Abstract
Context:
Closed-loop control (CLC) relies on an individual's open-loop insulin pump settings to initialize the system. Optimizing open-loop settings before using CLC usually requires significant time and effort.
Objective:
The objective was to investigate the effects of a one-time algorithmic adjustment of basal rate and insulin to carbohydrate ratio open-loop settings on the performance of CLC.
Design:
This study reports a multicenter, outpatient, randomized, crossover clinical trial.
Patients:
Thirty-seven adults with type 1 diabetes were enrolled at three clinical sites.
Interventions:
Each subject's insulin pump settings were subject to a one-time algorithmic adjustment based on 1 week of open-loop (i.e., home care) data collection. Subjects then underwent two 27-hour periods of CLC in random order with either unchanged (control) or algorithmic adjusted basal rate and carbohydrate ratio settings (adjusted) used to initialize the zone-model predictive control artificial pancreas controller. Subject's followed their usual meal-plan and had an unannounced exercise session.
Main Outcomes and Measures:
Time in the glucose range was 80–140 mg/dL, compared between both arms.
Results:
Thirty-two subjects completed the protocol. Median time in CLC was 25.3 hours. The median time in the 80–140 mg/dl range was similar in both groups (39.7% control, 44.2% adjusted). Subjects in both arms of CLC showed minimal time spent less than 70 mg/dl (median 1.34% and 1.37%, respectively). There were no significant differences more than 140 mg/dL.
Conclusions:
A one-time algorithmic adjustment of open-loop settings did not alter glucose control in a relatively short duration outpatient closed-loop study. The CLC system proved very robust and adaptable, with minimal (<2%) time spent in the hypoglycemic range in either arm.
The development of the artificial pancreas (AP) started almost 40 years ago with early designs that included subcutaneous insulin delivery and glucose sensing, but were not ready for widespread use (1, 2). Several promising control strategies have been evaluated in multiple clinical trials of AP technology, both in-clinic and in the ambulatory setting (3–17). However, current closed-loop control (CLC) is characterized by significant periods of hyperglycemia (18).
Many recent studies of CLC have included a period of clinician-led optimization of open-loop insulin pump settings because CLC relies on an individual's open-loop insulin pump settings to initialize the system (12, 14–17). The open-loop basal rate setting is often used when glucose is in the target range, adjusting up or down from that basal rate as needed. In addition, meal-time boluses are still used in CLC. Thus, if open-loop settings are not ideal, the AP controller may not be able to adequately adjust insulin dosing beyond preset constraints. This is important because there are many individuals on open-loop therapy who are taking too little basal insulin because of a fear of hypoglycemia, or too much basal insulin because of missed boluses or focus on an elevated glycated hemoglobin (HbA1c) (19).
To potentially obviate the need for an open-loop optimization period and thus facilitate future use of CLC, we performed a multicenter, outpatient, randomized crossover clinical trial in which we tested the effects of using a one-time algorithmic adjustment of open-loop basal rates and insulin to carbohydrate ratios (CRs) versus use of usual home parameters to initialize the closed-loop system to improve prespecified glycemic parameters in CLC (adjusted and control arm, respectively). We also performed a post hoc analysis comparing both arms of CLC to each subject's open-loop therapy. The algorithmic adjustment was based on 1 week of open-loop data collection. The closed-loop system used the zone-model predictive control (MPC) strategy combined with the Health Monitoring System (HMS) (20–22), which has shown excellent results with day-to-day challenges during previous in-clinic studies (7). This approach was updated to include a diurnal strategy (22) and asymmetric input costs to facilitate rapid insulin attenuation at times of predicted hypoglycemia (23).
Materials and Methods
Participants
A total of 37 subjects were enrolled at three sites: William Sansum Diabetes Center, Santa Barbara, CA; University of Virginia, Charlottesville, VA; and Mayo Clinic, Rochester, MN. Adult subjects (ages 21–65) with type 1 diabetes for at least 1 year, using an insulin pump for at least 6 months, with an HbA1c ≤10% (86 mmol/mol), were eligible to enroll as long as they did not meet the following exclusion criteria: pregnancy, diabetic ketoacidosis in the past 6 months, severe hypoglycemia resulting in seizure or loss of consciousness in the past 12 months, medical conditions or medication use that increase the risk of hypoglycemia or preclude participation in the study. Subjects provided written, informed consent. A physical examination and laboratory analysis were performed to ensure enrollment criteria were met. The study protocol was approved by each site's institutional review board and the Food and Drug Administration, and was registered at ClinicalTrials.gov (NCT01929798).
Data collection
Following enrollment, subjects were trained on a study CGM (Dexcom G4 Platinum, San Diego, CA). Subjects wore the CGM for 1 week at home and followed their usual routine for meals, insulin administration, and exercise. The CGM was blinded if the subject was not a previous CGM user. The CGMs were calibrated using a study glucometer (One Touch Ultra2, LifeScan). Subjects recorded known or estimated carbohydrate intake of all meals, snacks, and hypoglycemia treatments, and noted a subjective binary categorization of meal composition as “high” or “low” fat. All acknowledged meals from the data collection week were used by the initialization algorithm for the patient's CR.
Insulin parameter adjustment
Open-loop insulin pump setting adjustments were based on the data collection week that included data from the study CGM, insulin pump, glucometer, and subject diary. For each subject, these settings were adjusted only one time and were used only during the adjusted arm CLC session. The control arm CLC session used the subject's baseline open-loop settings to initialize the closed-loop system.
For the adjusted settings, changes to the basal rate profile were limited to 25% from baseline. CRs were adjusted to a profile with three time segments, beginning at 04:00, 11:00, and 17:00. The changes in CR were limited to be within 20% from baseline. The limits were enforced to prevent large insulin parameter changes. Specifically, the algorithm, based on estimated insulin sensitivity, glucose excursion, and infused insulin, calculates the optimal CR for each meal. The new CR was applied only if it was likely to improve glycemic control. Details on the basal rate and CR initialization procedures can be found in the Supplemental Materials and methods, initializers appendix. The study physician at each site reviewed and had to approve the profiles before implementation. After completion of the first eight subjects, there were two instances (duration: 5 and 15 minutes) of glucose less than 50 mg/dL that led to protocol changes in which insulin administered at the time of meals was adjusted based on CGM readings (see the Meals section), and the adjusted basal rate profile could be reduced, but not increased above, their baseline rate.
System equipment
The AP device is a CLC system consisting of a study tablet (HP Slate, Hewlett-Packard Company) running the zone-MPC algorithm and HMS on a portable artificial pancreas system platform from the University of California Santa Barbara (24). During CLC, subjects wore a One Touch Ping pump (Animas) and two study CGM transmitters/sensors. The CGM receiver and pump meter remote were connected to the tablet via USB cables. Subjects were not physically connected to the tablet, but were asked to remain within 10 m of the tablet for the CGM/receiver and pump/remote pairs to maintain wireless communication. Communication disruptions were few (see online Supplemental Table 7).
Outpatient protocol
Subjects were admitted twice, in random order, separated by 5 days but no more than 3 weeks apart: CLC with adjusted parameters and CLC with baseline parameters. Study procedures, meal times, exercise intensity, etc., of the first admission were replicated accurately during the second admission. Each admission lasted approximately 27 hours. The study sites were an off-site research house (University of Virginia), an outpatient area (William Sansum Diabetes Center), and a hotel (Mayo Clinic), with study staff on-site or nearby to supervise the subjects. Two days before the study date, two CGM sensors were placed. Subjects arrived at noon to the research site and were required to have a blood glucose level at or below 250 mg/dL for admission. Lunch was consumed at approximately 12:00 with an insulin dose determined using the subjects' open-loop insulin pump settings. At approximately 16:00, subjects were transitioned to the study pump. The subjects' insulin-dosing parameters were entered into the portable artificial pancreas system according to study phase: adjusted parameters or control parameters. The primary CGM was selected based on accuracy and performance. The secondary CGM was not used within the closed-loop system unless there was a failure of the primary CGM. There was one such instance during the entire study. The subjects consumed meals at defined times (±30 minutes), with dinner at 18:30; breakfast at 07:00, and lunch at 12:30, with discharge approximately 18:00. An exercise period of 45–60 minutes occurred and was designed to replicate the subjects' usual regimen in terms of timing, choice of activity and intensity (eg, brisk walking outside or on a treadmill, cycling on a stationary bike). Subjects consumed a snack (approximately 16 g of carbohydrate content) at the start of exercise if capillary blood glucose (BG) was less than 120 mg/dL. BG was assessed at: the time of CGM calibration (30 minutes before meals and bedtime), every 2 hours during the day, every 3 hours at night (midnight to 07:00), and any time the HMS issued a hypoglycemia alert. Consistent glycemic treatment guidelines were followed across the trial sites. After each HMS hypoglycemia alarm, BG was assessed by glucometer; 16 g of carbohydrates were consumed if BG less than 70 mg/dL or if hypoglycemia symptoms were present, depending on subject preference. Trials were stopped if the HMS did not alarm and instruct the subject to consume carbohydrates to prevent hypoglycemia or for persistent BG more than 300 mg/dL(>1 hour) with subsequent elevated ketones (>1.2 mmol/L).
Meals
Study meals were selected to match the subject's carbohydrate intake and caloric consumption during the data collection period (not exceeding 90 g per meal). Snacks were optional according to the subject's usual routine. All meals were announced to the system at mealtime, not before, with the subject's estimation of carbohydrate content using their usual method. Timing of the meal boluses was standardized such that the subjects started their meals just as the meal bolus delivery was completed. An insulin bolus for the meal was recommended by the system. The first eight subjects had the meal insulin calculated from the relevant CR (adjusted or control) without adjustment for the prevailing BG. For the subsequent 24 subjects, the insulin bolus at the time of a meal was adjusted by the controller based on CGM value at the time of the meal as follows: if CGM was less than 140 mg/dl, then 80% of meal bolus calculated by relevant CR at the time would be administered; if CGM was equal to or higher than 140 mg/dL, then the relevant CR would be used and in addition the correction factor would be used to provide additional correction to 140 mg/dL, capped at a maximum of 2 U.
Control algorithm
The zone-MPC algorithm was responsible for adjusting insulin delivery when glucose concentration was, or was predicted to be, outside the specific zone (80–140 mg/dL daytime and 110–170 mg/dL at nighttime). The algorithm transitioned from diurnal to nocturnal over a 2-hour period from 22:00 to midnight and conversely from 05:00 to 07:00 for the first eight subjects and 04:00–06:00 for the additional 24.
Impending hypoglycemia (<65 mg/dL in 15 minutes) as predicted by the HMS triggered the sending of short message service/multimedia messaging service alerts to both subjects and study staff. Subjects were required to perform a BG assessment, treat according to standard glycemic treatment guidelines, and inform the system of treatment.
Statistical analysis
Data from all 32 subjects who completed the protocol were included in the analysis. The primary outcome measures were percentage time spent between 80 and 140 mg/dL between adjusted and control conditions. Secondary outcomes included percent time overnight, 110–170 mg/dL; 5 hours after meal, 70–180 mg/dl; and during and 3 hours after exercise, 70–150 mg/dL. Additional secondary outcome measures were frequency, values, and duration of extremes of glucose, in particular if less than 70 and less than 60 mg/dL; adverse events; and outside interventions not following the recommendation of the HMS.
CGM data from both closed-loop periods (27 hours) as well as CGM data from a corresponding window covering day 2 of the open-loop week, were analyzed in post hoc analysis. The open-loop data for comparison was consistently selected as the second day of the data collection week and corresponded to the same duration and time of day as the closed-loop sessions. Furthermore, indices of glucose variability, low blood glucose index, and high blood glucose index (25) were also analyzed.
The crossover selection was conducted through a randomization process. Carryover effects were not considered significant with this trial design. There were no learning processes across each visit, regardless of which visit was randomized initially for each subject. In the case where it is not assumed that the two data samples are from populations with equal variances, the test statistic under the null hypothesis has an approximate Student's t distribution with a number of degrees of freedom given by Satterthwaite's approximation. Welch's modification of Student's two-sample paired t test was used to compare variables of interest (26). P < .05 was considered statistically significant.
Results
Subject characteristics
Thirty-seven subjects were enrolled and 32 subjects (18 females) completed both arms of the study. Five subjects dropped out of the study because of events not related to the study: ankle fracture at home (1), severe hypoglycemia event at home (1), scheduling conflicts (2), switched to a low-carbohydrate diet, and was unable to follow study meal plan (1).
The mean (sd) characteristics of the completed subjects were as follows: age 46.9 (10.9) years; HbA1c 7.5 (0.95)% (58 mmol/mol), body mass index 28.0 (4.8) kg/m2; and duration of diabetes 27.7 (12.1) years. Eight of these subjects completed the study before the protocol changes described previously and 24 subjects completed after. The median time (interquartile range) in CLC was 25.3 hours (24.6–25.4).
Glycemic control during day and night CLC
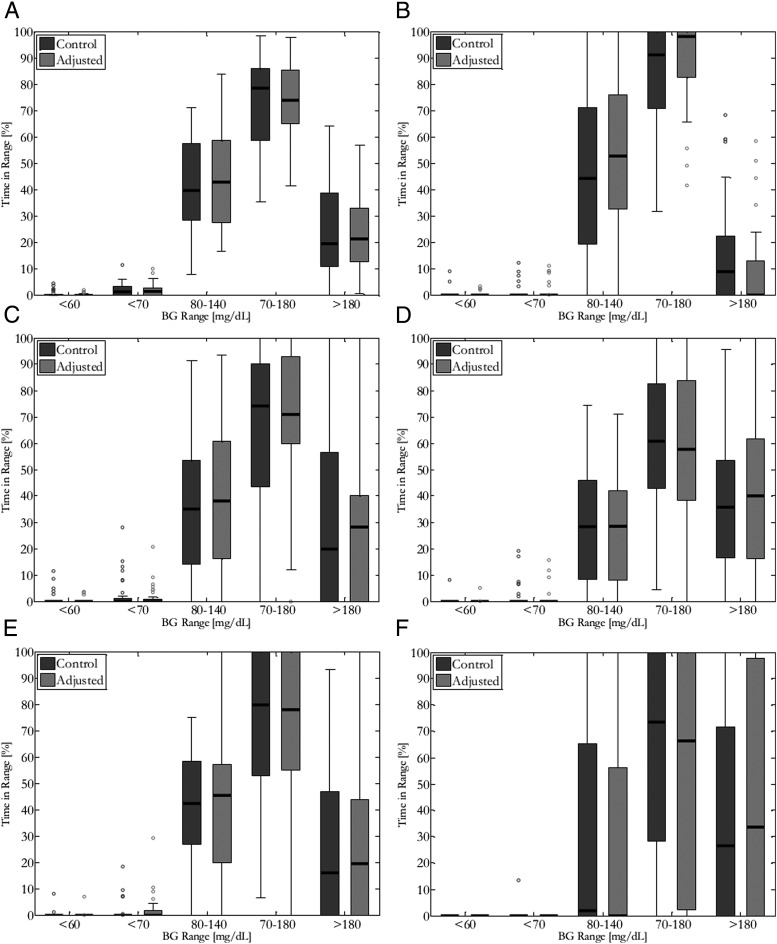
The primary study outcome of time in 80–140 mg/dL for tight glycemic control over the entire closed-loop period was not significantly different between the adjusted and control arms (Table 1 and Figure 1A; median time in target 44.2 versus 39.7%, P = NS). Overall, there were no significant differences in mean glucose and time in target ranges between adjusted and control arms of CLC (median percent time <70, 70–180, or 110–170 mg/dL).
Table 1.
Summary of Median Clinical Metrics that Characterize BG Control Performance of 32 Adult Subjects With Control or Adjusted Clinical Parameters, Overall, and Overnight Only
| Control | Adjusted | P Control versus Adjusted | |
|---|---|---|---|
| Overall | |||
| Mean glucose (mg/dL) | 142 (133–163) | 141 (130–158) | .772 |
| Glucose at 0700 (mg/dL) | 132 (106–162) | 133 (116–162) | .789 |
| % time <60 mg/dL | 0 (0–0.34) | 0 (0–0.34) | .22 |
| % time <70 mg/dL | 1.34 (0–3.17) | 1.37 (0–2.84) | .898 |
| <80% time <140 mg/dL | 39.7 (30.4–56.3) | 44.2 (30.1–58.6) | .686 |
| <140% time <180 mg/dL | 26.8 (24.1–33.1) | 24.9 (19.8–33.4) | .589 |
| % time >180 mg/dL | 19.2 (11–37.2) | 20.7 (13.3–31.6) | .901 |
| <70% time <180 mg/dL | 79.1 (60.4–86.1) | 75 (66.5–85.3) | .918 |
| LBGI | 0.53 (0.28–1.03) | 0.60 (0.33–0.91) | .986 |
| HBGI | 3.95 (2.32–7.07) | 4.02 (2.73–6.13) | .823 |
| Overnight only | |||
| Mean glucose (mg/dL) | 135 (121–159) | 128 (117–146) | .099 |
| % time <60 mg/dL | 0 (0–0) | 0 (0–0) | .804 |
| % time <70 mg/dL | 0 (0–0) | 0 (0–0) | .675 |
| <80% time <140 mg/dL | 46.3 (20–74) | 54.1 (33.3–71.8) | .573 |
| <140% time <180 mg/dL | 31.7 (15.3–56.7) | 29.9 (18.1–45.7) | .831 |
| % time >180 mg/dL | 7.78 (0–20.4) | 0 (0–12.4) | .184 |
| <70% time <180 mg/dL | 91.8 (78.8–100) | 98.8 (87.1–100) | .216 |
| LBGI | 0.05 (0–0.48) | 0.311 (0–1) | .147 |
| HBGI | 2.9 (0.76–4.83) | 1.73 (0.88–3.91) | .126 |
Abbreviations: HBGI, high blood glucose index; LBGI, low blood glucose index.
Parentheses represent interquartile range.
Figure 1.
Box and whisker plot representation of blood glucose control performance characterized by percent time in clinical range of 32 adult subjects with control or adjusted clinical parameters for (A) the entire trial period, (B) overnight, 5-hour postprandial periods for (C) dinner, (D) breakfast, (E) lunch, and duration of (F) exercise. The dark bars inside each box represent the median value, with the bounds of the box representing the 25%–75% interquartile range. The thin lines represent the minimum and maximum values.
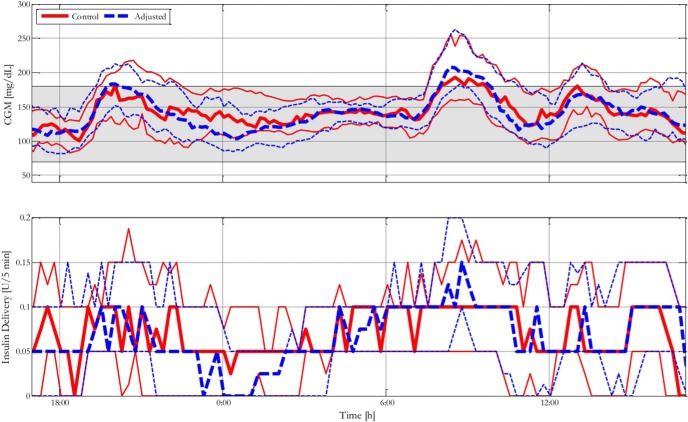
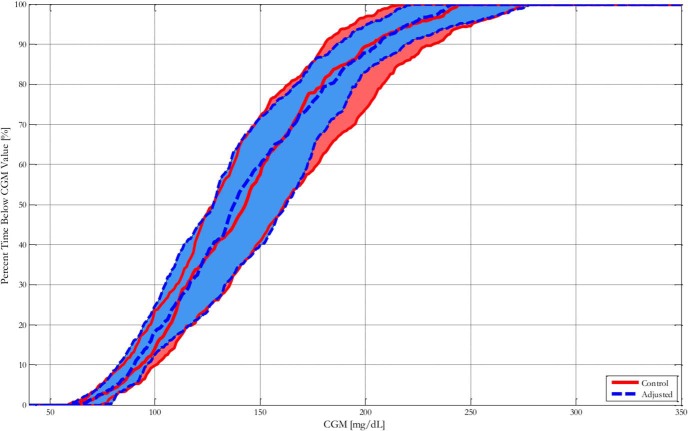
CGM traces and corresponding insulin delivery are presented in Figure 2. Cumulative time in blood glucose range as measured by CGM is presented in Figure 3. For individual traces, please see the Supplemental Results, all glucose and insulin traces appendix.
Figure 2.
Blood glucose control performance characterized by median and interquartile range glucose and insulin traces of % time in clinical range of 32 adult subjects with control or adjusted clinical parameters. The solid line represents subjects with control parameters; the dashed line represents subjects with adjusted parameters.
Figure 3.
Blood glucose control performance characterized by median and interquartile range of cumulative time in blood glucose of 32 adult subjects with control or adjusted clinical parameters. The solid line represents subjects with control parameters; the dashed line represents subjects with adjusted parameters.
Glycemic control during CLC overnight
There were no significant differences in glycemic control overnight between the adjusted and control conditions, with few HMS alerts for both designs (interquartile range of 0–0.5 adjusted and 0–0 control; Supplemental Table 6).
Glycemic control following meals and exercise
There were no differences in glycemic control 5 hours postprandially in the adjusted versus control conditions of closed loop (Figure 1C–E and Supplemental Tables 1–3). Adjustment of insulin parameters also did not change the glucose performance during exercise (Figure 1F) or 3 hours after exercise (Supplemental Tables 4–5).
Adverse events
Two of the first eight subjects had a BG less than 50 mg/dL on CLC with delayed HMS notifications that required the study to stop, as per protocol. The subjects experienced moderate hypoglycemic symptoms and were easily treated with oral glucose for recovery without sequelae. One subject had exercised intensely the day before the protocol as per the subject's usual routine, which contributed to the hypoglycemic event. The second subject did not follow his usual home meal plan that included a 20-g bedtime snack, and subsequently became hypoglycemic in the late evening. In both instances, the controller had attenuated insulin administration and the HMS issued a hypoglycemia alert. After adjustments were made to the protocol to change insulin dosing around meals in relation to CGM and to limit increases from subject's baseline basal rates (see Materials and Methods), no further study subjects (n = 24) had BG less than 50 mg/dL without prior alerts from the HMS.
One subject dropped out because of an ankle fracture after a fall at home 2 days after the first closed-loop session. This accident was unrelated to glucose control. One subject had a hypoglycemic episode requiring glucagon administration at home 6 days after completing the first CLC session. This episode was due to excessive meal insulin administration following exercise while using her usual home insulin parameters and personal insulin pump. There were no instances of severe hyperglycemia, diabetic ketoacidosis, or significant ketone formation requiring the study to be stopped or manual insulin to be administered during CLC.
Post hoc analyses of open loop data
Post hoc analysis was completed to compare CLC with open loop data collected at home before CLC. Overnight CLC significantly improved time in target ranges compared with open-loop therapy for percent time less than 70 and 70–180 mg/dL (see Supplemental Table 8). Fasting (07:00) glucose concentrations did not differ between closed-loop (median glucose 133 mg/dL adjusted arm versus 132 mg/dL control arm) and open-loop therapy (129 mg/dL).
There were significant differences comparing open loop with closed loop in either adjusted or control conditions for percent time less than 70 mg/dL with closed loop demonstrating reduced time in low glucose ranges compared with open loop (median time <70 was 1.34 and 1.37 in adjusted and control CLC versus 6.73% in open loop, P < .0001). Likewise, similar results were obtained for percent time less than 60 mg/dL between closed-loop and open-loop (median time 0 and 3.82%, respectively; P < .0001). The low blood glucose index was significantly lower in adjusted CLC compared with open loop (0.60 and 1.66, P < .0001).
Mean changes for basal rates and CRs as recommended by the algorithm are summarized in Supplemental Table 9.
Conclusions
The primary goal of this multicenter, single-hormone, CLC randomized outpatient crossover clinical trial using the zone-MPC controller was to determine the safety and efficacy of individualized adjustments of the insulin dosing parameters (adjusted) versus nonadjusted (control) in adults with type 1 diabetes. We have demonstrated that neither the primary nor the secondary outcome variables differed between the two approaches to insulin parameter initialization preceding CLC. Furthermore, it is noteworthy that during the nocturnal period, hypoglycemia (time <70 mg/dL) was very low overall and did not occur for some subjects with both CLC approaches. This is of critical importance and a win-win situation for patient safety that both approaches to initialization of the zone-MPC control algorithm essentially eliminated nocturnal hypoglycemia.
We hypothesized that a fusion algorithm (a method of combining the recommended changes in basal rate settings with changes in the CR to ensure the combined effect stays within a 20% deviation from the original insulin dose) that used open-loop data gathered under carefully controlled conditions could improve upon the residual hyperglycemia seen in current closed-loop studies (18). We therefore tested the adjusted settings in CLC under ambulatory conditions similar to those during the data gathering open-loop phase. Contrary to expectations, adjustment of the basal rates and CR settings did not alter indices of glucose control compared with the usual baseline parameters based on each participant's customary insulin pump settings. We had reasoned that informing the algorithm with individualized inputs based on each subject's behavioral and physiological requirements would make a difference to glucose control; that it did not could be due to multiple reasons.
First could be the time factor. It is possible that the 1-week duration of data collection may not have been sufficient to obtain adequate representative individualized data to set the initialization parameters. Given the significant day-to-day variability in carbohydrate metabolism (even during carefully controlled circumstances) (27) in type 1 diabetes, such variability could have been multiplied manifold in the free-living situation, making any generalization of initializing of insulin parameters for a “typical day” a formidable challenge. Therefore, future studies are necessary to test the efficacy of extending the data collection interval to derive a more representative estimate of average pump settings and parameters for initialization and to conduct “at home” CLC trials. Additionally, it is plausible that the relatively short duration of closed-loop periods may have been insufficient to permit these adjustments to take effect. Because we strongly believe that individualized adjustments of insulin delivery parameters are needed to improve performance of CLC, this concept should be further tested for longer durations with multiple cycles of small adjustments to assess safety and efficacy.
Second, for safety purposes, there were constraints placed on adjusted parameters (CR and basal rates) that prevented large changes to insulin dose settings. The limited changes may have had a modest impact in our randomized clinical trial because subjects were relatively controlled with mean HbA1c 7.5%; a larger effect may be seen in different populations with suboptimal baseline glycemic control. We reasoned that it would be safer to restrain insulin dosing, especially for meal boluses, because once a large meal bolus is administered, it is challenging to mitigate an over-bolus situation with a single hormone CLC. On the other hand, the zone-MPC algorithm could respond to rising glucose levels with additional insulin if the prandial bolus was not sufficient to attain target glucose concentrations. Our approach of avoiding overzealous use of prandial insulin while letting the algorithm handle any prandial hyperglycemia has been successful. This is evident from our observation that the median time spent less than 70 mg/dL was reduced to approximately 1.3% for the entire period, 0% for nocturnal period, 0.8–1.6% during 5-hour postmeals, and perhaps, most important, to 0% during and postexercise in CLC.
It is noteworthy that by design and for safety, the zone potentially greater changes: MPC relaxed the controller during the overnight period with the target glucose zone set between 110 and 170 mg/dL. This was by far successful in maintaining time spent (65–70%) within the prespecified nocturnal glucose target during both closed-loop conditions, with no difference between adjusted and control arms.
Like most studies, this study had strengths and limitations. Strengths include that we were able to individualize closed-loop initialization settings based on an outpatient data collection phase using a fusion algorithm that worked on both basal rate and CR settings. The algorithm was able to work with a short period (only 1 week) of data collection, and the study was completed at multiple centers. Limitations include that although the data collection period occurred at home under free-living conditions, the closed-loop periods were not at home but in a transitional environment. Although we attempted to replicate subjects' free-living conditions during the CLC period, the timing, intensity, and duration of exercise and meal conditions during CLC may have differed to an extent.
This randomized clinical trial establishes the feasibility, safety, and efficacy of algorithmic adjustment of individualized insulin dosing parameters for CLC. We have demonstrated that modest changes to initialization parameters within this system do not alter glucose control in a relatively short duration supervised outpatient closed-loop study. Additional multicenter trials are necessary for the paradigm shifting therapeutic approach of CLC to reach and benefit the diverse population of people with type 1 diabetes.
Acknowledgments
We are deeply indebted to the research participants. Our sincere thanks to the staff of the Mayo Clinic Center for Translational Science Activities and Mayo Clinic Clinical Research Unit; Ms Lauren Huyett and Dr Isuru Dasanayake from the University of California Santa Barbara; the staff of the Center for Diabetes Technology at the University of Virginia; Dr David Kerr, Dr Lois Jovanovič, Dr Alex Morf, Ms Jacqueline Wiley, Ms Maia Bradley, and the support staff at the William Sansum Diabetes Center for their assistance.
This work was supported by the National Institutes of Health Grants DP3DK094331, R01DK085628 (to UCSB), and UL1 TR000135 from the National Center for Advancing Translational Science and the Urdang Family Foundation to Mayo Clinic. C.C., C.D.M., and M.S. are partially funded by Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Progetto FIRB 2009).
Clinical trial reg. n.: NCT01929798.
Disclosure Summary: S.A.B. received material support from Roche Diagnostics and DexCom, Inc. C.D.M., M.S., A.B., Y.C.K., and C.C. have filed a patent application (PCT No. 13/661,755) related to estimation of insulin sensitivity from CGM readings. We also acknowledge product support from Animas Corp, Dexcom Inc. and Lifescan Inc. No other potential conflicts of interest relevant to this article were reported.
Footnotes
- AP
- artificial pancreas
- BG
- blood glucose
- CLC
- closed-loop control
- CR
- carbohydrate ratio
- HbA1c
- glycated hemoglobin
- HMS
- Health Monitoring System
- MPC
- model predictive control.
References
- 1. Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia. Br Med J. 1978;1:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. 1979;300:573–578. [DOI] [PubMed] [Google Scholar]
- 3. Turksoy K, Quinn L, Littlejohn E, Cinar A. Multivariable adaptive identification and control for artificial pancreas systems. IEEE Trans Biomed Eng. 2014;61:883–891. [DOI] [PubMed] [Google Scholar]
- 4. Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, Balliro C, Hillard MA, Nathan DM, Damiano ER. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nimri R, Phillip M. Artificial pancreas: fuzzy logic and control of glycemia. Curr Opin Endocrinol Diabetes Obes. 2014;21:251–256. [DOI] [PubMed] [Google Scholar]
- 6. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harvey RA, Dassau E, Bevier WC, et al. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther. 2014;16:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steil GM. Algorithms for a closed-loop artificial pancreas: the case for proportional-integral-derivative control. J Diabetes Sci Technol. 2013;7:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mauseth R, Hirsch IB, Bollyky J, et al. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther. 2013;15:628–633. [DOI] [PubMed] [Google Scholar]
- 11. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nimri R, Muller I, Atlas E, Miller S, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes. 2014;15:91–99. [DOI] [PubMed] [Google Scholar]
- 13. Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17–26. [DOI] [PubMed] [Google Scholar]
- 14. Cameron F, Niemeyer G, Wilson DM, et al. Inpatient trial of an artificial pancreas based on multiple model probabilistic predictive control with repeated large unannounced meals. Diabetes Technol Ther. 2014;16:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931–1937. [DOI] [PubMed] [Google Scholar]
- 17. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zisser H, Renard E, Kovatchev B, et al. , Control to Range Study Group. Multicenter closed-loop insulin delivery study points to challenges for keeping blood glucose in a safe range by a control algorithm in adults and adolescents with type 1 diabetes from various sites. Diabetes Technol Ther. 2014;16:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovatchev BP, Mendosa P, Anderson S, Hawley JS, Ritterband LM, Gonder-Frederick L. Effect of automated bio-behavioral feedback on the control of type 1 diabetes. Diabetes Care. 2011;34:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grosman B, Dassau E, Zisser HC, Jovanovič L, Doyle FJ., III Zone model predictive control: a strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Technol. 2010;4:961–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harvey RA, Dassau E, Zisser H, Seborg DE, Jovanovič L, Doyle FJ., III Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol. 2012;6:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gondhalekar R, Dassau E, Zisser HC, Doyle FJ., III Periodic-zone model predictive control for diurnal closed-loop operation of an artificial pancreas. J Diabetes Sci Technol. 2013;7:1446–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gondhalekar R, Dassau E, Doyle FJ., III MPC design for rapid pump-attenuation and expedited hyperglycemia response to treat T1DM with an artificial pancreas. Paper presented at: American Control Conference 2014; Portland, OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovič L, Doyle FJ., III Modular artificial β-cell system: a prototype for clinical research. J Diabetes Sci Technol. 2008;2:863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kovatchev BP, Straume M, Cox DJ, Farhy LS. Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes. Comput Math Methods Med. 2000;3:1–10. [Google Scholar]
- 26. Snedecor GW, Cochran WG. Statistical Methods. 8th ed Iowa State University Press; Ames, IA. [Google Scholar]
- 27. Hinshaw L, Dalla Man C, Nandy DK, et al. Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes. 2013;62:2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]