Abstract
Context:
Facial plethora is a clinical sign described since ancient times for a variety of diseases. In the 19th century, it was linked to increased blood volume or flow, but this has never been proven. Facial plethora is also one of the earliest described clinical features of Cushing's syndrome (CS).
Objective:
This study aimed to quantify facial plethora changes in CS as an early assessment of cure after surgery using noninvasive near-infrared multispectral imaging (MSI).
Design:
The longitudinal cohort study was initiated in August 2012 and completed in August 2014.
Setting:
Clinical research hospital, National Institutes of Health.
Patients:
Thirty-four of the 38 patients who received surgical treatment for CS under protocol 97CH0076 during this period were included.
Intervention(s):
MSI was performed on the right cheek of patients before surgery and 4.9 ± 3.1 days afterward.
Main Outcome Measure(s):
Average blood volume fraction as measured by MSI and serum cortisol.
Results:
All but four of the 28 patients (86%) who were assessed as cured by postoperative plasma cortisol measurements of < 3 μg/dL showed a decrease in blood volume fraction (17.7 ± 0.03 vs 15.8 ± 0.03%; P = .0019), whereas an increase was seen in patients with persistent CS (18.5 ± 0.03 vs 21.4 ± 0.04%; P = .0017). Change in blood volume fraction before and after surgery was correlated with postoperative cortisol (rs = 0.58; P = .0003).
Conclusions:
Clinical data obtained from 34 patients indicate that a decrease in facial plethora after surgery, as evidenced by a decrease in blood volume fraction, is correlated with CS outcome. This novel technology for the first time identified a physiological mechanism associated with an ancient clinical sign. Furthermore, as a proof of principle, MSI is a promising early marker of cure in patients with CS that complements biochemical and clinical data.
Cushing's syndrome (CS) is a rare disorder that results from chronic exposure to high levels of glucocorticoids (1). CS can be exogenous due to the administration of glucocorticoids or endogenous due to tumors that cause hypercortisolemia. These tumors can be classified as ACTH-secreting pituitary tumors (Cushing's disease [CD]), ectopic ACTH-secreting tumors, or alternatively, primary adrenal lesions leading to autonomous secretion of cortisol (2, 3).
Facial plethora is one of the oldest reported clinical signs in medicine, known from Hippocrates and linked to increased blood flow or volume; it is also a hallmark of CS. When Dr. Harvey Cushing wrote about Minnie G, the first recorded CS patient, he described his evaluation of the 23-year-old woman in 1910. On physical examination, Dr. Cushing noted that this patient's face was “… dusky and cyanosed and covered with a fine growth of hair… ” (4), thus, describing “facial plethora.” This is indeed a frequent clinical sign of CS, and based on our extensive clinical experience, its decrease in intensity and/or gradual disappearance is one of the first signs of its cure. Although it is true that facial plethora may be more prevalent among more severe cases of CS, variation exists among individual patients. It now becomes apparent that certain patients may be more susceptible to developing facial plethora, presumably due to genetic (or other) predisposition factors affecting circulation/vascularization. However, the pathophysiology of facial plethora in CS (or in any disease) remains unknown, and its link to increased blood flow or volume remains unproven. The heterogeneity of this clinical sign does vary between individuals; however, observing a decrease in plethora in the same individuals before and after treatment, ie, using the patients themselves as controls, has been thought to correlate with cure of CS based on our clinical experience. The present study not only investigated the pathophysiology of facial plethora; it also identified it as the early clinical sign of remission from CS, a disease that has a post-treatment relapse rate as high as 20% (5–7). Currently, there is no universally accepted approach to assess the status of the patient with CS after treatment (8). The most important criterion appears to be serum cortisol after surgery. However, even after a successful surgery, recurrence of the disease may occur within a few months, and in some cases, after several years (9, 10). The variability and uncertainty in the suggested criteria make it difficult to assess the final outcome of the treatment. Therefore, better criteria and more robust methods are necessary to evaluate the results of the surgery or other treatment.
In the near-infrared spectral region (690–900 nm), water absorption is minimal, and light can penetrate deeply inside the tissue (11). Furthermore, tissue chromophores (such as oxyhemoglobin, deoxyhemoglobin, and melanin) have different absorption spectrums; therefore, their concentration can be estimated by measuring the reflected light from the tissue at different wavelengths (12).
In the present study, we showed that increased blood flow in the superficial facial skin area was associated with the clinical presence of plethora. We then quantitatively followed the changes in the patient's facial blood volume fraction before and after surgery and compared its variations with the treatment outcome, as we did previously in patients with primary cutaneous diseases, such as Kaposi sarcoma (12–14). Our findings quantify for the first time facial plethora in a human disease; furthermore, it appears that we identified an early clinical sign of potential cure from the hypercortisolemia associated with CS. Our technology has the potential to standardize assessment of treatment efficacy in patients with CS at the early stages after surgery and provide a quantitative platform to complement clinical evaluations with characterization of tissue chromophores possibly in other diseases.
Patients and Methods
Clinical studies
The study was initiated in August 2012 and completed in August 2014. Thirty-four of the 38 patients who received surgical treatment for CS at the National Institutes of Health (NIH) during this period were included in the study. The patients presented here are unselected serial cases. A total of 38 patients with CS (26 females; mean age, 18.7 ± 11.5 y; 69% younger than 18 y) were recruited for the study. Among these, 32 patients had ACTH-secreting pituitary tumors (CD), four had ACTH-independent adrenocortical tumors, and two had an ectopic ACTH-secreting tumor—one a pulmonary carcinoid, and the other a thymic neuroendocrine tumor. Four patients with CD were excluded from the study—two due to severe facial acne, one due to fever on the day of imaging, and one due to variations in the quantity of facial hair at different time points. Therefore, 34 of the original 38 (90%) subjects were eligible for evaluation of facial plethora. Two of the patients had two transsphenoidal surgeries (TSSs) within 10–28 days; thus, 36 surgeries were included in the analysis.
All patients examined in this study were recruited through clinical protocols 97-CH-0076, 95-CH-0059, and 00-CH-0160 conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and gave written informed consent (and assent from older children). The diagnosis and etiology of CS were confirmed as previously described (15), as well as by histology. After surgery, serum cortisol was measured beginning on postoperative day 2 and was repeated daily until postoperative day 9 or 10. During this postoperative period, dexamethasone was administered to patients after adrenalectomy and to patients after TSS who required replacement. In post-TSS patients, 8 am serum cortisol and ACTH levels were monitored starting on postoperative day (POD) 3. Glucocorticoid replacement was withheld until POD 5 (or sooner if the patient was symptomatic for adrenal insufficiency); oral dexamethasone was started on POD 5 for patients with serum cortisol levels consistent with remission of CS (cortisol < 5 μg/dL). For patients with serum cortisol levels that were not consistent with remission of CS, dexamethasone replacement was withheld until POD 9 when results of the postoperative CRH stimulation test were available. Twenty-two CD patients were on 0.5 mg of dexamethasone per day at the time of postoperative multispectral imaging (MSI) assessment; these patients had been on dexamethasone for an average of 3.2 ± 2.4 days at the time of the imaging. Patients who underwent adrenalectomy (unilateral or bilateral) received a stress dose of glucocorticoid (soul-cortef) intra- and postoperatively, and cortef was tapered to a replacement dose over several days. Five patients were on hydrocortisone after adrenalectomy, at different doses depending on the length of time after surgery, their BSA, and their overall clinical status. One patient was on 20 mg of hydrocortisone every 8 hours, and three patients were on physiological replacement with 12 mg/m2/d hydrocortisone, with two-thirds in the morning and one-third in the afternoon. The clinical study conducted at NIH shows that all 66 children who remain in remission to date had a mean morning cortisol value after TSS < 6.5 μg/dL (179.3 nmol/L); specificity was 100% (66 of 66; confidence interval, 95–100%) (16). Because a number of the patients from the Batista et al paper (16) with postoperative cortisol levels between 3 and 6 have in the meantime recurred and all with levels below 3 achieved sustained remission (unpublished data), patients were considered to be cured of disease by postoperative measurements of plasma cortisol less than 3, and/or adrenocortical insufficiency for which they received replacement (17–19).
Imaging system and analysis
A charge-coupled device-based diffuse near-infrared MSI system was applied to obtain two-dimensional images of the patient's right cheek at four different wavelengths (700, 750, 800, and 850 nm), as previously described (12). Images of the region of interest (ROI) on the skin were preprocessed to remove spatial and spectral variations of the light source and spectral sensitivity of the charge-coupled device camera. A curvature correction method (20) was applied to remove the image artifacts generated by the curvature of the patient's cheek. Supplemental Video 1 illustrates how MSI is performed on a patient.
We applied the two-layer skin model (epidermis and dermis layers) to determine the fraction of blood volume through a best-fit multivariate analysis. For simplicity, reduced scattering coefficient of dermis and absorption coefficients of oxyhemoglobin and deoxyhemoglobin are considered as known constants. Melanin concentration and epidermal thickness were considered as known parameters (21). The Fitzpatrick skin type scale was used for classification of skin color and melanin concentration (21–23). Based on the studies in Refs. 23 and 24), we used 80 to 100 μm to estimate the epidermal thickness of the patient's cheek. Unlike oxyhemoglobin concentration of blood, blood volume fraction is not sensitive to epidermal thickness (25).
Data were collected before surgery and within 10 days after surgery. The time between the first imaging session and surgery varied from 1 week to 2 months, depending on the patient's physical condition.
Steps undertaken in the facial imaging are well defined (eg, focusing, exposure time) to maximize reproducibility. One observer took up to three blood fraction measurements on each individual patient. The intraclass correlation coefficients for blood volume fraction measurements before and after surgery were 0.997 and 0.999, respectively.
Frequency distributions and simple descriptive statistics described the data and are reported as number (percentage) or mean ± SD. The paired t test was used to compare pre- and postoperative (Δ) blood volume data, and a two-sample t test compared data between cured and noncured subjects. The binomial probability was computed for the outcome of decrease in blood volume fraction following surgery. Spearman's correlation coefficient was used to determine the correlation between change in blood volume fraction and postoperative cortisol levels. Percentage changes were computed by dividing the Δ blood volume fraction by preoperative blood volume fraction and were used to pictorially show the distribution of change. The receiver operating characteristic curve illustrated the performance of blood volume fraction in discriminating between cured and noncured patients. The F1 score and Matthew's correlation coefficient determined the accuracy of cured patients' classifications. Intraobserver reliability was measured with intraclass correlation using the ANOVA and mixed models frameworks. All data were assessed for their distributions, and appropriate tests were used accordingly; all tests were two-sided, with statistical significance defined as P < .05. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc).
Results
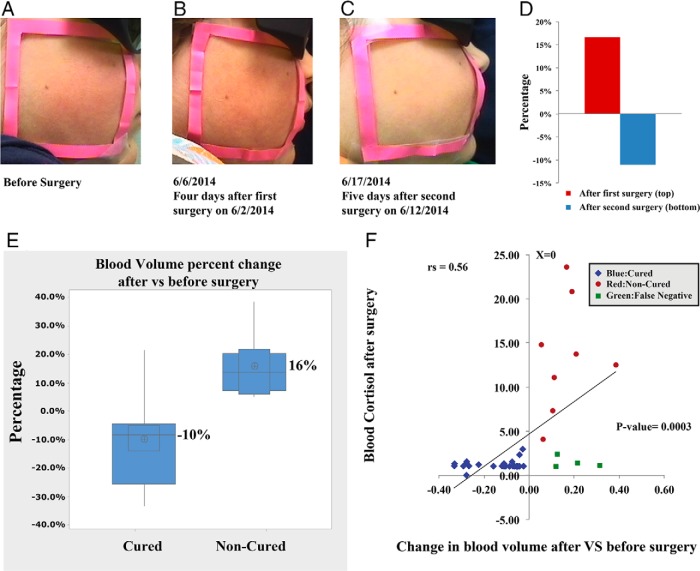
Baseline patient characteristics and pre- and postoperative biochemical results are presented in Table 1. Figure 1D shows the case that was studied before and after surgery and the characteristic reduction of facial plethora.
Table 1.
Baseline Patient Characteristics and Pre- and Postoperative Biochemical Results
| CD | Adrenocortical Tumor | Ectopic ACTH | |
|---|---|---|---|
| n | 28 | 4 | 2 |
| Age, y | 16.7 ± 9.9 (6–48) | 30 ± 16.1 (10–47) | 17 ± 5.7 (13–21) |
| Females/males, n (%) | 20 (71)/8 (29) | 3 (75)/1 (25) | 1 (50)/1 (50) |
| Race, n (%) | |||
| Asian | 2 (7) | 0 | 0 |
| Black | 1 (4) | 0 | 0 |
| White | 24 (85) | 4 (100) | 2 (100) |
| Other/unknown | 1 (4) | 0 | 0 |
| Ethnicity, n (%) | |||
| Latino or Hispanic | 5 (18) | 1 (25) | 0 |
| Not Latino or Hispanic | 23 (82) | 3 (75) | 2 (100) |
| Preoperative cortisol AM, μg/dL | |||
| All patients | 22 ± 15.6 (3.8–93) | 12.4 ± 8.3 (5.4–24.1) | 21.2 ± 11.8 (12.8–29.5) |
| Cured | 22.4 ± 17.6 (3.8–93) | 12.4 ± 8.3 (5.4–24.1) | 21.2 ± 11.8 (12.8–29.5) |
| Not cured | 20.6 ± 7.7 (7.5–30.9) | ||
| Postoperative cortisol AM, μg/dL | |||
| All patients | 4.6 ± 6.5 (1–23.6) | 5.6 ± 8.0 | 1.2 ± 0.2 (1–1.3) |
| Cured | 1.3 ± 0.6 (1–3) | <1 (1–1) | 1.2 ± 0.2 (1–1.3) |
| Not cured | 14.4 ± 6.4 (4.1–23.6) | ||
| Preoperative ACTH AM, pg/mL | |||
| All patients | 54.6 ± 77.7 (5–393) | 17.2 ± 10.5 (5–30.7) | 93.8 ± 13.1 (84.5–103) |
| Cured | 59.7 ± 80.3 (9.7–393) | 17.2 ± 10.5 (5–30.7) | 93.8 ± 13.1 (84.5–103) |
| Not cured | 39.4 ± 35.7 (5–112) | ||
| Postoperative ACTH AM, pg/mL | |||
| All patients | 13.7 ± 13.3 (5–50.5) | <5 (5–5) | 72.5 ± 95.5 (5–140) |
| Cured | 11.3 ± 12.0 (5–50.5) | <5 (5–5) | 72.5 ± 95.5 (5–140) |
| Not cured | 21.2 ± 15.3 (5–48.7) | ||
| Preoperative UFC, μg/24 h | |||
| All patients | 288 ± 446 (10.3–2355) | 108.1 ± 89 (6.4–212.3) | 1670.7 ± 1870.6 (348–2993.4) |
| Cured | 289.6 ± 494.4 (28.2–2355) | 108.1 ± 89 (6.4–212.3) | 1670.7 ± 1870.6 (348–2993.4) |
| Not cured | 283 ± 283.6 (10.3–836.9) | ||
| Postoperative UFC, μg/24 h | |||
| All patients | 9.3 ± 8.1 (1.3–26.4) | 1.4 ± 0.1 (1.3–1.5) | n/a |
| Cured | 8 ± 7.1 (1.3–21.6) | 1.4 ± 0.1 (1.3–1.5) | n/a |
| Not cured | 18.1 ± 11.7 (9.8–26.4) |
Abbreviations: UFC, urinary free cortisol; n/a, not available. Data are expressed as mean ± SD (range), unless stated otherwise.
Figure 1.
A—C, Facial plethora in a patient with CD before TSS, after the first TSS (noncured), and after the second TSS (cured). D, The blood volume fraction percentage change of the patient's left cheek in panels A–C at different imaging sessions. E, Boxplot of mean percentage change indicating change in blood volume after surgery, compared to before surgery, in each of the cured and noncured Cushing's patients. The distribution shows median confidence interval box and interquartile range. F, Change in blood volume from pre- to postsurgery vs blood cortisol level after surgery in 34 patients undergoing 36 surgical treatments for CS.
To quantify facial plethora, the average blood volume fraction in the ROI on the right cheek of each patient (inside the 6.2 × 6.2-cm2 marker) was calculated (Figure 1, A–C). All but four of the 28 patients (86%) who were assessed as cured by the standard postoperative plasma cortisol measurements of less than 3 μg/dL showed a decrease in blood volume fraction in ROI (P < .001). They also had a significantly lower blood volume fraction postoperatively than before surgery (17.7 ± 0.03 vs 15.8 ± 0.03%; Δ, −1.8% ± 0.03; P = .0019; Figure 1E). However, for all eight patients who had persistent CS after surgery (including those who required a second surgery), blood volume in ROI increased after surgery (18.5% ± 0.03 vs 21.4% ± 0.04; Δ, 2.9% ± 0.02; P = .0017). The change in blood volume was significantly different between the cured and noncured patients (P < .001). The distribution of percentage change in blood volume fraction is show in Figure 1E.
Two patients required two consecutive surgeries because the initial TSS was unsuccessful. In each case, the patient showed an increase in the cheek blood volume fraction after the first surgery. However, after the second curative surgery, the average blood volume fraction in the ROI decreased in both patients. Figure 1, A—D, shows the changes in facial plethora and blood volume fraction of one of these patients before and after both the first and second surgeries. The change in the patient's facial plethora and the corresponding blood volume fraction of the cheek was appreciable with the naked eye and was confirmed with quantitative measurements obtained from MSI.
The change in cheek blood volume fraction of each patient with CS in this study measured before and after surgery is presented in Supplemental Figure 1 There was a strong correlation between the change in ROI blood volume fraction before and after surgery and postoperative cortisol (rs = 0.58; P = .0003; Figure 1F). Physiological conditions potentially accounted for the inappropriately elevated blood volume fractions in two of the four cured patients who represent the false-negative cases. Patient no. 28 had a postoperative urinary tract infection and was receiving ceftriaxone therapy at the time of postsurgery imaging. Patient no. 29 had hypertension and was on amlodipine and atenolol (both may be associated with flushing). Supplemental Figure 2 illustrates the ROC curve (area = 0.91), confirming the ability of our method to correctly classify the cured and noncured patients into separate categories. The calculated F1 score and Matthew's correlation coefficient were 0.92 and 0.76, respectively, which suggests high accuracy of the treated patients' classification. No other covariates that could potentially impact results, such as patient temperature, were found to be associated with the evaluated blood volume fraction.
Discussion
Our investigation, for the first time quantified a pathophysiological mechanism that underlies one of the oldest clinical signs recorded in medicine: facial plethora. In addition, it appears that a reduction in plethora is an early sign of remission of CS. The results showed a decrease in the average blood volume fraction in the cheek area for patients whose serum cortisol level dropped rapidly to very low levels after surgery. To the contrary, we observed an increase in blood volume fraction in the cheeks of patients whose postsurgery cortisol levels did not decline to less than 3 μg/dL. Thirty-four patients were imaged before surgery and a few days after surgery.
We can only speculate about the reason for the unexpected apparent increase in blood volume fraction in noncured patients after surgery. This may have to do with the fact that noncured patients receive additional glucocorticoids in addition to endogenous levels or a delayed clearance of anesthesia given during surgery. Postoperative inflammation and/or cytokine release is another possibility.
Diffuse near-infrared MSI of skin allows quantitative assessment of its vascular parameters. This study suggests that the fraction of the cheek's blood volume measured by the MSI system can be used to characterize an early response to surgery in CS within a short time after surgery. This technique is safe, noninvasive, fast, and low cost, and it can be easily applied to monitor a disease or condition over time. Another potential advantage of this technique is that it can provide robust criteria for the definition of cure and remission by generating objective and quantitative data. Our method is not dependent on a threshold to define facial plethora because we are comparing preoperative to postoperative change in the individual. More studies will be necessary to further investigate whether a decrease in facial plethora (measured as blood volume fraction in the patient's cheek) after treatment is associated with lasting cure of CS. Our sample size in this study was small, and the findings should be considered as preliminary. Long-term follow-up imaging of larger group of patients is required to verify these results. It will take time to accumulate sufficient evidence for the confirmation of this technique; however, we are continuing to use MSI on our patients seen at the NIH and are accumulating additional data and experience. Limitations to having this technique become routine include the rarity of CS and the need for more data to be accumulated from separate observers; in addition, our cohort was largely representative of juvenile CS, which may limit the generalizability of the findings. Furthermore, additional studies of the effects on blood oxygenation may yield insights into the relationship of CS and facial blood oxygenation before and after therapy. In addition, there are potential future applications of this method—perhaps to use facial plethora change as a marker of recurrent disease, or to distinguish patients with pseudo-CS from those with true CS.
We conclude that facial plethora as measured by the cheek's vascular data (reconstructed from diffuse MSI) is indeed what ancient medical texts called it to be: associated with blood flow in superficial skin blood vessels. In CS, our novel technology quantified facial plethora and showed it to be a marker of early cure in patients with CS. It is possible that this technology will be applicable in a number of other diseases and may be used for assessment of the corresponding therapies. It should be noted, with the advent of mobile devices, that this kind of spectroscopy can be easily integrated as a handheld device, with easy access at outpatient as well as inpatient care.
Acknowledgments
This research was funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health (NIH). The NIH Clinical Center and the Graduate Partnership Program at the NIH and the Department of Engineering Management and Systems Engineering at The George Washington University are also acknowledged.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CD
- Cushing's disease
- CS
- Cushing's syndrome
- MSI
- multispectral imaging
- POD
- postoperative day
- ROI
- region of interest
- TSS
- transsphenoidal surgery.
References
- 1. Melmed S, Williams RH. Williams Textbook of Endocrinology. 12th ed Philadelphia, PA: Elsevier/Saunders; 2011. [Google Scholar]
- 2. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. 2015;386(14):913–927. [DOI] [PubMed] [Google Scholar]
- 3. Tsigos C, Chrousos GP. Differential diagnosis and management of Cushing's syndrome. Ann Rev Med. 1996;47:443–461. [DOI] [PubMed] [Google Scholar]
- 4. Lanzino G, Maartens NF, Laws ER., Jr Cushing's case XLV: Minnie G. J Neurosurg. 2002;97(1):231–234. [DOI] [PubMed] [Google Scholar]
- 5. McCance DR, Gordon DS, Fannin TF, et al. Assessment of endocrine function after transsphenoidal surgery for Cushing's disease. Clin Endocrinol (Oxf). 1993;38(1):79–86. [DOI] [PubMed] [Google Scholar]
- 6. Trainer PJ, Lawrie HS, Verhelst J, et al. Transsphenoidal resection in Cushing's disease: undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf). 1993;38(1):73–78. [DOI] [PubMed] [Google Scholar]
- 7. Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with Cushing's disease. J Clin Endocrinol Metab. 2013;98(3):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newell-Price J. Transsphenoidal surgery for Cushing's disease: defining cure and following outcome. Clin Endocrinol (Oxf). 2002;56(1):19–21. [DOI] [PubMed] [Google Scholar]
- 9. Mampalam TJ, Tyrrell JB, Wilson CB. Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med. 1988;109(6):487–493. [DOI] [PubMed] [Google Scholar]
- 10. Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M. Risk factors and long-term outcome in pituitary-dependent Cushing's disease. J Clin Endocrinol Metab. 1996;81(7):2647–2652. [DOI] [PubMed] [Google Scholar]
- 11. Arimoto H. Estimation of water content distribution in the skin using dualband polarization imaging. Skin Res Technol. 2007;13(1):49–54. [DOI] [PubMed] [Google Scholar]
- 12. Vogel A, Chernomordik VV, Riley JD, et al. Using noninvasive multispectral imaging to quantitatively assess tissue vasculature. J Biomed Opt. 2007;12(5):051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassan M, Little RF, Vogel A, et al. Quantitative assessment of tumor vasculature and response to therapy in Kaposi's sarcoma using functional noninvasive imaging. Technol Cancer Res Treat. 2004;3(5):451–457. [DOI] [PubMed] [Google Scholar]
- 14. Kainerstorfer JM, Amyot F, Demos SG, et al. Quantitative assessment of ischemia and reactive hyperemia of the dermal layers using multi - spectral imaging on the human arm. In: Proceedings from SPIE Conference; July 9, 2009; Munich, Germany; Vol 7369. [Google Scholar]
- 15. Batista DL, Riar J, Keil M, Stratakis CA. Diagnostic tests for children who are referred for the investigation of Cushing syndrome. Pediatrics. 2007;120(3):e575–e586. [DOI] [PubMed] [Google Scholar]
- 16. Batista DL, Oldfield EH, Keil MF, Stratakis CA. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab. 2009;94(8):2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lodish MB, Sinaii N, Patronas N, et al. Blood pressure in pediatric patients with Cushing syndrome. J Clin Endocrinol Metab. 2009;94(6):2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing's disease after transsphenoidal surgery. J Clin Endocrinol Metab. 2011;96(7):2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lodish M, Dunn SV, Sinaii N, Keil MF, Stratakis CA. Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing's disease. J Clin Endocrinol Metab. 2012;97(5):1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kainerstorfer JM, Amyot F, Ehler M, et al. Direct curvature correction for noncontact imaging modalities applied to multispectral imaging. J Biomed Opt. 2010;15(4):046013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pathak MA, Jimbow K, Szabo G, Fitzpatrick TB. Sunlight and melanin pigmentation. In: Smith KC, ed. Photochemical and Photobiological Reviews. New York: Plenum Press; 1976:211–239. [Google Scholar]
- 22. Jacques SL. Origins of tissue optical properties in the UVA, visible, and NIR regions. In: Alfano RR, Fujimoto JG, eds. The Optical Society, TOPS on Advances in Optical Imaging and Photon Migration. Vol 2 Washington, DC; 1996. [Google Scholar]
- 23. Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Dermato-Venereologica. 2003;83(6):410–413. [DOI] [PubMed] [Google Scholar]
- 24. Meglinski IV, Matcher SJ. Quantitative assessment of skin layers absorption and skin reflectance spectra simulation in the visible and near-infrared spectral regions. Physiol Meas. 2002;23(4):741–753. [DOI] [PubMed] [Google Scholar]
- 25. Yudovsky D, Pilon L. Rapid and accurate estimation of blood saturation, melanin content, and epidermis thickness from spectral diffuse reflectance. Appl Opt. 2010;49(10):1707–1719. [DOI] [PubMed] [Google Scholar]