Abstract
Mutations in a voltage-gated sodium channel (SCN1A) result in Dravet Syndrome (DS), a catastrophic childhood epilepsy. Zebrafish with a mutation in scn1Lab recapitulate salient phenotypes associated with DS, including seizures, early fatality, and resistance to antiepileptic drugs. To discover new drug candidates for the treatment of DS, we screened a chemical library of ∼1000 compounds and identified 4 compounds that rescued the behavioral seizure component, including 1 compound (dimethadione) that suppressed associated electrographic seizure activity. Fenfluramine, but not huperzine A, also showed antiepileptic activity in our zebrafish assays. The effectiveness of compounds that block neuronal calcium current (dimethadione) or enhance serotonin signaling (fenfluramine) in our zebrafish model suggests that these may be important therapeutic targets in patients with DS. Over 150 compounds resulting in fatality were also identified. We conclude that the combination of behavioral and electrophysiological assays provide a convenient, sensitive, and rapid basis for phenotype-based drug screening in zebrafish mimicking a genetic form of epilepsy.
Keywords: antiepileptic, drug discovery, epilepsy, high throughput, pharmacology, zebrafish
Significance Statement
Dravet syndrome is a catastrophic childhood epilepsy that is resistant to available medications. Current animal models for this disease are not amenable to high-throughput drug screening. We used a zebrafish model for Dravet syndrome and screened >1000 compounds. We report the identification of compounds with the ability to suppress seizure behavior and electrographic seizure activity. This approach provides an example of precision medicine directed to pediatric epilepsy.
Introduction
Dravet syndrome (DS) is a devastating genetic epileptic encephalopathy that has been linked to more than >300 de novo mutations in a neuronal voltage-gated sodium channel (SCN). Children with DS are at a higher risk for sudden unexplained death in epilepsy and episodes of uncontrolled status epilepticus (Dravet et al., 2005; Ceulemans et al., 2012). Delayed language development, disruption of autonomic function, and motor and cognitive impairment are also associated with this disease. Seizure management includes treatment with benzodiazepines, valproate, and/or stiripentol (Caraballo et al., 2005; Chiron and Dulac, 2011). Some reduction in seizure activity has been reported with the use of bromides and topiramate, or a ketogenic diet (Lotte et al., 2012; Wilmshurst et al., 2014; Dressler et al., 2015). Despite these options, available antiepileptic drugs (AEDs) do not achieve adequate seizure control in most DS patients (Dravet et al., 2005; Chiron and Dulac, 2011; Dressler et al., 2015), making the identification of new drugs a critical unmet need. High-throughput screening offers a powerful tool to identify new drug candidates for these patients. However, commonly available screening approaches rely on in vitro cell-based assays (Masimirembwa et al., 2001; Snowden and Green, 2008; Ko and Gelb, 2014) and do not recapitulate the complicated neural networks that generate seizures in vivo. Given the need for new treatments for children with DS, and the growing number of genetic epileptic encephalopathies that are medically intractable (Leppert, 1990; Epi4K Consortium, 2012; Ottman and Risch, 2012), we developed an alternative phenotype-based in vivo drug-screening strategy. While cell-based in vitro screening assays can efficiently identify compounds that bind specific targets, whole-organism-based screens are more likely to reliably predict therapeutic outcomes as they maintain the complex neural circuitry involved in the underlying disease process. Whole-organism screens do not require well validated targets to identify compounds that yield a desirable phenotypic outcome, but can be prohibitively costly and time consuming in mammals. As a simple vertebrate with significant genetic similarity to human, zebrafish are now recognized as an ideal cost-effective alternative to achieve rapid in vivo phenotype-based screening (Ali et al., 2011).
Using scn1a mutant zebrafish larvae with a gene homologous to human and spontaneously occurring seizures (Baraban et al., 2013), we screened, in a blinded manner, a repurposed library of ∼1000 compounds for drugs that inhibit unprovoked seizure events. We also screened two compounds (huperzine A and fenfluramine) that were discovered in rodent-based assays using acquired seizure protocols and that were recently suggested as potential treatments for DS (Boel and Casaer, 1996; Coleman et al., 2008; Ceulemans et al., 2012; Bialer et al., 2015). Only 20 compounds in the repurposed drug library reduced swim behavior to control levels. However, many of these compounds were toxic or were not confirmed on retesting, and only four compounds advanced to a second-stage in vivo electrophysiology assay. Of these compounds (cytarabine, dimethadione, theobromine, and norfloxacin) only dimethadione, a T-type calcium channel antagonist previously reported to have anticonvulsant activity (Lowson et al., 1990; Zhang et al., 1996), reduced ictal-like electrographic discharges seen in scn1Lab mutant larvae. This two-stage phenotype-based screening approach, using a genetic DS model with >75% genomic similarity to human, is a sensitive, rapid means to successfully identify compounds with antiepileptic activity.
Materials and Methods
Zebrafish
Zebrafish were maintained in a light- and temperature-controlled aquaculture facility under a standard 14:10 h light/dark photoperiod. Adult zebrafish were housed in 1.5 L tanks at a density of 5-12 fish per tank and fed twice per day (dry flake and/or flake supplemented with live brine shrimp). Water quality was continuously monitored: temperature, 28-30º C; pH 7.4-8.0; conductivity, 690-710 mS/cm. Zebrafish embryos were maintained in round Petri dishes (catalog #FB0875712, Fisher Scientific) in “embryo medium” consisting of 0.03% Instant Ocean (Aquarium Systems, Inc.) and 000002% methylene blue in reverse osmosis-distilled water. Larval zebrafish clutches were bred from wild-type (WT; TL strain) or scn1Lab (didys552) heterozygous animals that had been backcrossed to TL wild-type for at least 10 generations. Homozygous mutants (n = 6544), which have widely dispersed melanosomes and appear visibly darker as early as 3 d postfertilization (dpf; Fig. 1b ), or WT larvae (n = 71) were used in all experiments at 5 or 6 dpf. Embryos and larvae were raised in plastic petri dishes (90 mm diameter, 20 mm depth) and density was limited to ∼60 per dish. Larvae between 3 and 7 dpf lack discernible sex chromosomes. The care and maintenance protocols comply with requirements outlined in the Guide for the Care and Use of Animals (ebrary Inc., 2011) and were approved by the Institutional Animal Care and Use Committee (protocol #AN108659-01D).
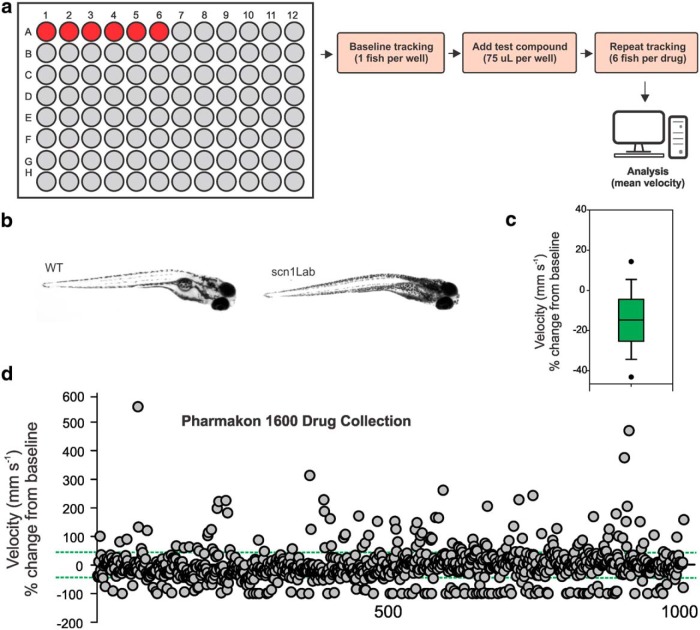
Figure 1.
Locomotion assay to identify drugs that rescue the scn1Lab mutant epilepsy phenotype. a, Schematic of the phenotype-based screening process. Chemical libraries can be coded and aliquoted in small volumes (75 µL) into individual wells containing one mutant fish. The 96-well microplate is arranged so that six fish are tested per drug; with one row of six fish maintained as an internal control (red circles) on each plate. b, Representative images for WT and scn1Lab mutant zebrafish larvae at 5 dpf. Note the morphological similarity but darker pigmentation in mutant larvae. c, Box plot of mean velocity (in millimeters per second) for two consecutive recordings of mutant larvae in embryo media. Experiments were performed by first placing the mutant larvae in embryo media and obtaining a baseline locomotion response; embryo media was then replaced with new embryo media (to mimic the procedure used for test compounds), and a second locomotion response was obtained. The percentage change in velocity from baseline (recording 1) versus experimental model (recording 2) is shown. In the box plot, the bottom and top of the box represent the 25th percentile and the 75th percentile, respectively. The line across the box represents the median value, and the vertical lines encompass the entire range of values. This plot represents normal changes in tracking activity in the absence of a drug challenge. d, Plot of locomotor seizure behavior for scn1Lab mutants at 5 dpf for the 1012 compounds tested. Threshold for inhibition of seizure activity (positive hits) was set as a reduction in mean swim velocity of ≥44%; the threshold for a proconvulsant or hyperexcitable effect was set at an increase in the mean swim velocity of ≥44% (green dashed lines).
Seizure monitoring
Zebrafish larvae were placed individually into 1 well of a clear flat-bottomed 96-well microplate (catalog #260836, Fisher Scientific) containing embryo media. Microplates were placed inside an enclosed motion-tracking device and acclimated to the dark condition for 10-15 min at room temperature. Locomotion plots were obtained for one fish per well at a recording epoch of 10 min using a DanioVision system running EthoVision XT software (DanioVision, Noldus Information Technology); threshold detection settings to identify objects darker than the background were optimized for each experiment. Seizure scoring was performed using the following three-stage scale (Baraban et al., 2005): Stage 0, no or very little swim activity; Stage I, increased, brief bouts of swim activity; Stage II, rapid “whirlpool-like” circling swim behavior; and Stage III, paroxysmal whole-body clonus-like convulsions, and a brief loss of posture. WT fish are normally scored at Stage 0 or I. Plots were analyzed for distance traveled (in millimeters) and mean velocity (in millimeters per second). As reported previously (Winter et al., 2008; Baraban et al., 2013), velocity changes were a more sensitive assay of seizure behavior. For electrophysiology studies, zebrafish larvae were briefly paralyzed with α-bungarotoxin (1 mg/ml) and immobilized in 1.2% agarose; field recordings were obtained from forebrain structures. Epileptiform events were identified post hoc in Clampfit (Molecular Devices) and were defined as multispike or polyspike upward or downward membrane deflections greater than three times the baseline noise level and >500 ms in duration. During electrophysiology experiments zebrafish larvae were continuously monitored for the presence (or absence) of blood flow and heart beat by direct visualization on an Olympus BX51WI upright microscope equipped with a CCD camera and monitor.
Drugs
Compounds for drug screening were purchased from MicroSource Discovery Systems, Inc. (PHARMAKON 1600) and were provided as 10 mm DMSO solutions (Table 1). Test compounds for locomotion or electrophysiology studies were dissolved in embryo media and were tested at an initial concentration of 100 µm, with a final DMSO concentration of <2%. In all drug library screen studies, compounds were coded and experiments were performed by investigators who were blind to the nature of the compound. Baseline recordings of seizure behavior were obtained from mutants bathed in embryo media, as described above; a second locomotion plot was then obtained following a solution change to a test compound and an equilibration period of 15–30 min. Criteria for a positive hit designation were as follows: (1) a decrease in mean velocity of ≥44% (e.g., a value based on the trial-to-trial variability measured in control tracking studies; Fig. 1c ); and (2) a reduction to Stage 0 or Stage I seizure behavior in the locomotion plot for at least 50% of the test fish. Each test compound classified as a “positive hit” in the locomotion assay was confirmed, under direct visualization on a stereomicroscope, as the fish being alive based on movement in response to external stimulation and a visible heartbeat following a 60 min drug exposure. Toxicity (or mortality) was defined as no visible heartbeat or movement in response to external stimulation in at least 50% of the test fish. Hyperexcitability was defined as a compound causing a ≥44% increase in swim velocity and/or Stage III seizure activity in at least 50% of the test fish. Hits identified in the primary locomotion screen were selected from the PHARMAKON 1600 library and rescreened using the method described above. Select compound stocks that were successful in two primary locomotion assays, and were not classified as toxic in two independent clutches of zebrafish, were then purchased separately from Sigma-Aldrich for further testing. Drug concentrations between 0.5 and 1 mm were used for electrophysiology assays to account for more limited diffusion in agar-embedded larvae.
Table 1.
List of compounds from the PHARMAKON 1600 library used in this screen.
| ABACAVIR SULFATE |
| ABAMECTIN (avermectin B1a shown) |
| ACADESINE |
| ACARBOSE |
| ACEBUTOLOL HYDROCHLORIDE |
| ACECLIDINE |
| ACECLOFENAC |
| ACENOCOUMAROL |
| ACETAMINOPHEN |
| ACETOHYDROXAMIC ACID |
| ACETOPHENAZINE MALEATE |
| ACETRIAZOIC ACID |
| ACETYLCHOLINE CHLORIDE |
| ACETYLCYSTEINE |
| ACIPIMOX |
| ACONITINE |
| ACRIFLAVINIUM HYDROCHLORIDE |
| ACRISORCIN |
| ACTARIT |
| ACYCLOVIR |
| ADAPALENE |
| ADELMIDROL |
| ADENINE |
| ADENOSINE |
| ADENOSINE PHOSPHATE |
| ADIPHENINE HYDROCHLORIDE |
| AKLOMIDE |
| ALAPROCLATE |
| ALBENDAZOLE |
| ALBUTEROL (+/-) |
| ALENDRONATE SODIUM |
| ALEXIDINE HYDROCHLORIDE |
| ALLANTOIN |
| ALLOPURINOL |
| ALMOTRIPTAN |
| alpha-TOCHOPHEROL |
| alpha-TOCHOPHERYL ACETATE |
| ALPRAZOLAM |
| ALRESTATIN |
| ALTHIAZIDE |
| ALTRETAMINE |
| ALVERINE CITRATE |
| AMANTADINE HYDROCHLORIDE |
| AMCINONIDE |
| AMIFOSTINE |
| AMIKACIN SULFATE |
| AMILORIDE HYDROCHLORIDE |
| AMINACRINE |
| AMINOCAPROIC ACID |
| AMINOGLUTETHIMIDE |
| AMINOHIPPURIC ACID |
| AMINOLEVULINIC ACID HYDROCHLORIDE |
| AMINOSALICYLATE SODIUM |
| AMITRIPTYLINE HYDROCHLORIDE |
| AMLEXANOX |
| AMLODIPINE BESYLATE |
| AMODIAQUINE DIHYDROCHLORIDE |
| AMOROLFINE HYDROCHLORIDE |
| AMOXICILLIN |
| AMPHOTERICIN B |
| AMPICILLIN SODIUM |
| AMPROLIUM |
| AMSACRINE |
| ANASTROZOLE |
| ANCITABINE HYDROCHLORIDE |
| ANETHOLE |
| ANIRACETAM |
| ANISINDIONE |
| ANTAZOLINE PHOSPHATE |
| ANTHRALIN |
| ANTIPYRINE |
| APOMORPHINE HYDROCHLORIDE |
| APRAMYCIN |
| ARGININE HYDROCHLORIDE |
| ARMODAFINIL |
| ARTEMETHER |
| ARTEMOTIL |
| ARTESUNATE |
| ASCORBIC ACID |
| ASPIRIN |
| ATENOLOL |
| ATORVASTATIN CALCIUM |
| ATOVAQUONE |
| ATROPINE SULFATE |
| AUROTHIOGLUCOSE |
| AVOBENZONE |
| AZACITIDINE |
| AZASERINE |
| AZATADINE MALEATE |
| AZATHIOPRINE |
| AZELAIC ACID |
| AZITHROMYCIN |
| AZLOCILLIN SODIUM |
| AZTREONAM |
| BACAMPICILLIN HYDROCHLORIDE |
| BACITRACIN |
| BACLOFEN |
| BALSALAZIDE DISODIUM |
| BECLOMETHASONE DIPROPIONATE |
| BEKANAMYCIN SULFATE |
| BEMOTRIZINOL |
| BENAZEPRIL HYDROCHLORIDE |
| BENDROFLUMETHIAZIDE |
| BENORILATE |
| BENSERAZIDE HYDROCHLORIDE |
| BENZALKONIUM CHLORIDE |
| BENZETHONIUM CHLORIDE |
| BENZOCAINE |
| BENZOIC ACID |
| BENZONATATE |
| BENZOYL PEROXIDE |
| BENZTHIAZIDE |
| BENZYL ALCOHOL |
| BENZYL BENZOATE |
| BEPRIDIL HYDROCHLORIDE |
| BERGAPTEN |
| beta-CAROTENE |
| BETAHISTINE HYDROCHLORIDE |
| BETAINE HYDROCHLORIDE |
| BETAMETHASONE |
| BETAMETHASONE 17,21-DIPROPIONATE |
| BETAMETHASONE VALERATE |
| BETAMIPRON |
| beta-NAPHTHOL |
| BETAZOLE HYDROCHLORIDE |
| BETHANECHOL CHLORIDE |
| BEZAFIBRATE |
| BICALUTAMIDE |
| BIOTIN |
| BISACODYL |
| BISOCTRIZOLE |
| BISORCIC |
| BITHIONATE SODIUM |
| BLEOMYCIN (bleomycin B2 shown) |
| BRETYLIUM TOSYLATE |
| BRINZOLAMIDE |
| BROMHEXINE HYDROCHLORIDE |
| BROMOCRIPTINE MESYLATE |
| BROMPHENIRAMINE MALEATE |
| BROXYQUINOLINE |
| BUDESONIDE |
| BUMETANIDE |
| BUPIVACAINE HYDROCHLORIDE |
| BUPROPION |
| BUSULFAN |
| BUTACAINE |
| BUTAMBEN |
| BUTOCONAZOLE |
| CAFFEINE |
| CAMPHOR (1R) |
| CANDESARTAN |
| CANDESARTAN CILEXTIL |
| CANDICIDIN |
| CANRENOIC ACID, POTASSIUM SALT |
| CANRENONE |
| CAPECITABINE |
| CAPREOMYCIN SULFATE |
| CAPSAICIN |
| CAPTAMINE |
| CAPTOPRIL |
| CARBACHOL |
| CARBENICILLIN DISODIUM |
| CARBENOXOLONE SODIUM |
| CARBETAPENTANE CITRATE |
| CARBIDOPA |
| CARBINOXAMINE MALEATE |
| CARBOPLATIN |
| CARISOPRODOL |
| CARMUSTINE |
| CARNITINE (dl) HYDROCHLORIDE |
| CARPROFEN |
| CARVEDILOL |
| CEFACLOR |
| CEFADROXIL |
| CEFAMANDOLE NAFATE |
| CEFAMANDOLE SODIUM |
| CEFAZOLIN SODIUM |
| CEFDINIR |
| CEFEPIME HYDROCHLORIDE |
| CEFMENOXIME HYDROCHLORIDE |
| CEFMETAZOLE SODIUM |
| CEFOPERAZONE |
| CEFORANIDE |
| CEFOTAXIME SODIUM |
| CEFOTETAN |
| CEFOXITIN SODIUM |
| CEFPIRAMIDE |
| CEFSULODIN SODIUM |
| CEFTIBUTEN |
| CEFTIOFUR HYDROCHLORIDE |
| CEFTRIAXONE SODIUM TRIHYDRATE |
| CEFUROXIME AXETIL |
| CEFUROXIME SODIUM |
| CELECOXIB |
| CEPHALEXIN |
| CEPHALOTHIN SODIUM |
| CEPHAPIRIN SODIUM |
| CEPHRADINE |
| CETYLPYRIDINIUM CHLORIDE |
| CHENODIOL |
| CHLORAMBUCIL |
| CHLORAMPHENICOL |
| CHLORAMPHENICOL HEMISUCCINATE |
| CHLORAMPHENICOL PALMITATE |
| CHLORCYCLIZINE HYDROCHLORIDE |
| CHLORHEXIDINE |
| CHLOROCRESOL |
| CHLOROGUANIDE HYDROCHLORIDE |
| CHLOROQUINE DIPHOSPHATE |
| CHLOROTHIAZIDE |
| CHLOROXINE |
| CHLOROXYLENOL |
| CHLORPHENIRAMINE (S) MALEATE |
| CHLORPROMAZINE |
| CHLORPROPAMIDE |
| CHLORPROTHIXENE HYDROCHLORIDE |
| CHLORTETRACYCLINE HYDROCHLORIDE |
| CHLORTHALIDONE |
| CHLORZOXAZONE |
| CHOLECALCIFEROL |
| CHOLESTEROL |
| CHOLINE CHLORIDE |
| CICLOPIROX OLAMINE |
| CILOSTAZOL |
| CIMETIDINE |
| CINCHOPHEN |
| CINNARAZINE |
| CINOXACIN |
| CINTRIAMIDE |
| CIPROFIBRATE |
| CIPROFLOXACIN |
| CISPLATIN |
| CITALOPRAM HYDROBROMIDE |
| CITICOLINE |
| CLARITHROMYCIN |
| CLAVULANATE LITHIUM |
| CLEMASTINE |
| CLIDINIUM BROMIDE |
| CLINAFOXACIN HYDROCHLORIDE |
| CLINDAMYCIN HYDROCHLORIDE |
| CLIOQUINOL |
| CLOBETASOL PROPIONATE |
| CLOFARABINE |
| CLOFIBRATE |
| CLOMIPHENE CITRATE |
| CLONIDINE HYDROCHLORIDE |
| CLOPIDOGREL SULFATE |
| CLORSULON |
| CLOSANTEL |
| CLOTRIMAZOLE |
| CLOXACILLIN SODIUM |
| CLOXYQUIN |
| CLOZAPINE |
| COENZYME B12 |
| COLCHICINE |
| COLFORSIN |
| COLISTIMETHATE SODIUM |
| CORTISONE ACETATE |
| COTININE |
| CRESOL |
| CROMOLYN SODIUM |
| CRYOFLURANE |
| CYCLAMIC ACID |
| CYCLIZINE |
| CYCLOBENZAPRINE HYDROCHLORIDE |
| CYCLOHEXIMIDE |
| CYCLOPENTOLATE HYDROCHLORIDE |
| CYCLOPHOSPHAMIDE HYDRATE |
| CYCLOSERINE (D) |
| CYCLOSPORINE |
| CYCLOTHIAZIDE |
| CYPERMETHRIN |
| CYPROTERONE ACETATE |
| CYSTEAMINE HYDROCHLORIDE |
| CYTARABINE |
| DACARBAZINE |
| DACTINOMYCIN |
| DANAZOL |
| DANTHRON |
| DANTROLENE SODIUM |
| DAPSONE |
| DAPTOMYCIN |
| DASATINIB |
| DAUNORUBICIN |
| DECIMEMIDE |
| DEFEROXAMINE MESYLATE |
| DEFLAZACORT |
| DEHYDROACETIC ACID |
| DEHYDROCHOLIC ACID |
| DEMECLOCYCLINE HYDROCHLORIDE |
| DERACOXIB |
| DESIPRAMINE HYDROCHLORIDE |
| DESOXYCORTICOSTERONE ACETATE |
| DESVENLAFAXINE SUCCINATE |
| DEXAMETHASONE |
| DEXAMETHASONE ACETATE |
| DEXAMETHASONE SODIUM PHOSPHATE |
| DEXIBUPROFEN |
| DEXLANSOPRAZOLE |
| DEXPROPRANOLOL HYDROCHLORIDE |
| DEXTROMETHORPHAN HYDROBROMIDE |
| DIAVERIDINE |
| DIBENZOTHIOPHENE |
| DIBUCAINE HYDROCHLORIDE |
| DICHLOROPHENE |
| DICHLORVOS |
| DICLAZURIL |
| DICLOFENAC SODIUM |
| DICLOXACILLIN SODIUM |
| DICUMAROL |
| DICYCLOMINE HYDROCHLORIDE |
| DIENESTROL |
| DIETHYLCARBAMAZINE CITRATE |
| DIETHYLSTILBESTROL |
| DIFLOXACIN HYDROCHLORIDE |
| DIFLUNISAL |
| DIGITOXIN |
| DIGOXIN |
| DIHYDROERGOTAMINE MESYLATE |
| DIHYDROSTREPTOMYCIN SULFATE |
| DILAZEP DIHYDROCHLORIDE |
| DIMENHYDRINATE |
| DIMERCAPROL |
| DIMETHADIONE |
| DIOXYBENZONE |
| DIPHENHYDRAMINE HYDROCHLORIDE |
| DIPHENYLPYRALINE HYDROCHLORIDE |
| DIPYRIDAMOLE |
| DIPYRONE |
| DIRITHROMYCIN |
| DISOPYRAMIDE PHOSPHATE |
| DISULFIRAM |
| DOBUTAMINE HYDROCHLORIDE |
| DOCETAXEL |
| DONEPEZIL HYDROCHLORIDE |
| DOPAMINE HYDROCHLORIDE |
| DOXEPIN HYDROCHLORIDE |
| DOXOFYLLINE |
| DOXYCYCLINE HYDROCHLORIDE |
| DOXYLAMINE SUCCINATE |
| DROFENINE HYDROCHLORIDE |
| DROPERIDOL |
| DROSPIRENONE |
| DYCLONINE HYDROCHLORIDE |
| DYPHYLLINE |
| ECAMSULE TRIETHANOLAMINE |
| ECONAZOLE NITRATE |
| EDETATE DISODIUM |
| EDITOL |
| EDOXUDINE |
| EMETINE |
| ENALAPRIL MALEATE |
| ENALAPRILAT |
| ENOXACIN |
| ENROFLOXACIN |
| ENTACAPONE |
| EPHEDRINE (1R,2S) HYDROCHLORIDE |
| EPINEPHRINE BITARTRATE |
| EPRINOMECTIN |
| ERGOCALCIFEROL |
| ERGONOVINE MALEATE |
| ERYTHROMYCIN |
| ERYTHROMYCIN ESTOLATE |
| ERYTHROMYCIN ETHYLSUCCINATE |
| ESCITALOPRAM OXALATE |
| ESOMEPRAZOLE POTASSIUM |
| ESTRADIOL |
| ESTRADIOL BENZOATE |
| ESTRADIOL CYPIONATE |
| ESTRADIOL DIPROPIONATE |
| ESTRADIOL VALERATE |
| ESTRAMUSTINE |
| ESTRIOL |
| ESTRONE |
| ESTROPIPATE |
| ETHACRYNIC ACID |
| ETHAMBUTOL HYDROCHLORIDE |
| ETHAVERINE HYDROCHLORIDE |
| ETHINYL ESTRADIOL |
| ETHIONAMIDE |
| ETHISTERONE |
| ETHOPROPAZINE HYDROCHLORIDE |
| ETHYL PARABEN |
| ETODOLAC |
| ETOPOSIDE |
| EUCALYPTOL |
| EUCATROPINE HYDROCHLORIDE |
| EUGENOL |
| EVANS BLUE |
| EXEMESTANE |
| EZETIMIBE |
| FAMCICLOVIR |
| FAMOTIDINE |
| FAMPRIDINE |
| FASUDIL HYDROCHLORIDE |
| FEBUXOSTAT |
| FENBENDAZOLE |
| FENBUFEN |
| FENDILINE HYDROCHLORIDE |
| FENOFIBRATE |
| FENOPROFEN |
| FENOTEROL HYDROBROMIDE |
| FENSPIRIDE HYDROCHLORIDE |
| FEXOFENADINE HYDROCHLORIDE |
| FIPEXIDE HYDROCHLORIDE |
| FIROCOXIB |
| FLOXURIDINE |
| FLUCONAZOLE |
| FLUDROCORTISONE ACETATE |
| FLUFENAMIC ACID |
| FLUINDAROL |
| FLUMEQUINE |
| FLUMETHASONE |
| FLUMETHAZONE PIVALATE |
| FLUNARIZINE HYDROCHLORIDE |
| FLUNISOLIDE |
| FLUNIXIN MEGLUMINE |
| FLUOCINOLONE ACETONIDE |
| FLUOCINONIDE |
| FLUOROMETHOLONE |
| FLUOROURACIL |
| FLUOXETINE |
| FLUPHENAZINE HYDROCHLORIDE |
| FLURANDRENOLIDE |
| FLURBIPROFEN |
| FLUROFAMIDE |
| FLUTAMIDE |
| FLUVASTATIN |
| FOLIC ACID |
| FOSCARNET SODIUM |
| FOSFOMYCIN CALCIUM |
| FTAXILIDE |
| FULVESTRANT |
| FURAZOLIDONE |
| FUROSEMIDE |
| FUSIDIC ACID |
| GABOXADOL HYDROCHLORIDE |
| GADOTERIDOL |
| GALANTHAMINE |
| GALLAMINE TRIETHIODIDE |
| GANCICLOVIR |
| GATIFLOXACIN |
| GEFITINIB |
| GEMFIBROZIL |
| GENTAMICIN SULFATE |
| GENTIAN VIOLET |
| GLIMEPIRIDE |
| GLUCONOLACTONE |
| GLUCOSAMINE HYDROCHLORIDE |
| GLUTAMINE (D) |
| GRAMICIDIN |
| GRANISETRON HYDROCHLORIDE |
| GRISEOFULVIN |
| GUAIFENESIN |
| GUANABENZ ACETATE |
| GUANETHIDINE SULFATE |
| HALAZONE |
| HALCINONIDE |
| HALOPERIDOL |
| HEPTAMINOL HYDROCHLORIDE |
| HETACILLIN POTASSIUM |
| HEXACHLOROPHENE |
| HEXYLRESORCINOL |
| HISTAMINE DIHYDROCHLORIDE |
| HOMATROPINE BROMIDE |
| HOMATROPINE METHYLBROMIDE |
| HOMOSALATE |
| HYCANTHONE |
| HYDRALAZINE HYDROCHLORIDE |
| HYDRASTINE (1R, 9S) |
| HYDROCHLOROTHIAZIDE |
| HYDROCORTISONE |
| HYDROCORTISONE ACETATE |
| HYDROCORTISONE BUTYRATE |
| HYDROCORTISONE HEMISUCCINATE |
| HYDROCORTISONE PHOSPHATE TRIETHYLAMINE |
| HYDROFLUMETHIAZIDE |
| HYDROQUINONE |
| HYDROXYAMPHETAMINE HYDROBROMIDE |
| HYDROXYCHLOROQUINE SULFATE |
| HYDROXYPROGESTERONE CAPROATE |
| HYDROXYTOLUIC ACID |
| HYDROXYUREA |
| HYDROXYZINE PAMOATE |
| HYOSCYAMINE |
| IBANDRONATE SODIUM |
| IBUPROFEN |
| IDOXURDINE |
| IDOXURIDINE |
| IMIPRAMINE HYDROCHLORIDE |
| IMIQUIMOD |
| INAMRINONE |
| INDAPAMIDE |
| INDOMETHACIN |
| INDOPROFEN |
| INOSITOL |
| IODIPAMIDE |
| IODIXANOL |
| IODOQUINOL |
| IOHEXOL |
| IOPANIC ACID |
| IOTHALAMIC ACID |
| IOVERSOL |
| IOXILAN |
| IPRATROPIUM BROMIDE |
| IRBESARTAN |
| ISONIAZID |
| ISOPROPAMIDE IODIDE |
| ISOPROTERENOL HYDROCHLORIDE |
| ISOSORBIDE DINITRATE |
| ISOSORBIDE MONONITRATE |
| ISOTRETINON |
| ISOXICAM |
| ISOXSUPRINE HYDROCHLORIDE |
| ITOPRIDE HYDROCHLORIDE |
| IVERMECTIN |
| KANAMYCIN A SULFATE |
| KETOCONAZOLE |
| KETOPROFEN |
| KETOROLAC TROMETHAMINE |
| KETOTIFEN FUMARATE |
| LABETALOL HYDROCHLORIDE |
| LACTULOSE |
| LAMIVUDINE |
| LANATOSIDE C |
| LANSOPRAZOLE |
| LEFLUNOMIDE |
| LETROZOLE |
| LEUCOVORIN CALCIUM |
| LEVAMISOLE HYDROCHLORIDE |
| LEVOCETIRIZINE DIHYDROCHLORIDE |
| LEVOFLOXACIN |
| LEVOMENTHOL |
| LEVONORDEFRIN |
| LEVONORGESTREL |
| LEVOSIMENDAN |
| LEVOTHYROXINE |
| LIDOCAINE HYDROCHLORIDE |
| LINCOMYCIN HYDROCHLORIDE |
| LINDANE |
| LINEZOLID |
| LIOTHYRONINE |
| LIOTHYRONINE (L- isomer) SODIUM |
| LISINOPRIL |
| LITHIUM CITRATE |
| LOBELINE HYDROCHLORIDE |
| LOFEXIDINE HYDROCHLORIDE |
| LOMEFLOXACIN HYDROCHLORIDE |
| LOMERIZINE HYDROCHLORIDE |
| LOMUSTINE |
| LORATADINE |
| LORNOXICAM |
| LOSARTAN |
| LOVASTATIN |
| LUMIRACOXIB |
| MAFENIDE HYDROCHLORIDE |
| MALATHION |
| MANGAFODIPIR TRISODIUM |
| MANIDIPINE HYDROCHLORIDE |
| MANNITOL |
| MAPROTILINE HYDROCHLORIDE |
| MEBENDAZOLE |
| MEBEVERINE HYDROCHLORIDE |
| MEBHYDROLIN NAPHTHALENESULFONATE |
| MECAMYLAMINE HYDROCHLORIDE |
| MECHLORETHAMINE |
| MECLIZINE HYDROCHLORIDE |
| MECLOCYCLINE SULFOSALICYLATE |
| MECLOFENAMATE SODIUM |
| MECLOFENOXATE HYDROCHLORIDE |
| MEDROXYPROGESTERONE ACETATE |
| MEDRYSONE |
| MEFENAMIC ACID |
| MEFEXAMIDE |
| MEFLOQUINE |
| MEGESTROL ACETATE |
| MEGLUMINE |
| MELOXICAM SODIUM |
| MELPERONE HYDROCHLORIDE |
| MELPHALAN |
| MEMANTINE HYDROCHLORIDE |
| MENADIONE |
| MEPARTRICIN |
| MEPENZOLATE BROMIDE |
| MEPHENESIN |
| MEPHENTERMINE SULFATE |
| MEPIVACAINE HYDROCHLORIDE |
| MEQUINOL |
| MERBROMIN |
| MERCAPTOPURINE |
| MEROPENEM |
| MESNA |
| MESO-ERYTHRITOL |
| MESTRANOL |
| METAPROTERENOL |
| METARAMINOL BITARTRATE |
| METAXALONE |
| METHACHOLINE CHLORIDE |
| METHACYCLINE HYDROCHLORIDE |
| METHAPYRILENE HYDROCHLORIDE |
| METHAZOLAMIDE |
| METHENAMINE |
| METHICILLIN SODIUM |
| METHIMAZOLE |
| METHOCARBAMOL |
| METHOTREXATE(+/-) |
| METHOXAMINE HYDROCHLORIDE |
| METHOXSALEN |
| METHSCOPOLAMINE BROMIDE |
| METHYCLOTHIAZIDE |
| METHYLBENZETHONIUM CHLORIDE |
| METHYLDOPA |
| METHYLERGONOVINE MALEATE |
| METHYLPREDNISOLONE |
| METHYLPREDNISOLONE SODIUM SUCCINATE |
| METHYLTHIOURACIL |
| METOCLOPRAMIDE HYDROCHLORIDE |
| METOPROLOL TARTRATE |
| METRONIDAZOLE |
| MEXILETINE HYDROCHLORIDE |
| MICONAZOLE NITRATE |
| MIDODRINE HYDROCHLORIDE |
| MIGLITOL |
| MILNACIPRAN HYDROCHLORIDE |
| MINAPRINE HYDROCHLORIDE |
| MINOCYCLINE HYDROCHLORIDE |
| MINOXIDIL |
| MITOMYCIN C |
| MITOTANE |
| MITOXANTRONE HYDROCHLORIDE |
| MOLSIDOMINE |
| MONENSIN SODIUM (monensin A is shown) |
| MONOBENZONE |
| MORANTEL CITRATE |
| MOXALACTAM DISODIUM |
| MOXIFLOXACIN HYDROCHLORIDE |
| MYCOPHENOLATE MOFETIL |
| MYCOPHENOLIC ACID |
| NABUMETONE |
| NADIDE |
| NADOLOL |
| NAFCILLIN SODIUM |
| NAFRONYL OXALATE |
| NALBUPHINE HYDROCHLORIDE |
| NALIDIXIC ACID |
| NALOXONE HYDROCHLORIDE |
| NALTREXONE HYDROCHLORIDE |
| NAPHAZOLINE HYDROCHLORIDE |
| NAPROXEN(+) |
| NAPROXOL |
| NATEGLINIDE |
| NEFAZODONE HYDROCHLORIDE |
| NEFOPAM |
| NELARABIN |
| NEOMYCIN SULFATE |
| NEOSTIGMINE BROMIDE |
| NEVIRAPINE |
| NIACIN |
| NICARDIPINE HYDROCHLORIDE |
| NICERGOLINE |
| NICLOSAMIDE |
| NICOTINYL ALCOHOL TARTRATE |
| NIFEDIPINE |
| NIFURSOL |
| NILUTAMIDE |
| NIMODIPINE |
| NITAZOXANIDE |
| NITRENDIPINE |
| NITROFURANTOIN |
| NITROFURAZONE |
| NITROMIDE |
| NOCODAZOLE |
| NOMIFENSINE MALEATE |
| NOREPINEPHRINE |
| NORETHINDRONE |
| NORETHINDRONE ACETATE |
| NORETHYNODREL |
| NORFLOXACIN |
| NORGESTREL |
| NORTRIPTYLINE |
| NOSCAPINE HYDROCHLORIDE |
| NOVOBIOCIN SODIUM |
| NYLIDRIN HYDROCHLORIDE |
| NYSTATIN |
| OCTOPAMINE HYDROCHLORIDE |
| OFLOXACIN |
| OLMESARTAN |
| OLMESARTAN MEDOXOMIL |
| OLSALAZINE SODIUM |
| OLSELTAMIVIR PHOSPHATE |
| OMEGA-3-ACID ESTERS (EPA shown) |
| ONDANSETRON |
| ORLISTAT |
| ORPHENADRINE CITRATE |
| OUABAIN |
| OXACILLIN SODIUM |
| OXALIPLATIN |
| OXCARBAZEPINE |
| OXETHAZAINE |
| OXIBENDAZOLE |
| OXIDOPAMINE HYDROCHLORIDE |
| OXOLINIC ACID |
| OXYBENZONE |
| OXYMETAZOLINE HYDROCHLORIDE |
| OXYPHENBUTAZONE |
| OXYPHENCYCLIMINE HYDROCHLORIDE |
| OXYQUINOLINE HEMISULFATE |
| OXYTETRACYCLINE |
| PACLITAXEL |
| PALIPERIDONE |
| PAPAVERINE HYDROCHLORIDE |
| PARACHLOROPHENOL |
| PARAROSANILINE PAMOATE |
| PARGYLINE HYDROCHLORIDE |
| PAROMOMYCIN SULFATE |
| PAROXETINE HYDROCHLORIDE |
| PEMETREXED |
| PENCICLOVIR |
| PENICILLAMINE |
| PENICILLIN G POTASSIUM |
| PENICILLIN V POTASSIUM |
| PENTOLINIUM TARTRATE |
| PENTOXIFYLLINE |
| PERGOLIDE MESYLATE |
| PERHEXILINE MALEATE |
| PERICIAZINE |
| PERINDOPRIL ERBUMINE |
| PERPHENAZINE |
| PHENACEMIDE |
| PHENAZOPYRIDINE HYDROCHLORIDE |
| PHENELZINE SULFATE |
| PHENINDIONE |
| PHENIRAMINE MALEATE |
| PHENOLPHTHALEIN |
| PHENTOLAMINE HYDROCHLORIDE |
| PHENYL AMINOSALICYLATE |
| PHENYLBUTAZONE |
| PHENYLEPHRINE HYDROCHLORIDE |
| PHENYLMERCURIC ACETATE |
| PHENYLPROPANOLAMINE HYDROCHLORIDE |
| PHENYTOIN SODIUM |
| PHTHALYLSULFATHIAZOLE |
| PHYSOSTIGMINE SALICYLATE |
| PILOCARPINE NITRATE |
| PIMOZIDE |
| PINDOLOL |
| PIOGLITAZONE HYDROCHLORIDE |
| PIPERACETAZINE |
| PIPERACILLIN SODIUM |
| PIPERAZINE |
| PIPERIDOLATE HYDROCHLORIDE |
| PIPERINE |
| PIPOBROMAN |
| PIRACETAM |
| PIRENPERONE |
| PIRENZEPINE HYDROCHLORIDE |
| PIROCTONE OLAMINE |
| PIROXICAM |
| PITAVASTATIN CALCIUM |
| PIZOTYLINE MALATE |
| POLYMYXIN B SULFATE |
| POTASSIUM p-AMINOBENZOATE |
| PRAMIPEXOLE DIHYDROCHLORIDE |
| PRAMOXINE HYDROCHLORIDE |
| PRASUGREL |
| PRAZIQUANTEL |
| PRAZOSIN HYDROCHLORIDE |
| PREDNICARBATE |
| PREDNISOLONE |
| PREDNISOLONE ACETATE |
| PREDNISONE |
| PRILOCAINE HYDROCHLORIDE |
| PRIMAQUINE DIPHOSPHATE |
| PRIMIDONE |
| PROADIFEN HYDROCHLORIDE |
| PROBENECID |
| PROBUCOL |
| PROCAINAMIDE HYDROCHLORIDE |
| PROCAINE HYDROCHLORIDE |
| PROCARBAZINE HYDROCHLORIDE |
| PROCHLORPERAZINE EDISYLATE |
| PROCYCLIDINE HYDROCHLORIDE |
| PROGESTERONE |
| PROGLUMIDE |
| PROMAZINE HYDROCHLORIDE |
| PROMETHAZINE HYDROCHLORIDE |
| PRONETALOL HYDROCHLORIDE |
| PROPAFENONE HYDROCHLORIDE |
| PROPANTHELINE BROMIDE |
| PROPIOLACTONE |
| PROPOFOL |
| PROPYLTHIOURACIL |
| PSEUDOEPHEDRINE HYDROCHLORIDE |
| PUROMYCIN HYDROCHLORIDE |
| PYRANTEL PAMOATE |
| PYRAZINAMIDE |
| PYRETHRINS |
| PYRIDOSTIGMINE BROMIDE |
| PYRILAMINE MALEATE |
| PYRIMETHAMINE |
| PYRITHIONE ZINC |
| PYRONARIDINE TETRAPHOSPHATE |
| PYRVINIUM PAMOATE |
| QUETIAPINE |
| QUINACRINE HYDROCHLORIDE |
| QUINAPRIL HYDROCHLORIDE |
| QUINESTROL |
| QUINETHAZONE |
| QUINIDINE GLUCONATE |
| QUININE SULFATE |
| QUIPAZINE MALEATE |
| RACEPHEDRINE HYDROCHLORIDE |
| RACTOPAMINE HYDROCHLORIDE |
| RAMIPRIL |
| RAMOPLANIN [A2 shown; 2mm] |
| RANITIDINE |
| RASAGILINE |
| RESERPINE |
| RESORCINOL |
| RESORCINOL MONOACETATE |
| RETAPAMULIN |
| RETINOL |
| RETINYL PALMITATE |
| RIBAVIRIN |
| RIFAMPIN |
| RITANSERIN |
| RITODRINE HYDROCHLORIDE |
| RITONAVIR |
| RIZATRIPTAN BENZOATE |
| ROFECOXIB |
| RONIDAZOLE |
| ROPINIROLE |
| ROSIGLITAZONE |
| ROSUVASTATIN CALCIUM |
| ROXARSONE |
| ROXATIDINE ACETATE HYDROCHLORIDE |
| ROXITHROMYCIN |
| RUFLOXACIN HYDROCHLORIDE |
| SACCHARIN |
| SALICIN |
| SALICYL ALCOHOL |
| SALICYLAMIDE |
| SALICYLANILIDE |
| SALINOMYCIN, SODIUM |
| SALSALATE |
| SANGUINARINE SULFATE |
| SCOPOLAMINE HYDROBROMIDE |
| SELAMECTIN |
| SEMUSTINE |
| SENNOSIDE A |
| SERATRODAST |
| SERTRALINE HYDROCHLORIDE |
| SEVOFLURANE |
| SIBUTRAMINE HYDROCHLORIDE |
| SILDENAFIL CITRATE |
| SIMVASTATIN |
| SIROLIMUS |
| SISOMICIN SULFATE |
| SODIUM DEHYDROCHOLATE |
| SODIUM NITROPRUSSIDE |
| SODIUM OXYBATE |
| SODIUM PHENYLACETATE |
| SODIUM PHENYLBUTYRATE |
| SODIUM SALICYLATE |
| SPARFLOXACIN |
| SPARTEINE SULFATE |
| SPECTINOMYCIN HYDROCHLORIDE |
| SPIPERONE |
| SPIRAMYCIN |
| SPIRAPRIL HYDROCHLORIDE |
| SPIRONOLACTONE |
| STAVUDINE |
| STREPTOMYCIN SULFATE |
| STREPTOZOSIN |
| SUCCINYLSULFATHIAZOLE |
| SULBACTAM |
| SULCONAZOLE NITRATE |
| SULFABENZAMIDE |
| SULFACETAMIDE |
| SULFACHLORPYRIDAZINE |
| SULFADIAZINE |
| SULFADIMETHOXINE |
| SULFAMERAZINE |
| SULFAMETER |
| SULFAMETHAZINE |
| SULFAMETHIZOLE |
| SULFAMETHOXAZOLE |
| SULFAMETHOXYPYRIDAZINE |
| SULFAMONOMETHOXINE |
| SULFANILATE ZINC |
| SULFANITRAN |
| SULFAPYRIDINE |
| SULFAQUINOXALINE SODIUM |
| SULFASALAZINE |
| SULFATHIAZOLE |
| SULFINPYRAZONE |
| SULFISOXAZOLE |
| SULINDAC |
| SULMAZOLE |
| SULOCTIDIL |
| SULPIRIDE |
| SUPROFEN |
| SURAMIN |
| TACROLIMUS |
| TAMOXIFEN CITRATE |
| TANDUTINIB |
| TANNIC ACID |
| TAZOBACTAM |
| TEGASEROD MALEATE |
| TELMISARTAN |
| TEMEFOS |
| TEMOZOLAMIDE |
| TENIPOSIDE |
| TENOXICAM |
| TERBUTALINE HEMISULFATE |
| TERCONAZOLE |
| TERFENADINE |
| TESTOSTERONE |
| TESTOSTERONE PROPIONATE |
| TETRACAINE HYDROCHLORIDE |
| TETRACYCLINE HYDROCHLORIDE |
| TETRAHYDROZOLINE HYDROCHLORIDE |
| TETROQUINONE |
| THALIDOMIDE |
| THEOBROMINE |
| THEOPHYLLINE |
| THIABENDAZOLE |
| THIAMPHENICOL |
| THIMEROSAL |
| THIOGUANINE |
| THIORIDAZINE HYDROCHLORIDE |
| THIOSTREPTON |
| THIOTEPA |
| THIOTHIXENE |
| THIRAM |
| THONZONIUM BROMIDE |
| THONZYLAMINE HYDROCHLORIDE |
| TIAPRIDE HYDROCHLORIDE |
| TIBOLONE |
| TIGECYCLINE |
| TILARGININE HYDROCHLORIDE |
| TILETAMINE HYDROCHLORIDE |
| TILMICOSIN |
| TIMOLOL MALEATE |
| TINIDAZOLE |
| TOBRAMYCIN |
| TODRALAZINE HYDROCHLORIDE |
| TOLAZAMIDE |
| TOLAZOLINE HYDROCHLORIDE |
| TOLBUTAMIDE |
| TOLMETIN SODIUM |
| TOLNAFTATE |
| TOLPERISONE HYDROCHLORIDE |
| TOSYLCHLORAMIDE SODIUM |
| TRANEXAMIC ACID |
| TRANYLCYPROMINE SULFATE |
| TRAZODONE HYDROCHLORIDE |
| TRETINOIN |
| TRIACETIN |
| TRIAMCINOLONE |
| TRIAMCINOLONE ACETONIDE |
| TRIAMCINOLONE DIACETATE |
| TRIAMTERENE |
| TRICHLORMETHIAZIDE |
| TRIFLUOPERAZINE HYDROCHLORIDE |
| TRIFLUPROMAZINE HYDROCHLORIDE |
| TRIFLURIDINE |
| TRIHEXYPHENIDYL HYDROCHLORIDE |
| TRILOSTANE |
| TRIMEPRAZINE TARTRATE |
| TRIMETHOBENZAMIDE HYDROCHLORIDE |
| TRIMETHOPRIM |
| TRIMETOZINE |
| TRIMIPRAMINE MALEATE |
| TRIOXSALEN |
| TRIPELENNAMINE CITRATE |
| TRIPROLIDINE HYDROCHLORIDE |
| TRISODIUM ETHYLENEDIAMINE TETRACETATE |
| TROLEANDOMYCIN |
| TROPICAMIDE |
| TROPISETRON HYDROCHLORIDE |
| TRYPTOPHAN |
| TUAMINOHEPTANE SULFATE |
| TUBOCURARINE CHLORIDE |
| TYROTHRICIN |
| URACIL |
| URAPIDIL HYDROCHLORIDE |
| UREA |
| URETHANE |
| URSODIOL |
| VALDECOXIB |
| VALGANCICLOVIR HYDROCHLORIDE |
| VALPROATE SODIUM |
| VALSARTAN |
| VANCOMYCIN HYDROCHLORIDE |
| VENLAFAXINE |
| VIDARABINE |
| VINBLASTINE SULFATE |
| VINORELBINE |
| VINPOCETINE |
| VIOMYCIN SULFATE |
| VORICONAZOLE |
| VORINOSTAT |
| WARFARIN |
| XYLAZINE |
| XYLOMETAZOLINE HYDROCHLORIDE |
| YOHIMBINE HYDROCHLORIDE |
| ZALCITABINE |
| ZAPRINAST |
| ZIDOVUDINE [AZT] |
| ZIPRASIDONE MESYLATE |
| ZOMEPIRAC SODIUM |
| ZOPICLONE |
Data analysis
Data are presented as the mean and SEM, unless stated otherwise. Pairwise statistical significance was determined with a Student’s two-tailed unpaired t test, ANOVA, or Mann–Whitney rank sum test, as appropriate, unless stated otherwise. Results were considered significant at p < 0.05, unless otherwise indicated.
Results
A first-stage behavioral screen for antiepileptic activity
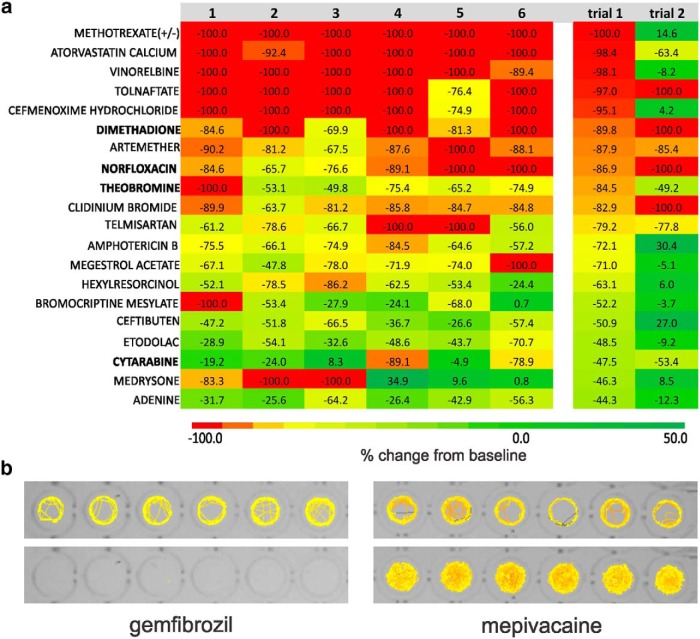
Locomotion tracking is a reliable and rapid strategy with which to monitor behavioral seizures in freely swimming larval zebrafish (Baraban et al., 2005, 2013; Winter et al., 2008). In these locomotion plots, high-velocity movements of ≥20 mm/s correspond to paroxysmal whole-body convulsions, referred to as Stage III, and are consistently observed in unprovoked scn1Lab mutant larvae but not in age-matched wild-type siblings. Using automated locomotion tracking, we performed a phenotype-based screen to identify compounds that significantly reduce mutant swim behavior to levels associated with Stage 0 or Stage I (e.g., activity equivalent to that seen in normal untreated WT zebrafish). In a 96-well format, we tracked mutant swim activity at baseline, and then again after addition of a test compound (100 µm); each compound was tested on six individual mutant larvae (Fig. 1a ), and larvae were sorted based on pigmentation differences (Fig. 1b ). Mutant swim activity between two consecutive recording epochs in embryo media is tracked on every plate as an internal control. A box plot showing the change in swim velocity in untreated mutants is shown in Figure 1c (n = 112) and defined as the control. Based on an SD of 21.8 for these data, we set the detection threshold as any compound that inhibits movement (measured as a change in mean velocity) by >2 SDs (or ≥44%). This approach was previously validated using standard antiepileptic drugs in this model (Baraban et al., 2013). Next, we screened a repurposed library in which all compounds have reached the clinical evaluation stage (PHARMAKON 1600 Collection; http://www.msdiscovery.com/pharma.html). Among the 1012 compounds screened (Fig. 1d ) only 20 (or 1.97%) were found to significantly inhibit spontaneous seizure behavior in scn1Lab mutants. All 20 compounds were subsequently retested in a separate clutch of scn1Lab mutants at a concentration of 100 µm (Fig. 2a , trial 2; N = 6 fish/compound). A total of 154 compounds were classified as “toxic” (Table 2); 55 compounds were classified as “hyperexcitable” (Table 3). Representative locomotion tracking raw data plots for gemfibrozil, a toxic nonsteroid nuclear receptor ligand, and mepivacaine, a hyperexcitable proconvulsant anesthetic, are shown in Figure 2b .
Figure 2.
Positive hits identified in the locomotion assay. a, Heat map showing the results of individual zebrafish trials (1-6) for compounds tested at a concentration of 100 µm in the locomotion-tracking assay. Raw data values for individual fish are shown within the color-coded boxes for one sample trial. Mean velocity data are shown at right for “trial 1” and “trial 2”; six fish per trial. Note: only drugs highlighted in bold type were classified as positive nontoxic hits in two independent trials and moved on to further testing. b, Representative raw locomotion data plots for six individual scn1Lab mutant larvae at baseline (top) and following the addition of a compound resulting in fatality (bottom, gemfibrozil) or hyperactivity (bottom, mepivacaine). Movement is color coded, with low-velocity movements shown in yellow, and high velocity movements shown in red.
Table 2:
List of compounds exhibiting toxicity.
| ABACAVIR SULFATE |
| ACIPIMOX |
| ADENOSINE PHOSPHATE |
| ALAPROCLATE |
| AMLEXANOX |
| AMOROLFINE HYDROCHLORIDE |
| ANTAZOLINE PHOSPHATE |
| ARTEMETHER |
| ASCORBIC ACID |
| ATORVASTATIN CALCIUM |
| AUROTHIOGLUCOSE |
| AZELAIC ACID |
| BENORILATE |
| BENZONATATE |
| BETAINE HYDROCHLORIDE |
| BETAMIPRON |
| BROMHEXINE HYDROCHLORIDE |
| BUDESONIDE |
| BUPIVACAINE HYDROCHLORIDE |
| BUSULFAN |
| BUTOCONAZOLE |
| CAPSAICIN |
| CARPROFEN |
| CEFORANIDE |
| CEFOTAXIME SODIUM |
| CEFOXITIN SODIUM |
| CEPHALEXIN |
| CHLORAMBUCIL |
| CHLORAMPHENICOL HEMISUCCINATE |
| CHLOROGUANIDE HYDROCHLORIDE |
| CHLORPHENIRAMINE (S) MALEATE |
| CINCHOPHEN |
| CINNARAZINE |
| CINTRIAMIDE |
| CIPROFLOXACIN |
| CLIDINIUM BROMIDE |
| CLOZAPINE |
| COLISTIMETHATE SODIUM |
| CRYOFLURANE |
| CYCLOPHOSPHAMIDE HYDRATE |
| CYCLOTHIAZIDE |
| CYPERMETHRIN |
| DAUNORUBICIN |
| DECIMEMIDE |
| DEXTROMETHORPHAN HYDROBROMIDE |
| DICHLOROPHENE |
| DIETHYLCARBAMAZINE CITRATE |
| DIOXYBENZONE |
| DIRITHROMYCIN |
| DISOPYRAMIDE PHOSPHATE |
| DISULFIRAM |
| ECONAZOLE NITRATE |
| EDETATE DISODIUM |
| EMETINE |
| ENALAPRILAT |
| ERYTHROMYCIN |
| ETHINYL ESTRADIOL |
| ETHIONAMIDE |
| ETHOPROPAZINE HYDROCHLORIDE |
| ETHYL PARABEN |
| EUGENOL |
| FIPEXIDE HYDROCHLORIDE |
| FLUMETHASONE |
| FLUNISOLIDE |
| FLUVASTATIN |
| GEMFIBROZIL |
| GENTAMICIN SULFATE |
| GLUCONOLACTONE |
| HALAZONE |
| HALCINONIDE |
| HETACILLIN POTASSIUM |
| HEXACHLOROPHENE |
| HOMATROPINE METHYLBROMIDE |
| HYDRASTINE (1R, 9S) |
| HYDROXYAMPHETAMINE HYDROBROMIDE |
| HYDROXYCHLOROQUINE SULFATE |
| IODIXANOL |
| IOHEXOL |
| IRBESARTAN |
| LEVOSIMENDAN |
| LISINOPRIL |
| LOMERIZINE HYDROCHLORIDE |
| MANGAFODIPIR TRISODIUM |
| MECLOFENOXATE HYDROCHLORIDE |
| MESTRANOL |
| METHACHOLINE CHLORIDE |
| METHYLERGONOVINE MALEATE |
| METRONIDAZOLE |
| MIGLITOL |
| MONENSIN SODIUM (monensin A is shown) |
| MONOBENZONE |
| MOXALACTAM DISODIUM |
| NADOLOL |
| NALBUPHINE HYDROCHLORIDE |
| NALTREXONE HYDROCHLORIDE |
| NAPHAZOLINE HYDROCHLORIDE |
| NAPROXEN(+) |
| NEOMYCIN SULFATE |
| NIFEDIPINE |
| NITAZOXANIDE |
| NITROMIDE |
| NORETHINDRONE |
| OLMESARTAN MEDOXOMIL |
| OXYMETAZOLINE HYDROCHLORIDE |
| PARACHLOROPHENOL |
| PAROMOMYCIN SULFATE |
| PERHEXILINE MALEATE |
| PHENTOLAMINE HYDROCHLORIDE |
| PHENYLBUTAZONE |
| PHENYLMERCURIC ACETATE |
| PHYSOSTIGMINE SALICYLATE |
| PIMOZIDE |
| PIPERACILLIN SODIUM |
| PIPERAZINE |
| PIRACETAM |
| PIRENZEPINE HYDROCHLORIDE |
| PIROCTONE OLAMINE |
| PITAVASTATIN CALCIUM |
| PRIMAQUINE DIPHOSPHATE |
| PROBENECID |
| PROCARBAZINE HYDROCHLORIDE |
| PROGLUMIDE |
| PROMETHAZINE HYDROCHLORIDE |
| PUROMYCIN HYDROCHLORIDE |
| QUININE SULFATE |
| RETINYL PALMITATE |
| RIFAMPIN |
| RITONAVIR |
| ROFECOXIB |
| RUFLOXACIN HYDROCHLORIDE |
| SACCHARIN |
| SALICIN |
| SENNOSIDE A |
| STAVUDINE |
| STREPTOMYCIN SULFATE |
| SULFADIAZINE |
| SULINDAC |
| SULOCTIDIL |
| TANNIC ACID |
| TELMISARTAN |
| TENOXICAM |
| THEOPHYLLINE |
| TILETAMINE HYDROCHLORIDE |
| TILMICOSIN |
| TIMOLOL MALEATE |
| TOLBUTAMIDE |
| TOLNAFTATE |
| TRAZODONE HYDROCHLORIDE |
| TRETINOIN |
| TRIFLUPROMAZINE HYDROCHLORIDE |
| TROPISETRON HYDROCHLORIDE |
| VALDECOXIB |
| VORINOSTAT |
| ZALCITABINE |
Table 3:
List of compounds exhibiting hyperexcitable or proconvulsant activity.
| ADENOSINE PHOSPHATE |
| ALBUTEROL (+/-) |
| ALEXIDINE HYDROCHLORIDE |
| AMANTADINE HYDROCHLORIDE |
| AMINOHIPPURIC ACID |
| AMINOLEVULINIC ACID HYDROCHLORIDE |
| AUROTHIOGLUCOSE |
| AZACITIDINE |
| BENZOYL PEROXIDE |
| BETAZOLE HYDROCHLORIDE |
| BROMHEXINE HYDROCHLORIDE |
| BUSULFAN |
| CEFSULODIN SODIUM |
| CEFUROXIME AXETIL |
| CHLOROGUANIDE HYDROCHLORIDE |
| CYSTEAMINE HYDROCHLORIDE |
| ECAMSULE TRIETHANOLAMINE |
| ECONAZOLE NITRATE |
| EDOXUDINE |
| ENROFLOXACIN |
| ESTRADIOL CYPIONATE |
| ETHINYL ESTRADIOL |
| ETHOPROPAZINE HYDROCHLORIDE |
| ETOPOSIDE |
| FASUDIL HYDROCHLORIDE |
| FEBUXOSTAT |
| FLUMETHASONE |
| FLUOROMETHOLONE |
| FURAZOLIDONE |
| GANCICLOVIR |
| GLUCONOLACTONE |
| GRANISETRON HYDROCHLORIDE |
| HALAZONE |
| HEXACHLOROPHENE |
| IODIPAMIDE |
| LABETALOL HYDROCHLORIDE |
| MEPIVACAINE HYDROCHLORIDE |
| MITOXANTRONE HYDROCHLORIDE |
| MORANTEL CITRATE |
| NOCODAZOLE |
| OFLOXACIN |
| PENTOLINIUM TARTRATE |
| PERINDOPRIL ERBUMINE |
| PIOGLITAZONE HYDROCHLORIDE |
| PRAMIPEXOLE DIHYDROCHLORIDE |
| PROGLUMIDE |
| RIFAMPIN |
| SERATRODAST |
| SERTRALINE HYDROCHLORIDE |
| SIBUTRAMINE HYDROCHLORIDE |
| SUCCINYLSULFATHIAZOLE |
| TACROLIMUS |
| TETROQUINONE |
| TIMOLOL MALEATE |
| URACIL |
A second-stage electrophysiology assay for antiepileptic activity
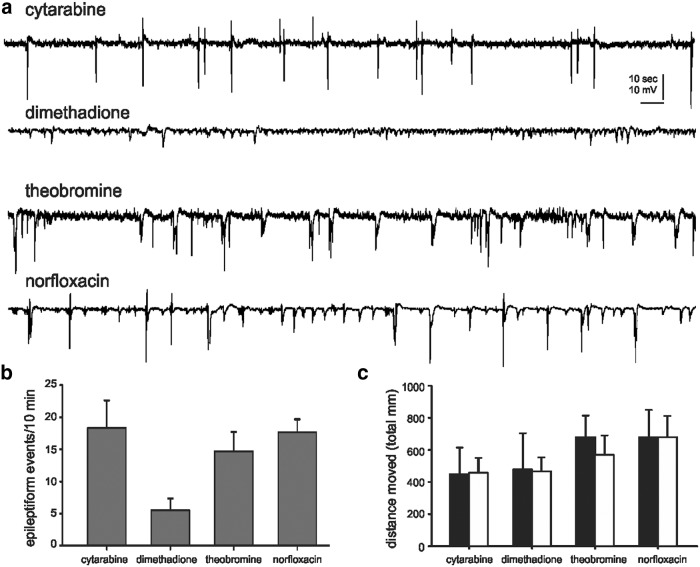
Extracellular recording electrodes are a reliable, reproducible, and sensitive approach to monitor electroencephalographic activity in agar-immobilized larval zebrafish (Baraban et al., 2005; Baraban, 2013). Field electrodes offer high a signal-to-noise ratio and can be placed, using direct visualization in transparent larvae, into specific CNS structures (i.e., telencephalon or optic tectum). Using a local field electrode, we can efficiently monitor the occurrence of electrographic seizure events in the same zebrafish that were previously tested in the locomotion assay. Based on a positive nontoxic result in two independent locomotion assays, four drugs moved on to electrophysiology testing at concentrations between 500 µm and 1 mm (Fig. 3). Consistent with a “false-positive” classification, spontaneous epileptiform discharge activity was observed for three of these drugs: norfloxacin, theobromine, and cytarabine. Dimethadione, previously shown to inhibit spontaneous epileptiform discharges in thalamocortical slices at concentrations between 1 and 10 mm (Zhang et al., 1996), suppressed burst discharge activity in scn1Lab mutant larvae (Fig. 3a,b ). To identify whether any of these four compounds exert nonspecific effects on behavior, they were also tested on freely swimming WT zebrafish larvae (5 dpf) at a concentration of 500 µm. Comparing the total distance moved during a 10 min recording epoch before, and after, the application of a test compound failed to reveal any significant changes in locomotor activity (Fig. 3c ).
Figure 3.
Electrophysiology assay to identify drugs that rescue the scn1Lab mutant epilepsy phenotype. a, Representative field electrode recording epochs (5 min in duration) are shown for the “positive” compounds identified in the locomotion assay. All recordings were obtained with an electrode placed in the forebrain of agar-immobilized scn1Lab larvae that was previously tested in the locomotion assay. A suppression of epileptiform electrographic discharge activity was noted in mutants exposed to dimethadione. b, Bar plot showing the mean number of epileptiform events in a 10 min recording epoch for scn1Lab larvae exposed to cytarabine (N = 6), dimethadione (N = 6), theobromine (N = 6), and norfloxacin (N = 6). The mean ± SEM is shown. The fish shown were tested in the locomotion assay first. c, Bar plot showing the total distance traveled before (black bars) and after (white bars) exposure to a test compound; 10 min recording epoch and six fish per drug. The mean ± SEM is shown.
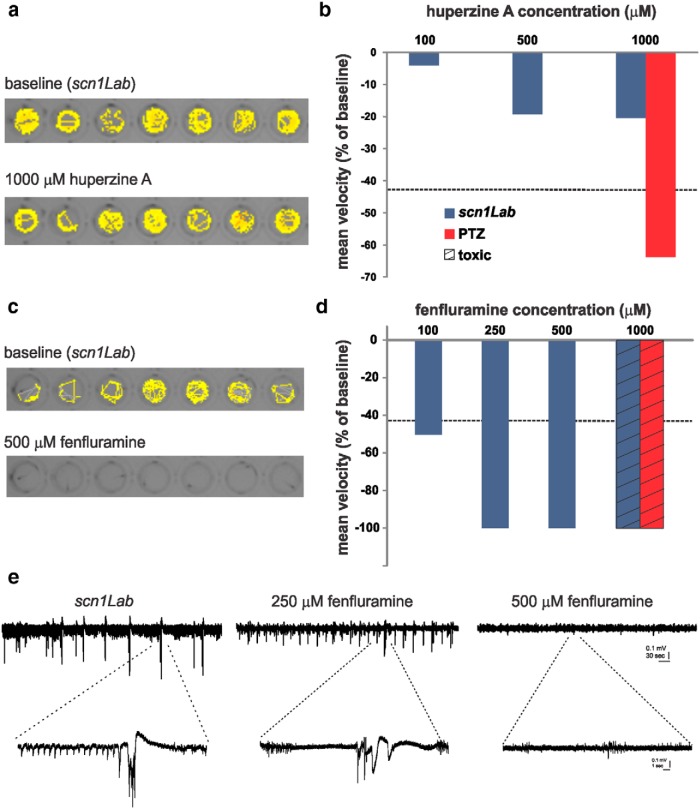
Assessment of huperzine A and fenfluramine for antiepileptic activity
Next, we tested two additional compounds that were not in our drug library, but have recently been described as potential antiepileptic treatments for DS. Huperzine A, a small-molecule alkaloid isolated from Chinese club moss with NMDA-type receptor blocking and anticholinesterase activity, has purported antiepileptic actions against NMDA- or soman-induced seizures (Tonduli et al., 2001; Coleman et al., 2008). In the locomotion assay, huperzine A failed to significantly alter scn1Lab seizure behavior at any concentration tested (Fig. 4a,b ). In contrast, huperzine A was effective at 1 mm in the acute pentylenetetrazole (PTZ) assay (Fig. 4b ). Fenfluramine is an amphetamine-like compound that has been reported to successfully reduce seizure occurrence in children with DS as a low-dose add-on therapy (Ceulemans et al., 2012). In the locomotion assay, fenfluramine significantly reduced mutant mean swim velocity at concentrations between 100 and 500 μm (Fig. 4c,d ); 1 mm fenfluramine was toxic in the scn1Lab and PTZ assays (Fig. 4d ). The fenfluramine-treated scn1Lab mutant exhibited a suppression of spontaneous electrographic seizure discharge to levels similar to controls at 500 μm, but only a partial reduction in electrographic activity at 250 μm (Fig. 4e ).
Figure 4.
Evaluation of putative antiepileptic drugs in scn1Lab mutants. a, Locomotion tracking plots for scn1Lab zebrafish at baseline and following huperzine A administration. Total movement is shown for a 10 min recording epoch. b, Plot showing the change in mean velocity for three different huperzine A concentrations (blue bars). Each bar is the mean change for six fish. The threshold for a positive hit is shown as a dashed line. WT fish exposed to PTZ and huperzine A are shown in red (N = 7). c, d, Same for fenfluramine. Note that 1 mm fenfluramine was toxic, as indicated. e, Representative field recordings from scn1Lab mutant larvae at 5 dpf. Electrographic activity is shown for a 5 min recording epoch (top traces); high-resolution traces are shown below, as indicated. Note that abnormal burst discharge activity persists in scn1Lab mutants exposed to 250 µm fenfluramine. The fish shown were tested in the locomotion assay first.
Discussion
Zebrafish and humans share extensive genomic similarity. With regard to disease, 84% of genes known to be associated with disease states in humans have a zebrafish homolog (Howe et al., 2013). This genetic similarity and the characteristic of zebrafish larvae to exhibit quantifiable seizure behaviors or electrographic seizure discharge that is fundamentally similar to that recorded in humans (Jirsa et al., 2014) make this an ideal system for drug discovery. Behavioral assays customized for automated evaluation of locomotion (Winter et al., 2008; Creton, 2009; Baxendale et al., 2012; Baraban et al., 2013; Raftery et al., 2014) make moderate-to-high-throughput phenotype-based drug screening in zebrafish possible. Using this approach and a zebrafish scn1 mutant (Baraban et al., 2013), we successfully identified antiepileptic compounds. Here we report results from screening ∼1000 compounds from a repurposed drug library and present data that will be periodically updated on-line using this open-access publishing mechanism.
As a model system, the scn1Lab mutant zebrafish has many advantages. First, in contrast to transient and variable knockdown of gene expression using antisense morpholino oligonucleotides (Teng et al., 2010; Finckbeiner et al., 2011; Mahmood et al., 2013), scn1Lab mutants carry a stable and heritable amino acid substitution at position 1208 in the third domain of SCN1A that shares 76% homology with humans (Schoonheim et al., 2010; Baraban et al., 2013). Mutations in this channel are one of the primary genetic causes underlying DS (Claes et al., 2003; Escayg and Goldin, 2010; De Jonghe, 2011; Saitoh et al., 2012). As zebrafish possess two scn1 genes (Novak et al., 2006), homozygous mutants for scn1Lab are comparable to the haploinsufficient clinical condition, and there is no variability from larvae to larvae, or clutch to clutch, with respect to gene inactivation, as is commonly observed with morpholino injections (Kok et al., 2015). Although crosses of heterozygotes produce only one-quarter homozygous scn1Lab mutants per mating, there are virtually no limitations on maintaining a large colony of healthy, adult breeders for these types of large-scale screens. Second, it is possible to observe and monitor seizure-like behavior consisting of rapid movements and whole-body convulsions in freely swimming scn1Lab mutants as early as 4 dpf that persist for the life of the larvae (∼12 dpf). These behaviors are comparable to those observed with exposure to a common convulsant agent (PTZ) and classified as Stage III (Baraban et al., 2005). In addition, clear evidence for epileptiform discharge generated in the CNS of immobilized scn1Lab mutant larvae has been obtained at ages between 4 and 8 dpf (Baraban et al., 2013). Both zebrafish measures of seizure activity are sensitive to inhibition by AEDs commonly prescribed to children with DS (e.g., valproate, benzodiazepines, and stiripentol), but are resistant to many antiepileptic compounds (e.g., phenytoin, carbamazepine, ethosuximide, decimemide, tiletamine, primidone, phenacemide, and vigabatrin). Pharmacoresistance is defined as the inability to control seizure activity with at least two different AEDs (Berg, 2009), and, with demonstrated resistance to eight AEDs, our model clearly fits this definition. This level of model validation has not been possible with morpholinos probably owing to the high degree of variability, or off-target effects, associated with this technique (Kok et al., 2015).
Our screening results highlight the stringency of our approach with a positive hit rate of only 1.97% on the first-stage locomotion assay, and successful identification of 1 compound (of 1012 compounds) with known antiepileptic activity (i.e., dimethadione, a T-type channel antagonist). In additional testing, we confirmed an antiepileptic action for fenfluramine (serotonin uptake inhibitor). Similar to ethosuximide, a reduction in regenerative burst discharges associated with neuronal T-type calcium currents could be the underlying mechanism for dimethadione in DS mutants; however, it is worth noting that T-type channel blockers ethosuximide and flunarizine were not similarly effective (Baraban et al. 2013; this article), and that dimethadione can cause arrhythmia owing to blockade of cardiac human ether-a-go-go-related gene potassium channels (Azarbayjani and Danielsson, 2002; Danielsson et al., 2007). Modulation of serotonin [5-hydroxytryptamine (5-HT)] signaling by blocking uptake or increasing release from neurons by acting as substrates for 5-HT transporter (sertraline) proteins (Fuller et al., 1988; Gobbi and Mennini, 1999; Baumann et al., 2000; Rothman et al., 2010) may be the mechanism of action for fenfluramine in patients with DS, though a detailed analysis of precisely how fenfluramine modulates excitability via this signaling pathway has not been performed. Nonetheless, both drugs probably exert a direct effect on network excitability (at neuronal or synaptic levels, respectively) to suppress electrographic discharge and the associated high-velocity seizure behavior seen in scn1Lab mutants, and may be potential targets for clinical use. In contrast, three other drugs identified in the primary locomotion assay were not effective in suppressing electrical events and were designated as false positives. This is not altogether surprising given that the xanthine alkaloid (theobromine), chemotherapeutic (cytarabine), and antibiotic (norfloxacin) mechanisms for these compounds would not be consistent with seizure inhibition. Moreover, the variability inherent in behavioral experiments performed on different zebrafish larvae, in different microplates, and on different days may contribute to these false-positive designations in locomotion assays, and is evident in the range of mean velocity values seen during tracking episodes from control studies (Fig. 1c ) or in the failure of many of the initial 20 lead compounds to be confirmed on subsequent retesting (see Fig. 2a ). This is a limitation of locomotion-based screening assays and is another reason why a secondary electrophysiology assay on the same zebrafish is a critical advantage of our approach.
An additional advantage of in vivo screening with zebrafish larvae is the simultaneous identification of compounds resulting in toxicity. Zebrafish-based anticonvulsant drug-screening assays based primarily on in situ hybridization detection of early gene expression at 2 dpf (Baxendale et al., 2012) do not routinely monitor spontaneous swim behavior, heart rate, or response to external stimuli. Lacking these real-time measures of toxicity, compounds observed to induce fatality in a freely swimming scn1Lab-based behavioral assay (e.g., gemfibrozil, suloctidil, pimozide, or dioxybenzone) were mistakenly classified as seizure-suppressing compounds in the PTZ-based c-Fos in situ hybridization assay. Indeed, 41% of the “anticonvulsant” compounds positively identified at 2 dpf in Baxendale et al. (2012) were toxic, proconvulsant, or simply not effective in scn1Lab mutant assays at 5-6 dpf. Similarly, it is critical to monitor blood flow and heart activity even in the agar-immobilized electrophysiology assay as compounds effective in suppressing electrical activity can also be toxic. These discrepancies highlight the potential problems associated with zebrafish drug-screening strategies that do not encompass multiple readouts and suggest the need for a note of caution when comparing screening results from different laboratory groups. While any lead compound identified in a zebrafish-based screening assay will, ultimately, need to be independently replicated and/or validated in additional mammalian model systems, the ability to rapidly identify such compounds, while simultaneously identifying potential negative side effects, makes genetically modified zebrafish a unique resource for drug discovery in an age of personalized medicine.
Acknowledgments
Acknowledgments: We thank B. Grone and D. Lowenstein for comments on earlier versions of this manuscript.
Synthesis
The decision was a result of the Reviewing Editor Elizabeth Powell and the peer reviewers coming together and discussing their recommendations until a consensus was reached. A fact-based synthesis statement explaining their decision and outlining what is needed to prepare a revision is listed below. The following reviewers agreed to reveal their identity: Karen Wilcox, Rosane Da Silva
Synthesis
The manuscript describes a high throughput study in which a zebrafish model of Dravet syndrome is uses to screen more than 1000 compounds in the search for new drugs for treatment in human patients. The reviewers stated strong enthusiasm for the work and the potential for advancing the field of human epilepsy and new therapeutic compounds. The format of the eNeuro journal is appropriate, as the data sets can be later updated to include new findings. Publishing all the data, the effective and ineffective or harmful compounds, is an important resource for the field.
Some major and minor issues were raised by the reviewers. These issues should be addressed prior to the manuscript being considered for publication. The reviewers' comments follow.
Reviewer #1
This is a well described, effective screen that has identified a number of potential anti-seizure drugs (ASDs) that may be efficacious for the treatment of Dravet's syndrome and other types of epilepsy. This approach should streamline ASD discovery and provide the field with useful positive and perhaps more importantly, negative results (so as to not waste time and effort on ineffective compounds). There are some issues that should be addressed.
Major concern:
There seems to be considerable variability in trial to trial effectiveness of compounds (e.g. Figure 3, with a stated 4/20 compounds showing comparable reductions in movement velocity from trial 1 to trial 2). The authors are to be commended for performing independent validation of each trial. However, the variability is not discussed in the context of being a limitation of this approach. Also, the threshold for considering whether a compound is indeed a positive hit is not well described. For example it is not clear why Theobromine was considered a hit in Fig 3, but Tolnaftate and Artemether was not. These issues should be discussed.
Minor Concerns:
In Figure 4, it would be helpful to provide baseline recording segments prior to the administration of compounds, especially any that prove efficacious (e.g. dimethadione).
In the discussion, towards the end of the second paragraph, the author states "...and successful identification of two compounds.....and fenfluramine". However, in the results section, it was mentioned that fenfluramine was not part of the original screen but was tested as a consequence of cited literature. In the discussion, the way that sentence is phrased, it sounds like fenfluramine was found in the screen of the library.
Figure 2 is somewhat confusing. At what point is it determined that velocity and distance are not correlated. It is unclear why the drugs selected were plotted, as none were viewed as significant hits. Also, as a positive hit needed to demonstrate >44% reduction in velocity, it is not clear why estradiol was not considered a positive hit? Were the 5 drugs in part B part of the library?
Reviewer #2
This manuscript describes a large-scale drug screening to identify antiepileptic drugs based in locomotor activity and electroencephalography registration using zebrafish. The study is well conducted and its publication will be a huge contribution to the use of zebrafish as a model to investigate antiepileptic properties and a contribution to the study of refractory epilepsy. While I am enthusiastic about the results of this manuscript, my enthusiasm became a little bit vanished while reading the text. In this way, I strongly recommend a careful revision of the text, especially in relation to the points mentioned below:
Major points:
1. While the methodological approach is one of the main contributions of the study the authors do not discuss the results on the perspectives of the disease. The authors should contextualize the results in the end of the abstract and through the discussion session in order to show how the drugs identified could contribute to the study of the Dravet Syndrome.
2. Also, the authors do not discuss the findings on the mechanistic level of the drugs identified with antiepileptic properties.
3. On the introduction, the authors should enrich this session with data from the Dravet syndrome, treatments available...
4. There is an important problem in the distribution of the text through the sessions. There are elements of material and methods, results and discussion sessions on the introduction. There is lot of comments in the material and methods results sessions that should be avoided or transferred to the discussion session. The material and methods should not include results (e.g: last sentence of Drugs subtitle). The result session is exclusively to the results reached from this specific study, please delete comments, justifications,...
5. The material and methods session is very poorly written and there are several methodological information through the others sessions. See examples below:
5.1 What is the guideline used to base the animal care?
5.2 What conditions the animals were maintained (temperature, pH, conductivity, light/dark conditions, density, tanks dimension, 96 wells plate ...)?
5.3 The age of larvae during the seizure monitoring experiments is lacking (this information appears just in the legend of the figure 1)
5.4 How the heart beating and blood flow were registered?
5.5 How the animals were exposed to the drugs? How long was the exposition? Is the 15-30 minutes of equilibration period the time of exposure?
5.6 Was the final DMSO concentration in the electrophysiology experiments the same of locomotor experiments?
5.7 The concept of "% changes in the mean velocity" should be stated in the material and methods and not in the result session.
5.8 The criteria to define a drug as "toxic" should be stated in the material and methods and not in the result session.
5.9 Please, explain the criteria to consider a drug as an anticonvulsant or a proconvulsant at the material and methods and not at the results session.
5.10 Please, was the mortality registered?
6. In the results session, the authors mentioned that the animals were sorted based on the pigmentation. What were the parameters considered? Please, at least mention that in the material and methods session.
7. Also in the results session, the authors should show the results of heart beat and blood flow, since at the discussion they mentioned that as an important data to avoid misunderstandings on the interpretation of data. This would help to differentiate the animals from gemfibrozil (Figure 3b) from fenfluramine (figure 5c), since the first was fatal and the second not.
8. The figures should include the data from WT animals and not just the mutant at the free-drug condition (locomotion and electrophysiology data).
9. At figure 1d, it is hard to identify the "1.97%" of compounds at the figure 1. The figure is a little bit crowded.
10. At the figure 4, why just 3 animals were used to the electrophysiology assay (cytarabine, theobromine and norfloxacin) since the locomotor assay was performed with 6 animals?
11. Why the statistic of the results is not shown in the figures? For example, it is hard to identify how the authors differentiated the groups at the figure 2.
12. Why the raw data were show just to the first trial?
Minor points:
1. Please, avoid the words very little, higher, increased swimming, when the basal data is not available. If possible, show the real data.
2. There are no figures 1e or 3c. Please, correct to 1d and 3b.
3. Please, organize the legend of figure 5, there is some repetition.
4. Why sometimes the total number of drugs is 1012, sometimes 1013, 1014..?
References
- Ali S, Champagne DL, Spaink HP, Richardson MK (2011) Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res C Embryo Today 93:115-133. 10.1002/bdrc.20206 [DOI] [PubMed] [Google Scholar]
- Azarbayjani F, Danielsson BR (2002) Embryonic arrhythmia by inhibition of HERG channels: a common hypoxia-related teratogenic mechanism for antiepileptic drugs? Epilepsia 43:457-468. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA (2013) Drug screening in Scn1a zebrafish mutant identifies clemizole as a potential Dravet syndrome treatment. Nat Commun 4:2410. 10.1038/ncomms3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H (2005) Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 131:759-768. 10.1016/j.neuroscience.2004.11.031 [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB (2000) Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36:102-113. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Holdsworth CJ, Meza Santoscoy PL, Harrison MR, Fox J, Parkin CA, Ingham PW, Cunliffe VT (2012) Identification of compounds with anti-convulsant properties in a zebrafish model of epileptic seizures. Dis Model Mech 5:773-784. 10.1242/dmm.010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT (2009) Identification of pharmacoresistant epilepsy. Neurol Clin 27:1003-1013. 10.1016/j.ncl.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2015) Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res 111:85-141. 10.1016/j.eplepsyres.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Boel M, Casaer P (1996) Add-on therapy of fenfluramine in intractable self-induced epilepsy. Neuropediatrics 27:171-173. 10.1055/s-2007-973781 [DOI] [PubMed] [Google Scholar]
- Caraballo RH, Cersosimo RO, Sakr D, Cresta A, Escobal N, Fejerman N (2005) Ketogenic diet in patients with Dravet syndrome. Epilepsia 46:1539-1544. 10.1111/j.1528-1167.2005.05705.x [DOI] [PubMed] [Google Scholar]
- Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, Lagae L (2012) Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia 53:1131-1139. 10.1111/j.1528-1167.2012.03495.x [DOI] [PubMed] [Google Scholar]
- Chiron C, Dulac O (2011) The pharmacologic treatment of Dravet syndrome. Epilepsia 52 Suppl 2:72-75. 10.1111/j.1528-1167.2011.03007.x [DOI] [PubMed] [Google Scholar]
- Claes L, Ceulemans B, Audenaert D, Smets K, Löfgren A, Del-Favero J, Ala-Mello S, Basel-Vanagaite L, Plecko B, Raskin S, Thiry P, Wolf NI, Van Broeckhoven C, De Jonghe P (2003) De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat 21:615-621. 10.1002/humu.10217 [DOI] [PubMed] [Google Scholar]
- Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, Nambiar MP (2008) [+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats. Chem Biol Interact 175:387-395. 10.1016/j.cbi.2008.05.023 [DOI] [PubMed] [Google Scholar]
- Creton R (2009) Automated analysis of behavior in zebrafish larvae. Behav Brain Res 203:127-136. 10.1016/j.bbr.2009.04.030 [DOI] [PubMed] [Google Scholar]
- Danielsson BR, Danielsson C, Nilsson MF (2007) Embryonic cardiac arrhythmia and generation of reactive oxygen species: common teratogenic mechanism for IKr blocking drugs. Reprod Toxicol 24:42-56. 10.1016/j.reprotox.2007.04.005 [DOI] [PubMed] [Google Scholar]
- De Jonghe P (2011) Molecular genetics of Dravet syndrome. Dev Med Child Neurol 53 Suppl 2:7-10. 10.1111/j.1469-8749.2011.03965.x [DOI] [PubMed] [Google Scholar]
- Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O (2005) Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol 95:71-102. [PubMed] [Google Scholar]
- Dressler A, Trimmel-Schwahofer P, Reithofer E, Mühlebner A, Gröppel G, Reiter-Fink E, Benninger F, Grassl R, Feucht M (2015) Efficacy and tolerability of the ketogenic diet in Dravet syndrome - Comparison with various standard antiepileptic drug regimen. Epilepsy Res 109:81-89. 10.1016/j.eplepsyres.2014.10.014 [DOI] [PubMed] [Google Scholar]
- ebrary Inc. (2011) Guide for the care and use of laboratory animals. Washington, DC: National Academy Press. [Google Scholar]
- Epi4K Consortium (2012) Epi4K: gene discovery in 4,000 genomes. Epilepsia 53:1457-1467. 10.1111/j.1528-1167.2012.03511.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escayg A, Goldin AL (2010) Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51:1650-1658. 10.1111/j.1528-1167.2010.02640.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finckbeiner S, Ko PJ, Carrington B, Sood R, Gross K, Dolnick B, Sufrin J, Liu P (2011) Transient knockdown and overexpression reveal a developmental role for the zebrafish enosf1b gene. Cell Biosci 1:32. 10.1186/2045-3701-1-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD, Robertson DW (1988) Mechanisms of effects of d-fenfluramine on brain serotonin metabolism in rats: uptake inhibition versus release. Pharmacol Biochem Behav 30:715-721. [DOI] [PubMed] [Google Scholar]
- Gobbi M, Mennini T (1999) Release studies with rat brain cortical synaptosomes indicate that tramadol is a 5-hydroxytryptamine uptake blocker and not a 5-hydroxytryptamine releaser. Eur J Pharmacol 370:23-26. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsa VK, Stacey WC, Quilichini PP, Ivanov AI, Bernard C (2014) On the nature of seizure dynamics. Brain 137:2210-2230. 10.1093/brain/awu133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HC, Gelb BD (2014) Concise review: drug discovery in the age of the induced pluripotent stem cell. Stem Cells Transl Med 3:500-509. 10.5966/sctm.2013-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND (2015) Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 32:97-108. 10.1016/j.devcel.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert MF (1990) Gene mapping and other tools for discovery. Epilepsia 31 [Suppl 3]:S11–S18. [DOI] [PubMed] [Google Scholar]
- Lotte J, Haberlandt E, Neubauer B, Staudt M, Kluger GJ (2012) Bromide in patients with SCN1A-mutations manifesting as Dravet syndrome. Neuropediatrics 43:17-21. 10.1055/s-0032-1307454 [DOI] [PubMed] [Google Scholar]
- Lowson S, Gent JP, Goodchild CS (1990) Anticonvulsant properties of propofol and thiopentone: comparison using two tests in laboratory mice. Br J Anaesth 64:59-63. [DOI] [PubMed] [Google Scholar]
- Mahmood F, Mozere M, Zdebik AA, Stanescu HC, Tobin J, Beales PL, Kleta R, Bockenhauer D, Russell C (2013) Generation and validation of a zebrafish model of EAST (epilepsy, ataxia, sensorineural deafness and tubulopathy) syndrome. Dis Model Mech 6:652-660. 10.1242/dmm.009480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masimirembwa CM, Thompson R, Andersson TB (2001) In vitro high throughput screening of compounds for favorable metabolic properties in drug discovery. Comb Chem High Throughput Screen 4:245-263. [DOI] [PubMed] [Google Scholar]
- Novak AE, Jost MC, Lu Y, Taylor AD, Zakon HH, Ribera AB (2006) Gene duplications and evolution of vertebrate voltage-gated sodium channels. J Mol Evol 63:208-221. 10.1007/s00239-005-0287-9 [DOI] [PubMed] [Google Scholar]
- Ottman R, Risch N (2012) Genetic epidemiology and gene discovery in epilepsy In: Jasper's basic mechanisms of the epilepsies (Noebels JL, Avoli M, Rogawski M, Olsen R, Delgado-Escueta A, eds). New York: Oxford UP. pp. 651–658. [PubMed] [Google Scholar]
- Raftery TD, Isales GM, Yozzo KL, Volz DC (2014) High-content screening assay for identification of chemicals impacting spontaneous activity in zebrafish embryos. Environ Sci Technol 48:804-810. 10.1021/es404322p [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Blough BE, Jacobson AE, Rice KC, Partilla JS (2010) Evidence for noncompetitive modulation of substrate-induced serotonin release. Synapse 64:862-869. 10.1002/syn.20804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Shinohara M, Hoshino H, Kubota M, Amemiya K, Takanashi JL, Hwang SK, Hirose S, Mizuguchi M (2012) Mutations of the SCN1A gene in acute encephalopathy. Epilepsia 53:558-564. 10.1111/j.1528-1167.2011.03402.x [DOI] [PubMed] [Google Scholar]
- Schoonheim PJ, Arrenberg AB, Del Bene F, Baier H (2010) Optogenetic localization and genetic perturbation of saccade-generating neurons in zebrafish. J Neurosci 30:7111-7120. 10.1523/JNEUROSCI.5193-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M, Green DV (2008) The impact of diversity-based, high-throughput screening on drug discovery: “chance favours the prepared mind”. Curr Opin Drug Discov Devel 11:553-558. [PubMed] [Google Scholar]
- Teng Y, Xie X, Walker S, Rempala G, Kozlowski DJ, Mumm JS, Cowell JK (2010) Knockdown of zebrafish Lgi1a results in abnormal development, brain defects and a seizure-like behavioral phenotype. Hum Mol Genet 19:4409-4420. 10.1093/hmg/ddq364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonduli LS, Testylier G, Masqueliez C, Lallement G, Monmaur P (2001) Effects of Huperzine used as pre-treatment against soman-induced seizures. Neurotoxicology 22:29-37. [DOI] [PubMed] [Google Scholar]
- Wilmshurst JM, Berg AT, Lagae L, Newton CR, Cross JH (2014) The challenges and innovations for therapy in children with epilepsy. Nat Rev Neurol 10:249-260. 10.1038/nrneurol.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin JP, Hutchinson TH (2008) Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early-stage development drugs. J Pharmacol Toxicol Methods 57:176-187. 10.1016/j.vascn.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Zhang YF, Gibbs JW 3rd, Coulter DA (1996) Anticonvulsant drug effects on spontaneous thalamocortical rhythms in vitro: ethosuximide, trimethadione, and dimethadione. Epilepsy Res 23:15-36. 10.1016/0920-1211(95)00079-8 [DOI] [PubMed] [Google Scholar]