Abstract
Drug-induced liver injury is a prominent reason for premarketing and postmarketing drug withdrawal and can be manifested in a number of ways, such as cholestasis, steatosis and fibrosis. The mechanisms driving these toxicological processes have been well characterized and have been emdedded in adverse outcome pathway frameworks in recent years. This paper reviews these constructs and simultaneously illustrates their use in the preclinical testing of drug-induced liver injury.
Keywords: AOP, DILI, steatosis, cholestasis, fibrosis, read-across, IATA
1. Introduction
Drug-induced liver injury (DILI) is of high clinical concern, as it is the leading cause of acute liver failure.1,2 Indeed, DILI accounts for about half of the cases of acute liver failure in the US and in the UK.1,3-6 Furthermore, DILI is also of clear relevance to pharmaceutical industry, since it underlies the withdrawal of a considerable number of drugs during premarketing and postmarketing phases of drug development.7 DILI can be predictable or unpredictable, designated as intrinsic or idiosyncratic hepatotoxicity, respectively. The former depends on the dose and becomes manifested within a few days. It mainly results from direct toxicity of a drug or its metabolites and can be reproduced in animal models. Idiosyncratic hepatotoxicity, which on its turn is subdivided into immune idiosyncrasy and metabolic idiosyncrasy, does not necessarily depend on the dose and occurs with variable latency.6,8-12 Idiosyncratic hepatotoxicity occurs in 1 in 10.000 to 100.000 individuals.1,3,4,6,10,13-15 Immunoallergic drug reactions are seen in about 25% to 30% of all DILI patients.16
Predictive toxicology, based upon mechanistic information, has become a critical aspect in the safety evaluation of drugs in the last decade. A major tool introduced in this regard is the adverse outcome pathway (AOP) framework, which refers to a conceptual construct that portrays existing knowledge concerning the linkage between a direct molecular initiating event (MIE) and an adverse outcome via a number of key events (KEs) at a biological level of organization relevant to risk assessment.17-21 AOPs have yet been designed for several human-relevant toxicological endpoints. In response to the increasing use of AOPs, the Organization for Economic Cooperation and Development together with the US Environmental Protection Agency, the US Army Engineer Research and Development Center and the European Joint Research Center has initiated a project to facilitate the use of AOPs in assessing the safety of chemicals, called the AOP Knowledge Base. The AOP Knowledge Base consists of four modules, namely the AOP Xplorer, Effectopedia, the Intermediate Effects Database and the AOP Wiki. The latter provides an open-source interface for rapid, widely accessible and collaborative sharing of established AOPs and building new AOPs.21 The AOP Wiki was launched in 2014 and thus far contains as much as fifty AOPs for diverse types of toxicity, including hepatotoxicity. Among those, a number are of clear relevance to the DILI field and will be scrutinized in this paper. Particular focus will be put on how these AOPs can form the basis for preclinical DILI testing.
2. Established AOPs on DILI
2.1. Liver fibrosis
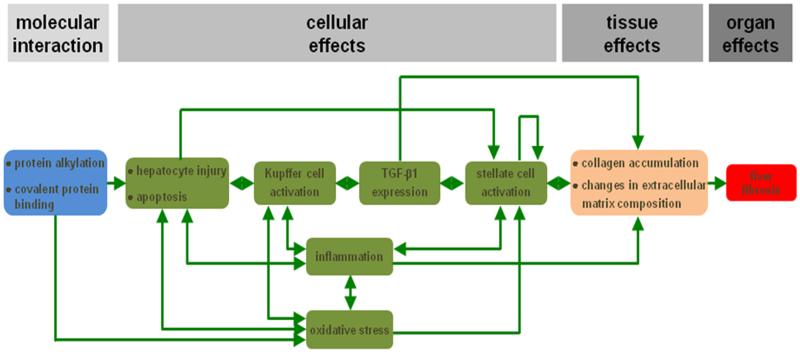
Liver fibrosis is a reversible wound healing response to either acute or chronic cellular injury that reflects a balance between liver repair and scar formation. It can be activated by a number of drugs, such as methotrexate. A central event in liver fibrosis is the activation of hepatic stellate cells, which occurs in two phases, namely the initiation stage and the perpetuation stage.20,22-27 In the initiation phase, quiescent hepatic stellate cells become responsive to growth factors. This may be triggered by a variety of signals, including reactive oxygen species and apoptotic bodies originating from dying hepatocytes. In the perpetuation phase, the primed hepatic stellate cells undergo several changes related to proliferation, contractility, fibrogenesis, chemotaxis, extracellular matrix degradation and retinoid loss, whereby they adopt a myofibroblast-like phenotype. Hepatic stellate cell activation may be counteracted in a resolution phase through apoptosis, senescence or reversion to the quiescent phenotype.20,25,26 Protein alkylation is considered as the MIE in an established AOP on liver fibrosis, whereas the obvious adverse outcome at the organ level is liver fibrosis (Figure 1). Different KEs at the cellular and tissue level have been defined, including hepatocyte injury and cell death, activation of Kupffer cells, expression of transforming growth factor beta 1, activation of hepatic stellate cells, oxidative stress and chronic inflammation, collagen accumulation and changes in hepatic extracellular matrix composition.20-23,28
Figure 1. AOP for drug-induced liver fibrosis.
The MIE (blue) is considered protein alkylation and covalent protein binding in the liver. This serves as a trigger to provoke hepatocyte injury, including apoptosis, which in turn activates Kupffer cells. As a result, transforming growth factor beta 1 (TGF-β1) expression is induced, which is a key factor for stellate cell activation. The latter goes hand in hand with the occurrence of inflammation and oxidative stress. The different KEs at the cellular level (green) are interconnected in several ways. The overall end result is accumulation of collagen and changes in the extracellular matrix composition in the liver (orange), which becomes clinically manifested as the adverse outcome, namely liver fibrosis (red) (reproduced with permission from20).
2.2. Liver steatosis
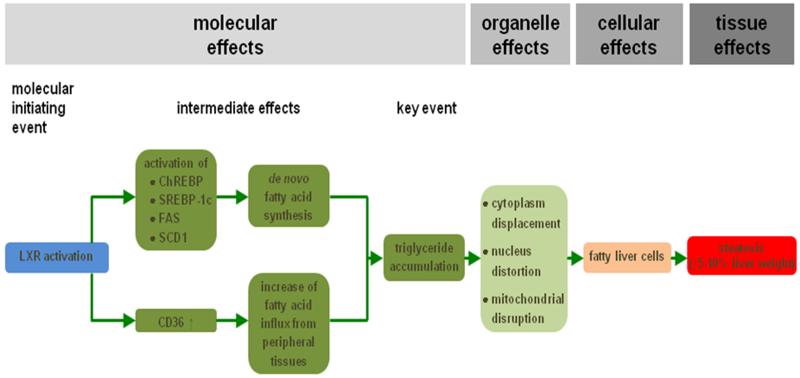
Steatosis is a prototypical type of drug-induced liver injury that refers to the process of abnormal retention of lipids, mainly triglycerides, within hepatocytes. It reflects the impairment of normal synthesis and elimination of triglycerides, and is triggered by a plethora of drugs, such as valproic acid.20,22,23,27 Steatosis can develop further into non-alcoholic steatohepatitis, which is characterized by hepatocellular injury and inflammation.20,29,30 Liver steatosis may occur in a microvesicular or in a macrovesicular pattern. In microvesicular steatosis, numerous small lipid droplets are present in the hepatocyte cytoplasm, which do not displace the cell nucleus. By contrast, large droplets that move the hepatocyte nucleus to the periphery are observed in macrovesicular steatosis.20,27,31-33 Since interaction of drugs with nuclear receptors is a frequent mechanism observed in liver steatosis, it has been considered as the main MIE in an established liver steatosis AOP (Figure 2). In particular, activation of the liver X receptor induces an array of effects, such as enhanced transcription of genes encoding mediators of cholesterol and lipid metabolism. This leads to the increased influx of fatty acids from peripheral tissues into the liver and equally drives do novo synthesis of fatty acids. Consequently, triglycerides tend to accumulate in hepatocytes, which is considered as a KE in this AOP. At the organelle level, hepatocellular lipid accumulation may provoke cytoplasm displacement, nucleus distortion, mitochondrial toxicity and endoplasmic reticulum stress. All together, these effects underlie the acquisition of the typical fatty liver cell phenotype, which in turn causes a clinically relevant increase in liver weight.20-23,34
Figure 2. AOP for drug-induced liver steatosis.
Activation of the liver X receptor (LXR), which is the MIE (blue), induces a number of transcriptional changes, including activation of the expression of carbohydrate response element binding protein (ChREBP), sterol response element binding protein 1c (SREBP-1c), fatty acid synthase (FAS) and stearoyl-coenzyme A desaturase 1 (SCD1). As a result, de novo synthesis of fatty acids is enhanced in the liver. At the same time, fatty acid translocase (CD36) production is upregulated, which mediates increased hepatic influx of fatty acids from peripheral tissues. All together, these steps drive the accumulation of triglycerides, which is considered a KE (dark green). At the organelle level, this evokes cytoplasm displacement, distortion of the nucleus and mitochondrial disruption. This ultimately burgeons into the appearance of fatty liver cells (orange) and further into the clinical diagnosis of liver steatosis (red) (reproduced with permission from20).
2.3. Cholestasis
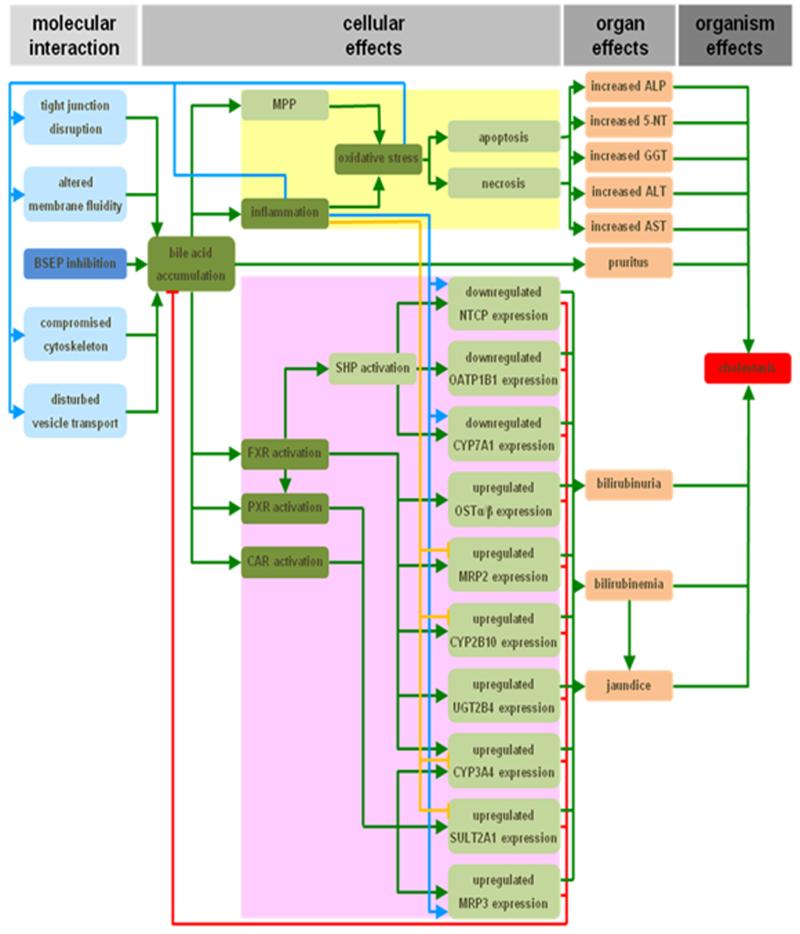
Cholestasis accounts for 47% of all reported DILI cases35 and can be caused by drugs such as bosentan. In the established cholestasis AOP, the MIE is the direct cis-inhibition of the bile salt export pump (Figure 3). As a result of this, toxic bile acids accumulate into hepatocytes or bile canaliculi. These bile salts trigger a direct deteriorative response and an adaptive response.20,27 At the cellular level, the deteriorative response is accompanied by the formation of the mitochondrial permeability pore, which leads to mitochondrial impairment, inflammation, the production of reactive oxygen species and ultimately to the onset of cell death by both apoptotic and necrotic mechanisms.20,36,37 Because of the latter, cytosolic enzymes start to leak from hepatocytes and cholangiocytes, and become measurable in the serum.20,38,39 A hallmark of cholestasis at the cellular level includes the induction of an adaptive response, which is aimed at counteracting bile accumulation and thus cholestatic liver injury. Accordingly, a complex machinery of transcriptionally coordinated mechanisms involving nuclear receptors is activated by bile acids, which collectively decrease the uptake and increase the export of bile acids and bilirubin into and from hepatocytes, respectively. Simultaneously, detoxification of bile acids is enhanced, while their synthesis becomes downregulated.20,40-42 The increased effort of cholestastic hepatocytes to remove bilirubin causes bilirubinuria and hyperbilirubinemia. As a result, yellowish pigmentation of the skin and the conjunctival membranes over the sclera becomes visible, known as jaundice. Furthermore, the elevated presence of bile acids in the serum is thought to account for the typical skin itching in cholestasis patients.20,38,39,42 Proposed KEs are the accumulation of bile, the induction of oxidative stress and inflammation, and the activation of nuclear receptors. Furthermore, the AOP distinguishes direct adverse and indirect adaptive effects, and takes a number of alternative MIEs mechanisms into account.20,21,43
Figure 3. AOP for drug-induced cholestasis.
The response matrix between the MIE (dark blue) and adverse outcome (red), the inhibition of the bile salt export pump (BSEP) and cholestasis, respectively, spans over the cellular and organ levels. Identified KEs (dark green) include the accumulation of bile, the induction of oxidative stress and inflammation, and the activation of the nuclear receptors pregnane X receptor (PXR), farnesoid X receptor (FXR) and constitutive androstane receptor (CAR). Together with a number of other steps, these KEs drive both a deteriorative cellular response (yellow), which underlies directly caused cholestatic injury, and an adaptive cellular response (purple), which is aimed at counteracting the primary cholestatic insults. Direct inducing and inhibiting effects are indicated with green and red arrows, respectively. Secondary inducing and inhibiting effects of oxidative stress and/or inflammation are indicated with blue and orange arrows, respectively (5′-NT, 5′-nucleotidase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CYP2B10/3A4/7A1, cytochrome P450 2B10/3A4/7A1; GGT, gamma-glutamyl transpeptidase; MPP, mitochondrial permeability pore; MRP2/3, multidrug resistance-associated protein 2/3; NTCP, sodium/taurocholate cotransporter; OATP1B1, organic anion transporter 1B1; OSTα/β organic solute transporter α/β; SHP, small heterodimeric partner; SULT2A1, dehydroepiandrosterone sulfotransferase; UGT2B4, uridine 5′-diphosphate-glucuronosyltransferase 2B4) (reproduced with permission from20).
3. Applications of AOPs and DILI testing
3.1. Read-across and chemical grouping
The term read-across refers to the process of reading information from a set of toxicologically well-characterized chemicals and presuming that a compound with unknown adverse properties will show similar effects because of its structural or mechanistic similarity to those chemicals.44,45 This inherently necessitates the establishment of chemical categories, such as based on structural alerts. This can be accomplished by addressing several in silico tools, including expert systems, relying on human expert knowledge, and statistical systems, using automated algorithms.46 Among those are quantitative structure-activity relationships, and using this approach, it has been demonstrated that chemicals with an ester bound to a carbon atom of a heterocyclic group or carbocyclic systems with a least one aromatic ring positively contribute to bile salt export pump inhibition, being the MIE in the AOP on drug-induced cholestasis, while the presence of hydroxyl groups bound to aliphatic carbon atoms has a negative contribution.47,48 In silico modeling further showed the role of hydroxyl groups in the interaction of chemicals with the bile salt export pump.49 Twodimensional and tridimensional quantitative structure-activity relationships studies have also been performed on ligands of the liver X receptor, which constitutes the MIE in the AOP on drug-induced steatosis. By doing so, a number of chemical features, such as the presence of phenyl rings, chloro groups and methyl moieties, have been identified as determinants of liver X receptor binding and activity.50 While such approaches typically start from toxicological mechanisms and then search for chemical structures that can trigger them, other strategies may work the other way around. For instance, using a dataset of over nine hundred drugs, sixteen structural alerts for hepatotoxicity in humans have been developed and a mechanistic rationale has been proposed to hypothesize MIEs leading to DILI. This kind of alerts have the potential to be used in the hepatotoxicity screening of drug candidates, while the chemical categories as such are important in applying read-across approaches.51 Thus, specific MIEs, in casu related to DILI AOPs, can be related to these chemical classes and may assist in predicting toxicity. However, it should be kept in mind that the ability to participate in a chemistry-dependent MIE does not lead to a toxicological outcome per se.44
3.2. Integrated approaches to testing and assessment
Integrated approaches to testing and assessment (IATA) are structured concepts used for hazard identification (i.e. potential), hazard characterization (i.e. potency) and/or safety assessment (i.e. potential/potency and exposure) of a chemical or group of chemicals, which strategically integrate and weight all relevant data required for regulatory decision-making, while reducing reliance on animal testing.45,52 IATA is considered a generic approach and may encompass integrated testing strategies and weight-of-evidence considerations.52 An integrated testing strategy for candidate pharmaceuticals has been proposed.53 AOPs can be envisaged as the mechanistic backbone of IATA. In fact, IATA typically focus on different information blocks of AOPs, such as the MIE and selected KEs.45 AOP-driven IATA not only consist of non-testing (i.e. in silico) approaches, but also of in chemico, in vitro and in vivo testing elements.52 In chemico assays aid in the characterization of the intrinsic reactivity of chemicals to a specific biological target embodied by MIEs and differ from in vitro and in vivo testing in that they usually do not involve cellular systems. The majority of in chemico tests that intend to predict toxicity study the interaction of an electrophilic molecule (i.e. the toxicant) with a model nucleophile that represents the biological target.54 A plethora of in vitro tests can be used to test MIEs and/or specific KEs.52 Their testing principle and nature can be diverse, and hence these in vitro tests should be selected on a case-by-case basis. DILI in vitro tests typically rely on the use of liver-based testing platforms, which can range from acellular systems, such as microsomes, to artificial liver bioreactors.55 An obvious yet challenging prerequisite to be implemented in IATA is that the addressed liver-based in vitro model should reproduce the MIE or KE of interest at an in vivo-like level. This specifically holds true when studying liver fibrosis, whereby in vitro settings consisting of hepatic stellate cells, hepatocytes and Kupffer cells should be used in order to appropriately simulate activation of the former.20,24-27 Furthermore, IATA can also make use of limited in vivo testing, such as on Zebrafish embryos.52 In some cases, predictivity of these models is higher than in vitro systems, given that they accommodate the complexity of a whole organism.56 State-of-the-art technologies can be used in all these testing approaches, including high-throughput screening methodologies, such as receptor binding and cellular reporter assays, as well as toxicogenomics strategies, in particular transcriptomics, proteomics and metabonomics.52 Basically, a drug candidate should be run through this battery of in silico, in chemico, in vitro and in vivo approaches, each which test the MIE and one or more KEs of a specific DILI AOP. This may be embedded in a tiered testing approach based on prioritization of AOP information blocks. This can be combined with weigh-of-evidence evaluation of testing results and can be anticipated to yield a reliable outcome eligible for regulatory purposes. Obviously, such proposed strategy inherently raises the question how many AOPs should be tested in order to ensure decisive results and thus to avoid false negatives while running toxicity assays.
4. Conclusions and perspectives
Because of its unique function and localization in the body, the liver is a primary target of toxicity induced by drugs. DILI can be manifested in a number of prominent ways, such as cholestasis, steatosis and fibrosis, and is a major reason for discontinuation of drug development or withdrawal of drugs from the market.7 Nevertheless, it still remains challenging to detect DILI. Indeed, standard animal studies conducted during routine drug development usually pick up about half of all human hepatotoxic compounds7,57,58, whilst human-based in vitro testing identifies up to 60% of the in vivo human hepatotoxic drugs.58 AOPs are promising tools in this respect, as they may allow to predict DILI in a more accurate and mechanistically-anchored way. In fact, AOPs can not only be applied in such bottom-up approach, targeted towards predicting an adverse outcome after determination of a MIE and downstream KEs, but equally in a top-down scenario, which aims at explaining an adverse outcome in terms of a MIE and KEs.59 Despite the well-recognized and broad potential of AOPs for the safety evaluation of chemicals in general and although guidance on AOP development and evaluation has been introduced by the Organization for Economic Cooperation and Development60, this area is still in its infancy and will greatly benefit from fine-tuning in the upcoming years. A criticism on AOPs nowadays still is their simplicity and thus their poor reflection of complex toxicological processes, including adaptive responses and homeostatic modifications. AOPs are indeed presented as stand-alone linear events, yet the reality is likely to be much less straightforward, as parallel cascades and crossing of pathways may be involved. Furthermore, AOPs are to be considered as open and flexible structures that should be continuously refined by feeding in old and new data. During such iterative refinement exercises, particular attention should be paid to quantification of AOPs, which is an absolute conditio sine qua non for their implementation into regulatory risk assessment. This can be achieved in several ways, such as by establishing dose/concentration-effect relationships for the MIE and/or KEs. Simultaneously, kinetic features should be included in AOPs, which may be critical for determining overall exposure and KE relationships. Another aspect that deserves further scrutiny relates to the introduction of uncertainty factors in AOPs. Several worldwide efforts are currently ongoing to tackle these challenges, including at the level of the Organization for Economic Cooperation and Development21,60, the US Hamner Institutes of Health61, the US Center for Alternatives to Animal Testing62 and the European research program called Safety Evaluation Ultimately Replacing Animal Testing.63,64 Such projects are expected to produce robust and reliable AOP tools that can be used for a variety of purposes pertinent to toxicology and risk assessment, including the prediction of DILI during early drug development.
Acknowledgments
Funding sources
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel - Belgium (Willy Gepts Fonds UZ - VUB), the University of São Paulo - Brazil (USP), the São Paulo Research Foundation - Brazil (FAPESP), the Fund for Scientific Research - Flanders (FWO - Vlaanderen), the European Research Council (project CONNECT), the European Union (FP7) and Cosmetics Europe (projects DETECTIVE and HeMiBio).
Abbreviations
- AOP(s)
adverse outcome pathway(s)
- DILI
drug-induced liver injury
- IATA
integrated approaches to testing and assessment
- KE(s)
key event(s)
- MIE(s)
molecular initiating event(s)
References
- (1).Przybylak KR, Cronin MT. In silico models for drug-induced liver injury: current status. Expert Opin. Drug Metab. Toxicol. 2012;8:201–217. doi: 10.1517/17425255.2012.648613. [DOI] [PubMed] [Google Scholar]
- (2).Faa G, Ekstrom J, Castagnola M, Gibo Y, Ottonello G, Fanos V. A developmental approach to drug-induced liver injury in newborns and children. Curr. Med. Chem. 2012;19:4581–4594. doi: 10.2174/092986712803306385. [DOI] [PubMed] [Google Scholar]
- (3).Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8:E48–E54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009;58:1555–1564. doi: 10.1136/gut.2008.163675. [DOI] [PubMed] [Google Scholar]
- (5).Andrade RJ, Agundez JA, Lucena MI, Martinez C, Cueto R, Garcia-Martin E. Pharmacogenomics in drug induced liver injury. Curr. Drug Metab. 2009;10:956–970. doi: 10.2174/138920009790711805. [DOI] [PubMed] [Google Scholar]
- (6).Andrade RJ, Robles M, Ulzurrun E, Lucena MI. Drug-induced liver injury: insights from genetic studies. Pharmacogenomics. 2009;10:1467–1487. doi: 10.2217/pgs.09.111. [DOI] [PubMed] [Google Scholar]
- (7).Blomme EA, Yang Y, Waring JF. Use of toxicogenomics to understand mechanisms of drug-induced hepatotoxicity during drug discovery and development. Toxicol. Lett. 2009;186:22–31. doi: 10.1016/j.toxlet.2008.09.017. [DOI] [PubMed] [Google Scholar]
- (8).Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Uetrecht J. Immunoallergic drug-induced liver injury in humans. Semin. Liver Dis. 2009;29:383–392. doi: 10.1055/s-0029-1240007. [DOI] [PubMed] [Google Scholar]
- (10).Guengerich FP. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011;26:3–14. doi: 10.2133/dmpk.dmpk-10-rv-062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J. Clin. Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- (12).Zimmerman HJ. Drug-induced liver disease. Clin. Liver Dis. 2000;4:73–96. doi: 10.1016/s1089-3261(05)70097-0. [DOI] [PubMed] [Google Scholar]
- (13).Tujios S, Fontana RJ. Mechanisms of drug-induced liver injury: from bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 2011;8:202–211. doi: 10.1038/nrgastro.2011.22. [DOI] [PubMed] [Google Scholar]
- (14).Chalasani N, Bjornsson E. Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology. 2010;138:2246–2259. doi: 10.1053/j.gastro.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cui Y, Paules RS. Use of transcriptomics in understanding mechanisms of drug-induced toxicity. Pharmacogenomics. 2010;11:573–585. doi: 10.2217/pgs.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bjornsson E. Drug-induced liver injury in clinical practice. Aliment. Pharmacol. Ther. 2010;32:3–13. doi: 10.1111/j.1365-2036.2010.04320.x. [DOI] [PubMed] [Google Scholar]
- (17).Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- (18).Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol. Sci. 2014;142:312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway development II: best practices. Toxicol. Sci. 2014;142:321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Vinken M. The adverse outcome pathway concept: a pragmatic tool in toxicology. Toxicology. 2013;312:158–165. doi: 10.1016/j.tox.2013.08.011. [DOI] [PubMed] [Google Scholar]
- (21).2015 Jun; https://aopkb.org/ consulted.
- (22).Willett C, Caverly Rae J, Goyak KO, Landesmann B, Minsavage G, Westmoreland C. Pathway-based toxicity: history, current approaches and liver fibrosis and steatosis as prototypes. ALTEX. 2014;31:407–421. doi: 10.14573/altex.1401283. [DOI] [PubMed] [Google Scholar]
- (23).Willett C, Caverly Rae J, Goyak KO, Minsavage G, Westmoreland C, Andersen M, Avigan M, Duché D, Harris G, Hartung T, Jaeschke H, Kleensang A, Landesmann B, Martos S, Matevia M, Toole C, Rowan A, Schultz T, Seed J, Senior J, Shah I, Subramanian K, Vinken M, Watkins P. Building shared experience to advance practical application of pathway-based toxicology: liver toxicity mode-of-action. ALTEX. 2014;31:500–519. doi: 10.14573/altex.1401281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract. Res. Clin. Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J. Hepatol. 2015;62:S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- (26).Friedman SL. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- (27).Vinken M, Maes M, Vanhaecke T, Rogiers V. Drug-induced liver injury: mechanisms, types and biomarkers. Curr. Med. Chem. 2013;20:3011–3021. doi: 10.2174/0929867311320240006. [DOI] [PubMed] [Google Scholar]
- (28).Landesmann B, Lostia A, Horvat T, Vinken M, Munn S, Whelan M. Adverse outcome pathway development from protein alkylation to liver fibrosis. PLoS One. 2015 doi: 10.1007/s00204-016-1814-8. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B. Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011;54:773–794. doi: 10.1016/j.jhep.2010.11.006. [DOI] [PubMed] [Google Scholar]
- (30).Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Amacher DE. The mechanistic basis for the induction of hepatic steatosis by xenobiotics. Expert Opin. Drug Metab. Toxicol. 2011;7:949–965. doi: 10.1517/17425255.2011.577740. [DOI] [PubMed] [Google Scholar]
- (32).Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J. Clin. Pathol. 2009;62:481–492. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- (33).Zimmerman HJ. Drug-induced liver disease. Clin. Liver Dis. 2000;4:73–96. doi: 10.1016/s1089-3261(05)70097-0. [DOI] [PubMed] [Google Scholar]
- (34).Landesmann B, Goumenou M, Munn S, Whelan M. Description of prototype modes-of-action related to repeated dose toxicity. JRC Scientific and Policy Report. 2012;75689 [Google Scholar]
- (35).Oorts M, Richert L, Annaert P. Drug-induced cholestasis detection in cryopreserved rat hepatocytes in sandwich culture. J. Pharmacol. Toxicol. Methods. 2015;73:63–71. doi: 10.1016/j.vascn.2015.03.002. [DOI] [PubMed] [Google Scholar]
- (36).Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–1573. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- (37).Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hofmann AF. Bile acids and the enterohepatic circulation. In: Arias IM, Alter HJ, Boyer JL, Cohen DE, Fausto N, Shafritz DA, Wolkoff AW, editors. The liver: biology and pathobiology. Wiley-Blackwell; Oxford: 2009. pp. 289–304. [Google Scholar]
- (39).Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology. 2011;53:1377–1387. doi: 10.1002/hep.24229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zollner G, Trauner M. Molecular mechanisms of cholestasis. Wien. Med. Wochenschr. 2006;156:380–385. doi: 10.1007/s10354-006-0312-7. [DOI] [PubMed] [Google Scholar]
- (41).Zollner G, Trauner M. Mechanisms of cholestasis. Clin. Liver Dis. 2008;12:1–26. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- (42).Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J. Hepatol. 2009;51:565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- (43).Vinken M, Landesmann B, Goumenou M, Vinken S, Shah I, Jaeschke H, Willett C, Whelan M, Rogiers V. Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol. Sci. 2013;136:97–106. doi: 10.1093/toxsci/kft177. [DOI] [PubMed] [Google Scholar]
- (44).Ellison CM, Enoch SJ, Cronin MT. A review of the use of in silico methods to predict the chemistry of molecular initiating events related to drug toxicity. Expert Opin. Drug Metab. Toxicol. 2011;7:1481–1495. doi: 10.1517/17425255.2011.629186. [DOI] [PubMed] [Google Scholar]
- (45).Patlewicz G, Simon TW, Rowlands JC, Budinsky RA, Becker RA. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regul. Toxicol. Pharmacol. 2015;71:463–477. doi: 10.1016/j.yrtph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- (46).Przybylak KR, Cronin MT. In silico models for drug-induced liver injury: current status. Expert Opin. Drug Metab. Toxicol. 2012;8:201–217. doi: 10.1517/17425255.2012.648613. [DOI] [PubMed] [Google Scholar]
- (47).Hirano H, Kurata A, Onishi Y, Sakurai A, Saito H, Nakagawa H, Nagakura M, Tarui S, Kanamori Y, Kitajima M, Ishikawa T. High-speed screening and QSAR analysis of human ATP-binding cassette transporter ABCB11 (bile salt export pump) to predict drug-induced intrahepatic cholestasis. Mol. Pharm. 2006;3:252–265. doi: 10.1021/mp060004w. [DOI] [PubMed] [Google Scholar]
- (48).Saito H, Osumi M, Hirano H, Shin W, Nakamura R, Ishikawa T. Technical pitfalls and improvements for high-speed screening and QSAR analysis to predict inhibitors of the human bile salt export pump (ABCB11/BSEP) AAPS J. 2009;11:581–589. doi: 10.1208/s12248-009-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Warner DJ, Chen H, Cantin LD, Kenna JG, Stahl S, Walker CL, Noeske T. Mitigating the inhibition of human bile salt export pump by drugs: opportunities provided by physicochemical property modulation, in silico modeling, and structural modification. Drug Metab. Dispos. 2012;40:2332–2341. doi: 10.1124/dmd.112.047068. [DOI] [PubMed] [Google Scholar]
- (50).Honório KM, Salum LB, Garratt RC, Polikarpov I, Andricopulo AD. Two- and three-dimensional quantitative structure-activity relationships studies on a series of liver X receptor ligands. Open Med. Chem. J. 2008;2:87–96. doi: 10.2174/1874104500802010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Hewitt M, Enoch SJ, Madden JC, Przybylak KR, Cronin MT. Hepatotoxicity: a scheme for generating chemical categories for read-across, structural alerts and insights into mechanism(s) of action. Crit. Rev. Toxicol. 2013;43:537–558. doi: 10.3109/10408444.2013.811215. [DOI] [PubMed] [Google Scholar]
- (52).Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G. Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA) Regul. Toxicol. Pharmacol. 2014;70:629–640. doi: 10.1016/j.yrtph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- (53).Balls M, Combes RD, Bhogal N. The use of integrated and intelligent testing strategies in the prediction of toxic hazard and in risk assessment. Adv. Exp. Med. Biol. 2012;745:221–253. doi: 10.1007/978-1-4614-3055-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Aptula AO, Roberts DW. Mechanistic applicability domains for nonanimal-based prediction of toxicological end points: general principles and application to reactive toxicity. Chem. Res. Toxicol. 2006;19:1097–1105. doi: 10.1021/tx0601004. [DOI] [PubMed] [Google Scholar]
- (55).Lin C, Ballinger KR, Khetani SR. The application of engineered liver tissues for novel drug discovery. Expert Opin. Drug Discov. 2015;10:519–540. doi: 10.1517/17460441.2015.1032241. [DOI] [PubMed] [Google Scholar]
- (56).Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. Zebrafish embryos as an alternative to animal experiments: a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- (57).Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- (58).Laverty HG, Antoine DJ, Benson C, Chaponda M, Williams D, Park BK. The potential of cytokines as safety biomarkers for drug-induced liver injury. Eur. J. Clin. Pharmacol. 2010;66:961–976. doi: 10.1007/s00228-010-0862-x. [DOI] [PubMed] [Google Scholar]
- (59).Burden N, Sewella F, Andersen ME, Boobis A, Chipman JK, Cronin MTD, Hutchinson TH, Kimberg I, Whelan M. Adverse outcome pathways can drive non-animal approaches for safety assessment. J. Appl. Toxicol. 2015 doi: 10.1002/jat.3165. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).OECD Proposal for a template and guidance on developing and assessing the completeness of adverse outcome pathways. Series on Testing and Assessment. 2013;184:1–45. [Google Scholar]
- (61).Andersen ME, Clewell R, Bhattacharya S. Developing in vitro tools sufficient by themselves for 21st century risk assessment. In: Gocht T, Schwarz M, editors. Towards the replacement of in vivo repeated dose systemic toxicity testing. Vol. 2. Imprimerie Mouzet; France: 2012. pp. 347–360. [Google Scholar]
- (62).2015 Jun; http://caat.jhsph.edu/ consulted.
- (63).2015 Jun; http://www.seurat-1.eu/ consulted.
- (64).Vinken M, Pauwels M, Ates G, Vivier M, Vanhaecke T, Rogiers V. Screening of repeated dose toxicity data present in SCC(NF)P/SCCS safety evaluations of cosmetic ingredients. Arch. Toxicol. 2012;86:405–412. doi: 10.1007/s00204-011-0769-z. [DOI] [PubMed] [Google Scholar]