Abstract
Context:
Steroid sex hormones and SHBG may modify metabolism and diabetes risk, with implications for sex-specific diabetes risk and effects of prevention interventions.
Objective:
This study aimed to evaluate the relationships of steroid sex hormones, SHBG and SHBG single-nucleotide polymorphisms (SNPs) with diabetes risk factors and with progression to diabetes in the Diabetes Prevention Program (DPP).
Design and Setting:
This was a secondary analysis of a multicenter randomized clinical trial involving 27 U.S. academic institutions.
Participants:
The study included 2898 DPP participants: 969 men, 948 premenopausal women not taking exogenous sex hormones, 550 postmenopausal women not taking exogenous sex hormones, and 431 postmenopausal women taking exogenous sex hormones.
Interventions:
Participants were randomized to receive intensive lifestyle intervention, metformin, or placebo.
Main Outcomes:
Associations of steroid sex hormones, SHBG, and SHBG SNPs with glycemia and diabetes risk factors, and with incident diabetes over median 3.0 years (maximum, 5.0 y).
Results:
T and DHT were inversely associated with fasting glucose in men, and estrone sulfate was directly associated with 2-hour post-challenge glucose in men and premenopausal women. SHBG was associated with fasting glucose in premenopausal women not taking exogenous sex hormones, and in postmenopausal women taking exogenous sex hormones, but not in the other groups. Diabetes incidence was directly associated with estrone and estradiol and inversely with T in men; the association with T was lost after adjustment for waist circumference. Sex steroids were not associated with diabetes outcomes in women. SHBG and SHBG SNPs did not predict incident diabetes in the DPP population.
Conclusions:
Estrogens and T predicted diabetes risk in men but not in women. SHBG and its polymorphisms did not predict risk in men or women. Diabetes risk is more potently determined by obesity and glycemia than by sex hormones.
Associations of steroid sex hormones and SHBG with diabetes have long been recognized. A systematic analysis encompassing literature up to 2005 (1) using case-control and prospective data, found that in men lower T was associated with elevated diabetes risk. Conversely, in women, higher T was associated with increased diabetes risk. In men and women, combined estradiol was higher in diabetes cases than controls. In both sexes, lower SHBG was associated cross sectionally and prospectively with diabetes, but the magnitude of this effect was larger in women. These populations were not at elevated diabetes risk, and in most (2–7) but not all studies (8) these associations were attenuated but remained significant after accounting for concurrent effects of obesity and/or insulin resistance. The relationship of SHBG and SHBG gene variants with diabetes has been evaluated using an instrumental variable approach (7, 9), finding that the genetically determined variations in SHBG concentrations were associated with effects on diabetes risk that were proportional to the effect to alter SHBG concentrations. Again, these observations were made in populations without average elevated diabetes risk.
In this literature, there is a relative paucity of prospective studies, and limited capacity to evaluate effects of sex steroids and SHBG concurrently with obesity, dysglycemia, and other diabetes risk factors. The Diabetes Prevention Program (DPP) was a multicenter randomized clinical trial that assessed the effects of lifestyle or metformin to delay progression to diabetes. By design, participants had elevated diabetes risk, based on overweight or obesity and dysglycemia. The population was multiethnic and included men, premenopausal women, and postmenopausal women. We have measured SHBG, SHBG single-nucleotide polymorphisms (SNPs), and steroid sex hormones in this population, testing the hypothesis that sex hormones are prospectively associated with diabetes incidence in the high-risk DPP population.
Materials and Methods
The DPP study population has been previously described (10). The eligibility criteria included age at least 25 years, body mass index (BMI) at least 24 kg/m2 (22 kg/m2 in people of Asian descent), fasting plasma glucose 95–125 mg/dL (100–139 mg/dL prior to June 1997), and glucose 2 hours after a 75-g oral glucose load of 140–199 mg/dL. All participants provided written informed consent. Those included in the current analyses approved the use of their blood samples for secondary analyses including genetics. Each participating institution was overseen by its respective ethics review board.
At study entry, participants were randomly assigned to intensive lifestyle intervention, metformin, or placebo. The average followup for diabetes incidence in the DPP was 3.2 years.
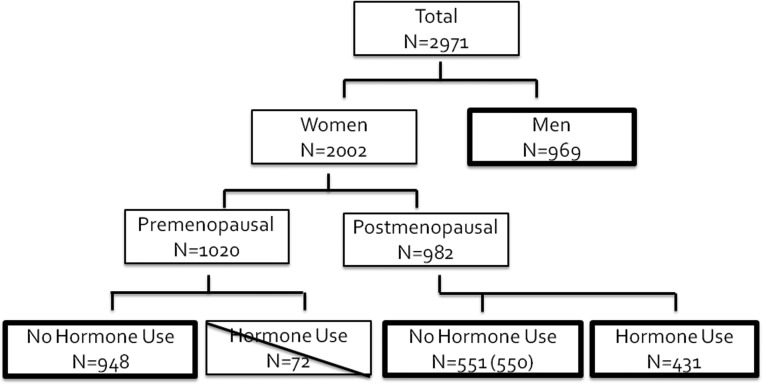
The population analyzed included DPP participants with prerandomization samples available for steroid sex hormone and SHBG measurements (Figure 1). We divided our population a priori into groups based on sex and hormone status: Men (n = 969), premenopausal women not taking exogenous sex hormones (n = 948), postmenopausal women not taking exogenous sex hormones (n = 551), and postmenopausal women taking exogenous sex hormones (n = 431). Due to the small sample size (n = 72), the group of premenopausal women taking hormone therapy was excluded. Women were classified as postmenopausal if their age at enrollment was greater than 55 years or they self reported a natural or surgical menopause, according to the questions, “Are you still having periods (menstrual bleeding),” “Did you ever have an operation to have both your ovaries taken out,” and “Did you ever have an operation to remove your uterus (womb) (Hysterectomy).” Hormone use was also determined by self report. Data from one postmenopausal woman not reporting hormone use but demonstrating a very high measured estradiol level was also excluded, providing a total study population of 2898 (Figure 1).
Figure 1.
Consort diagram representing participants with available data for the current analyses. Premenopausal women taking exogenous hormones were excluded from the current analyses due to the small sample size (n = 72; exclusion indicated by diagonal line). One postmenopausal woman reporting no hormone use was found to have elevated estradiol levels, and was excluded from analysis.
Diabetes ascertainment
Diabetes ascertainment was performed with 6-monthly fasting glucose measures and annual 75-g oral glucose tolerance testing, using the 1997 American Diabetes Association criteria of fasting glucose at least 126 mg/dL (measured every 6 mo) and/or 2-hour postload glucose at least 200 mg/dL (measured annually). We also conducted post-hoc analyses using a diabetes diagnosis based on glycosylated hemoglobin (HbA1c) at least 6.5% (11), measured every 6 months. The primary outcome evaluated was time to diabetes diagnosis. In the DPP the median time of followup was 3.0 years; maximum, 5.0 years.
Sex hormone measurements
Blood samples for sex hormone measurements were collected fasting, before 1000 h, at random with regard to any endogenous hormonal cycle or exogenous hormone exposure. Sex hormones were measured by Endoceutics. SHBG was measured using an ELISA (Bioline) with interassay coefficients of variation of 7.8% and 5.0% at 18.2 and 63.1 nmol/L, respectively. Sex steroids were measured using gas chromatography/mass spectrometry (GC/MS) (12). The lower limits of detection for dehydroepiandrosterone (DHEA), dihydrotestosterone (DHT), T, estradiol, estrone, and estrone sulfate were 100, 2, 10, 0.2, 0.8, and 10 pg/mL, respectively, and 20 ng/mL for DHEA sulfate. The lower limits of quantification for DHEA, DHT, T, estradiol, estrone, and estrone sulfate were 500, 10, 50, 1, 4, and 50 pg/mL, respectively, and 100 ng/mL for DHEA sulfate. Interassay variation (coefficient of variation) was 10.2, 10.7, 7.0, 12.5% for DHEA, T, estradiol, and estrone, respectively at the lower limits of quantification level. Values were extrapolated below the lower limit of quantitation using Mass Hunter. In sensitivity analyses we assigned these values the steroid-specific lower limit of quantitation and found a similar pattern of results (not shown).
Genotyping
DNA genotyping was performed using the Sequenom iPLEX platform (Illumina) as we have previously done (13, 14). The mean genotyping success rate was 99.6%. We evaluated three SNPs at the SHBG locus [rs6259 (15), rs6257 (16), and rs1799941 (17)] that have been previously associated with SHBG levels and with diabetes outcomes (7, 9). All three SNPs were in Hardy-Weinberg equilibrium in each of the five DPP ethnic groups (P > .05).
Statistical methods
Parallel analyses were performed in the four prespecified groups, evaluating cross-sectional and prospective relationships within each group independently. Descriptive statistics for variables that were approximately normally distributed were reported as mean (SD); otherwise we reported median and [25th, 75th] percentile ranges. The sex hormone distributions were skewed. Therefore, Spearman partial correlations [adjusting for age, race/ethnicity, smoking, alcohol consumption and leisure activity; the latter two ascertained using the Food Frequency Questionnaire and the Modified Activity Questionnaire, respectively (10)] were performed to evaluate cross-sectional associations of sex hormones with baseline fasting glucose and 2-hour glucose. Associations of SHBG with glycemia and other metabolic variables were also assessed with Spearman partial correlations adjusted as above. Cox proportional hazards regression analyses were performed to evaluate relationships with diabetes incidence. Interactions between randomization treatment group and sex steroid level were evaluated first, and found to be nonsignificant. Subsequently, adjusted analyses were performed with treatment groups combined.
Stepwise Cox proportional hazards modeling was performed incorporating adjustments for known diabetes risk factors, including obesity (waist circumference), insulin resistance (1/fasting insulin), and β-cell function (insulinogenic index; increment in insulin from 0–30 min divided by increment in glucose from 0–30 min with the oral glucose tolerance test at baseline) (18).
Analyses of relationships of SHBG genotype with SHBG concentrations and with incident diabetes initially examined the simple univariate association, using a gene-dose model and expressing the differences per minor allele. Models were constructed adjusting for randomization arm, demographic covariates, waist circumference, and 1/fasting insulin (an estimate of insulin sensitivity).
Analyses were performed using the Statistical Analysis Software (SAS) version 9.2 (SAS Institute) and all tests were two sided with statistical significance was set at P < .05.
Results
Table 1 presents descriptive statistics by group at entry into the DPP. Among the postmenopausal women, fewer nonwhite women used hormones, and hormone users reported less alcohol use and had higher BMI, fasting glucose, insulin resistance, and β-cell function (Table 1). Sex-steroid levels differed in expected ways across groups. SHBG concentrations differed markedly by sex and hormone use, with lower levels in men (median, 38.8 nmol/L) and postmenopausal women not taking hormones (35.1 nmol/L), and higher levels in postmenopausal women taking hormones (75.3 nmol/L).
Table 1.
Baseline Characteristics and Sex Hormone Levels of Subjects Entering DPP With Measurable Sex Hormone Concentrations
| Characteristic | Men (n = 969) | Women |
P (ANOVA) | ||
|---|---|---|---|---|---|
| Premenopausal (n = 948) | Postmenopausal, On HRT (n = 551) | Postmenopausal, Not on HRT (n = 431) | |||
| Age, y | 54.0 ± 11.1 | 42.3 ± 6.0 | 56.0 ± 7.2 | 56.4 ± 9.5 | <.001 |
| Race, C/AA/H/A/NA, n | 569/151/162/77/10 | 479/210/178/29/52 | 289/70/53/14/5 | 274/167/88/13/9 | <.001 |
| Alcohol, g/d | 0.94 [0, 83] | 0 [0, 35] | 0 [0, 36] | 0 [0, 33] | <.001 |
| Leisure activity, met-h per wk | 14.8 [0, 683] | 8.0 [0, 253] | 9.5 [0, 241] | 7.9 [0, 195] | <.001 |
| Weight, kg | 98.7 ± 19.4 | 95.6 ± 21.4 | 87.2 ± 17.7 | 91.8 ± 19.4 | <.001 |
| Waist, cm | 108.1 ± 13.3 | 104.9 ± 15.5 | 100.8 ± 13.8 | 104.3 ± 14.3 | <.001 |
| BMI, kg/m2 | 32.1 ± 5.6 | 35.9 ± 7.2 | 33.2 ± 6.5 | 34.9 ± 6.7 | <.001 |
| FPG, mg/dL | 108 [102, 114] | 104 [100, 111] | 103 [99, 108] | 105 [101, 111] | <.001 |
| 2hG, mg/dL | 162 [149, 177] | 162 [150, 178] | 165 [151.00, 180] | 162 [149, 178] | .384 |
| 1/FI, mL/μU | 0.04 [0.03, 0.06] | 0.04 [0.03, 0.06] | 0.05 [0.03, 0.07] | 0.04 [0.03, 0.06] | <.001 |
| IGI, μU · dL/mg | 99.0 [58.6, 153.8] | 109.1 [72.7, 164.4] | 98.6 [66.7, 143.8] | 102.4 [67.1, 157.6] | <.001 |
| DHEA, pg/mL | 3068 [2461, 4103] | 2080 [1395, 3052] | 1390 [920, 2140] | 1640 [1050, 2429] | <.001 |
| DHEA-S, ng/mL | 999 [603, 1551] | 866 [556, 1234] | 515 [305, 794] | 594 [365, 914] | <.001 |
| Estrone, pg/mL | 38.2 [31.5, 47.4] | 86.6 [54.4, 129.7] | |||
| Estrone-S, pg/mL | 630 [427, 918] | 1179 [593, 2182] | 1359 [640, 2577] | 419 [209, 761] | <.001 |
| Estradiol, pg/mL | 22.3 [18.2, 27.3] | 53.5 [23.1, 10] | 17.5 [11.9, 27.2] | 9.4 [6.0, 17.8] | <.001 |
| T, pg/mL | 10 953 [8579, 13 658] | 611 [462, 864] | 520 [347, 728] | 514 [347, 729] | <.001 |
| DHT, pg/mL | 234.32 [169.25, 317.45] | ||||
| SHBG, nmol/L | 38.8 [26.4, 55.8] | 44.1 [30.4, 68.3] | 75.3 [43.9, 121.3] | 35.1 [25.7, 46.5] | <.001 |
Abbreviations: 2hG, 2-h post-challenge glucose; A, Asian American; AA, African American; C, Caucasian; FI, fasting insulin; FPG, fasting plasma glucose; H, Hispanic American; HRT, hormone replacement therapy; IGI, insulinogenic index; NA, Native American; -S, sulfate.
Results are presented as mean ± sd for data that were normally distributed, or median [25th, 75th percentile] for nonnormally distributed data.
The sample sizes differ slightly for each individual measurement; the minimum N for a given cell is 369 for T in postmenopausal women on HRT, 491 for T in postmenopausal women not on HRT, 934 for estradiol in premenopausal women, and 615 for DHEA in men.
Empty cells indicate too few evaluable samples (estrone in postmenopausal women), or measurement not performed (DHT in women).
The statistical testing evaluated the means (or medians) across all four groups by ANOVA or the corresponding nonparametric test.
Cross-sectional associations between sex hormones, glucose, and diabetes risk factors
Overall, steroid sex hormones did not relate to glucose concentrations at study baseline in the women in these analyses (Table 2). Exceptions were an association of DHEA sulfate with fasting glucose in postmenopausal women not taking exogenous hormones and of estrone sulfate with 2-hour glucose in premenopausal women. In men, estrone, T, and DHT were associated with fasting glucose, and estrone sulfate was associated with 2-hour glucose. Estrone, estrone sulfate, T (all subjects combined), and DHT (men only) were associated with BMI, waist circumference, and insulinogenic index after similar adjustments (Supplemental Table 2). DHT and estrone were associated with waist circumference and with inverse fasting insulin (Supplemental Table 2).
Table 2.
Correlations Relating Sex Hormones and Glycemia
| Hormone | Men n = 917 |
Women |
Women |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal (n = 914) |
Postmenopausal, Not on HRT (n = 424) |
Postmenopausal, On HRT (n = 312) |
||||||||||
| FPG | 2hG | SHBG | FPG | 2hG | SHBG | FPG | 2hG | SHBG | FPG | 2hG | SHBG | |
| DHEA | 0.04 (0.28) | 0.02 (0.60) | 0.08 (0.05) | −0.03 (0.36) | 0.01 (0.88) | −0.10 (0.002) | −0.04 (0.36) | 0.06 (0.24) | 0.02 (0.67) | −0.07 (0.22) | −0.08 (0.17) | −0.07 (0.24) |
| DHEA-S | 0.03 (0.31) | 0.01 (0.76) | −0.09 (0.005) | −0.04 (0.27) | 0.06 (0.08) | −0.06 (0.06) | −0.11 (0.03) | 0.08 (0.11) | −0.04 (0.42) | −0.04 (0.460 | −0.06 (0.31) | −0.15 (0.007) |
| Estrone | 0.05 (0.16) | 0.06 (0.06) | 0.07 (0.03) | −0.02 (0.62) | 0.03 (0.48) | 0.09 (0.02) | ||||||
| Estrone-S | 0.01 (0.80) | 0.07 (0.03) | −0.11 (0.001) | −0.01 (0.85) | 0.08 (0.01) | −0.11 (0.0007) | −0.06 (0.25) | 0.08 (0.10) | −0.04 (0.43) | 0.02 (0.67) | 0.04 (0.49) | 0.16 (0.005) |
| Estradiol | 0.03 (0.44) | 0.010 (.78) | 0.23 (<0.0001) | 0.01 (0.76) | 0.00 (0.99) | 0.00 (0.88) | −0.08 (0.11) | 0.01 (0.90) | 0.12 (0.01) | −0.01 (0.84) | 0.04 (0.43) | 0.16 (0.006) |
| T | −0.09 (0.004) | −0.06 (0.09) | 0.50 (<0.0001) | −0.04 (0.18) | 0.06 (0.06) | 0.14 (<0.0001) | −0.07 (0.16) | −0.04 (0.42) | 0.25 (<0.0001) | −0.04 (0.51) | 0.01 (0.80) | 0.11 (0.05) |
| DHT | −0.11 (0.001) | −0.06 (0.07) | 0.48 (<0.0001) | |||||||||
| SHBG | −0.06 (0.07) | −0.03 (0.31) | −0.16 (<0.0001) | −0.04 (0.27) | 0.06 (0.25) | −0.09 (0.07) | −0.22 (<0.0001) | 0.08 (0.16) | ||||
Abbreviations: 2hG, 2-h post-challenge glucose; FPG, fasting plasma glucose; HRT, hormone replacement therapy; -S, sulfate.
Results are presented as ρ (p).
Cells present partial Spearman correlations after adjustment for age, race, smoking status, alcohol consumption, and physical activity at the time of enrollment into DPP.
Statistically significant results are presented in boldface for convenience.
Analyte units are as presented in Table 1.
SHBG was significantly associated with fasting glucose in premenopausal women and in postmenopausal women taking hormones, but not in the other groups (Table 2). SHBG was not related to 2-hour glucose in any subgroup (Table 2 and Supplemental Table 1). SHBG was associated with waist circumference (Spearman r = −0.15; P < .0001) and with inverse fasting insulin in all subjects combined (r = +0.18; P < .0001; Supplemental Table 2). SHBG was also inversely associated with the insulinogenic index in all subjects combined (r = −0.12; P < .0001; Supplemental Table 2).
Estrone, estrone sulfate, DHEA, and T were related to SHBG concentrations in premenopausal women (Table 2). SHBG, estradiol, and T were associated in postmenopausal women not taking hormones, and SHBG was associated with DHEA sulfate, estrone sulfate, and estradiol in postmenopausal women taking hormones. In men all measured sex hormones except DHEA were associated with SHBG.
Prospective relationships with diabetes incidence
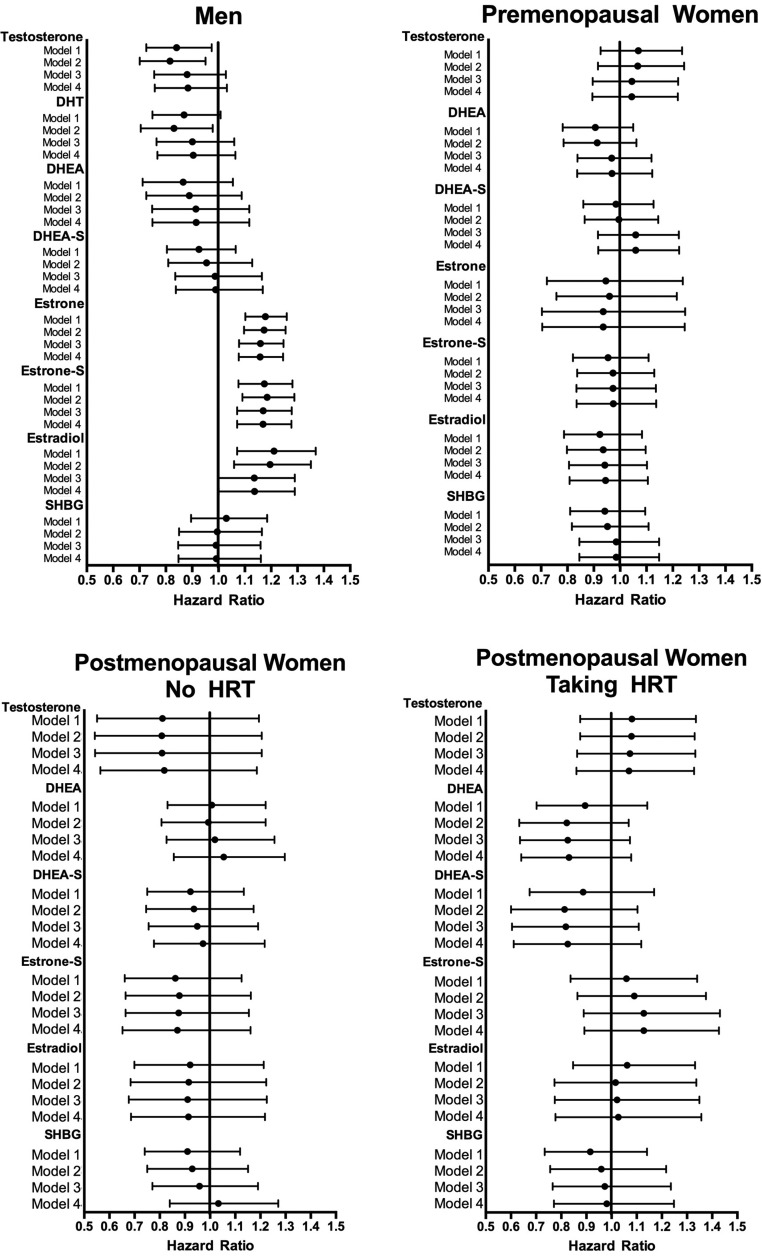
The cumulative incidence of diabetes at the end of DPP within each study group as defined in the current analyses was 23% (men), 23% (premenopausal women), 22% (postmenopausal women not taking hormones), and 19% (postmenopausal women taking hormones) (P = .31 comparing the four groups). Steroid sex hormones were not associated with diabetes risk in any group of women in our population (Figure 2). In analyses adjusted only for treatment arm in men, diabetes risk was directly associated with estrone (hazard ratio [HR], 1.101; 95% confidence interval [CI], 1.059–1.145), estrone sulfate (HR, 1.003; 95% CI, 1.001–1.005), and estradiol (HR, 1.130 95 % CI, 10.45–1.221), and diabetes risk was inversely associated with T (HR, 0.996; 0.993–0.999). Demographic adjustments brought out an inverse association with DHT in men (Model 2: HR, 0.986; 95% CI, 0.974–0.998) that did not survive further adjustments. The associations with estrogens but not T persisted after adjustment for waist circumference, insulin resistance and β-cell function (Model 4: estrone HR, 1.081 95% CI, 1.038–1.125; estrone sulfate HR, 1.003 95% CI. 1.001–1.005; estradiol HR, 1.094 95% CI, 1.011–1.184; T HR, 0.997; 95% CI, 0.993–1.000).
Figure 2.
Cox proportional hazards modeling of the relationships between sex hormones and diabetes incidence in the DPP. Model 1 is adjusted for treatment arm, without adjustment for demographic, anthropometric, or metabolic factors. Model 2 is adjusted for age, race/ethnicity, smoking, alcohol consumption, and leisure activity. Model 3 is further adjusted for waist circumference. Model 4 is further adjusted for inverse fasting insulin and the insulinogenic index. For each model, the midpoint symbol indicates the HR point estimate, and the line extends to the lower and upper 95% CIs for the estimate. Lines that do not cross the line of unity achieve statistical significance. HRs are expressed per SD difference of each variable within the treatment group.
SHBG concentration was not associated with diabetes risk in any of the models, in men (HR = 1.03 per SD difference in SHBG in models adjusting only for treatment group assignment, P = .68), or in women (HR = 0.910–0.942; P = .37–0.44) (Figure 2). We undertook post-hoc testing using SHBG quartiles (defined within each population subgroup), and found no differences in diabetes incidence by quartile trend, in any of the models. An inconsistent effect of increased risk in the lowest quartile of SHBG concentrations was seen in men, reaching significance in models with demographic adjustments (HR, 0.64; 95% CI, 0.42–0.95 comparing second vs first quartile; P = .02) and with full adjustments (HR, 0.61; 95% CI, 0.41–0.93; P = .02) (not shown). Comparisons of other quartiles against the first quartile did not show this same pattern in men. In women pairwise comparisons among the quartiles did not reveal any diabetes risk effect (not shown).
Among participants who had HbA1c less than or equal to 6.5% at baseline (n = 2516), time to progression to HbA1c greater than 6.5% was evaluated as an alternate definition of diabetes (11). Here the point estimates of the HRs, 0.97; 95% CI, 0.79–1.19 per SD difference in SHBG (P = .77) in men, HR, 0.81; 95% CI, 0.65–1.01 (P = .06) in premenopausal women, HR, 1.06; 95% CI, 0.89–1.27 (P = .52) in postmenopausal women not using hormones and HR, 0.66; 95% CI, 0.47–0.93 (P = .02) in postmenopausal women taking hormones (results presented for models adjusted only for treatment group). In this latter group, the point estimate was essentially unaffected by sequential adjustments, although the significance of the relationship was lost with full adjustment (not shown).
Three SHBG SNPs were analyzed for relationships with SHBG concentrations, and with diabetes incidence (rs6259: minor allele frequencies, 8.4% for the A allele; rs6257: 7.7%, C allele; and rs179941: 18.5%, A allele). The distribution of these SNPs differed significantly by race/ethnicity (all P < .0001; Supplemental Table 3). Distributions did not differ across groups for rs6259 (P = .41) and rs6257 (P = .81). The distribution of rs179941 differed across the groups (P = .048), with a lower minor allele frequency among postmenopausal women taking hormones (15.97%) than the other groups (18.39–20.95%).
In premenopausal women and postmenopausal women not taking hormones, the rs1799941 SNP was associated with SHBG concentrations (Table 3), but rs6259 and rs6257 were not associated with SHBG concentrations in any group of women. In men, rs6259 and rs1799941 were associated with SHBG concentrations, with persisting effects after adjustments.
Table 3.
Associations of SHBG Single-Nucleotide Polymorphisms With Log-Transformed SHBG Concentrations and With Diabetes Outcomes in the DPP Population
| Model | Association With SHBG Concentrations |
Association With DM Outcomes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6259 |
rs6257 |
rs1799941 |
rs6259 |
rs6257 |
rs1799941 |
|||||||
| β | P | β | P | β | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Men | ||||||||||||
| Baseline | 0.17 | .0002 | −0.07 | .17 | 0.06 | .08 | 1.21 (0.86–1.69) | .28 | 1.27 (0.91–1.78) | .16 | 0.98 (0.76–1.27) | .88 |
| Model 1 | 0.15 | .0004 | −0.08 | .08 | 0.08 | .007 | 1.21 (0.86–1.71) | .27 | 1.30 (0.93–1.84) | .13 | 0.95 (0.73–1.25) | .72 |
| Model 2 | 0.10 | .005 | −0.04 | .27 | 0.06 | .04 | 1.24 (0.87–1.78) | .24 | 1.26 (0.89–1.78) | .19 | 0.98 (0.75–1.29) | .89 |
| Model 3 | 0.10 | .01 | −0.05 | .22 | 0.06 | .04 | 1.21 (0.84–1.74) | .30 | 1.23 (0.87–1.73) | .25 | 0.99 (0.75–1.29) | .91 |
| Model 4 | 0.10 | .01 | −0.05 | .22 | 0.06 | .04 | 1.27 (0.88–1.83) | .20 | 1.24 (0.87–1.76) | .24 | 0.99 (0.76–1.31) | .97 |
| Women | ||||||||||||
| Premenopausal | ||||||||||||
| Baseline | 0.03 | .58 | −0.11 | .06 | 0.05 | .18 | 0.89 (0.61–1.29) | .53 | 1.04 (0.72–1.50) | .85 | 0.76 (0.58–0.99) | .04 |
| Model 1 | 0.05 | .35 | −0.08 | .16 | 0.09 | .03 | 0.912 (0.62–1.34) | .65 | 1.04 (0.71–1.50) | .86 | 0.80 (0.60–1.06) | .12 |
| Model 2 | 0.06 | .30 | −0.09 | .14 | 0.09 | .03 | 0.95 (0.64–1.39) | .77 | 1.02 (0.70–1.49) | .91 | 0.81 (0.61–1.08) | .15 |
| Model 3 | 0.06 | .28 | −0.07 | .20 | 0.08 | .04 | 0.94 (0.64–1.38) | .73 | 1.02 (0.69–1.48) | .94 | 0.82 (0.62–1.09) | .17 |
| Model 4 | 0.06 | .28 | −0.07 | .20 | 0.08 | .046 | 1.000 (0.67–1.48) | .99 | 1.04 (0.71–1.52) | .84 | 0.80 (0.60–1.05) | .11 |
| Postmenopausal, not on HRT | ||||||||||||
| Baseline | −0.01 | .87 | 0.02 | .71 | 0.08 | .07 | 0.84 (0.48–1.49) | .54 | 1.09 (0.67–1.79) | .72 | 0.93 (0.65–1.32) | .68 |
| Model 1 | 0.02 | .78 | 0.03 | .58 | 0.12 | .005 | 0.83 (0.47–1.46) | .51 | 0.94 (0.55–1.58) | .81 | 0.87 (0.60–1.27) | .48 |
| Model 2 | −0.01 | .83 | 0.05 | .44 | 0.10 | .03 | 0.90 (0.50–1.63) | .74 | 1.08 (0.64–1.82) | .78 | 0.88 (0.60–1.28) | .49 |
| Model 3 | −0.01 | .86 | 0.02 | .76 | 0.09 | .03 | 0.87 (0.48–1.56) | .64 | 1.17 (0.68–1.99) | .57 | 0.87 (0.59–1.26) | .45 |
| Model 4 | −0.03 | .67 | 0.002 | .97 | 0.07 | .08 | 0.91 (0.51–1.64) | .76 | 1.03 (0.59–1.81) | .91 | 0.87 (0.60–1.27) | .47 |
| Postmenopausal, on HRT | ||||||||||||
| Baseline | 0.00 | .97 | −0.02 | .82 | 0.06 | .34 | 1.12 (0.66–1.90) | .69 | 1.37 (0.82–2.31) | .23 | 0.88 (0.59–1.32) | .54 |
| Model 1 | 0.00 | 1.00 | −0.02 | .85 | 0.05 | .40 | 1.16 (0.67–1.20) | .61 | 1.34 (0.79–2.26) | .28 | 0.91 (0.59–1.39) | .65 |
| Model 2 | −0.05 | .61 | 0.06 | .55 | 0.04 | .51 | 1.21 (0.66–2.23) | .54 | 1.75 (0.94–3.29) | .08 | 0.94 (0.59–1.49) | .78 |
| Model 3 | −0.03 | .71 | 0.06 | .57 | 0.05 | .40 | 1.21 (0.66–2.23) | .53 | 1.73 (0.92–3.23) | .09 | 0.93 (0.59–1.49) | .77 |
| Model 4 | −0.02 | .84 | 0.06 | .56 | 0.05 | .42 | 1.19 (0.64–2.21) | .59 | 1.79 (0.96–3.35) | .07 | 0.91 (0.57–1.46) | .69 |
Abbreviation: HRT, hormone replacement therapy.
The increment in log-SHBG concentration per minor allele is interpretable as an exponential multiplier relative to the group-specific mean (eg, β of +0.17 = e[+0.17]-fold difference in SHBG per allele). The increment in diabetes hazard is expressed per minor allele.
The baseline model includes adjustment for randomized treatment only.
Model 1, adjusted for age, race/ethnicity, smoking, alcohol consumption, and leisure activity. Model 2 is further adjusted for T and estradiol. Model 3 is further adjusted for waist circumference. Model 4 is further adjusted for inverse fasting insulin and the insulinogenic index.
Statistically significant results are bolded for convenience.
The rs1799941 SNP was associated with diabetes risk, with borderline significance in premenopausal women in unadjusted analyses (HR = 0.76 per allele, P = .04) that did not persist after adjusting for baseline covariates.
Discussion
We evaluated the associations of SHBG and sex steroids with diabetes risk factors and with diabetes incidence in the DPP, an overweight or obese glucose-intolerant population. Among men, higher estrogen and lower T were associated with increased diabetes risk. Among women, sex steroids were not associated with diabetes risk. SHBG and SHBG gene variants were not associated with diabetes incidence in men or women.
The cross-sectional relationships of sex steroids and SHBG with glycemic and metabolic variables were overall as previously reported in other populations (19–23), except for associations of sex steroids with fasting and 2-hour postchallenge glucose readings, which were evident for androgens in men, but not evident overall for estrogens in men or in women. These observations may reflect the population definition, which was based in part on glycemia. However, this explanation should arguably apply also to the relationship of sex hormones with BMI, which was clearly evident in our population. Our overall interpretation is that the biology relating sex steroids and SHBG to dysmetabolism was evident in this population, as previously described in groups with broader distributions of glycemia and weight.
Sex steroids and diabetes incidence
Using high-sensitivity GC/MS methods for measuring steroid hormone concentrations, we observed associations of sex steroids with diabetes risk in men, but not in any group of women in this cohort. These estimates are likely superior to those related to measures of sex steroids (particularly estrone, estrone sulfate, and estradiol) using older methods, as the sensitivity and precision at low ranges are superior with GC/MS.
In men estrone, estrone sulfate, and estradiol were directly associated with diabetes risk and T was inversely associated with diabetes risk. Such associations have been noted in prior literature (1–6, 19, 20, 24). Importantly, our observations are made in the setting of absent associations with SHBG, and are therefore free of potential confounding by differences in hormone binding. Our observations confirm the relationships of these sex steroids with diabetes risk in men even in the setting of obesity and dysglycemia.
In prior literature, menopausal and hormone status relate to diabetes risk in women, but the relationships of sex steroid concentrations with diabetes are quantitatively modest and influenced by body composition (1, 25–27). In our population we see no such relationships in any of the defined hormone status groups. These observations suggest that sex steroids and SHBG contribute little to the aggregate risk of diabetes among women with obesity and dysglycemia.
SHBG and diabetes incidence
In previously studied populations not selected for increased diabetes risk including individuals with normal weight and glucose tolerance, SHBG relates inversely to diabetes risk, and adjustments for effects of obesity and insulin sensitivity attenuate the apparent effect of SHBG (1–8, 20). In the DPP population we find no relationship of SHBG with prospective diabetes incidence. Similarly, the evaluated SHBG gene polymorphisms, associated with diabetes risk in other populations via effects on SHBG, have no relationship with diabetes incidence in the DPP.
Our results differ from prior published reports. Important differences may include features that define our population, our approach to diabetes ascertainment, and that our observations are made in the context of a prospective randomized clinical trial. On this latter point, although we see no treatment group difference in the relationships of SHBG with diabetes incidence, this does not exclude an unmeasured effect of participation in an active treatment study on this underlying relationship. Other differences in the strengths and weaknesses between case-control, prospective cohort, and prospective randomized trial studies may also have contributed.
The DPP participants were selected on the basis of features defining a high risk for diabetes, and it is possible that the risk relationships reflected in SHBG pertain only to stages of obesity or dysglycemia prior to those used to identify DPP participants. It is evident that obesity and insulin resistance are potential confounders, and it may be that the effect of our inclusion criteria to focus these risk factors produced different relationships than does adjustment in statistical models. Alternatively, the diabetes risk related to our defined range of glucoses may be the material difference between our population and those previously evaluated. Our current observations raise questions regarding the potency of SHBG as a risk factor in the setting of elevated risk based on dysglycemia plus overweight/obesity.
In the DPP we systematically identified diabetes at an earlier stage than is possible in population-based studies. With this approach we have previously demonstrated relationships of diabetes risk with established and novel risk factors, including confirming discoveries of genetic markers of diabetes risk (14, 18, 28–31). These observations suggest that the sensitive or early diabetes ascertainment within the DPP does not likely account for the absent relationship with SHBG.
On balance our observations suggest while SHBG relationships with diabetes risk are evident in unselected populations, in the setting of focused risk based on the potent risk factors of dysglycemia in overweight/obese individuals there is no evident additional effect of SHBG. This argues that elevated glucose and weight are more important than SHBG as a means of identifying diabetes risk.
Limitations
The DPP study population was selected for enrichment of diabetes risk, which might have influenced the distributions of diabetes risk factors relevant to the diabetes associations with sex hormones. Nevertheless, our population exhibited a range of SHBG and sex-steroid concentrations similar to that reported in other populations in which risk associations were seen. We stratified our population by sex menopausal status and hormone use, reducing the sample size for each set of comparisons. Nevertheless our overall sample size is on par with many studies in this literature (3, 8, 20, 26, 32–34) that have reported associations of diabetes risk with SHBG, and larger-than-case-control studies using subsamples of larger populations (6, 7, 35), suggesting that the current negative findings do not result from reduced statistical power.
Summary
In the DPP cohort we have observed expected relationships of sex steroids with glycemia, and of SHBG with physiologic phenomena underlying diabetes risk. However, relationships of sex steroids with diabetes risk were seen only in men, and SHBG did not relate to diabetes risk in any subgroup of this cohort. These observations argue that effects of SHBG on diabetes risk are modest compared with traditional diabetes risk factors among adults who are already overweight and glucose intolerant.
Acknowledgments
We thank the commitment and dedication of the participants of the Diabetes Prevention Program.
This study was registered in ClinicalTrials.gov as trial number NCT00004992.
Author contributions: K.J.M., C.K., C.A.C., S.E.K., W.C.K., and J.C.F., conceived and designed the current analyses; K.J.M., C.K., C.A.C., V.R.A., W.C.K., S.E.E., J.C.F., F.L., S.E.K., R.B.G., and E.B.-C. contributed to acquisition of data, interpretation of data and drafting of the manuscript. C.A.C. and S.E.E. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. C.A.C. conducted and is responsible for data analysis. K.J.M., C.K., C.A.C., V.R.A., W.C.K., S.E.E., J.C.F., F.L., S.E.K., R.B.G., and E.B.-C. provided final approval of the manuscript and acknowledge that they are accountable for all aspects of the work.
This work was supported by National Institutes of Health/National Institute of Diabetes, Digestive and Kidney Disorders, National Institute of Child Health and Human Development, National Institute of Aging, Office of Research in Minority Health and Health Disparities, Office of Research in Womens' Health Indian Health Service, Centers for Disease Control Clinical Genetics Research Program, National Center for Research Resources, American Diabetes Association, Bristol-Meyers Squibb, Lipha, Parke-Davis, R01 DK072041.
Disclosure Summary: F.L. is an employee of Endoceutics Inc., which provided hormone measurements on a contractual basis. K.J.M., C.K., C.A.C., V.R.A., W.C.K., S.E.E., J.C.F., S.E.K., R.B.G., and E.B.-C. have nothing to disclose.
Footnotes
- CI
- confidence interval
- DHEA
- dehydroepiandrosterone
- DPP
- Diabetes Prevention Program
- GC/MS
- gas chromatography/mass spectrometry
- HbA1c
- glycosylated hemoglobin
- HR
- hazard ratio
- HRT
- hormone replacement therapy
- SNPs
- single nucleotide polymorphisms.
References
- 1. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. [DOI] [PubMed] [Google Scholar]
- 2. Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: Results from the Third National Health and Nutrition Examination Survey (NHANES III). Diabetes Care. 2007;30(2):234–238. [DOI] [PubMed] [Google Scholar]
- 3. Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci. 2010;65(5):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atlantis E, Lange K, Martin S, et al. Testosterone and modifiable risk factors associated with diabetes in men. Maturitas. 2011;68(3):279–285. [DOI] [PubMed] [Google Scholar]
- 5. Schipf S, Haring R, Friedrich N, et al. Low total testosterone is associated with increased risk of incident type 2 diabetes mellitus in men: Results from the Study of Health in Pomerania (SHIP). Aging Male. 2011;14(3):168–175. [DOI] [PubMed] [Google Scholar]
- 6. Goto A, Morita A, Goto M, et al. Associations of sex hormone-binding globulin and testosterone with diabetes among men and women (the Saku Diabetes study): A case control study. Cardiovasc Diabetol. 2012;11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen BH, Brennan K, Goto A, et al. Sex hormone-binding globulin and risk of clinical diabetes in American black, Hispanic, and Asian/Pacific Islander postmenopausal women. Clin Chem. 2012;58(10):1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perry JR, Weedon MN, Langenberg C, et al. Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet. 2010;19(3):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diabetes Prevention Program Research Group. HbA1c as a predictor of diabetes and as an outcome in the diabetes prevention program: A randomized clinical trial. Diabetes care. 2015;38(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ke Y, Bertin J, Gonthier R, Simard JN, Labrie F. A sensitive, simple and robust LC-MS/MS method for the simultaneous quantification of seven androgen- and estrogen-related steroids in postmenopausal serum. J Steroid Biochem Mol Biol. 2014;144(Pt B):523–534. [DOI] [PubMed] [Google Scholar]
- 13. McCaffery JM, Jablonski KA, Franks PW, et al. TCF7L2 polymorphism, weight loss and proinsulin:insulin ratio in the diabetes prevention program. PloS One. 2011;6(7):e21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355(3):241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Power SG, Bocchinfuso WP, Pallesen M, Warmels-Rodenhiser S, Van Baelen H, Hammond GL. Molecular analyses of a human sex hormone-binding globulin variant: Evidence for an additional carbohydrate chain. J Clin Endocrinol Metab. 1992;75(4):1066–1070. [DOI] [PubMed] [Google Scholar]
- 16. Hardy DO, Cariño C, Catterall JF, Larrea F. Molecular characterization of a genetic variant of the steroid hormone-binding globulin gene in heterozygous subjects. J Clin Endocrinol Metab. 1995;80(4):1253–1256. [DOI] [PubMed] [Google Scholar]
- 17. Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol Cell Endocrinol. 2010;316(1):60–65. [DOI] [PubMed] [Google Scholar]
- 18. Kitabchi AE, Temprosa M, Knowler WC, et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes. 2005;54(8):2404–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brand JS, Wareham NJ, Dowsett M, et al. Associations of endogenous testosterone and SHBG with glycated haemoglobin in middle-aged and older men. Clin Endocrinol. 2011;74(5):572–578. [DOI] [PubMed] [Google Scholar]
- 20. Colangelo LA, Ouyang P, Liu K, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: Multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(6):1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: A population-based study. Eur J Endocrinol. 2003;149(6):601–608. [DOI] [PubMed] [Google Scholar]
- 22. Goodman-Gruen D, Barrett-Connor E. Sex hormone-binding globulin and glucose tolerance in postmenopausal women. The Rancho Bernardo Study. Diabetes Care. 1997;20(4):645–649. [DOI] [PubMed] [Google Scholar]
- 23. Haffner SM, Dunn JF, Katz MS. Relationship of sex hormone-binding globulin to lipid, lipoprotein, glucose, and insulin concentrations in postmenopausal women. Metabolism. 1992;41(3):278–284. [DOI] [PubMed] [Google Scholar]
- 24. Cao J, Li J, Hao W, et al. Correlation of sex hormone and androgen receptor with diabetes mellitus in elderly men. Aging Male. 2011;14(3):162–167. [DOI] [PubMed] [Google Scholar]
- 25. Krentz AJ, von Mühlen D, Barrett-Connor E. Adipocytokines, sex hormones, and cardiovascular risk factors in postmenopausal women: Factor analysis of the Rancho Bernardo study. Horm Metab Res. 2009;41(10):773–777. [DOI] [PubMed] [Google Scholar]
- 26. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94(11):4127–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Genugten RE, Utzschneider KM, Tong J, et al. Effects of sex and hormone replacement therapy use on the prevalence of isolated impaired fasting glucose and isolated impaired glucose tolerance in subjects with a family history of type 2 diabetes. Diabetes. 2006;55(12):3529–3535. [DOI] [PubMed] [Google Scholar]
- 28. Mather KJ, Funahashi T, Matsuzawa Y, et al. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008;57(4):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldberg RB, Temprosa MG, Mather KJ, et al. Lifestyle and metformin interventions have a durable effect to lower CRP and tPA levels in the diabetes prevention program except in those who develop diabetes. Diabetes Care. 2014;37(8):2253–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Billings LK, Jablonski KA, Ackerman RJ, et al. The influence of rare genetic variation in SLC30A8 on diabetes incidence and β-cell function. J Clin Endocrinol Metab. 2014;99(5):E926–E930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hivert MF, Jablonski KA, Perreault L, et al. Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60(4):1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Huang X, Liao M, et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: Results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes Metab Res Rev. 2013;29(5):391–397. [DOI] [PubMed] [Google Scholar]
- 33. Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: Prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23(4):490–494. [DOI] [PubMed] [Google Scholar]
- 34. Lindstedt G, Lundberg PA, Lapidus L, Lundgren H, Bengtsson C, Björntorp P. Low sex-hormone-binding globulin concentration as independent risk factor for development of NIDDM. 12-yr follow-up of population study of women in Gothenburg, Sweden. Diabetes. 1991;40(1):123–128. [DOI] [PubMed] [Google Scholar]
- 35. Bonnet F, Balkau B, Malécot JM, et al. Sex hormone-binding globulin predicts the incidence of hyperglycemia in women: Interactions with adiponectin levels. Eur J Endocrinol. 2009;161(1):81–85. [DOI] [PubMed] [Google Scholar]