Abstract
Context:
Loss of function (LoF) mutations in more than 20 genes are now known to cause isolated GnRH deficiency (IGD) in humans. Most causal IGD mutations are typically private, ie, limited to a single individual/pedigree. However, somewhat paradoxically, four IGD genes (GNRH1, TAC3, PROKR2, and GNRHR) have been shown to harbor LoF founder mutations that are shared by multiple unrelated individuals. It is not known whether similar founder mutations occur in other IGD genes.
Objective:
The objective of the study was to determine whether shared deleterious mutations in IGD-associated genes represent founder alleles.
Setting:
This study was an international collaboration among academic medical centers.
Methods:
IGD patients with shared mutations, defined as those documented in three or more unrelated probands in 14 IGD-associated genes, were identified from various academic institutions, the Human Gene Mutation Database, and literature reports by other international investigators. Haplotypes of single-nucleotide polymorphisms and short tandem repeats surrounding the mutations were constructed to assess genetic ancestry.
Results:
A total of eight founder mutations in five genes, GNRHR (Q106R, R262Q, R139H), TACR3 (W275X), PROKR2 (R85H), FGFR1 (R250Q, G687R), and HS6ST1 (R382W) were identified. Most founder alleles were present at low frequency in the general population. The estimated age of these mutant alleles ranged from 1925 to 5600 years and corresponded to the time of rapid human population expansion.
Conclusions:
We have expanded the spectrum of founder alleles associated with IGD to a total of eight founder mutations. In contrast to the approximately 9000-year-old PROKR2 founder allele that may confer a heterozygote advantage, the rest of the founder alleles are relatively more recent in origin, in keeping with the timing of recent human population expansion and any selective heterozygote advantage of these alleles requires further evaluation.
Isolated GnRH deficiency (IGD) is a rare reproductive disorder (1:29 000 in males, 1:130 000 in females) with considerable genetic and allelic heterogeneity (1, 2). More than 20 genes have been implicated in the etiology of this condition (1). Given that the typical phenotype of IGD involves reproductive failure, it would be anticipated that any underlying causal genetic mutations would be loss of function (LoF) and hence subject to negative selection. In keeping with this notion, most IGD-associated mutations have proven to be private and nonrecurrent. However, haplotype analyses have demonstrated recurrent mutations in four IGD genes: GNRH1 (c.18_19insA) (3), TAC3 (c.209-1G>C) (4), PROKR2 (L173R) (5), and GNRHR (R139H) (6) that descend from a common founder. These intriguing observations of potential founder mutations in reproductive genes suggest that they may confer a selective advantage (5).
Given that reproductive fitness is a crucial determinant of species survival and evolution, the identification of such founder alleles has begun to provide valuable insight into the role of these genes in various adaptations of the mammalian reproductive axis during evolution. For example, we recently described the presence of heterozygosity for such a founder IGD mutation, PROKR2 L173R, in women with hypothalamic amenorrhea (HA), whose reproductive axes are susceptible to a functional suppression during periods of stress, excessive exercise, and/or malnutrition (7). Hence, we entertained the hypothesis that the ability of such mutated alleles to suppress reproduction during periods of environmental stress, malnutrition, and prolonged migration might actually confer a survival advantage during periods of environmental stresses (5). Despite the insights from the above-mentioned studies, the precise frequency, nature, and spectrum of similar founder alleles in other IGD genes remain unknown. This study constitutes a systematic search among a cohort of 147 patients with IGD and family members from our own center and from those of other international collaborators for recurrent causal mutations causing IGD that might also represent examples of founder alleles.
Subjects and Methods
Subject selection
DNA sequencing data of 14 IGD genes (GNRH1, GNRHR, KISS1, KISS1R, TAC3, TACR3, FGF8, FGFR1, PROK2, PROKR2, KAL1, NSMF, CHD7, and HS6ST1) were reviewed in a total of 1227 probands from the Reproductive Endocrine Unit of the Massachusetts General Hospital (MGH) with a diagnosis of IGD (Kallmann syndrome or normosmic idiopathic hypogonadotropic hypogonadism; n = 1126); or functional IGD in the form of HA (n = 101). Criteria for the diagnosis of IGD/HA and the DNA sequencing analysis for each of the 14 genes has been previously described (8, 9). We also downloaded mutational data for the same 14 genes from 328 IGD subjects reported in the Human Gene Mutation Database (HGMD) (http://www.hgmd.org/). The diagnostic criteria for the HGMD IGD cohort have been published previously and are referenced within the database (http://www.hgmd.org/). All subjects harboring mutations in the examined genes that are present at a minor allele frequency (MAF) less than 1% in the Exome Aggregation Consortium (ExAC) browser (http://exac.broadinstitute.org/) and that have been previously demonstrated to be LoF alleles by functional analyses (10, 11) were considered for selection as potential founder mutations.
Selection criteria for potential founder mutations and haplotype analysis for ascertaining founder alleles
Shared mutations, defined as those documented in three or more unrelated probands, were selected as candidates for potential founder mutations. Although a minimum of two unrelated probands could have been considered for founder mutations, we chose three or more probands as selection criteria to avoid any cryptic relatedness between probands and strengthen the possibility of a true common founder. A total of 11 mutations in six genes fulfilled these criteria for putative founder events (cf. Table 1). DNA samples were then obtained for haplotype analysis from the MGH subjects harboring the 11 mutations. In addition, for subjects harboring these mutations in the HGMD, individual reporting investigators were contacted by the MGH investigators and DNA samples were requested. A total of 147 DNA samples were made available for haplotype analysis (Table 1).
Table 1.
MAFs of Candidate Mutations for Haplotype Analyses
| Samples, n |
IGD Patients |
MAF From ExAc Browser |
|||||
|---|---|---|---|---|---|---|---|
| Probands, n | Family Members, n | Screened Probands, n | MAF | European (Non-Finnish) | African | Total | |
| GNRHR | |||||||
| Q106R | 32 | 27 | 1098 | 0.016 | 0.004 | 0.0005 | 0.003 |
| R262Q | 12 | 10 | 1095 | 0.006 | 0.002 | 0.0003 | 0.002 |
| R139H | 15 | 11 | 1096 | 0.009 | 0.0002 | 0.0 | 0.0002 |
| TACR3 | |||||||
| W275X | 7 | 3 | 760 | 0.004 | 0.0004 | 9.614 × 10−5 | 0.0002 |
| PROKR2 | |||||||
| R85H | 3 | 1 | 1182 | 0.002 | 0.001 | 0.0003 | 0.0007 |
| FGFR1 | |||||||
| R250Q | 5 | 1 | 1222 | 0.002 | ND | ND | ND |
| G687R | 4 | 1 | 1222 | 0.001 | ND | ND | ND |
| HS6ST1 | |||||||
| R382W | 5 | 0 | 777 | 0.003 | 0.0005 | 0.0006 | 0.002 |
| KAL1 | |||||||
| R423X | 8 | 2 | 1188 | 0.002 | ND | ND | ND |
| R424X | 6 | 1 | 1188 | 0.003 | 0 | 0 | 1.208 × 10−5 |
| R457X | 3 | 1 | 1189 | 0.002 | ND | ND | ND |
Abbreviation: ND, not detected.
Founder mutations reside on haplotypes that are shared by all carriers of the mutation because they are inherited from a common ancestor (12). Haplotypes inherit a disease mutation from a shared founder and thus exhibit allele sharing at markers near the mutation locus by linkage disequilibrium (13). A region from a common ancestor shares a chromosomal segment derived from a common ancestor, ie, a segment that is identical by descent (14). Hence, to identify founder mutations, haplotype analysis for each gene was performed using single-nucleotide polymorphism (SNP) and/or short tandem repeat (STR) markers within a genomic region encompassing at least three recombination hot spots on both sides of the mutation(s). The STR markers were selected from the UniSTS database (http://www.ncbi.nlm.nih.gov/unists). The 5′ end of forward primers was labeled with fluorescein amidite. PCR amplification was performed under standard conditions in a total volume of 50 μL containing 50 ng of genomic DNA. PCR products were mixed with denaturing loading buffer for gel electrophoresis and analyzed with capillary electrophoresis by the ABI3730XL DNA Analyzer (Applied Biosystems). Amplicon sizes were determined using ABI GeneMapper software version 4.0 (Applied Biosystems).
SNPs with an MAF greater than 0.01 and no violation of the Hardy-Weinberg equilibrium (P > .05) were selected from the International HapMap CEU (samples collected by the Centre d'Etude du Polymorphism Humain [CEPH] from Utah residents with Northern and Western European ancestry) panel (http://hapmap.ncbi.nlm.nih.gov/). Tag SNPs were determined using the tagger function in Haploview version 4.2 (http://www.broad.mit.edu/mpg/haploview/; Broad Institute, Cambridge, Massachusetts). The tag SNP selection was performed done using the pairwise tagging algorithm (15) to retain SNP markers with a linkage disequilibrium coefficient (r2) greater than 0.8. We genotyped 459 tag SNPs from six candidate genes (Supplemental Table 1) using the Sequenom MassARRAY iPLEX Gold (Sequenom) (16). Allele-specific products were generated as described before (17) and one HapMap DNA sample was additionally genotyped for each gene to assess genotyping accuracy. To determine familial segregation of mutations and to establish phasing, haplotype analysis also included available DNA from parents and siblings from each pedigree with potential founder mutations when available (Table 1). In total, 93 unrelated IGD probands (88 with IGD and five with HA) and 54 family members were subjected to haplotype analysis (Table 1 and Supplemental Table 1).
Chromosomal phasing was used to identify the alleles that are colocated on the same chromosome and helped determine on which of the two parental chromosomes, or haplotypes, a particular allele falls. Haplotypes were established manually for those families where phase could be determined directly. When parental genotypes were not available, we determined homozygosity haplotypes (14). For affected individuals in whom chromosomal phase could not be determined, both alleles are given (Supplemental Tables 2–7). For unphased samples, both alleles were considered to ascertain founder haplotype status. Haplotypes shared in at least three unrelated individuals were considered to be due to founder events. The frequency of the identified haplotypes in the CEU (Northern Europeans from Utah) population was obtained using Haploview 4.2 and the HapMap database (http://hapmap.ncbi.nlm.nih.gov/). In addition, we calculated the probability of a hot spot mutation that arises recurrently as independent de novo events on the same haplotype using a binomial probability formula as previously described (5). This study was approved by the Partners Institutional Review Board at the MGH, and informed consent was obtained from all participants.
Estimation of mutation age
Given the expected decay of haplotypes over generations since the time of the most recent common ancestor due to recombination (18), the age of the mutations was estimated using the DMLE+ 2.3 software program (http://www.dmle.org/) (19, 20) using either the mutation genotype or its genomic location as input. We ran this program twice for each mutation, first with its genomic location and second with its genotype information specified as input and subsequently confirmed that the age estimation was consistent under both settings (21). The population growth rate was set as 0.025 (22), with an intergenerational time interval of 25 years (23).
Results
Founder mutations identified in patients with IGD
Among the 11 mutations identified as shared by multiple IGD patients, eight mutations involving five IGD genes were found to share a common haplotype consistent with a founder event: GNRHR, Q106R, R262Q, R139H; TACR3, W275X; PROKR2, R85H; FGFR1, R250Q, G687R; and HS6ST1, R382W. The number of individuals, ethnicities, size of the ancestral haplotype, binomial probabilities, and their estimated allelic age are shown in Table 2. For two of these mutations (GNRHR R262Q and R139H), a sufficient number of homozygous and phased samples were available for analysis. Hence, unequivocal founder core haplotypes could be defined in all subjects harboring these variants (Figures 1 and 2, Supplemental Figure 1, and Supplemental Table 2). For the remaining six mutations, the analysis of available phased and unphased samples using homozygosity haplotypes (14) revealed that these mutational events may have occurred on a single core founder haplotype in at least three IGD subjects. The DMLE program estimated that the age of each of these mutant alleles since the most recent common ancestor to be 1925–5600 years (Table 2). These ages of alleles are in keeping with the recently reported age of other rare LoF alleles in humans that correspond to a period of accelerated human population growth. Whereas a precise geographical origin was not evident for most of these founder mutations, eight probands sharing the GNRHR R139H founder haplotype were of Polish origin, suggesting that the potential origin of this mutation was in an ancestral founding Polish population. A shared, common haplotype was not identified for the shared LoF mutations in the KAL1 gene, suggesting that each of these mutations have arisen recurrently on independent haplotypes (Supplemental Table 7).
Table 2.
The Number of Individuals, Ethnicities, Size of the Haplotype, Binomial Probabilities, and Estimated Age of Each Founder Mutation
| Mutations | Subjects Sharing Founder Haplotype, na | Ethnicities | Size of Founder Haplotype, kb | Frequency of Haplotype in CEU | Binomial Probabilityb | Generations (95% Confidence Interval) | Years (95% Credible Set) |
|---|---|---|---|---|---|---|---|
| GNRHR Q106R | 26/32 | Caucasian, African American, South Asian | 83 | 0.326 | 1.874 × 10−8 | 224 (137–337) | 5600 (3425–8425) |
| GNRHR R262Q | 12/12 | Caucasian, South Asian | 135 | 0.029 | 3.538 × 10−19 | 159 (98–271) | 3975 (2450–6775) |
| GNRHR R139H | 15/15 | Caucasian | 220 | 0.097 | 6.333 × 10−16 | 160 (109–289) | 4000 (2725–7225) |
| PROKR2 R85H | 3/3 | Caucasian | 33 | 0.045 | 9.113 × 10−5 | 104 (24–222) | 2600 (600–5550) |
| TACR3 W275X | 3/7 | Caucasian | 251 | 0.011 | 4.457 × 10−5 | 77 (16–176) | 1925 (400–4400) |
| FGFR1 R250Q | 4/5 | Caucasian, South Asian | 137 | 0.344 | 0.046 | 116 (45–242) | 2900 (1125–6050) |
| FGFR1 G687R | 4/4 | Caucasian, African American, South Asian | 70 | 0.546 | 0.089 | 108 (41–220) | 2700 (1025–5500) |
| HS6ST1 R382W | 5/5 | Caucasian, South Asian | 150 | 0.020 | 3.2 × 10−9 | 104 (42–252) | 2600 (1050–6300) |
Abbreviation: CEU refers to Northern Europeans from Utah.
Number of subjects who bear founder core haplotype per total number of subjects with the mutation who were included in this study.
Probability of a hot spot mutation that arises recurrently as independent de novo events on the same haplotype (5).
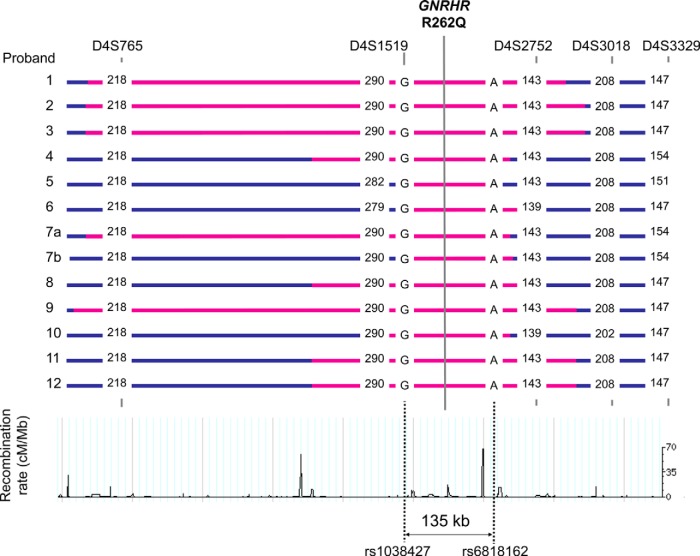
Figure 1.
Haplotypes of probands with GNRHR R262Q. For each STR marker, the number of dinucleotide repeats is shown. Seven probands (numbers 1–3, 6, 7, 11, and 12) had the shared haplotype deduced from fully phased samples. Most probands were Caucasians except proband number 2 (Mexican) and proband number 3 (South Asian). Two probands (numbers 3 and 7) had hypothalamic amenorrhea, whereas the remaining probands had Kallmann syndrome or normosmic idiopathic hypogonadotropic hypogonadism. Blue horizontal lines display the individual haplotypes, with pink horizontal lines showing the haplotype regions identical with each other. The minimum common haplotype shared by all probands is marked with vertical dotted lines. Subject 7 was homozygous for this mutation and his two haplotypes are labeled a and b.
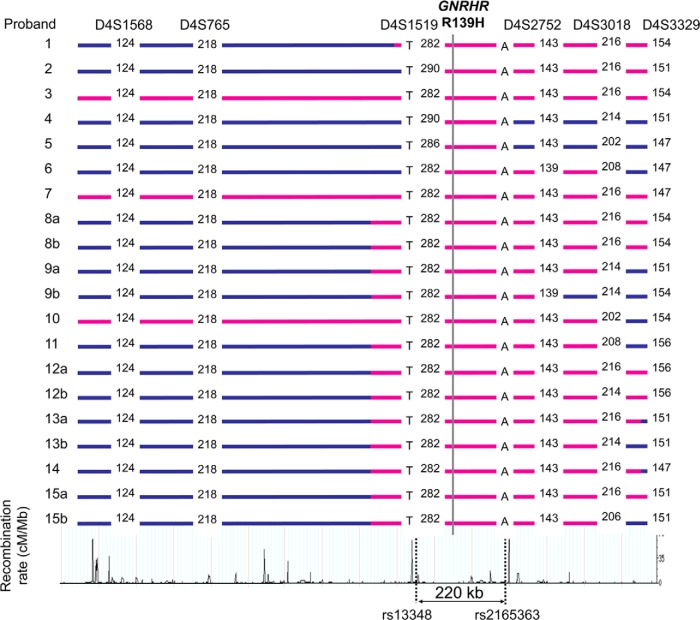
Figure 2.
Haplotypes of probands with GNRHR R139H. In 10 probands (numbers 1, 3, 6–10, 12, 13, and 15), full phasing with both parents samples were possible and they shared common haplotypes. All probands were Caucasians, and eight of them (proband numbers 6–10, 12, 13, and 15) were from Poland. Blue horizontal lines display the individual haplotypes, with pink horizontal lines showing the haplotype regions identical with each other. The common minimum haplotype shared by all probands is marked with vertical dotted lines. In each of the probands homozygous for the mutation (subjects 8, 9, 12, 13, and 15), their two respective haplotypes are labeled a and b.
Discussion
In this study, 11 shared LoF mutations were identified from a large international cohort of IGD patients and each was subjected to founder mutation analysis. By performing rigorous haplotype analysis on these candidate mutations, we defined a founder haplotype for eight mutations. Of these eight founder mutations, seven are novel founder alleles (GNRHR [Q106R, R262Q]; TACR3 [W275X]; PROKR2 [R85H]; FGFR1 [R250Q, G687R]; HS6ST1 [R382W]), and one previously reported founder mutation (GNRHR R139H) (6) was also confirmed to be a founder allele. The presence of sufficient numbers of phased mutation carriers showed unequivocal evidence of a core ancestral haplotype shared across all mutation carriers for two GNRHR mutations (R262Q and R139H) (binomial probability 3.54 × 10−19 and 6.33 × 10−16, respectively) (Table 2), whereas the remaining six alleles with fewer phased samples showed haplotypes that were compatible with a founder effect.
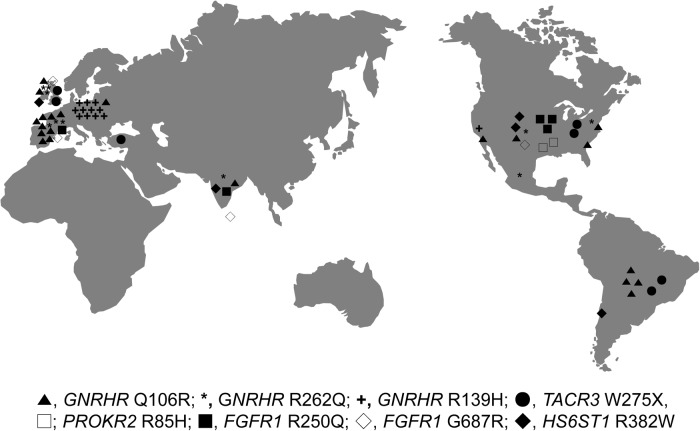
Founder mutations have generated considerable interest in human genetics because their study can facilitate detailed analyses of population evolution (24), human migration pathways, and survival patterns (25). Founder mutations are embedded in a large stretch of DNA identical to the ancestor, ie, a shared haplotype that surrounds a founder mutation. When a disease allele first occurs in the population by mutation or migration, the particular set of DNAs linked to the disease locus by linkage disequilibrium constitute a disease-containing haplotype (13, 26). Of the previously known founder alleles in IGD genes, both the GNRH1 (3) and TAC3 (4) founder mutations were specific to certain ethnic groups (Romanian and Congo/Haiti, respectively), representing regional founder events. Similarly, among the founder mutations identified in this study, 8 of 10 carriers with the GNRHR R139H ancestral haplotype originated from Poland, suggesting that this particular founder mutation is also likely to be a regional founder mutation in the Polish population. Interestingly, the GNRHR R139H mutation was also recently identified as a Brazilian founder mutation (6). In contrast, other founder mutations were documented in patients with diverse ethnicity (Caucasian, Mexican, and Asian ancestry) without any specific geographical proclivity similar to the diverse ethnic origins (Caucasian, Brazilian, Mexican, and Maghrebian) of PROKR2 L173R founder mutation, as we previously reported (Figure 3) (5).
Figure 3.
A world map indicating the geographic location of the patients with the founder mutations.
Although some of the reported founder alleles may cause dominantly inherited IGD (eg, FGFR1 [R250Q, G687R]) and may be subject to negative selection due to the inherent reproductive failure, other mutations represent heterozygous alleles of recessive genes and hence are less subject to purifying selection than dominant mutations and consequently can remain fixed in populations at a low frequency. Nonetheless, the persistence of the LoF alleles in diverse populations might reflect a potential heterozygote advantage to the carriers (5). Persistence of regional founder alleles (TAC3 c.209-1G>C, GNRH1 c.18_19insA, and GNRHR R139H) may also indicate selective advantage that may be relevant to adaptation of the reproductive axis during a period of local environmental limitations such as impaired nutrition, migration, and stress. In keeping with a potential selective heterozygote advantage for these founder events, two of the newly identified founder alleles, PROKR2 R85H and GNRHR R262Q, were also reported in women with HA (7). Similarly, another previously reported founder mutation in PROKR2 (L173R) was also documented in women with HA (7). Taken together, these observations could imply a potential role for these founder alleles in adaptive evolution of the GnRH neurons to stressful environments. These founder alleles may also underlie other evolutionary adaptations such as seasonal breeding in animals, but these hypotheses require further confirmation.
Whereas the heterozygote advantage hypothesis is intriguing and requires additional experimental validation, several alternate explanations for these founder events are worth considering. First, it is possible that the haplotype per se may favor the same mutation, possibly due to cis-acting cryptic regulatory mechanisms. Second, recent data from whole exome sequencing data sets suggest that most deleterious Mendelian disease alleles appear to have originated within the last 5000 years. This time frame corresponds to the estimated period of rapid acceleration of human population growth and contributes to the genetic and allelic heterogeneity of severe Mendelian traits (27). In keeping with this notion, previous founder alleles in GNRH1 (240–1500 y) and TAC3 (600 y) as well as the founder mutations identified in the current study all appear to have originated within this time frame. These observations suggest that the rapid population expansion may account for the persistence of these founder alleles without any selective advantage and that these alleles may be subject to purifying selection and/or genetic drift in subsequent generations (28). Finally, it is possible that these LoF mutations may have low penetrance and may be subject to either environmental or genetic modifiers (oligogenicity) as has been described previously (8). Consistent with this hypothesis, these alleles are also found in normative databases (Table 1), albeit at lower frequencies than in IGD, suggesting that these are weakly penetrant, especially in the heterozygous state.
This study has several limitations. The lack of parental DNA in several families did not allow appropriate phasing of all samples to determine the precise haplotype bearing the mutations in some individuals. However, in such instances, the limited analysis available for such unphased genotypes was generally compatible with the possibility that these mutations descended from a common founder. Although only 14 of the more than 20 known IGD genes were examined in this study, this collaborative effort represents the most comprehensive analysis of founder mutations conducted to date in IGD patients.
In conclusion, the current study identified eight founder mutations, each of which joins the expanding lists of IGD-associated global or regional founder alleles. In contrast to the ancient PROKR2 founder allele, these identified LoF founder alleles are more recent in origin, coincident with recent human population expansion, and their population prevalence suggests that they may represent low penetrance alleles in the heterozygous state without any selective heterozygote advantage.
Acknowledgments
We thank all the patients and families who contributed to the genetic material used in this research and their respective referring physicians for collaborating on this project; Pardis Sabeti, MD, PhD (Associate Professor at the Department of Organismic and Evolutionary Biology, Center for Systems Biology, Broad Institute of Massachusetts Institute of Technology and Harvard University), for helpful discussions about this project; and Kimberly H. Cox, PhD (Reproductive Endocrine Unit, Massachusetts General Hospital), for her critical comments on this manuscript.
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health Grants R01 HD15788 and U54 HD028138 (to W.F.C.), and HD33004 (to L.C.L.). R.B. is supported by a K23 Career Development award from National Institute of Child Health and Human Development (K23 HDHD077043).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ExAC
- Exome Aggregation Consortium
- HA
- hypothalamic amenorrhea
- HGMD
- Human Gene Mutation Database
- IGD
- isolated GnRH deficiency
- LoF
- loss-of-function
- MAF
- minor allele frequency
- MGH
- Massachusetts General Hospital
- SNP
- single-nucleotide polymorphism
- STR
- short tandem repeat.
References
- 1. Balasubramanian R, Crowley WF., Jr Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol Cell Endocrinol. 2011;346:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laitinen EM, Vaaralahti K, Tommiska J, et al. Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J Rare Dis. 2011;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouligand J, Ghervan C, Tello JA, et al. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360:2742–2748. [DOI] [PubMed] [Google Scholar]
- 4. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95:2287–2295. [DOI] [PubMed] [Google Scholar]
- 5. Avbelj Stefanija M, Jeanpierre M, Sykiotis GP, et al. An ancient founder mutation in PROKR2 impairs human reproduction. Hum Mol Genet. 2012;21:4314–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beneduzzi D, Trarbach EB, Min L, et al. Role of gonadotropin-releasing hormone receptor mutations in patients with a wide spectrum of pubertal delay. Fertil Steril. 2014;102:838–846.e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caronia LM, Martin C, Welt CK, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sykiotis GP, Plummer L, Hughes VA, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci USA. 2010;107:15140–15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw ND, Seminara SB, Welt CK, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96:E566–E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Roux N, Young J, Misrahi M, et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. [DOI] [PubMed] [Google Scholar]
- 11. Costa EM, Bedecarrats GY, Mendonca BB, Arnhold IJ, Kaiser UB, Latronico AC. Two novel mutations in the gonadotropin-releasing hormone receptor gene in Brazilian patients with hypogonadotropic hypogonadism and normal olfaction. J Clin Endocrinol Metab. 2001;86:2680–2686. [DOI] [PubMed] [Google Scholar]
- 12. Liu K, Martini M, Rocca B, et al. Evidence for a founder effect of the MPL-S505N mutation in eight Italian pedigrees with hereditary thrombocythemia. Haematologica. 2009;94:1368–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu K, Martin RB, Whittemore AS. Classifying disease chromosomes arising from multiple founders, with application to fine-scale haplotype mapping. Genet Epidemiol. 2004;27:173–181. [DOI] [PubMed] [Google Scholar]
- 14. Miyazawa H, Kato M, Awata T, et al. Homozygosity haplotype allows a genomewide search for the autosomal segments shared among patients. Am J Hum Genet. 2007;80:1090–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. [DOI] [PubMed] [Google Scholar]
- 16. Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009;Chapter 2:Unit 2 12. [DOI] [PubMed] [Google Scholar]
- 17. Storm N, Darnhofer-Patel B, van den Boom D, Rodi CP. MALDI-TOF mass spectrometry-based SNP genotyping. Methods Mol Biol. 2003;212:241–262. [DOI] [PubMed] [Google Scholar]
- 18. Slatkin M, Rannala B. Estimating allele age. Annu Rev Genomics Hum Genet. 2000;1:225–249. [DOI] [PubMed] [Google Scholar]
- 19. Rannala B, Reeve JP. High-resolution multipoint linkage-disequilibrium mapping in the context of a human genome sequence. Am J Hum Genet. 2001;69:159–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reeve JP, Rannala B. DMLE+: Bayesian linkage disequilibrium gene mapping. Bioinformatics. 2002;18:894–895. [DOI] [PubMed] [Google Scholar]
- 21. Cornes BK, Tang CS, Leon TY, et al. Haplotype analysis reveals a possible founder effect of RET mutation R114H for Hirschsprung's disease in the Chinese population. PLoS One. 2010;5:e10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borroni B, Bonvicini C, Galimberti D, et al. Founder effect and estimation of the age of the Progranulin Thr272fs mutation in 14 Italian pedigrees with frontotemporal lobar degeneration. Neurobiol Aging. 2011;32:555 e551–558. [DOI] [PubMed] [Google Scholar]
- 23. Slatkin M, Rannala B. Estimating the age of alleles by use of intraallelic variability. Am J Hum Genet. 1997;60:447–458. [PMC free article] [PubMed] [Google Scholar]
- 24. Zeegers MP, van Poppel F, Vlietinck R, Spruijt L, Ostrer H. Founder mutations among the Dutch. Eur J Hum Genet. 2004;12:591–600. [DOI] [PubMed] [Google Scholar]
- 25. Henn BM, Cavalli-Sforza LL, Feldman MW. The great human expansion. Proc Natl Acad Sci USA. 2012;109:17758–17764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wall JD, Pritchard JK. Haplotype blocks and linkage disequilibrium in the human genome. Nat Rev Genet. 2003;4:587–597. [DOI] [PubMed] [Google Scholar]
- 27. Fu W, O'Connor TD, Jun G, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai JJ, Petrov DA. Relaxed purifying selection and possibly high rate of adaptation in primate lineage-specific genes. Genome Biol Evol. 2010;2:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]