Abstract
Context:
Human embryonic stem cells (hESCs) differentiated toward β-cells and fetal human pancreatic islet cells resemble each other transcriptionally and are characterized by immaturity with a lack of glucose responsiveness, low levels of insulin content, and impaired proinsulin-to-insulin processing. However, their response to stimuli that promote functionality have not been compared.
Objective:
The objective of the study was to evaluate the effects of our previous strategies for functional maturation developed in rodents in these two human models of β-cell immaturity and compare their responses.
Design, Settings, Participants, and Interventions:
In proof-of-principle experiments using either adenoviral-mediated overexpression of V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA) or the physiologically driven path via thyroid hormone (T3) and human fetal islet-like cluster (ICC) functional maturity was evaluated. Then the effects of T3 were evaluated upon the functional maturation of hESCs differentiated toward β-cells.
Main Outcome Measures:
Functional maturation was evaluated by the following parameters: glucose responsiveness, insulin content, expression of the mature β-cell transcription factor MAFA, and proinsulin-to-insulin processing.
Results:
ICCs responded positively to MAFA overexpression and T3 treatment as assessed by two different maturation parameters: increased insulin secretion at 16.8 mM glucose and increased proinsulin-to-insulin processing. In hESCs differentiated toward β-cells, T3 enhanced MAFA expression, increased insulin content (probably mediated by the increased MAFA), and increased insulin secretion at 16.8 mM glucose.
Conclusion:
T3 is a useful in vitro stimulus to promote human β-cell maturation as shown in both human fetal ICCs and differentiated hESCs. The degree of maturation induced varied in the two models, possibly due to the different developmental status at the beginning of the study.
Immature human β-cells are characterized by their lack of glucose responsiveness (1–3), low insulin content, lack or low expression of the mature β-cell transcription factor V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA) (4), and impaired proinsulin-to-insulin processing (5, 6). These characteristics have been described in human fetal pancreas and are shared by insulin positive cells produced by most in vitro protocols for generating β-cells from stem/progenitor cells (1, 2). From a transcriptional point of view, differentiated human stem cells resemble fetal, not adult, β-cells (4). Therefore, understanding β-cell immaturity and developing strategies to further maturation to adult-like β-cells is of paramount importance for successful replacement therapies using stem cells.
As with full-term human infants, neonatal rodents have a delayed and blunted glucose-induced insulin secretion (3, 7), making the rodent model a useful tool to study β-cell maturation. Using the rodent model, we have previously reported that overexpression of Mafa, a glucose-responsive transcription factor selectively expressed in β-cells, enhances the glucose-induced insulin secretion of P2 rat islets to near-normal adult levels (8). Furthermore, we showed that thyroid hormone (T3) acts as a physiological regulator of maturation by directly up-regulating Mafa transcription in rodent islets (9). Two very recent papers (10, 11) effectively translated into human embryonic stem cell (hESC) differentiation protocols the success of using T3 to promote functional β-cell maturation. In these new protocols T3, in conjunction with other factors, was shown to enhance the coexpression of NKX6.1 and insulin and increase expression of insulin and other mature β-cell markers at the protein and mRNA levels (10). Moreover, T3 was used to enhance the generation of glucose-sensing, insulin-secreting human β-cells in vitro (11).
Even with the success of using T3 along with other factors in hESC differentiation protocols, its sole effect on human β-cell immaturity using either fetal human pancreas or differentiated hESCs has not been reported. We hypothesized that MAFA overexpression or treatment with T3 on its own would enhance the maturation of immature human β-cells in both models and allow a comparison of their responses with maturation interventions. Thus, our aim was to test the isolated effects of T3 on the maturation in these two models of immature human β-cells.
Our results show that MAFA overexpression and treatment with T3 effectively enhance several maturation parameters in cells derived both from fetal pancreas and from differentiated hESCs. However, the responses in these two models differed, suggesting that despite their transcriptional similarity, important differences that determined their response to maturation stimuli remained.
Materials and Methods
ICC preparation
Anonymously donated 19- to 22-week-old fetal human pancreas (Advanced Biosciences Resources, Inc) were minced and then digested following a modified collagenase method of Gotoh et al (12) and handpicked to ensure high purity. For immunostaining, ICCs recovered after culture and similar pancreases (n = 2, aged 19 and 21 wk of gestation) were either fixed for 2 hours in 4% paraformaldehyde for paraffin embedding or were enrobed in Tissue-Tek O.C.T. and frozen without fixation in chilled isopentane for frozen sections. All tissues were approved for research and had institutional review board exemption status.
hESC differentiation protocol and processing
The Viacyte surface-attached β-cell differentiation protocol (1, 13) using CyT49 hESCs was used with two modifications: 1) cells were plated on Matrigel-coated silicon rubber and 2) stage 3 was lengthened to 7 days. In experimental samples, T3 was added at 10 or 100 nM throughout stage 5. At the end of each stage, cells were extracted for RNA for quantitative PCR (qPCR) analysis. At the end of stage 5, cells were assayed for glucose-stimulated insulin secretion or collected for qPCR, immunostaining, or fluorescence-activated cell sorter (FACS) analysis. Except for cells used for glucose-stimulated insulin secretion, plates of cells were washed in PBS and then enzymatically dissociated to single-cell suspension using trypsin (VWR). Cells were pelleted and then resuspended in PBS. For immunostaining, 1.5105 cells/well were then plated on a 96-well plate in basal media for 24 hours before fixing. The remaining cells were used for either RNA extraction or FACS analysis.
ICC culture: hormone treatment and adenoviral infection
ICCs were cultured 4 days in RPMI 1640 supplemented with 10% charcoal-stripped fetal bovine serum (CS-FBS) and T3 (150 pM, equivalent to 7.5 pM free T3 in 10% CS-FBS [14]), followed by RNA extraction or insulin secretion assays. For adenoviral infection, human MAFA coding sequence was used to generate Adv-CMV-hMAFA-IRES-GFP (Adv-MAFA) based on a pShuttle vector (Stratagene) (8); Adv-CMV-IRES-GFP was used as a control. ICCs were infected overnight with adenovirus and cultured 4 days in RPMI 1640 with 10% CS-FBS. Clusters were then retrieved for qPCR or sequential insulin secretion.
Quantitative real-time PCR
Total RNA was isolated with a PicoRNA extraction kit (Arcturus) or an RNeasy kit (QIAGEN) and reverse transcribed (SuperScript reverse transcriptase; Invitrogen). Real-time quantitative PCR used SYBR green detection and specific primers (see Supplemental Table 1). Samples were normalized to a control gene (S18 and TBP for ICCs and TBP for hESCs), and the comparative threshold cycle method used to calculate gene expression levels.
Insulin secretion in vitro
Insulin secretion for both ICCs and hESCs was measured by static incubation sequentially in 2.6 mM and 16.8 mM glucose in Krebs-Ringer bicarbonate buffer (16 mM HEPES and 0.1% BSA, pH 7.4) as previously described (15). Supernatants and cells were frozen until measured with human insulin or proinsulin enzyme-linked immunosorbent assay (ALPCO).
FACS analysis
Before fixing, cells were incubated with a 1:1000 dilution of live/dead violet staining (Invitrogen) in PBS for 30 minutes on ice. After the staining, the cells were pelleted and then resuspended in 4% (wt/vol) paraformaldehyde and fixed for 20 minutes on ice. Cells were stored in PBS at 4°C while awaiting staining. Cells were permeabilized with perm buffer (PBS, 0.2% [wt/vol] Triton X-100, 5% [vol/vol] normal donkey serum) for 30 minutes on ice and then washed with wash buffer (PBS, 0.1% [wt/vol] BSA). Cells were incubated with primary antibodies diluted with block buffer (PBS, 0.1% [wt/vol] Triton X-100, 5% [vol/vol] normal donkey serum) overnight at 4°C. Cells were washed in wash buffer and then incubated with appropriate secondary antibodies for 60 minutes at 4°C, followed by three washes with wash Buffer. Cells were resuspended in wash buffer for flow acquisition. Flow cytometry data were acquired with a FACS LSR HTS (BD Biosciences), using excitation lines at 405, 488, and 635 nm. Data were analyzed using FlowJo software (Tree Star).
Immunostaining
Pancreatic sections were immunostained for insulin, glucagon, NKX6.1, MAFA, pancreatic and duodenal homeobox-1 (PDX1), NGN3, and SRY-box9 (SOX9) (see Supplemental Table 2 for antibodies). Sectioned agar pellets containing ICCs were immunostained for insulin and E-cadherin.
For hESCs, 96-well plates of cells were gently washed with warmed PBS twice before being fixed with 4% paraformaldehyde for 20 minutes. After fixing, cells were stored in PBS at 4°C until immunostaining. Then cells were first permeabilized with perm buffer for 30 minutes, washed with PBS, and then incubated with primary antibodies diluted with block buffer overnight at 4°C. After a wash in PBS, appropriate secondary antibodies were applied and incubated for 2 hours in the dark, followed by PBS washes and incubation with 4′,6-diamino-2-phenylindole for nuclear visualization. Images of hESCs were acquired with Zeiss epifluorescent microscope using excitation lines at 405, 488, and 561 nm; those of fetal tissues were obtained either on an Olympus epifluorescent or in confocal mode on Zeiss LSM710 microscope.
Statistical analysis
A normality test was done for all data using D'Agostino and Pearson omnibus normality test or Shapiro-Wilk normality test included in the GraphPad Prism software. When the distribution was normal, the mean and SE are shown, and an analysis was done with parametric statistics, usually a Student's t test. However, if values did not pass a normality test, the median was plotted and nonparametric statistics were used, specifically Wilcoxon matched-pairs signed rank test. The tests used are indicated in the figure legends.
Results
Proof-of-principle experiments
Our aim was to test whether enhancing MAFA expression in fetal ICCs would induce the expression of downstream targets and promote functional maturation of the tissue.
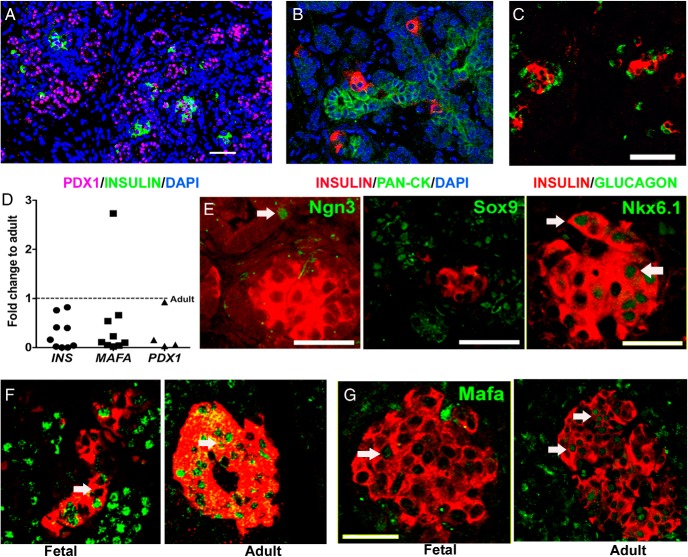
First, we evaluated developmental markers for islet and duct cells in midgestation human fetal pancreas (HFP). At 19–21 weeks, the fetal human pancreas had many insulin-positive cells as singlets or small clusters associated with ductal cells (Figure 1, A and B) as well as small islets (Figure 1C). ICCs were isolated from similarly aged pancreases. To evaluate the composition and consistency of ICC preparations, we collected them after 4 days in culture and stained them for E-cadherin, insulin, and glucagon. We found consistently E-cadherin marked all the cells, whereas 37% of them expressed insulin and 7% expressed glucagon (Supplemental Figure 1, A and B). Key β cell-genes were expressed at significantly lower levels in ICCs than adult human islets (Figure 1D), having only 30% PDX1, 50% MAFA, and 30% insulin mRNA that of adult human islets. As found in perinatal rodents, the main isoform of the thyroid hormone receptor expressed in ICCs was thyroid hormone receptor (THR)-A, with THRB being expressed at very low levels. When mRNA levels for each THR isoform were quantified, THRA levels were 27 times higher than those of THRB (n = 4, P = .0009). Therefore, in experiments using ICCs, we analyzed only changes in the THRA expression.
Figure 1.
Midgestation (19–21 wk) HFP had many insulin-positive cells as singlets or small clusters associated with ductal cells (A and B) and with glucagon-positive cells (C). D, Key β-cell genes had significantly lower expression in fetal ICCs than in adult human islets by qPCR. Dotted line indicates the adult levels (n = 4 for Pdx1 [*, P = .01]; n = 9 for insulin [*, P = .000 003], and Mafa [*, P = .05]) with an unpaired t test. E, At this age, insulin-positive cells were NKX6.1 positive (arrows) and SOX9 negative. Ngn3-positive cells (arrow) were still present in ductal structures. β-Cells from fetal human pancreas had lower levels of PDX1 (F) and MAFA (G) (arrows) when compared with adult pancreas (19 wk fetal human pancreas, representative of two blocks each from two HFP). Magnification bar, 50 μm, except in panel E for PDX1 and SOX9 in which the magnification bar was 25 μm.
Immunostaining for important β-cell developmental transcription factors also showed that endocrine differentiation was incomplete (Figure 1, E–G). At this stage insulin-positive cells had lower levels of MAFA and PDX1 than adult cells (Figure 1, F and G) but were Sox9negative and NKX6.1positive (Figure 1E). The NGN3-positive cells were still present in ductal structures. Thus, ICCs isolated from this age of human fetal pancreas include differentiated but immature β-cells.
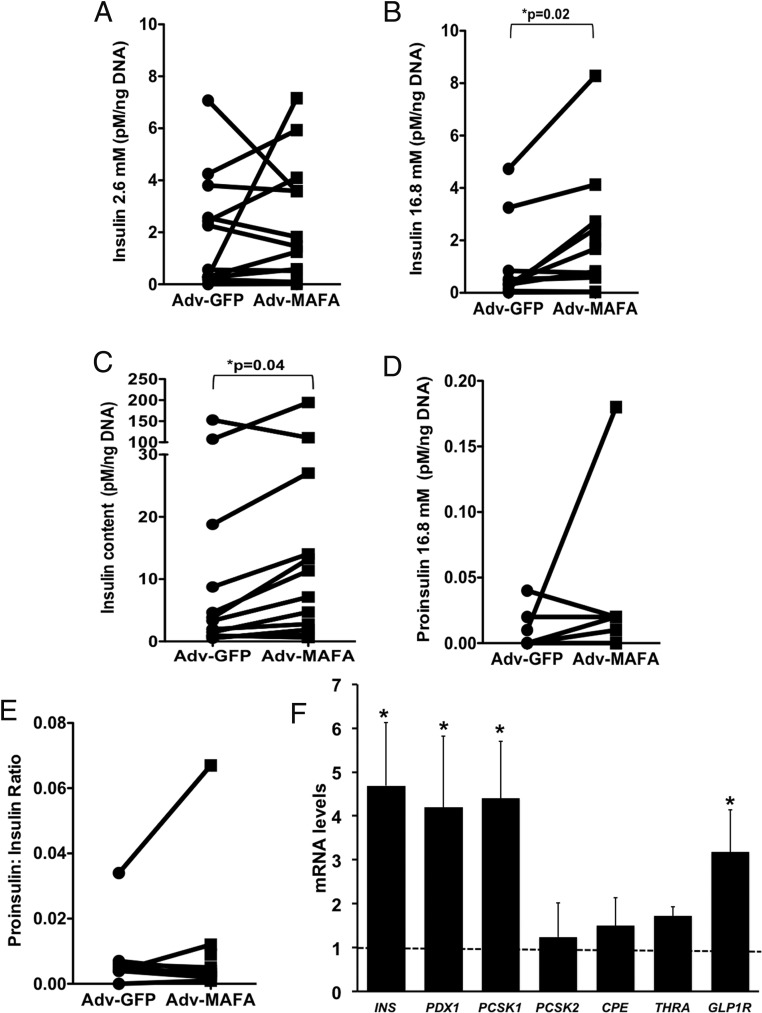
Four days after infection with adenovirus carrying MAFA or GFP, the infection rates were 43% ± 5% for Adv-CMV-IRES-GFP and 41% ± 9% for Adv-MAFA (n = 5 independent experiments). In ICCs adenoinfected with MAFA, compared with green fluorescent protein-infected controls, insulin secretion was unchanged at 2.6 mM glucose (Figure 2A) but increased at 16.8 mM glucose (Figure 2B), with greater than 90% of total secreted insulin as processed insulin (Figure 2B vs Figure 2D). Adv-MAFA also increased insulin content (Figure 2C) but did not change the proinsulin secreted at 16.8 mM glucose concentration (Figure 2D) or the ratio of secreted proinsulin to insulin at 16.8 mM glucose (Figure 2E). Changes in β-cell function induced by Adv-MAFA were accompanied by an increased expression of putative MAFA target genes, with 4-fold increases in insulin, PDX1, and PCSK1 mRNA, a 3-fold increase in GLP1R, and a 50% increase in THRA (Figure 2F).
Figure 2.
Functional effects of adenoviral-mediated overexpression of MAFA in ICCs. A, Insulin secretion at 2.6 mM glucose. B, Increased insulin secretion to 16.8 mM glucose. C, Increased insulin content. There was no change in proinsulin secretion (D) or proinsulin to insulin ratio (E). F, Key β-cell genes had increased expression as measured by qPCR and compared with untreated samples (dotted line). For panels A–E, n = 12 pancreas of 19–22 weeks' gestation; connector lines represent experimental and control data for individual pancreases; for panel F, n = 3–4 of 20 weeks' gestation. Nonparametric statistics were used for panels B and C; paired t test was used for the others.
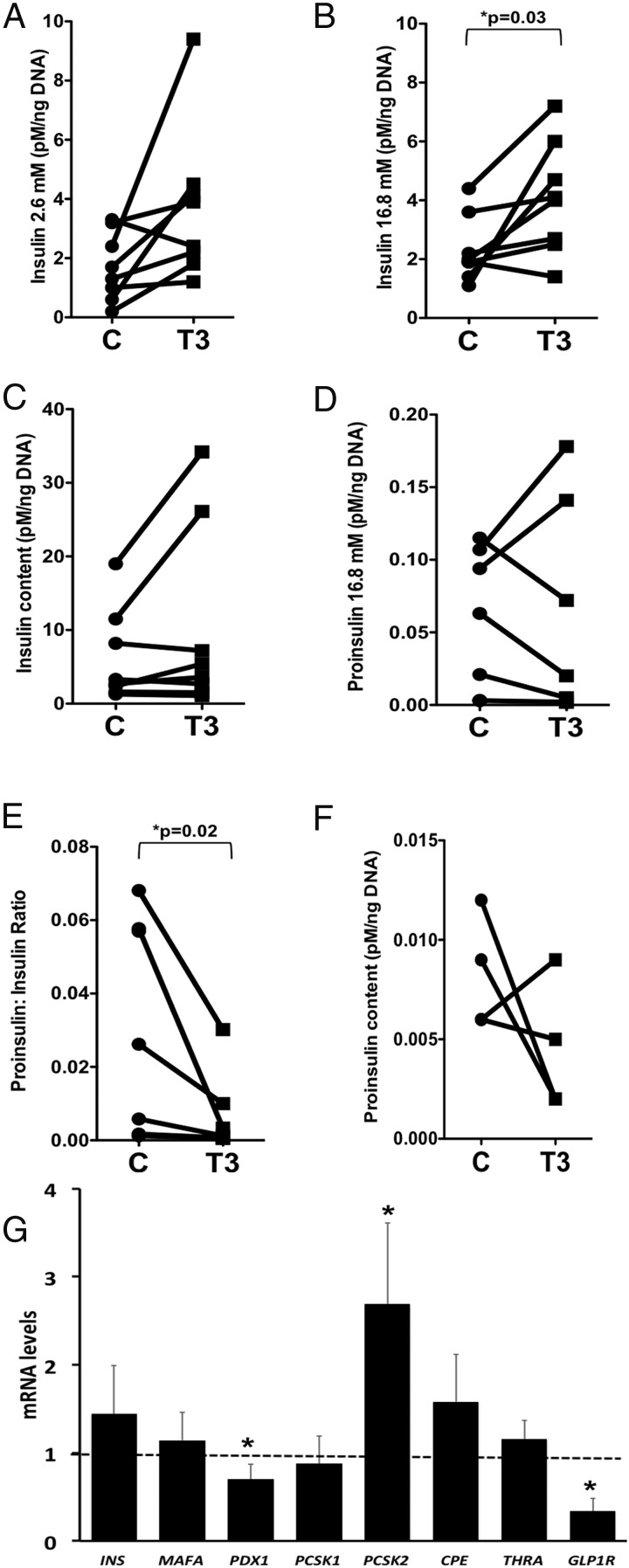
Because we previously showed that T3 treatment is an effective in vitro strategy to induce Mafa expression of immature rodent cells, we tested the effects of T3 on the maturation processes in the fetal human ICCs. After 4 days of culture with T3, insulin secretion was unchanged at 2.6 mM glucose (Figure 3A) but increased at 16.8 mM glucose (Figure 3B), no change in cellular insulin content (Figure 3C), and no change in the proportion of secreted insulin as proinsulin (Figure 3D), thus decreasing the proinsulin to insulin ratio (Figure 3E). These results were similar to those observed with Adv-MAFA treatment (Figure 2) with an additional positive effect on proinsulin processing. In fetal ICCs the high basal insulin secretion at 2.6 mM glucose, another trait of β-cell immaturity, blunts the stimulation index; neither MAFA nor T3 was effective in altering this parameter (Figures 2 and 3). T3-treated ICCs were characterized by an improved proinsulin-to-insulin processing as shown by the ratio. Consistent with this finding, T3 increased the mRNA expression of proinsulin processing enzyme PCSK2 mRNA by 2.5-fold (Figure 3G).
Figure 3.
T3 effects on ICC function and gene expression. A, Insulin secretion at 2.6 mM glucose. At 16.8 mM glucose, insulin secretion was increased (B). there was no change in insulin content (C) or proinsulin secreted at 16.8 mM glucose (D). The proinsulin to insulin ratio (E) is shown, with nonsignificant changes in proinsulin (F) cellular content. G, T3 effects on gene expression of ICCs as measured by qPCR and compared with untreated samples (dotted line) (n = 8 human fetal pancreas of 20–22 wk gestation); connector lines represent experimental and control data for individual pancreases. *, P ≤ .05, nonparametric statistics used for panels C and E and a Student t test for the others. C, control.
Do cells from differentiated hESCs respond similarly to T3 as fetal ICCs?
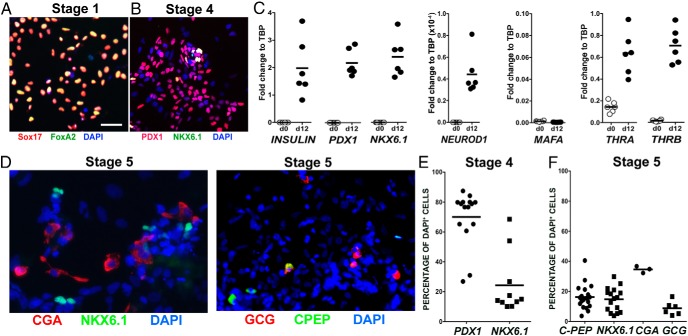
Gene profiling of differentiated human stem cells showed that the resultant endocrine cells resembled fetal rather than adult β-cells (2, 4). We confirmed these results by testing the expression of key β-cell genes at the end of stage 4, prior to treatment with T3. By immunostaining, 70% cells expressed PDX1 protein and 24% expressed NKX6.1 (Figure 4, B and E). By qPCR at this stage, the cells expressed insulin and some important β-cell-specific transcription factors, PDX1, NKX6.1, and NEUROD1 but, characteristic of all immature beta cells, lacked MAFA expression (Figure 4C) (16). They also expressed mRNA for both thyroid receptor isoforms, THRA and THRB (Figure 4C), which would render them responsive to T3. At the end of stage 5 under control conditions, the expression of C-peptide, NKX6.1, chromogranin A (CGA), and glucagon mRNA were examined (Figure 4, D and F). Under control conditions, 16% of differentiated hESCs expressed C-peptide, whereas 9% expressed glucagon. As shown in the scatter plots (Figure 4, C, E, and F), there was consistency within and between experiments.
Figure 4.
hESCs after differentiation express transcription factors characteristic of endocrine progenitors and some important β-cell-specific transcription factors but not MAFA. Stage 1 (A) represents cells that are differentiated to definitive endoderm and stage 4 (B) further differentiated to pancreatic endoderm but before exposure to T3. Magnification bar, 100 μm. C, Scatter plots of mRNA levels of different genes before the differentiation protocol (d0) and at the end of stage 4 (d12). Individual points represent independent samples from a single experiment and are expressed in relation to housekeeping gene TBP. D, Representative pictures of cells at the end of the differentiation at stage 5 (control conditions) immunostained for CGA, NKX 6.1, glucagon (GCG), C-peptide (CPEP), and 4′,6-diamino-2-phenylindole (DAPI) to counterstain nuclei. E, FACS quantification at the end of stage 4. F, FACS quantification at the end of stage 5.
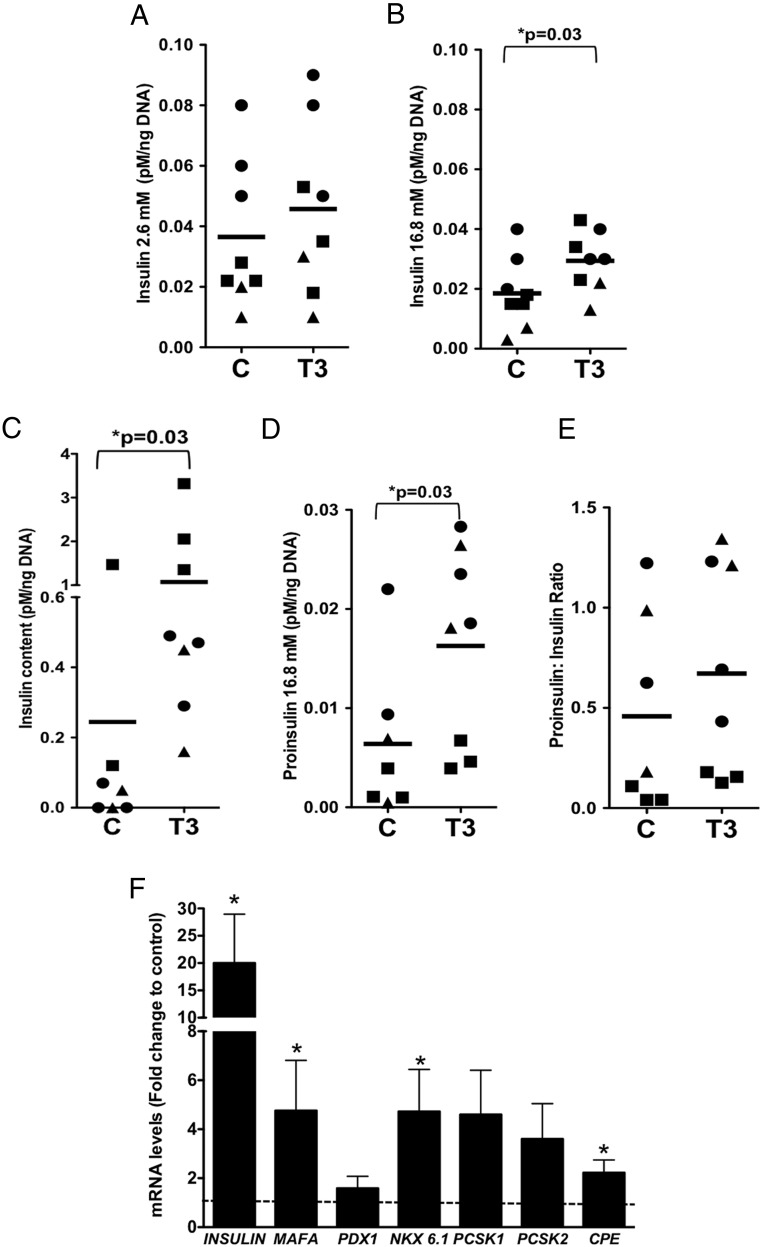
When T3 was added at the end of stage 4 and the cells studied at the end of stage 5, insulin secretion did not change at 2.6 mM glucose (Figure 5A) but was enhanced in response to 16.8 mM glucose (Figure 5B) as was the cellular insulin content (5-fold) (Figure 5C). The secreted proinsulin at 16.8 mM glucose also increased (Figure 5D); however, there was no overall change in proinsulin to insulin ratio (Figure 5E).
Figure 5.
T3 effects on hESC-derived cell function and gene expression. At the end of stage 5, T3 (100 nM)-treated cells were assessed for insulin secretion at 2.6 mM glucose (A), at 16.8 mM glucose (B), insulin cell content (C), proinsulin (D), and proinsulin to insulin ratio (E). For functional experiments, n = 7–8 samples from three independent experiments. F, qPCR was used to evaluate the expression of the insulin gene and other key β-cell genes (dotted line, control = 1). All data are expressed as fold change to untreated control samples. For gene expression, n = 5–9 samples from two to four independent experiments and normalized to levels of TBP as an endogenous control. Student t test was used for all panels. C, control.
Some potential mechanisms behind the differences in the glucose-stimulated insulin secretion, insulin processing, and insulin synthesis in fetal ICCs and hESC-derived cells after T3 treatment were revealed by transcript analysis. As shown in Figure 3E, T3-treated ICCs were characterized by an improved proinsulin-to-insulin processing, perhaps due to the increased expression of the proinsulin-processing enzyme proprotein convertase subtilisin/kexin (PCSK)-2. In contrast, T3-treated hESCs were characterized by a large increase in total insulin content, with concordant increases in proinsulin. At the end of stage 5, T3 resulted in a 20-fold increase insulin mRNA, a 5-fold increase in transcription factors MAFA and NKX6.1, a 2-fold increase in CPE, and a nonsignificant tendency for an increase for both PCSK1 and PCSK2 (Figure 5F).
To address whether the developmental stage of the β-cells could explain the differential response to maturation stimuli, we overexpressed MAFA in fetal ICCs from differently aged pancreas and evaluated the effects on expression of the known MAFA targets, insulin and PDX1. The response to MAFA was age dependent, such that as β-cells developed, their response to MAFA became more robust (Figure 6, A and B). Additionally, the increased insulin secretion at 16.8 mM glucose (Figure 6C) moderately correlated to gestational age.
Figure 6.
ICCs obtained at different gestational ages respond differently to MAFA overexpression. mRNA of insulin (A) and PDX1 (B) as well as insulin secretion at 16.8 mM glucose (C) increased in an age-dependent manner after adenovirally mediated MAFA overexpression. Each dot represents an independent sample from independent experiments.
A limitation of this study is that both preparations, human fetal pancreas and differentiated hESCs, were not pure β-cells but rather mixed pancreatic cells, and thus, the measured parameters may be underestimated, even though the ratios should remain constant. The low proportion of cells in ICCs after 4 days in culture immunostained for insulin and glucagon (Supplemental Figure 1, A and B) suggest that the effects of Adv-MAFA and T3 on β-cell transcription and secretion were underestimated. After a correction for the percentage of Ins+ cells in 4-day cultured ICCs (Supplemental Figure 1, C and D), insulin and PDX1 mRNA levels were similar to those of adult human islets, whereas insulin secretion at 16.8 mM was about 10% of that reported by adult human islets (17).
Discussion
Our proof-of-principle experiments in fetal ICCs suggest that immature human β-cells respond positively to MAFA overexpression, with both increased insulin secretion at high glucose levels and increased insulin content. These results were reproduced by T3 in ICCs, with an additional effect of increasing proinsulin-to-insulin processing. In contrast, T3 treatment of differentiated hESCs had no effect on insulin processing but resulted in both increased insulin content and increased insulin secretion at 16.8 mM glucose.
Similar to Adv-MAFA-treated ICCs, those treated with T3 in culture improved insulin secretion at 16.8 mM glucose, yet surprisingly their MAFA levels were unchanged and the effects on insulin processing seemed to be a direct effect of T3 on the expression of the insulin-processing enzyme PCSK2. A possible explanation for the lack of T3-induced MAFA expression on ICCs is that as stated in Results, the main isoform of the receptor expressed at this developmental stage is THRA, whereas work in our laboratory suggests that THRB is the isoform that mediates MAFA expression (18). T3 during stage 5 differentiation of hESC-derived cells to islet cells increased MAFA, NKX6.1, and insulin mRNA, increased insulin protein content, and increased insulin secreted at 16.8 mM glucose. Yet there was no net glucose-stimulated insulin secretion because there was higher basal insulin release at 2.6 mM glucose. Such a high basal release is seen both in immature islets and dysfunctional adult islets (19–21). Thus, T3 in culture is useful in promoting human β-cell maturity, although the response varied between cells derived from fetal pancreas and those obtained from differentiated hESCs.
The observed differences in the effects of T3 on fetal ICCs and hESCs could be due to a different stage in their developmental or differences in the concentration of T3 used. A proper dose-response curve was difficult to perform in the fetal tissue due to the tissue's scarcity, so a suboptimal concentration of T3 may have been used. However, with differentiated hESCs, the effective T3 concentration was selected using MAFA expression as a readout (Supplemental Table 3). The stage of maturity of the insulin-expressing cells may also differ. Using insulin processing as a gauge, cells derived from differentiated human stem cells (proinsulin to insulin ratio 0.5) were more immature than cells obtained from fetal pancreas (proinsulin to insulin ratio 0.03) but were comparable with some published ratios for primary adult β-cells (0.03 reported elsewhere [11]) yet markedly differed from others (1.8 in another report [22]). The differences in the reported cellular proinsulin to insulin ratio from humans may be due to differences in the isolation and culture processes. The notion of differing developmental stages is additionally supported by the lower insulin content in cells obtained after an in vitro differentiation protocol (0.02 pM/ng DNA) (Figures 5C and 3C) than ICCs (5 pM/ng DNA); the magnitude of this difference cannot be accounted for by the differences in the percentage of insulin/C-peptide in each preparation (16% vs 37%). Another argument in favor of a less developed β-cell comes from only 5% colocalization between CGA and NKX6.1 in stage 5 cells. Current protocols using differing oxygen concentrations during the differentiation protocols address this, now obtaining up to 30% of colocalization; however, this was not known at the time the presented experiments were performed.
Interestingly, in the more developed fetal ICCs, T3 enhanced the insulin-processing efficiency, one of the final stages of the β-cell differentiation process, whereas the same stimulus increased insulin content and MAFA expression in cells obtained from the in vitro differentiation of hESCs. In T3-treated hESCs, the increase of proinsulin and the proinsulin to insulin ratio is likely an effect on insulin transcription, which was increased almost 20-fold with a parallel increase in insulin protein (Figures 5C and 7F). T3 has been reported to have direct effects on the human insulin gene (23). This raises the possibility that T3 is in fact a true maturation factor by enhancing the normal development of β-cells, regardless of their stage of development, as long as THRs are expressed. However, it cannot be ruled out that tissues at different stages of development respond differently to a given stimulus. Higher proinsulin values in T3-treated hESCs might be secondary to the fact that the increase in the processing enzyme was not to the same extent as insulin, thus resulting in higher proportions of the prohormone.
In summary, we report that interventions shown to be effective in promoting rodent β-cell maturity are applicable to two human models of immature β-cells. Specifically, T3 offers the advantage of being used in vitro, being readily available, and seems to have positive effects, regardless of the maturation stage of the tissue being treated.
Acknowledgments
Author contributions included the following: C.A.-M. and S.B.-W. conceived the project and wrote the manuscript; C.A.-M., A.D., J.H.-L., and C.H. researched the data; and G.W., C.C., and A.S. provided critical discussions and edited the manuscript. All authors reviewed the manuscript.
This work was supported by National Institutes of Health Grant R01 DK093909 (to S.B.-W.), Grant P30 DK036836 for the Joslin Diabetes Research Center (to G.L.K.), and by Juvenile Diabetes Research Foundation Grant 1-2011-591 (to S.B.-W.). This work also was supported by the Diabetes Research and Wellness Foundation and an important group of private donors.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Adv-MAFA
- Adv-CMV-hMAFA-IRES-GFP
- CGA
- chromogranin A
- CS-FBS
- charcoal-stripped fetal bovine serum
- FACS
- fluorescence-activated cell sorter
- hESC
- human embryonic stem cell
- HFP
- human fetal pancreas
- MAFA
- V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog A
- PCSK
- proprotein convertase subtilisin/kexin
- Pdx1
- pancreatic duodenal homeobox-1
- qPCR
- quantitative PCR
- SOX9
- SRY-box9
- THR
- thyroid hormone receptor.
References
- 1. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. [DOI] [PubMed] [Google Scholar]
- 2. Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aynsley-Green A, Bloom SR, Williamson DH, Turner RC. Endocrine and metabolic response in the human newborn to first feed of breast milk. Arch Dis Childh. 1977;52(4):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hrvatin S, O'Donnell CW, Deng F, et al. Differentiated human stem cells resemble fetal, not adult, beta cells. Proc Natl Acad Sci USA. 2014;111(8):3038–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet β-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113(3 Pt 1):537–541. [DOI] [PubMed] [Google Scholar]
- 6. Baetge EE. Production of β-cells from human embryonic stem cells. Diabetes Obesity Metab. 2008;10(suppl 4):186–194. [DOI] [PubMed] [Google Scholar]
- 7. Bliss CR, Sharp GW. Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am J Physiol. 1992;263(5 Pt 1):E890–E896. [DOI] [PubMed] [Google Scholar]
- 8. Aguayo-Mazzucato C, Koh A, El Khattabi I, et al. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat β cells. Diabetologia. 2011;54(3):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, et al. Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62(5):1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–1133. [DOI] [PubMed] [Google Scholar]
- 11. Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159(2):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gotoh M, Maki T, Satomi S, et al. Reproducible high yield of rat islets by stationary in vitro digestion following pancreatic ductal or portal venous collagenase injection. Transplantation. 1987;43(5):725–730. [DOI] [PubMed] [Google Scholar]
- 13. D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. [DOI] [PubMed] [Google Scholar]
- 14. Samuels HH, Stanley F, Casanova J. Relationship of receptor affinity to the modulation of thyroid hormone nuclear receptor levels and growth hormone synthesis by L-triiodothyronine and iodothyronine analogues in cultured GH1 cells. J Clin Invest. 1979;63(6):1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schuppin GT, Bonner-Weir S, Montana E, Kaiser N, Weir GC. Replication of adult pancreatic-β cells cultured on bovine corneal endothelial cell extracellular matrix. In Vitro Cell Dev Biol Animal. 1993;29A(4):339–344. [DOI] [PubMed] [Google Scholar]
- 16. Nishimura W, Kondo T, Salameh T, et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol. 2006;293(2):526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ling Z, Pipeleers DG. Prolonged exposure of human β cells to elevated glucose levels results in sustained cellular activation leading to a loss of glucose regulation. J Clin Invest. 1996;98(12):2805–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aguayo-Mazzucato CM, Ramados P, Scanlan T, Hollenberg A, Sharma A, Bonner-Weir S. T3 has dual effects upon pancreatic beta cells inducing markers of both maturation and aging. Diabetes. 2014;63(suppl 1):A54. [Google Scholar]
- 19. Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A, Melton DA. Functional β-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30(3):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leahy JL, Cooper HE, Weir GC. Impaired insulin secretion associated with near normoglycemia. Study in normal rats with 96-h in vivo glucose infusions. Diabetes. 1987;36(4):459–464. [DOI] [PubMed] [Google Scholar]
- 21. Leahy JL, Bonner-Weir S, Weir GC. Abnormal insulin secretion in a streptozocin model of diabetes. Effects of insulin treatment. Diabetes. 1985;34(7):660–666. [DOI] [PubMed] [Google Scholar]
- 22. Hostens K, Pavlovic D, Zambre Y, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark AR, Wilson ME, London NJ, James RF, Docherty K. Identification and characterization of a functional retinoic acid/thyroid hormone-response element upstream of the human insulin gene enhancer. Biochem J. 1995;309(Pt 3):863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]