Abstract
Context:
Rapid bone accrual and calcium demands during puberty may result in compensatory increases in PTH and 1,25-dihydroxyvitamin D [1,25(OH)2D] levels; however, these relations have not been established in longitudinal studies.
Objective:
To determine whether greater bone accrual velocity is associated with greater PTH and 1,25(OH)2D levels in healthy children and adolescents.
Design:
Prospective cohort study with baseline PTH, 25-hydroxyvitamin D [25(OH)D], and 1,25(OH)2D levels and dual-energy x-ray absorptiometry whole-body bone mineral content (BMC) accrual over 12 months. Secondary analyses examined bone biomarkers and tibia quantitative computed tomography midshaft cortical-BMC.
Participants:
A total of 594 healthy participants, ages 5–21 years, with longitudinal measures in a subset of 145 participants.
Main Outcome Measures:
PTH and 1,25(OH)2D levels.
Results:
PTH levels were higher during Tanner stages 3 and 4 compared to Tanner 1 (P < .05) in males and females and were inversely and significantly associated with 25(OH)D levels and dietary calcium intake. In multivariable analyses, greater bone accrual [measured directly as change in dual-energy x-ray absorptiometry-BMC (P < .001) or quantitative computed tomography-BMC (P < .05), or indirectly as growth velocity (P < .05) or greater bone-formation biomarker level (P < .01)] was associated with higher PTH levels, independent of 25(OH)D level and dietary calcium intake. Similar associations were observed between these direct and indirect indices of bone accrual and 1,25(OH)2D levels.
Conclusions:
PTH levels rise in midpuberty, in association with multiple measures of bone accrual. This is consistent with compensatory increases in PTH to drive 1,25(OH)2D production and calcium absorption during periods of increased calcium demands. Additional studies are needed to address PTH effects on bone modeling and remodeling during growth and development.
Puberty marks a time of rapid linear growth and bone accrual (1, 2). Approximately 40% of whole-body bone mineral content (BMC) is accrued during the circumpubertal years in males and females (3). This rapid increase in BMC depends on the availability of calcium and phosphate from the systemic circulation. The parathyroid gland responds to lower serum calcium concentrations by increasing synthesis and release of PTH. In turn, PTH increases calcium reabsorption and phosphate excretion in the kidney and increases the activity of the renal 1-α-hydroxylase to convert 25-hydroxyvitamin D [25(OH)D] to 1,25-dihydroxyvitamin D [1,25(OH)2D]. Higher 1,25(OH)2D levels promote gastrointestinal absorption and may promote renal retention of calcium and phosphate based on preclinical studies (4, 5).
Vitamin D deficiency results in compensatory increases in PTH levels as a consequence of low calcium levels. The inverse association between PTH and 25(OH)D levels is well established in children and adults (6–9). A cross-sectional study of 944 adults in Iceland addressed the relative impact of dietary calcium intake and vitamin D levels on PTH levels; a lower calcium intake (<800 mg/d) was associated with higher PTH levels only among individuals with 25(OH)D levels <10 ng/mL (10). Ionized calcium levels were not associated with calcium intake, suggesting that compensatory increases in PTH, and presumably 1,25(OH)2D levels, served to maintain calcium levels.
Hyperparathyroidism contributes to bone loss in older adults (11), largely through decreases in cortical bone mineral density (BMD) and thickness (12). However, the relations between calcium requirements and vitamin D and PTH metabolism are more complex during growth. A recent cross-sectional study in early-pubertal children demonstrated that black children had higher PTH and 1,25(OH)2D levels and greater total cortical bone area, compared with white children (9). The authors hypothesized that the significantly higher PTH levels observed in the black children may have exerted an anabolic effect on cortical bone, contributing to their significantly greater total bone area. An alternative explanation is that greater calcium requirements due to accrual of larger cortical bone, combined with lower 25(OH)D levels, resulted in greater secondary hyperparathyroidism and greater 1,25(OH)2D levels in the black children. Abrams et al (13) conducted a calcium absorption study using stable isotopes in adolescents and demonstrated that calcium absorption was positively associated with serum 1,25(OH)2D and PTH levels but was not associated with 25(OH)D levels. Furthermore, PTH levels were positively associated with osteocalcin levels (a biomarker of bone formation) but were not associated with N-telopeptide (a biomarker of bone resorption). The authors concluded that the independent associations of serum osteocalcin and 25(OH)D with PTH levels indicated that rapid bone formation during midpuberty and low 25(OH)D levels both contributed to a rise in PTH in order to increase 1,25(OH)2D levels and meet calcium needs. The measures of vitamin D and mineral metabolism were not associated with dual-energy x-ray absorptiometry (DXA) BMC in that cross-sectional analysis. To our knowledge, no studies have examined the relations of rates of bone mineral accrual with PTH, 25(OH)D, and 1,25(OH)2D levels in children and adolescents.
The objective of this study was to determine whether greater whole-body BMC accrual velocity, as measured by DXA, was associated with greater PTH and 1,25(OH)2D levels (as the study outcomes), independent of 25(OH)D levels and dietary calcium intake. Secondary analyses considered peripheral quantitative computed tomography (pQCT) cortical BMC and BMD accrual velocity, height velocity, and biomarkers of bone formation as additional indices of bone accrual.
Subjects and Methods
Study subjects
A total of 920 children and adolescents, ages 5 to 21 years, were enrolled as healthy control subjects for multiple concurrent bone studies at the Children's Hospital of Philadelphia (CHOP), as previously described (14). All data collection mechanisms were identical across the entire cohort with the exception of dietary calcium data, which were not collected in participants more than 18 years of age. Participants were recruited from general pediatric clinics and from the community using advertisements. Exclusion criteria included chronic diseases or medications known to affect growth, nutrition, or bone health. Use of mineral or vitamin supplements was not an exclusion criterion. Nonfasting laboratory studies were obtained at the baseline visit and were optional; 594 participants provided specimens. The participants that declined phlebotomy were significantly younger, on average, than those that provided specimens (11.4 vs 12.2 y); other demographic variables did not differ.
A total of 300 of the participants were recruited for a longitudinal substudy with repeat anthropometry, DXA, and pQCT scans after 6 and 12 months (15). Of these, 210 provided blood specimens. Similarly, the participants that declined phlebotomy were significantly younger than those that provided specimens (11.4 vs 12.9 y) (15). The study presented here includes cross-sectional analyses in the larger sample of 594 participants and longitudinal analyses in the 145 participants with baseline laboratory results and DXA and pQCT results at 12 months.
The protocols were approved by the Institutional Review Board at CHOP. Informed consent was obtained from young adult participants and the parents or guardians of those younger than 18 years of age. Assent was obtained from those younger than 18 years.
Assessment of anthropometrics and pubertal development
Weight and height were measured using a digital scale (Scaltronix) and stadiometer (Holtain Ltd), respectively. Pubertal development was determined using a validated self-assessment questionnaire, with parental assistance as necessary (16). Tanner stage was assigned based on breast development for females and genital development for males. Participants self-identified race according to the National Institute of Health categories.
DXA scans
Whole-body DXA scans were obtained at each visit with a Hologic Delphi densitometer (software version 12.4; Hologic). The measurements were obtained with standard positioning techniques and were analyzed to generate estimates of whole body less head (subtotal) BMC (g). The DXA instrument was calibrated using a hydroxyapatite spine phantom daily and with a whole body phantom three times per week. The in vitro coefficient of variation (CV) at our institution is <0.6%, and the in vivo CV is <1%.
pQCT scans
Tibia bone outcomes were measured by pQCT using a Stratec XCT-2000 device (Orthometrix, Inc) with a 12-detector unit, 0.4-mm voxel size, 2.3-mm slice thickness, 25 mm/s scan speed, and software version 5.5, as previously described (17). Tibia length was measured with a sliding caliper (Rosscraft). A scout view was obtained to guide placement of the reference line at the medial proximal border of the distal growth plate in participants with open growth plates and at the medial proximal border of the endplate in participants with fused growth plates. Cortical BMC (mg) and volumetric BMD (mg/cm3) were assessed in the diaphysis at 38% of tibia length proximal to the reference line. The CV was <2% for these outcomes in children and adolescents.
Laboratory tests
Serum and a second morning urine specimen were stored in aliquots at −70°C and shipped in batches to Quest Diagnostics' Nichols Institute for analysis of serum intact PTH using the Nichols chemiluminescence assay (CV, 7–9%), serum bone-specific alkaline phosphatase (BSAP; μg/L) using a two-site immunoradiometric assay (CV, 8.5%), and the urine ratio of deoxypyridinoline (DPD) to creatinine (nmol/mmol creatinine) using HPLC (CV, 6–8%). BSAP was chosen as a biomarker of bone formation because of its high bone specificity, specimen stability, and lack of circadian variability (18, 19). Urine DPD was chosen as the biomarker of bone resorption of its long-term stability in urine and osteoclast specificity (18, 19). 25(OH)D and 1,25(OH)2D were quantified by RIA with I125-labeled tracer; the interassay CV ranged from 2–9% (20).
The relations among baseline bone biomarkers and bone accrual have been reported (15). Briefly, greater linear growth and whole-body bone mineral accrual velocity were significantly associated with greater BSAP (as μg/L) and DPD levels (as nmol/mmol creatinine). Height velocity alone explained 68 and 58% of the variability in BSAP and DPD levels, respectively.
The longitudinal substudy used the vitamin D assays described above in all participants that provided blood samples. The larger protocol used the Nichols vitamin D assays, which changed over the course of the study; therefore, vitamin D levels are not available in all participants with PTH levels. Serum 25(OH)D levels were reported in a subset of the larger study, showing that levels were significantly lower in older children and adolescents, black participants (compared with non-black), those enrolled during winter months, and those with lower dietary vitamin D intake (8).
Dietary calcium intake
Dietary calcium intake was assessed using three separate 24-hour dietary recalls, administered by interview with a research dietitian on two separate weekdays and one weekend day. On each occasion, study participants were asked to recall and describe the kinds and amounts of all foods consumed during the preceding full day. Data were analyzed by the Minnesota Nutritional Data System (21).
Analysis plan
Statistical analyses were performed using SAS version 9.4 (SAS Institute) and Stata version 13.0 (Stata Corporation) software. Two-sided tests of hypotheses were used, and a P value <.05 was considered statistically significant. Differences of means were assessed with the Student's t test when the data were normally distributed. The Wilcoxon rank-sum test was used when the data were not normally distributed. Non-normally distributed variables, including PTH, 25(OH)D, and 1,25(OH)2D levels, were natural log-transformed using the lnskew0 function in Stata. Group differences in categorical variables were assessed with the χ2 test. Pearson's correlation coefficients were used to describe relationships among the log-transformed PTH, 25(OH)D, and 1,25(OH)2D levels. Winter season was defined as November through March, inclusive. Height and body mass index (BMI) were converted to age- and sex-specific Z-scores using the National Center for Health Statistics 2000 Centers for Disease Control reference data (22).
Cross-sectional analyses of the associations between PTH levels and Tanner stage and between PTH levels and age were conducted in the larger sample of 594 participants, including multiplicative interaction terms to test for sex interactions with Tanner stage and with age. Cross-sectional analyses of serum 25(OH)D levels considered age (as a continuous variable), season, and race. All of the remaining analyses were limited to the participants in the longitudinal substudy.
PTH and 1,25(OH)2D levels were the primary outcomes in the longitudinal models. These multivariate models assessed the impact of sex, race, 25(OH)D levels, and dietary calcium intake on PTH and 1,25(OH)2D levels, along with the impact of multiple indices of bone accrual. First, DXA subtotal-BMC accrual velocity (g/y), adjusted for baseline subtotal-BMC (g), was the primary measure of bone accrual. Second, the skeleton is approximately 80% cortical bone; therefore, pQCT cortical-BMC accrual velocity (mg/y), adjusted for baseline cortical-BMC (mg), was used as an additional measure of bone mineral accrual. Third, BSAP was used as an index of bone formation.
PTH has an anabolic effect on bone. Therefore, two strategies were used to support the hypothesis that greater bone accrual resulted in higher PTH levels, rather than greater PTH levels promoting bone accrual. First, height velocity (cm/y) was used as an additional measure of bone accrual that is presumably not affected by PTH or 1,25(OH)2D levels. Second, prior studies have shown that factors that promote bone formation in children resulted in gains in cortical area but decreases in cortical BMD (23–25). Therefore, we examined associations of changes in cortical BMD with PTH levels.
Results
Participant characteristics
Table 1 summarizes participant characteristics at the baseline visit in the larger sample of 594 participants, in the 210 enrolled in the longitudinal substudy, and in the 145 that completed the 12-month visit with DXA and pQCT data. The 65 participants in the substudy that did not complete the 12-month visit were younger, and a greater proportion were black. Serum PTH, 25(OH)D, and 1,25(OH)2D levels; height and BMI Z-scores; dietary calcium intake; and laboratory parameters did not differ between those that did and did not complete the follow-up visit.
Table 1.
Participant Characteristics
| All Participants at Baseline | Longitudinal Substudy |
|||
|---|---|---|---|---|
| Participants Without 12-mo Data at Baseline | Participants With 12-mo Data |
|||
| Baseline | 12 mo | |||
| n | 594 | 210 | 145 | 145 |
| Age, y | 12.3 ± 4.0 | 13.7 ± 4.4 | 14.1 ± 4.3 | |
| Age range, y | 5.0–21.9 | 5.3–21.9 | 5.3–21.9 | |
| Female sex, n (%) | 291 (49) | 101 (48) | 71 (49) | |
| Race, n (%) | ||||
| White | 290 (49) | 133 (63) | 101 (70) | |
| Black | 242 (41) | 57 (27) | 30 (21) | |
| Other | 62 (10) | 20 (10) | 14 (9) | |
| Tanner stage, n (%) | ||||
| I | 187 (31) | 51 (24) | 29 (20) | 19 (13) |
| II | 69 (12) | 22 (11) | 16 (11) | 12 (8) |
| III | 80 (13) | 19 (9) | 7 (5) | 15 (11) |
| IV | 139 (23) | 56 (27) | 47 (33) | 35 (24) |
| V | 119 (20) | 61 (29) | 45 (31) | 64 (44) |
| Height, cm | 150.6 ± 18.9 | 155.7 ± 18.0 | 157.7 ± 16.7 | 161.2 ± 14.8c |
| Height Z-score | 0.28 ± 0.93 | 0.34 ± 0.88 | 0.36 ± 0.83 | 0.37 ± 0.82 |
| BMI, kg/m2 | 20.0 (17.5, 23.3) | 20.7 (17.6, 23.3) | 20.0 (17.6, 23.1) | 20.6 (18.3, 23.2)c |
| BMI Z-score | 0.38 ± 1.02 | 0.32 ± 1.03 | 0.27 ± 0.96 | 0.27 ± 0.98 |
| Winter season, n (%) | 214 (36) | 85 (41) | 59 (41) | |
| Calcium intake, mg/da | 799 (577, 1057) | 856 (629, 1117) | 857 (629, 1098) | |
| Laboratory studies | ||||
| PTH, pg/mL | 30 (21, 42) | 29 (22, 41) | 29 (22, 43) | |
| 25(OH)D, ng/mL | b | 23.1 (16.6, 28.0) | 23.1 (17.4, 27.9) | |
| 25(OH)D < 20 ng/mL, n (%) | b | |||
| Black race | 41 (72) | 24 (80) | ||
| Non-black race | 40 (26) | 32 (28) | ||
| 1,25(OH)2D, pg/mL | b | 39.2 (34, 47.3) | 39.3 (33.5, 46.8) | |
| BSAP, μg/L | 69.2 (31.2, 94.2) | 47.7 (16.7, 84.2) | 34.5 (16.1, 80.7) | |
| DPD, nmol/mmol creatinine | 54.8 (28.1, 71.4) | 47.0 (21.1, 66.4) | 42.5 (21.6, 65.6) | |
| Imaging studies | ||||
| DXA subtotal BMC, g | 1120 (680, 1535) | 1334 (819, 1721) | 1386 (957, 1731) | 1465 (1198, 1818)c |
| pQCT cortical BMC, mg | 256 (184, 321) | 274 (217, 333) | 275 (228, 329) | 299 (251, 356)c |
| pQCT cortical BMD, mg/cm3 | 1098 (1064, 1143) | 1115 (1067, 1162) | 1116 (1066, 1162) | 1136 (1076, 1171)c |
Data are presented as mean ± SD or median (interquartile range), unless stated otherwise.
Dietary calcium intake data available in 530 of the overall sample, 155 of the 210 participants in the substudy at baseline, and 102 of those with longitudinal data.
Vitamin D data available in only the 210 participants in the longitudinal substudy due to differences in assays.
P < .001 compared to baseline measure.
Baseline laboratory parameters
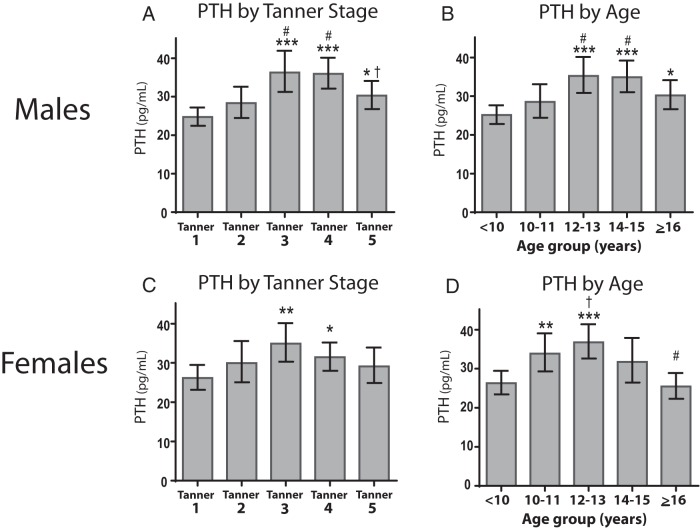
Figure 1 illustrates serum PTH levels according to sex, Tanner stage, and age range among the 594 children and adolescents. In males, PTH levels were higher in Tanner stages 3, 4, and 5, compared to Tanner 1. In females, PTH levels were higher in stages 3 and 4 compared to stage 1. In an analysis of males and females together, PTH levels were significantly higher in Tanner 3 (36 pg/mL; 95% confidence interval [CI], 32, 39; P < .0001), Tanner 4 (33 pg/mL; 95% CI, 31, 36; P < .0001), and Tanner 5 (30 pg/mL; 95% CI, 27, 33; P < .05) compared to Tanner 1 (25 pg/mL; 95% CI, 23, 27). Analysis by age revealed similar results, with the exception of females demonstrating higher levels of PTH by 10–11 years of age (as compared to < 10 y), consistent with earlier onset of puberty. The test for an interaction between sex and Tanner stage was not significant; however, the test for an interaction between sex and age was significant (P = .002) for PTH levels.
Figure 1.
Levels of PTH by sex, Tanner stage, and age. Mean levels and 95% CIs of PTH for males (A and B) and females (C and D) from unadjusted regression analysis of natural log-transformed values, back-calculated to original units. Comparison to Tanner 1: *, P < .05; **, P < .01; ***, P < .001; comparison to Tanner 2: #, P < .05; comparison to Tanner 4: †, P < .05.
The following analyses were limited to the 210 participants in the substudy. In multivariable models, serum 25(OH)D levels were independently lower in black participants and during winter months, and were inversely related to age (all P < .001), consistent with our prior study (8). The univariate relations among laboratory parameters are shown in Table 2. PTH levels were negatively associated with 25(OH)D and positively associated with 1,25(OH)2D and BSAP levels. PTH levels were not associated with DPD levels. 1,25(OH)2D levels did not differ according to sex or race. Lower dietary calcium intake was associated with higher PTH levels (R = −0.21; P < .001). Only nine (4%) of 210 participants had PTH levels above 65 pg/mL, the upper limit of the assay.
Table 2.
Univariate Correlations Among Laboratory Parameter in All Participants in the Longitudinal Substudy
| PTH | 25(OH)D | 1,25(OH)2D | BSAP | |
|---|---|---|---|---|
| 25(OH)D | R = −0.29 | |||
| P < .001 | ||||
| 1,25(OH)2D | R = 0.23 | R = 0.13 | ||
| P < .001 | P = .05 | |||
| BSAP | R = 0.11 | R = −0.01 | R = 0.32 | |
| P = .02 | P = .86 | P < .001 | ||
| DPD | R = 0.02 | R = 0.06 | R = 0.24 | R = 0.69 |
| P = .57 | P = .41 | P < .001 | P < .001 |
Results represent Pearson correlations between natural log-transformed results.
In a multivariable analysis adjusted for age, lower 25(OH)D (P < .05), greater BSAP levels (P < .01), and lower dietary calcium intake (P < .05) were significantly associated with greater PTH levels. Greater PTH (P < .001) and 25(OH)D (P < .01) levels were significantly and positively associated with 1,25(OH)2D levels. When either BSAP or DPD levels were added to the 1,25(OH)2D model, each was significantly associated with greater 1,25(OH)2D levels (P < .001); however, neither was significant when both were included.
As previously reported, there was a significant sex-by-Tanner interaction for BSAP and DPD levels (15). Among males, biomarker levels were comparable across Tanner stages 1 to 4 and significantly lower in Tanner stage 5 (P < .001). Among females, biomarker levels were comparable across Tanner stages 1 to 3, were significantly lower in Tanner stage 4 compared with earlier stages (P < .001), and were even lower in Tanner stage 5—consistent with the earlier cessation of growth in females. BSAP and DPD levels were both positively correlated with 12-month changes in height, DXA subtotal-BMC, and pQCT cortical-BMC (all R = 0.60 to 0.75; P < .001), but were not correlated with changes in pQCT cortical-BMD (R ≤ 0.10).
Associations between laboratory parameters and indices of bone accrual velocity
We examined direct (change in DXA and pQCT BMC) and indirect (baseline BSAP and change in height) measures of bone acquisition as potential correlates of PTH and 1,25(OH)2D levels. The models for PTH were adjusted for 25(OH)D levels and dietary calcium intake, based on the analyses above. Table 3 shows the four models with PTH as the outcome, demonstrating consistent and significant results with each estimate of bone accrual; greater increases in height, DXA subtotal-BMC, and pQCT cortical-BMC, and higher BSAP levels were associated with higher PTH levels. In addition, changes in pQCT cortical-BMD (P = .01) were positively associated with PTH levels, independent of 25(OH)D levels and dietary calcium intake. Table 4 summarizes the models with 1,25(OH)2D levels as the outcome. These models were adjusted for 25(OH)D levels, based on the analyses above, and showed consistent and significant results across the four separate models; greater increases in height, DXA subtotal-BMC, and pQCT cortical-BMC, and higher BSAP levels were associated with higher 1,25(OH)2D levels.
Table 3.
Regression Models With Baseline PTH as Outcome
| Coefficient | SE | P | |
|---|---|---|---|
| Change in subtotal BMC (0–12 mo), g | 0.51 | 0.14 | <.001 |
| Baseline subtotal BMCa | 0.15 | 0.07 | .05 |
| 25(OH)Da | −1.18 | 0.27 | <.001 |
| Dietary Ca intakea | −0.24 | 0.11 | .03 |
| Constant | 6.22 | 1.60 | <.001 |
| Change in cortical BMC (0–12 mo), mg | 0.34 | 0.14 | .02 |
| Baseline cortical BMCa | 0.23 | 0.10 | .03 |
| 25(OH)Da | −1.35 | 0.28 | <.001 |
| Dietary Ca intakea | −0.23 | 0.12 | .05 |
| Constant | 8.36 | 1.52 | <.001 |
| Change in height (0–12 mo), cm | 0.029 | 0.012 | .02 |
| Baseline heighta | 0.92 | 0.29 | .002 |
| 25(OH)Da | −1.37 | 0.27 | <.001 |
| Dietary Ca intakea | −0.20 | 0.12 | .09 |
| Constant | 15.19 | 1.98 | <.001 |
| Baseline BSAP, μg/La | 0.43 | 0.15 | .006 |
| 25(OH)Da | −0.74 | 0.27 | .007 |
| Dietary Ca intakea | −0.21 | 0.12 | .07 |
| Constant | 6.06 | 1.39 | <.001 |
Greater bone accrual velocity, height velocity, and BSAP levels are associated with significantly higher PTH levels as an outcome. All analyses were adjusted for serum 25(OH)D levels and dietary calcium intake. The models for bone accrual and growth velocity were adjusted for the baseline values. P values <.05 are shown in bold.
Natural log of value used to produce normality.
Table 4.
Regression Models With Baseline 1,25(OH)2D as Outcome
| Coefficient | SE | P | |
|---|---|---|---|
| Change in subtotal BMC (0–12 mo), g | 0.40 | 0.09 | <.001 |
| Baseline subtotal BMCa | −0.007 | 0.053 | .90 |
| 25(OH)Da | 0.23 | 0.19 | .22 |
| Constant | 0.32 | 1.15 | .78 |
| Change in cortical BMC (0–12 mo), mg | 0.24 | 0.10 | .02 |
| Baseline cortical BMCa | −0.043 | 0.071 | .55 |
| 25(OH)Da | 0.22 | 0.20 | .27 |
| Constant | 1.99 | 1.06 | .06 |
| Change in height (0–12 mo), cm | 0.030 | 0.009 | <.001 |
| Baseline heighta | 0.25 | 0.20 | .23 |
| 25(OH)Da | 0.11 | 0.19 | .54 |
| Constant | 4.24 | 1.23 | <.001 |
| Baseline BSAP, μg/La | 0.41 | 0.01 | <.001 |
| 25(OH)Da | 0.386 | 0.17 | .024 |
| Constant | −0.07 | 0.87 | .94 |
Greater bone accrual velocity, height velocity, and BSAP levels are associated with significantly higher 1,25(OH)2D levels as an outcome. All models were adjusted for serum 25(OH)D levels. The models for bone accrual and growth velocity were adjusted for the baseline values. P values <.05 are shown in bold.
Natural log-transformed.
Discussion
Greater bone accrual rates as assessed by gains in BMC and height, and higher levels of biomarkers of bone formation (but not bone resorption) were consistently and significantly associated with greater PTH levels. These relations persisted after adjustment for the expected associations of lower 25(OH)D levels and dietary calcium intake with greater PTH levels. On average, PTH levels were 44% higher among adolescents in midpuberty compared to prepubertal children. Similar positive associations were observed between 1,25(OH)2D levels and the four indices of bone accrual. Taken together, these data are consistent with a compensatory rise in PTH levels in response to calcium uptake during periods of peak bone accrual, thereby increasing production of 1,25(OH)2D to promote calcium absorption. To our knowledge, this is the first study to examine the associations between PTH and 1,25(OH)2D levels and bone accrual. These data have implications for the interpretation of mild elevations of PTH levels during puberty and for studies of calcium requirements during growth. Of note, the PTH levels were within the normal range in 96% of participants; therefore, these findings are not generalizable to adolescents with secondary hyperparathyroidism in the setting of chronic disease.
PTH and 1,25(OH)2D are important for bone formation. Therefore, the positive association of these hormones with bone accrual may represent direct effects on bone, a compensatory response to calcium demands during growth, or a combination of both processes. Abrams et al (13) reported that calcium absorption (measured using stable isotopes) in adolescents was positively associated with serum 1,25(OH)2D and PTH levels, and PTH levels were positively associated with a biomarker of bone formation but not a biomarker of bone resorption. We observed the same associations between PTH levels and bone biomarkers, and our longitudinal data provide further evidence to support their conclusion that rapid bone formation during midpuberty contributed to a rise in PTH in order to increase 1,25(OH)2D levels and promote calcium absorption (13). Furthermore, our observation that greater height velocity was associated with higher PTH and 1,25(OH)2D levels is consistent with this interpretation, because one would not suggest that these hormones promote linear growth.
The impact of anabolic agents on bone metabolism in children and adolescents is best illustrated in a study of GH therapy. Schweizer et al (23) reported that 1 year of GH therapy was associated with significant increases in biomarkers of bone formation and resorption, increases in total cortical bone area, and decreases in cortical volumetric BMD. The authors attributed these associations to bone remodeling and undermineralization of newly formed bone. Therefore, if PTH was having an anabolic effect on bone, one might expect to see gains in BMC, but decreases in cortical BMD and increases in biomarkers of bone resorption. We did not observe the latter associations. Although PTH may exert an important anabolic effect on bone modeling and remodeling during growth and development, the associations observed here suggest that the effects of bone accrual and increased calcium demands on PTH levels predominate.
The rapid bone accrual during puberty is driven by striking increases in sex hormones, with greater estrogen-mediated gains in trabecular bone volume fraction and endocortical bone in females, and greater T-mediated gains in periosteal circumference in males (26, 27). Pubertal sex hormone release is also linked to GH and subsequent IGF-1 surges that result in rapid linear growth, with further bone formation and calcium demands (26). A prior preclinical study demonstrated that estradiol stimulated PTH secretion through a direct effect on parathyroid cells (28); however, a small study in postmenopausal women did not show effects of estrogen treatment or withdrawal on basal or stimulated PTH secretion (29). Additional studies are needed to determine whether increases in estrogen levels during puberty contribute to increases in PTH levels.
This study has multiple limitations. First, we used a validated self-assessment of Tanner staging instead of examination by a pediatric endocrinologist. Some participants with higher adiposity may have inaccurately assessed a higher degree of breast development, whereas relatively few subjects identified themselves as Tanner 3 for unclear reasons. Second, PTH and vitamin D levels were only collected at baseline; therefore, we could not demonstrate a temporal association between greater bone accrual and subsequent increases in PTH and 1,25(OH)2D levels. Our implicit assumption is that bone accrual velocity in the 12 months after the PTH and 1,25(OH)2D samples were collected was representative of the bone accrual velocity at the time of the laboratory measures. This is a reasonable assumption, given our prior report that growth and bone accrual velocity over the year explained 80% of the variability in baseline BSAP levels in this cohort (15). That is to say that the strength of this association suggests that this ensuing growth is an adequate indicator of accrual velocity at the time of biomarker measurement. Furthermore, any imprecision in the temporal assessment of these relations would only serve to weaken the associations; we saw strong and consistent associations across multiple indices of bone accrual (Tables 3 and 4). Dietary calcium recall may not fully capture variability in calcium intake. However, the demonstrated inverse association with PTH levels speaks to the utility of this measure in this cohort. Use of pQCT does not provide information on material density or cortical porosity, and bone biomarkers in children and adolescents do not distinguish between the effects of modeling and remodeling. Furthermore, the use of the urinary DPD to creatinine ratio may underestimate bone resorption in children and adolescents with greater muscle mass. Finally, we do not have measures of serum calcium and phosphorus levels or measures of calcium absorption. However, to our knowledge this is the first study to examine the relations between bone accrual velocity and PTH and vitamin D levels, including adjustments for dietary calcium intake.
In conclusion, we found that greater bone accrual velocity was associated with higher PTH and 1,25(OH)2D levels in children and adolescents. These data are consistent with a compensatory response to increased calcium demand during growth. Future studies are needed to determine the direct effects of PTH and 1,25(OH)2D on bone modeling and remodeling during growth.
Acknowledgments
This work was supported by Grants R01-DK060030, R01-HD040714, K24-DK076808, K23-DK093556, K08-HD060739, and UL1-RR-024134 from the National Center for Research Resources, National Institutes of Health.
Disclosure Summary: None of the authors has any conflicts to declare.
Footnotes
- BMC
- bone mineral content
- BMD
- bone mineral density
- BMI
- body mass index
- BSAP
- bone-specific alkaline phosphatase
- CV
- coefficient of variation
- DPD
- deoxypyridinoline
- DXA
- dual-energy x-ray absorptiometry
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D
- pQCT
- peripheral quantitative computed tomography.
References
- 1. Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134:696S–700S. [DOI] [PubMed] [Google Scholar]
- 2. McKay HA, Bailey DA, Mirwald RL, Davison KS, Faulkner RA. Peak bone mineral accrual and age at menarche in adolescent girls: a 6-year longitudinal study. J Pediatr. 1998;133:682–687. [DOI] [PubMed] [Google Scholar]
- 3. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26:1729–1739. [DOI] [PubMed] [Google Scholar]
- 4. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 5. Kumar R, Tebben PJ, Thompson JR. Vitamin D and the kidney. Arch Biochem Biophys. 2012;523:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. [DOI] [PubMed] [Google Scholar]
- 7. Ginde AA, Wolfe P, Camargo CA, Jr, Schwartz RS. Defining vitamin D status by secondary hyperparathyroidism in the U.S. population. J Endocrinol Invest. 2012;35:42–48. [DOI] [PubMed] [Google Scholar]
- 8. Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–158. [DOI] [PubMed] [Google Scholar]
- 9. Warden SJ, Hill KM, Ferira AJ, et al. Racial differences in cortical bone and their relationship to biochemical variables in black and white children in the early stages of puberty. Osteoporos Int. 2013;24:1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294:2336–2341. [DOI] [PubMed] [Google Scholar]
- 11. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. [DOI] [PubMed] [Google Scholar]
- 12. Lewiecki EM, Miller PD. Skeletal effects of primary hyperparathyroidism: bone mineral density and fracture risk. J Clin Densitom. 2013;16:28–32. [DOI] [PubMed] [Google Scholar]
- 13. Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab. 2005;90:5576–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. [DOI] [PubMed] [Google Scholar]
- 17. Augustine MV, Leonard MB, Thayu M, et al. Changes in vitamin D-related mineral metabolism after induction with anti-tumor necrosis factor-α therapy in Crohn's disease. J Clin Endocrinol Metab. 2014;99:E991–E998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cremers S, Garnero P. Biochemical markers of bone turnover in the clinical development of drugs for osteoporosis and metastatic bone disease: potential uses and pitfalls. Drugs. 2006;66:2031–2058. [DOI] [PubMed] [Google Scholar]
- 19. Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–294. [DOI] [PubMed] [Google Scholar]
- 20. Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–186. [DOI] [PubMed] [Google Scholar]
- 21. Smiciklas-Wright HA, Mitchell DC, Norton LD, Derr JA. Interviewer reliability of nutrient intake data from 24-hour recalls collected using the Minnesota Nutrition Data System (NDS). J Am Diet Assoc. 1991;91:A28. [Google Scholar]
- 22. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190. [PubMed] [Google Scholar]
- 23. Schweizer R, Martin DD, Schwarze CP, et al. Cortical bone density is normal in prepubertal children with growth hormone (GH) deficiency, but initially decreases during GH replacement due to early bone remodeling. J Clin Endocrinol Metab. 2003;88:5266–5272. [DOI] [PubMed] [Google Scholar]
- 24. Mostoufi-Moab S, Brodsky J, Isaacoff EJ, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97:3584–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Griffin LM, Thayu M, Baldassano RN, et al. Improvements in bone density and structure during anti-TNF-α therapy in pediatric Crohn's disease. J Clin Endocrinol Metab. 2015;100:2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Callewaert F, Sinnesael M, Gielen E, Boonen S, Vanderschueren D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J Endocrinol. 2010;207:127–134. [DOI] [PubMed] [Google Scholar]
- 27. Callewaert F, Venken K, Kopchick JJ, et al. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25:617–626. [DOI] [PubMed] [Google Scholar]
- 28. Greenberg C, Kukreja SC, Bowser EN, Hargis GK, Henderson WJ, Williams GA. Parathyroid hormone secretion: effect of estradiol and progesterone. Metabolism. 1987;36:151–154. [DOI] [PubMed] [Google Scholar]
- 29. Vincent A, Riggs BL, Atkinson EJ, Oberg AL, Khosla S. Effect of estrogen replacement therapy on parathyroid hormone secretion in elderly postmenopausal women. Menopause. 2003;10:165–171. [DOI] [PubMed] [Google Scholar]