested to be related to activation of the inflammatory system.122 In future, randomised controlled trials should focus on specific patient groups characterised by, for example, resistance to conventional antipsychotics, presence of a defined pattern of immune activation, such as a predominantly proinflammatory molecular signature, or symptom profile, such as predominant negative symptoms or cognitive dysfunction.
Table. Anti-inflammatory agents in the treatment of schizophrenia and related psychosis.
| Design and setting | Sample and source | Outcome and exposure measurement | Main findings | Strengths | Limitations | |
|---|---|---|---|---|---|---|
| Longitudinal studies of treatment with NSAIDs and schizophrenia risk | ||||||
| Laan et al (2007)102 | Nested case-control design; compares incidence of schizophrenia in users and non-users of prescribed NSAIDs | 82 patients with schizophrenia and 359 controls from routine register | Outcome: new prescription for antipsychotics for ≥3 months as proxy for incident schizophrenia; Exposure: prescription for NSAIDs in the preceding 4 years | No association overall, but after adjustment for age and prescription frequency, protective effect of NSAID use in men, OR for schizophrenia 0·41 (95% CI 0·17–0·97) | Large population-based study | No direct assessment for schizophrenia or NSAID use; short follow-up; included young individuals |
| Stolk et al (2007)103 | Nested case-control design; compares exacerbation of schizophrenia in users and non-users of prescribed NSAIDs | Exacerbation of psychotic symptoms (case event; n=1443) compared with non-exacerbation (control event; n=1443) in ICD-9 schizophrenia cases receiving antipsychotic therapy with or without NSAIDs | Case event: change of antipsychotic, increase in dose, use of combination or parenteral treatment; Exposure: NSAID use 90 days before case event | No association overall; COX2 inhibitors led to exacerbation of symptoms, OR 2·56 (95% CI 1·35–4·87) | Large population-based study | No direct assessment for exacerbation of symptoms, prescription as proxy for NSAIDs use |
| RCTs of NSAIDs as adjunct of antipsychotic treatment in schizophrenia | ||||||
| Laan et al (2010)104 | Double-blind RCT of antipsychotic plus aspirin (1 g daily) or placebo; Trial duration: 90 days; Analysis: ITT | 37 aspirin vs 33 placebo; Inclusion criteria: DSM-IV schizophrenia, aged 18–55 years, illness duration <10 years; Exclusion criteria: contraindication to or chronic use of NSAIDs, stomach illness, corticosteroid use, pregnancy | Outcome measures: PANSS total, positive, negative, and general psychopathology subscores, cognitive tests, immunological markers | In the aspirin group, significant improvement in total and positive PANSS score (Cohen’s d 0·47 and 0·39, respectively) | Double-blind RCT, included both inpatient and outpatient cases, and various clinical outcome measures | Small sample, short duration, high attrition |
| Baheti et al (2013)105 | Open-label trial of celecoxib (400 mg daily); Trial duration: 42 days; Analysis: study completers | 31 celecoxib plus olanzapine vs 31 olanzapine only; Inclusion criteria: ICD-10 schizophrenia with acute relapse; Exclusion criteria: comorbid physical or other psychiatric illness, treatment-resistant schizophrenia, PANSS score <80 or >120 | Outcome measures: PANSS total, positive, negative, and general psychopathology subscores | Significantly lower positive, negative and general psychopathology scores at 3 and 6 weeks in the celecoxib, than olanzapine only group | Objective outcome measure | Small sample, short duration, no placebo or blinding of assessment |
| Muller et al (2002)106*; Muller et al (2004)107*; Muller et al (2005)108* | Double-blind RCT of risperidone (2–6 mg daily) plus celecoxib (400 mg daily) or placebo; Trial duration: 35 days; Analysis: ITT | 25 celecoxib vs 25 placebo; Inclusion criteria: DSM-IV schizophrenia, aged 18–65 years | Outcome measures: PANSS scores; cognitive factor of the PANSS scale; serum immunological measures | Total PANSS score lower in the celecoxib arm; no difference on cognitive measures; lower baseline TNF-R1 predicted better response to celecoxib | Double-blind RCT, use of plasma drug levels for monitoring | Small sample, and short duration |
| Rapaport et al (2005)109†; Bresee et al (2006)110† | Double blind RCT of risperidone/olanzapine plus celecoxib (400 mg daily) or placebo; Trial duration: 56 days | 18 celecoxib vs 17 placebo; Inclusion criteria: stable outpatient cases of DSM-IV schizophrenia Exclusion criteria: use of other antipsychotics, other criteria similar to Laan et al (2010) | Outcome measures: PANSS, SANS, CGI, CDS, HAM-A scales; serum cytokine levels | No difference between groups on any measures | RCT, used various outcome measures | Only included stable cases, analysed trial completers, small sample, and short duration |
| Akhondzadeh et al (2007)74 | Double blind RCT of risperidone (6 mg daily) plus celecoxib (400 mg daily) or placebo; Trial duration: 56 weeks; Analysis: ITT | 30 celecoxib vs 30 placebo; Inclusion criteria: inpatient DSM-IV schizophrenia, aged 19–44 years Exclusion criteria: organic disease or substance dependence, other psychotic illness, peptic ulcer, gastrointestinal bleeding, pregnancy or lactation | Outcomes: PANSS scores | Significant improvement in PANSS total, positive, and general scores in the celecoxib arm | Double-blind RCT, low drop out | Small sample, short duration of trial |
| Muller et al (2010)75 | Double blind RCT of amisulpride plus celecoxib (400 mg daily) or placebo; Trial duration: 42 days | 25 celecoxib vs 25 placebo; Inclusion criteria: inpatient cases of DSM-IV schizophrenia or schizophreniform disorder, aged 19–44 years, illness duration <2 years | Outcomes: PANSS and CGI; | Significant global clinical improvement in the celecoxib arm using ITT analysis | Double-blind RCT | Small sample, short duration, and high attrition |
| RCTs of minocycline as an adjunct of antipsychotic treatment in schizophrenia | ||||||
| Levkovitz et al (2010)111 | Double-blind RCT of antipsychotic plus minocycline (200 mg daily) or placebo; Trial duration: 26 weeks | 36 minocycline vs 18 placebo; Inclusion criteria: DSM-IV schizophrenia, aged 18–35 years, illness duration <5 years; Exclusion criteria: contraindication to or use of minocycline in last 6 months, compulsory admission to hospital, others similar to Akhondzadeh et al74 | Outcomes: SANS, PANSS, CGI, CDS, ITAQ, CANTAB; | In the minocycline arm, significant improvement in negative symptoms, executive function, clinical status, and general functioning starting from week 14 | Double-blind RCT, use of clinical, cognitive, and functional outcome measures, long duration | Small sample, high attrition |
| Chaudhry et al (2012)112 | Double blind RCT of antipsychotic treatment plus minocycline (200 mg daily) or placebo; Trial duration: 52 weeks; Analysis: ITT | 71 minocycline vs 73 placebo; Inclusion criteria: DSM-IV in or outpatient cases of schizophrenia, and related psychosis, aged 18–65 years, diagnosis <5 years, stable on medication; Exclusion criteria: similar to Levkovitz et al107 | Outcomes: PANSS, CGI, GAF, CANTAB | In the minocycline arm, significant improvement in negative symptoms | Double-blind RCT, use of clinical, cognitive, and functional outcome measures, long trial duration | Small sample, high attrition |
| Case reports of minocycline and aspirin as an adjunct of antipsychotic treatment in schizophrenia | ||||||
| Webb et al (2013)113 | Aspirin (650 mg daily) plus aripiprazole 20 mg daily | A 13-year-old Hispanic male with schizophrenia | Outcome: PANSS score and clinical impression | Hallucinations, and social communication improved in a few days | Early-onset case | Only one case, no control group |
| Jhamnani et al (2013)114 | Minocycline (10 mg daily) plus atypical antipsychotic for 2–3 months | Two patients with ICD-10 schizophrenia with persistent negative symptoms | Outcome: SANS and SAPS | Improvement in negative symptoms and serum CRP levels compared with baseline | Use of CRP and psychopathology measures | No control group |
| Miyaoko et al (2008)115 | Minocycline (200–450 mg daily) plus atypical antipsychotic for 4 weeks | 22 inpatient or outpatient cases of DSM-IV schizophrenia not responding to current treatment | Outcome: PANSS | Significant decrease in positive and negative symptoms after 4–8 weeks | Large case series | No control group |
| Miyaoko et al (2007)116 | Minocycline (150 mg daily) plus haloperidol/risperidone for 10–11 weeks | Two male patients with DSM-IV schizophrenia with prominent catatonic features and concurrent infection | Outcome: clinical impression | Clinical improvement in both cases | · · | Only two cases, no control group |
| Chaves et al (2010)117 | Minocycline (200 mg daily) plus haloperidol for 8 weeks | A 19-year-old man with a 5-year history of treatment resistant DSM-IV schizophrenia | Outcome: PANSS score, and brain scan | Improvement in positive symptoms, and decrease in hyperperfusion of posterior cingulate gyrus | Use of neuroimaging data | Only one case, no control group |
| Kelly et al (2011)118 | Minocycline (200 mg daily) plus clozapine for up to 16 weeks | Two cases of schizophrenia with catatonic symptoms | Outcome: BPRS, SANS, CDS | In both cases, improvement in negative and positive symptoms | · · | Only two cases, no control group |
RCT=randomised controlled trial. PANSS=positive and negative syndrome scale. DSM-IV=diagnostic and statistical manual of mental disorders, fourth edition. ITT=intention to treat. TNFα=tumour necrosis factor α. SANS=scale for the assessment of negative symptoms. SAPS=scale for assessment of positive symptoms. CGI=clinical global impressions scale. HAM-A=Hamilton anxiety rating scale. CDS=Calgary depression scale for schizophrenia. ITAQ=insight and treatment attitude questionnaire. CANTAB=Cambridge neuropsychological test automated battery. GAF=global assessment of functioning. BPRS=Brief psychiatric rating scale.
Articles share the same dataset but focus on different outcomes.
Articles share the same sample but focus on different outcomes. NSAID=non-steroidal anti-inflammatory drug.
Identification of specific inflammatory pathways for neuropsychiatric symptoms would provide novel targets for therapeutic intervention.123 Mouse studies44 suggest a mechanistic role for quinolonic acid, an NMDA receptor agonist, in inflammation-induced depression. Furthermore, randomised controlled trials of NMDA receptor antagonists, ketamine, and compound AZD6765 have shown promising results for treatment-resistant depression.124–126 Although the safety and tolerability of ketamine might restrict its applicability in clinical setting, these findings provide further support for a role for inflammation in major mental illness.
New therapeutics for immunologically stratified psychosis might be based on molecules and targets that are already well known in other therapeutic areas, allowing repurposing of existing anti-inflammatory drugs.127 Collaboration between industry and academia would be important to realise the potential of immune-modulatory molecules for the treatment of major mental illness. In future, studies should assess the prophylactic potential of immunological drugs in individuals at high risk of developing psychosis. Longitudinal associations between higher concentrations of interleukin 6 and subsequent risks of psychosis,2 depression,2 heart disease,128 and type 2 diabetes129 suggest that control of inflammation might reduce the risk of several chronic adult diseases, and thus, have a huge beneficial effect at the population level.
Conclusion
Inflammation and immune dysfunction might contribute to cognitive, negative, and positive symptoms in schizophrenia. We have described several hypotheses and potential areas of interest for future research regarding the immunological aspects of schizophrenia. Addressing these issues would contribute to understanding the disease mechanism and development of new effective interventions. However, success would need collaborative work between several disciplines. Although animal model studies have much to offer in terms of understanding specific biological systems, these findings need to be confirmed in human beings. This underscores the scope for translational research in schizophrenia over the coming years, encompassing immune, genetic, microbiological, and other biomarkers.
Panel 1. The link between immune system and psychosis has a long history.
1876—Alexander Rosenblum suggests that typhoid or malaria fever might cure psychosis.
1926—Karl Menninger publishes 200 cases of postinfluenzal psychosis; a third of which were reported to resemble dementia praecox (conceptual predecessor of schizophrenia).
1927—Julius Wagner-Jauregg is awarded the Nobel prize for medical inoculation of malarial parasites as a treatment for syphilitic psychosis.
1929—Moritz Tramer reports an association between schizophrenia and winter or spring birth.
1937—Lehman Facius describes autoantibodies against brain structures in the cerebrospinal fluid of patients with schizophrenia.
1988—Sarnoff Mednick and colleagues report increased risk of schizophrenia in adult offspring of women pregnant during the 1957 influenza pandemic. Although subsequent meta-analysis of ecological studies do not find an association between pandemic influenza and schizophrenia, these findings spur on a great deal of research, leading to some important discoveries regarding early-life infection and schizophrenia by use of serological data for prenatal maternal infection.
1992—Ronald Smith proposes a macrophage-T-lymphocyte theory of schizophrenia.
Panel 2. Key research questions about the immunological aspects of schizophrenia and other psychiatric disorders.
Is increased serum concentration of proinflammatory cytokines a cause or consequence of schizophrenia?
Could systemic inflammatory markers be used to identify prodromal cases of schizophrenia, to predict disease progression, response to antipsychotic treatment and recovery?
Could inflammatory processes explain cognitive deficits, progressive cognitive decline and brain volume loss in some cases of schizophrenia?
Could inflammatory processes explain the association between early-life adversity and risk of psychiatric disorders in adult life?
Does activation of microglia correspond with clinical severity of schizophrenia and response to antipsychotic treatment?
What is the role of neuronal cell surface autoantibodies in schizophrenia and other psychiatric disorders?
What is the prevalence of neuronal cell surface antibodies in schizophrenia, other psychiatric disorders, and healthy controls?
Does the prevalence of neuronal cell surface antibodies depend on the phase of the illness? Are there clinical signs that predict a positive test?
Are there other peripheral biomarkers associated with neuronal cell surface antibodies which could form therapeutic targets?
What is the association between intestinal microbiota and inflammatory, behavioural, cognitive, and neurochemical phenotypes seen in schizophrenia and other psychiatric disorders?
What is the response of antibody-associated psychosis to immunosuppression or antipsychotic treatment?
Does control of inflammation lead to clinical improvement in psychosis and other mental illness?
Is stratification of patients based on their immune phenotype helpful with respect to prediction of response to conventional and novel treatments?
Search strategy and selection criteria.
For this Review, each contributing author selected references that they deemed most relevant for their particular topic. We used systematic reviews and meta-analyses, where available, in addition to expert reviews, classical articles, and recent articles that demonstrate cutting-edge advances in the field. GMK searched the PubMed database from its inception until Sept 22, 2014, for studies of anti-inflammatory agents in schizophrenia and related psychosis. Search terms included the following Mesh terms: “schizophrenia” (Mesh) or “psychotic disorders” (Mesh), and “anti-inflammatory agents, non-steroidal” (Mesh), or “anti-inflammatory agents” (Mesh), or “Minocycline” (Mesh). Selected studies were published in English, included cases of schizophrenia and related psychotic disorders, and were observational, clinical trials, case series, or case reports by design. Studies that did not report primary data, such as reviews or trial protocols, were excluded. Studies of anti-inflammatory agents in disorders other than psychosis were also excluded. The PubMed search identified 157 studies, 19 of which were selected after screening of title, abstract, and an examination of full article content.
For a video on microenvironment surveillance by microglia see http://www.nature.com/news/microglia-the-constant-gardeners-1.10732
Acknowledgments
We thank Claire Dibben (Norfolk and Suffolk NHS Foundation Trust, Norwich, UK) for her helpful comments on a previous version of this manuscript. This work was supported by a clinical research fellowship grant from the Wellcome Trust to GMK (094790/Z/10/Z; 2010-’13), grants from the Stanley Medical Research Institute and the National Institute of Mental Health (MH-94268) to RY, and grants from the Wellcome Trust (095844/Z/11/Z and 088869/Z/09/Z), and the National Institute for Health Research (RP-PG-0606–1335) to PBJ.
Footnotes
Declaration of interests
We declare no competing interests regarding the content of this study. RY is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. PBJ received an honorarium that he donated to his department from Roche for taking part in an advisory board to advise on education about schizophrenia for psychiatrists.
Contributor Information
Golam M Khandaker, Department of Psychiatry, University of Cambridge, Cambridge, UK; National Institute for Health Research Cambridge Biomedical Research Centre, Cambridge, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK.
Lesley Cousins, Department of Psychiatry, University of Cambridge, Cambridge, UK; National Institute for Health Research Cambridge Biomedical Research Centre, Cambridge, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK.
Julia Deakin, Department of Psychiatry, University of Cambridge, Cambridge, UK; National Institute for Health Research Cambridge Biomedical Research Centre, Cambridge, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK.
Belinda R Lennox, Department of Psychiatry, University of Oxford, Oxford, UK.
Prof Robert Yolken, Stanley Division of Developmental Neurovirology, Johns Hopkins University, Baltimore, MD, USA.
Prof Peter B Jones, Department of Psychiatry, University of Cambridge, Cambridge, UK; National Institute for Health Research Cambridge Biomedical Research Centre, Cambridge, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, UK.
References
- 1.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin-6 and c-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitdunal study. JAMA Psychiatry. 2014;71:1121–28. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–19. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 4.Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zandi MS, Irani SR, Lang B, et al. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. 2011;258:686–88. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter RHS, editor. Neurophysiology. 4th edn Hodder Arnold; London: 2002. [Google Scholar]
- 8.Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology: the immune system in health and disease. 5th edn Garland Science; New York: 2001. [Google Scholar]
- 9.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 11.Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other psychoses in England, 1950–2009: a systematic review and meta-analyses. PloS One. 2012;7:e31660. doi: 10.1371/journal.pone.0031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khandaker GM, Clarke M, Cannon M, Jones PB. Life course approach to specific mental disorders: schizophrenia and related psychosis. In: Koenen K, Rudenstine S, Susser E, Galeo S, editors. Life course epidemiology of mental disorders. 1st edn Oxford University Press; New York, NY: 2014. pp. 61–75. [Google Scholar]
- 13.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139:161–68. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43:239–57. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canetta S, Sourander A, Surcel HM, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171:960–68. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner RM, Dalman C, Wicks S, Lee BK, Karlsson H. Neonatal levels of acute phase proteins and later risk of non-affective psychosis. Transl Psychiatry. 2013;3:e228. doi: 10.1038/tp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandaker GM, Stochl J, Zammit S, Lewis G, Jones PB. Childhood Epstein-Barr Virus infection and subsequent risk of psychotic experiences in adolescence: A population-based prospective serological study. Schizophr Res. 2014;158:19–24. doi: 10.1016/j.schres.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull (Bp) 2012;38:642–47. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khandaker GM, Zammit S, Lewis G, Jones PB. A population-based study of atopic disorders and inflammatory markers in childhood before psychotic experiences in adolescence. Schizophr Res. 2014;152:139–45. doi: 10.1016/j.schres.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 23.Eaton WW, Byrne M, Ewald H, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–28. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- 24.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–57. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–47. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–27. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andreassen OA, Harbo HF, Wang Y, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.195. published online Jan 28. http://dx.doi.org/10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–08. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Wium-Andersen MK, Orsted DD, Nordestgaard BG. Elevated C-reactive protein associated with late- and very-late-onset schizophrenia in the general population: a prospective study. Schizophr Bull (Bp) 2014;40:1117–27. doi: 10.1093/schbul/sbt120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguli R, Brar JS, Chengappa KN, Yang ZW, Nimgaonkar VL, Rabin BS. Autoimmunity in schizophrenia: a review of recent findings. Ann Med. 1993;25:489–96. doi: 10.3109/07853899309147317. [DOI] [PubMed] [Google Scholar]
- 33.Garver DL, Tamas RL, Holcomb JA. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28:1515–20. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- 34.Hayes LN, Severance EG, Leek JT, et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull (Bp) 2014;40:963–72. doi: 10.1093/schbul/sbu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stojanovic A, Martorell L, Montalvo I, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32. doi: 10.1016/j.psyneuen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 36.de Witte L, Tomasik J, Schwarz E, et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res. 2014;154:23–29. doi: 10.1016/j.schres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Maes M, Meltzer HY, Bosmans E. Immune-inflammatory markers in schizophrenia: comparison to normal controls and effects of clozapine. Acta Psychiatr Scand. 1994;89:346–51. doi: 10.1111/j.1600-0447.1994.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 38.Maes M, Bosmans E, Calabrese J, Smith R, Meltzer HY. Interleukin-2 and interleukin-6 in schizophrenia and mania: effects of neuroleptics and mood stabilizers. J Psychiatr Res. 1995;29:141–52. doi: 10.1016/0022-3956(94)00049-w. [DOI] [PubMed] [Google Scholar]
- 39.Maes M, Bosmans E, Kenis G, De Jong R, Smith RS, Meltzer HY. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr Res. 1997;26:221–25. doi: 10.1016/s0920-9964(97)00057-1. [DOI] [PubMed] [Google Scholar]
- 40.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral Inflammation Acutely Impairs Human Spatial Memory via Actions on Medial Temporal Lobe Glucose Metabolism. Biol Psychiatry. 2014;76:585–93. doi: 10.1016/j.biopsych.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eraly SA, Nievergelt CM, Maihofer AX, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–31. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker AK, Budac DP, Bisulco S, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichenberg A, Yirmiya R, Schuld A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 46.Santambrogio L, Belyanskaya SL, Fischer FR, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 48.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 49.van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–22. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 50.Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–07. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 51.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–25. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehmann-Facius H. Über die Liquordiagnose der Schizophrenien. Klinische Wochenschrift. 1937;16:1646–48. (in German) [Google Scholar]
- 54.Lachance LR, McKenzie K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis. Schizophr Res. 2014;152:521–27. doi: 10.1016/j.schres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav Immun. 2001;15:319–39. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- 56.Deakin J, Lennox BR, Zandi MS. Antibodies to the N-methyl-D-aspartate receptor and other synaptic proteins in psychosis. Biol Psychiatry. 2014;75:284–91. doi: 10.1016/j.biopsych.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Lennox BR, Vincent A. Antibody-mediated encephalitis: a treatable cause of schizophrenia? Br J Psychiatry. 2012;200:92–94. doi: 10.1192/bjp.bp.111.095042. [DOI] [PubMed] [Google Scholar]
- 58.Parthasarathi UD, Harrower T, Tempest M, et al. Psychiatric presentation of voltage-gated potassium channel antibody-associated encephalopathy. Case report. Br J Psychiatry. 2006;189:182–83. doi: 10.1192/bjp.bp.105.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steiner J, Walter M, Glanz W, et al. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–78. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 60.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res. 2014;157:249–58. doi: 10.1016/j.schres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Pomarol-Clotet E, Honey GD, Murray GK, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–79. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikkila HV, Muller K, Ahokas A, Rimon R, Andersson LC. Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 2001;49:99–105. doi: 10.1016/s0920-9964(99)00218-2. [DOI] [PubMed] [Google Scholar]
- 66.Nikkila H, Muller K, Ahokas A, Miettinen K, Andersson LC, Rimon R. Abnormal distributions of T-lymphocyte subsets in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 1995;14:215–21. doi: 10.1016/0920-9964(94)00039-b. [DOI] [PubMed] [Google Scholar]
- 67.Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26:1273–79. doi: 10.1016/j.bbi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–99. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–34. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 70.Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull (Bp) 1990;16:425–32. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- 71.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 72.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 73.Schwieler L, Erhardt S, Erhardt C, Engberg G. Prostaglandin-mediated control of rat brain kynurenic acid synthesis—opposite actions by COX-1 and COX-2 isoforms. J Neural Transm. 2005;112:863–72. doi: 10.1007/s00702-004-0231-y. [DOI] [PubMed] [Google Scholar]
- 74.Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–85. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Muller N, Krause D, Dehning S, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–24. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 76.Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain Behav Immun. 2010;24:525–28. doi: 10.1016/j.bbi.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J Neuroimmunol. 2005;169:161–71. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. Eur J Immunol. 2001;31:3504–12. doi: 10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 79.Ilani T, Ben-Shachar D, Strous RD, et al. A peripheral marker for schizophrenia: Increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci USA. 2001;98:625–28. doi: 10.1073/pnas.021535398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagai Y, Ueno S, Saeki Y, Soga F, Hirano M, Yanagihara T. Decrease of the D3 dopamine receptor mRNA expression in lymphocytes from patients with Parkinson’s disease. Neurology. 1996;46:791–95. doi: 10.1212/wnl.46.3.791. [DOI] [PubMed] [Google Scholar]
- 81.Woods BT. Is schizophrenia a Progressive Neurodevelopmental Disorder? Toward a Unitary Pathogenetic Mechanism. Am J Psychiatry. 1998;155:1661–70. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- 82.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 83.Veijola J, Guo JY, Moilanen JS, et al. Longitudinal changes in total brain volume in schizophrenia: relation to symptom severity, cognition and antipsychotic medication. PloS One. 2014;9:e101689. doi: 10.1371/journal.pone.0101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–09. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809–16. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khandaker GM, Jones PB. Cognitive and functional impairment after severe sepsis. JAMA. 2011;305:673–74. doi: 10.1001/jama.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? BMJ (Clin Res Ed) 1987;295:681–82. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–69. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 90.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 91.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–30. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 93.Nielsen PR, Laursen TM, Mortensen PB. Association Between Parental Hospital-Treated Infection and the Risk of Schizophrenia in Adolescence and Early Adulthood. Schizophr Bull (Bp) 2013;39:230–37. doi: 10.1093/schbul/sbr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–42. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 95.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 96.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–55. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Severance EG, Alaedini A, Yang S, et al. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr Res. 2012;138:48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–88. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 100.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 101.Schwarz E, van Beveren NJ, Ramsey J, et al. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull (Bp) 2014;40:787–95. doi: 10.1093/schbul/sbt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laan W, Selten JP, Grobbee DE, Smeets H, Kahn RS, Burger H. Nonsteroidal anti-inflammatory drugs and the risk of psychosis. Eur Neuropsychopharmacol. 2007;17:309–11. doi: 10.1016/j.euroneuro.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Stolk P, Souverein PC, Leufkens HG, Weil JG, Egberts AC, Heerdink ER. The association between exposure to COX-2 inhibitors and schizophrenia deterioration. A nested case-control study. Pharmacopsychiatry. 2007;40:111–15. doi: 10.1055/s-2007-977714. [DOI] [PubMed] [Google Scholar]
- 104.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–27. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 105.Baheti T, Nischal A, Nischal A, et al. A study to evaluate the effect of celecoxib as add-on to olanzapine therapy in schizophrenia. Schizophr Res. 2013;147:201–02. doi: 10.1016/j.schres.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 106.Muller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–34. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 107.Muller N, Ulmschneider M, Scheppach C, et al. COX-2 inhibition as a treatment approach in schizophrenia: immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 108.Muller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255:149–51. doi: 10.1007/s00406-004-0548-4. [DOI] [PubMed] [Google Scholar]
- 109.Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–96. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 110.Bresee CJ, Delrahim K, Maddux RE, Dolnak D, Ahmadpour O, Rapaport MH. The effects of celecoxib augmentation on cytokine levels in schizophrenia. Int J Neuropsychopharmacol. 2006;9:343–48. doi: 10.1017/S1461145705005808. [DOI] [PubMed] [Google Scholar]
- 111.Levkovitz Y, Mendlovich S, Riwkes S, et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–49. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 112.Chaudhry IB, Hallak J, Husain N, et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J Psychopharmacol. 2012;26:1185–93. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
- 113.Webb JR, Stubbe DE, Poncin YB. Aspirin as an adjunctive treatment for childhood onset schizophrenia. J Child Adolesc Psychopharmacol. 2013;23:585–86. doi: 10.1089/cap.2013.0033. [DOI] [PubMed] [Google Scholar]
- 114.Jhamnani K, Shivakumar V, Kalmady S, Rao NP, Venkatasubramanian G. Successful use of add-on minocycline for treatment of persistent negative symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2013;25:E06–07. doi: 10.1176/appi.neuropsych.11120376. [DOI] [PubMed] [Google Scholar]
- 115.Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol. 2008;31:287–92. doi: 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- 116.Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Possible antipsychotic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:304–07. doi: 10.1016/j.pnpbp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 117.Chaves C, de Marque CR, Wichert-Ana L, et al. Functional neuroimaging of minocycline’s effect in a patient with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:550–52. doi: 10.1016/j.pnpbp.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 118.Kelly DL, Vyas G, Richardson CM, et al. Adjunct minocycline to clozapine treated patients with persistent schizophrenia symptoms. Schizophr Res. 2011;133:257–58. doi: 10.1016/j.schres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–19. doi: 10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 120.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–59. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 121.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carvalho LA, Torre JP, Papadopoulos AS, et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord. 2013;148:136–40. doi: 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 123.Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18:1236–41. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zarate CA, Jr., Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 125.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zarate CA, Jr, Mathews D, Ibrahim L, et al. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–64. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bullmore ET, Lynall ME. Immunologic therapeutics and psychotic disorders. Biol Psychiatry. 2014;75:260–61. doi: 10.1016/j.biopsych.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 128.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]