Abstract
Context:
Limited data suggest that interrupting sedentary behaviors with activity improves metabolic parameters in adults.
Objective:
We tested whether interrupting sitting with short, moderate-intensity walking bouts improved glucose tolerance in children.
Design:
Participants underwent two experimental conditions in random order on different days: continuous sitting for 3 hours or sitting interrupted by walking (3 min of moderate-intensity walking every 30 min). Insulin, C-peptide, glucose, and free fatty acids were measured every 30 minutes for 3 hours during an oral glucose tolerance test. Area under the curve (AUC) was calculated from hormone and substrate measurements. Children were given a buffet meal after each condition.
Setting:
The study was conducted at the National Institutes of Health Hatfield Clinical Research Center.
Participants:
Twenty-eight normal-weight 7–11 year olds participated.
Main Outcomes:
Patterns of substrate/hormone secretion and AUC, as well as energy intake, were examined by experimental condition.
Results:
Interrupting sitting resulted in a 32% lower insulin AUC (P < .001), 17% lower C-peptide AUC (P < .001), and 7% lower glucose AUC (P = .018) vs continuous sitting. Mixed model results indicated that insulin (P = .036) and free fatty acid concentrations (P = .009) were significantly lower in the interrupted vs the continuous sitting condition. Lunchtime buffet meal energy intake did not significantly differ between the conditions (975 ± 387 vs 963 ± 309 kcal; P = .85).
Conclusions:
Interrupting sedentary time with brief moderate-intensity walking improved short-term metabolic function in non-overweight children without increasing subsequent energy intake. These findings suggest that interrupting sedentary behavior may be a promising prevention strategy for reducing cardiometabolic risk in children.
Excessive time used for sedentary behaviors is associated with pediatric obesity and metabolic risk (1). Sedentary behaviors are low-intensity (intensity < 1.5 metabolic equivalent, or the ratio of the working metabolic rate to the resting metabolic rate) (2) activities with limited body movement in a seated or reclining position (3). National Health and Nutrition Examination Survey data indicate that children spend about 6 hours per day sedentary (4). Many studies (5–7), although not all (8), suggest that sedentary behaviors are positively associated with obesity and insulin resistance, independent of fat mass (9), and predict gains in body mass index (BMI) from 9–15 years of age, independent of moderate to vigorous physical activity (10). Thus, sedentary behaviors may contribute to the high obesity prevalence and increased metabolic risk of children in the United States and Europe.
Sedentary behaviors are consistent from childhood through adolescence. Tracking coefficients are moderate-to-large for sedentary behaviors (and highest for TV viewing) (11). Thus, preventing excessive sedentary behaviors in young children could decrease sedentary behaviors in adults, which are well linked to metabolic consequences (12). Recent studies have investigated acute metabolic effects of interrupting sitting as a potential prevention strategy. Dunstan et al (13) conducted the first randomized crossover study in overweight adults where prolonged sitting was interrupted with 2 minutes of light- and moderate-intensity walking every 20 minutes for 5 hours. Interrupting sitting significantly lowered glucose and insulin responses. However, recent findings from similar in-laboratory studies with young adults and adolescents are mixed (14, 15).
We therefore conducted a randomized crossover study to evaluate the effects of prolonged vs interrupted sitting on metabolism and energy intake in healthy 7–11 year olds. We hypothesized that, compared to prolonged sitting, interrupted sitting would result in lower insulin and glucose area under the curve (AUC) during an oral glucose tolerance test (OGTT). Our secondary hypotheses were that, compared to prolonged sitting, interrupted sitting would also result in salutary changes in triglyceride, free fatty acid (FFA), cortisol, and C-peptide concentrations, without changing postintervention dietary intake.
Subjects and Methods
Study overview
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board approved the randomized crossover trial (ClinicalTrials.gov registration no. NCT01888939). Participants were seen at the National Institutes of Health (NIH) Clinical Research Center on three separate occasions. After a baseline screening visit to assess participant eligibility, those who qualified and were willing to adhere to study procedures returned twice more to complete the two experimental conditions (uninterrupted sitting, and sitting interrupted with walking), in random order. The washout period between visits was 7 to 30 days to avoid any potential carryover effects of acute bouts of activity (16).
Participants
Study participants were recruited between June 2013 and December 2014 via flyers, listservs, word of mouth, and social media from the surrounding geographical area. Inclusion criteria were: good general health, age of 7–11 years, fasting plasma glucose < 100 mg/dL, and BMI between the fifth and 84th percentiles on the age- and sex-specific Centers for Disease Control growth standards (17). Exclusion criteria were: overweight or obesity (BMI ≥ 85th percentile); the presence of significant cardiac or pulmonary disease that could result in hypoxia or decreased perfusion; impaired glucose tolerance or type 2 diabetes; the presence of other endocrine disorders leading to obesity (eg, Cushing syndrome); use of medications that would affect metabolism (eg, antipsychotic medications), cognitive outcomes, or body habitus; medical treatment other than diet for hypertension or dyslipidemia; undue advancement of pubertal development; and a below-average age-adjusted standard score (<85) on the NIH Toolbox Picture Vocabulary Test (18).
Screening visit
Participants and parents gave assent/consent and were screened for eligibility. Participants underwent a physical examination, and parents provided a medical history. Pubertal stage was assessed at this visit. Blood obtained from participants after an overnight fast was used to examine metabolic, hematological, renal, and hepatic function. Fasting plasma glucose was used to exclude those with impaired fasting glucose or diabetes. Dual-energy x-ray absorptiometry (Lunar iDXA; GE Healthcare) and software (GE Encore 11.10) assessed body composition. A 12-lead electrocardiogram, anthropometric measures (waist and hip circumference), and a maximum oxygen uptake (VO2max) fitness test using a modified Balke continuous ramp protocol (19) were performed. The 10-point Borg scale of Rating of Perceived Exertion was used to assess exercise tolerance. Ventilatory threshold (VT) estimated by gas exchange, was determined using the V-slope method and dual criteria graphs (20). The walking speed and the treadmill grade for the interrupted sitting condition were determined as the speed corresponding with VO2 and heart rate at 80% of the VT.
Receptive vocabulary (a verbal IQ proxy) was assessed via a Picture Vocabulary Test using the NIH Toolbox (www.nihtoolbox.org).
Study protocol
Experimental design
The experimental conditions were:
Uninterrupted sitting (SIT): Participants remained seated for 3 hours, with limited movement, and were allowed to rise only to use the bathroom.
Sitting interrupted with walking (SIT+WALK): Participants walked on a treadmill at a customized walking speed (80% of VT) for 3 minutes every 30 minutes (eg, during min 27–30, 57–60, etc). Heart rate was monitored, and the walking speed was increased or decreased so as to keep the participant near the target heart rate (as determined from the VO2max test). After walking, the participants returned to sitting. Participants walked six times during the 3-hour test, for a total of 18 minutes.
Eligible participants were randomized 1:1 using random permutations (www.randomization.com) to experimental order (SIT, then SIT+WALK; or SIT+WALK, then SIT). Randomization was stratified by sex. Before interventions were assigned, the randomization sequence was concealed from participants but not from investigators. Subjects were informed of their experimental order when they participated in the first experimental condition. Study team members (S.M.B., B.R.B.) enrolled participants and assigned them to the two experimental orders.
Data collection
Participants wore a triaxial accelerometer (Actigraph GT3X+; Actigraph) on the nondominant wrist for 1 week before each study visit to measure habitual, free-living activity and sleep. Data were recorded from the three axes at a rate of 80 Hz and later filtered and integrated using the manufacturer's software (Actilife, version 6.11) into 1-minute epochs yielding activity and step counts. The values from each axis were summed to create a minute-based vector magnitude.
For the experimental visits, participants reported to the NIH Metabolic Clinical Research Unit between 7:30 and 8 am, after an overnight fast. Members of the research team supervised participants throughout the experimental conditions to ensure they adhered to the protocol. Nurses collected vital signs, weight (model 5702 scale; Scale-Tronix), and height (electronic height measure, model 242; Seca). During these visits, participants wore two Actigraph GT3X+ accelerometers on the nondominant wrist and right hip to quantify in-lab movement. Heart rate was measured using a Polar RS800CX Watch plus chest-band electrode (Polar Electro Inc). Hip and wrist accelerometer data were aligned with 1-second heart rate data based on the position of maximum cross-correlation between the two series using custom software written in Matlab (The Mathworks Inc). The aligned activity series was further integrated into 60-second epochs, and vector magnitude was calculated on a per-minute basis. Step counts were cumulated in 60-second epochs and were based on hip-mounted data on the vertical axis. A trained nutrition staff member conducted a computer-assisted, multiple-pass, 24-hour dietary recall (Nutrition Data System for Research, Nutrition Coordinating Center) at each experimental visit.
At approximately 9 am, an iv catheter was inserted in the antecubital vein. Two fasting blood samples were obtained at −10 minutes and 0 minutes, and then participants were instructed to consume a dextrose oral solution (Glucola; 1.75 g/kg dextrose, maximum 75 g). Participants watched movies, read, colored, or did homework while seated. Blood samples were drawn every 30 minutes for 3 hours. In the SIT+WALK condition, samples were obtained immediately after each 3-minute walking bout; samples were obtained in the SIT condition at the same times relative to consumption of the dextrose oral solution.
Participants ate from a standardized 9835-kcal multiple-item buffet test meal after the OGTT was completed. The meal consisted of an array of macronutrients (12% protein, 51% carbohydrate, and 37% fat) from foods frequently consumed by children (21). Amounts consumed were calculated by measuring the difference in the weight of each item before and after the meal. Caloric and macronutrient consumption was calculated using the USDA National Nutrient Database for Standard Reference (Agricultural Research Service) and nutrient information from the manufacturer.
Assays
Insulin, C-peptide, glucose, cortisol, FFA, and triglyceride concentrations were measured at −10, 0, 30, 60, 90, 120, 150, and 180 minutes. Baseline values were calculated as the average of the −10- and 0-minute results. The NIH Clinical Center Department of Laboratory Medicine assayed the samples. Plasma for glucose was collected in tubes containing powdered sodium fluoride and was kept on ice until centrifuged; glucose was measured using a Hitachi 917 analyzer (Roche Diagnostics). Total cholesterol and triglycerides were also measured on a Hitachi 917 analyzer using reagents from Roche Diagnostics. Insulin concentrations were determined using a commercially available immunochemiluminometric assay purchased from Diagnostic Products Corporation and calibrated against insulin reference preparation 66/304. The insulin assay used a monoclonal anti-insulin antibody and was run on an Immulite 2000 machine (Diagnostic Products Corp). The cross-reactivity of the insulin assay was 8% with proinsulin and 1% with C-peptide; sensitivity was 2 μU/mL, and the mean inter- and intra-assay coefficients of variation were 5.8 and 3.6%, respectively. C-peptide was measured using the Roche Cobas 6000 analyzer (Roche Diagnostics). The mean intra- and interassay coefficients of variation were 1.1 and 2.7%, respectively. Plasma FFA was measured using the Wako HR Series NEFA kit (Wako Chemicals USA, Inc,). The mean intra- and interassay coefficients of variation were 8.7 and 5.1%, respectively. Cortisol was measured on the Immulite 2000 XPi Analyzer (Siemens USA). The mean intra- and interassay coefficients of variation were 10.2 and 9.9%, respectively.
Statistical analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute) and GraphPad Prism 6 (GraphPad Software, Inc). Sample size calculations were based on the one extant adult trial at the time (13), pilot data from Dr. Mai Chin A. Paw, and previously collected data from our laboratory. These calculations were based on the mean change in insulin AUC between experimental conditions, assuming a moderate effect size (Cohen's d of 0.40) and correlation coefficient of 0.5 between repeated measures. We estimated that 27 paired observations were needed to achieve a power of 0.80 for one-sided hypothesis testing and P < .025. We aimed to randomize at least 30 children (15 per crossover order group).
Paired t-tests assessed any significant differences in descriptive baseline mean ± SD values for serum measures, dietary intake, activity, and sleep outcomes between the two experimental visits. The crossover study design allows for the examination of within-individual comparisons of test effects and within-individual longitudinal test effects.
For our primary outcomes (insulin and glucose AUC), a repeated measures ANOVA assessed differences in mean serum values by condition, controlling for randomization order. For the exploratory outcomes, repeated measures ANOVA controlling for randomization order assessed differences by condition in mean C-peptide, triglyceride, cortisol, and FFA AUC. Paired t-tests assessed differences in mean energy intake from the buffet meal. Mixed models assessed the effect of the experimental condition on changes in log-transformed insulin, glucose, and exploratory outcome hormone concentrations measured at 30-minute intervals over the 3 hours. Variance components were estimated using the restricted maximum likelihood estimate method to control for within-individual correlations and an unstructured covariance structure. Time-invariant covariates were: baseline serum values, age (years), sex, fat mass (kilograms), randomization order (ie, SIT, SIT+WALK order vs SIT+WALK, SIT), visit condition (SIT and SIT+WALK experimental conditions), and pubertal stage. Time-variant variables were time (minutes) and serum values. Including pubertal status in the models did not change the direction or significance of any finding.
Results
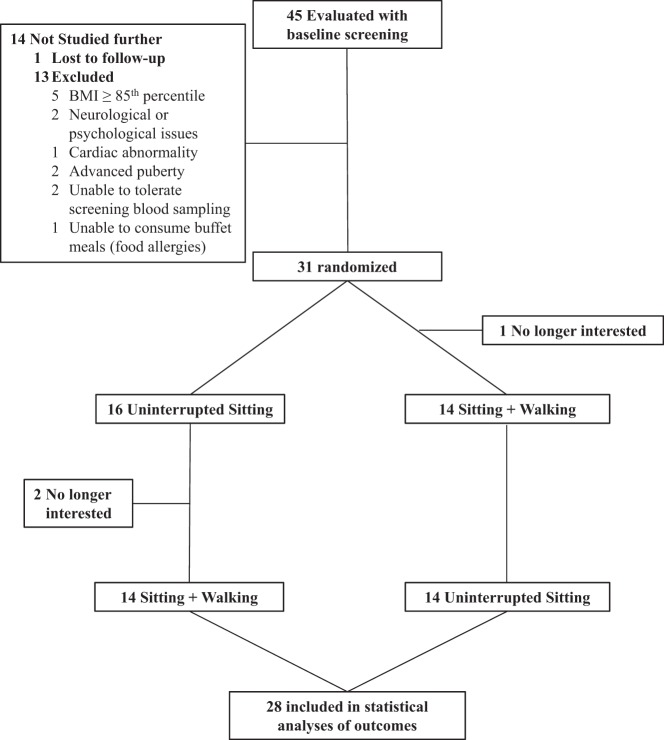
Figure 1 presents participant flow through the protocol. Of the 31 children randomized, 28 completed all assessments. Table 1 presents baseline sample characteristics of the children who completed the study. Participants had a mean ± SD age of 10.2 ± 1.5 years, were prepubertal or in early puberty (12 were in stage 1, 15 were in stage 2, and one was in stage 3), were of healthy weight, and had normal fasting glucose concentrations. There were no statistically significant differences in mean baseline values by randomization order, with the exception of fasting glucose, which was slightly higher in participants randomized to SIT followed by SIT+ WALK experimental order (89 ± 2.9 vs 86 ± 4.0; P = .02). There was an average interval of 28 days between the screening and the first experimental visit, and an average of 20 days between the two experimental visits. During the week before experimental visits, free-living energy intake, activity, sleep time, and sleep efficiency did not significantly differ by condition (Supplemental Table 1). Participants consumed the Glucola over an average of 5.6 minutes, with no significant difference between conditions (P = .403). There were no adverse events recorded during the study.
Figure 1.
Participant flow through the study.
Table 1.
Baseline Characteristics of Participants (n = 28)
| Variable | Mean (SD) |
|---|---|
| Sex, n (%) | |
| Boys | 15 (54) |
| Girls | 13 (46) |
| Race, n (%) | |
| White | 17 (61) |
| Black/AA | 4 (14) |
| Asian | 4 (14) |
| Multiracial | 3 (11) |
| Ethnicity, n (%) | |
| Non-Hispanic/Latino | 25 (89) |
| Hispanic/Latino | 3 (11) |
| Age, y | 10.2 (1.5) |
| Pubertya | |
| Boys, testis volume, mL | 3 (1–5) |
| Girls, Tanner breast stage | 1 (1–3) |
| Picture vocabulary score (age-adjusted percentile) | 113 (10.4) |
| Fat mass, kg | 7.2 (2.4) |
| Lean mass, kg | 22.2 (3.8) |
| Waist-height ratio | 0.4 (0.03) |
| BMI, kg/m2 | 16.2 (1.5) |
| BMI z-score | −0.3 (0.6) |
| Fasting glucose, mg/dL | 88 (3.8) |
| Systolic BP, mm Hg | 105 (8.3) |
| Diastolic BP, mm Hg | 58 (7.1) |
Abbreviations: AA, African American; BP, blood pressure. Results are expressed as mean (SD) unless otherwise indicated.
Median (range); breast for girls, testicular volume for boys.
During the OGTT, the mean heart rate, wrist- and hip-mounted triaxial vector sum magnitude, and step counts over the entire 3 hours were significantly higher in the SIT+WALK condition vs the SIT condition (Supplemental Table 2). When we compared the sitting and walking periods during the SIT+WALK condition for all subjects, we found that the mean ± SD heart rate was 89 ± 8 beats per minute during sitting vs 121 ± 9 beats per minute during walking (P < .001); the mean wrist-mounted triaxial vector magnitude counts were 1012 ± 671 during sitting vs 4826 ± 2527 during walking (P < .001); the mean hip-mounted triaxial vector magnitude counts were 282 ± 173 during sitting vs 3037 ± 891 during walking (P < .001); and the mean step count per minute was 4 ± 11 during sitting vs 260 ± 557 during walking (P < .001). These data indicate that the experimental conditions were implemented correctly. Supplemental Figure 1 shows representative results from one participant for heart rate and wrist- and hip-mounted accelerometer data over the 3-hour experimental period.
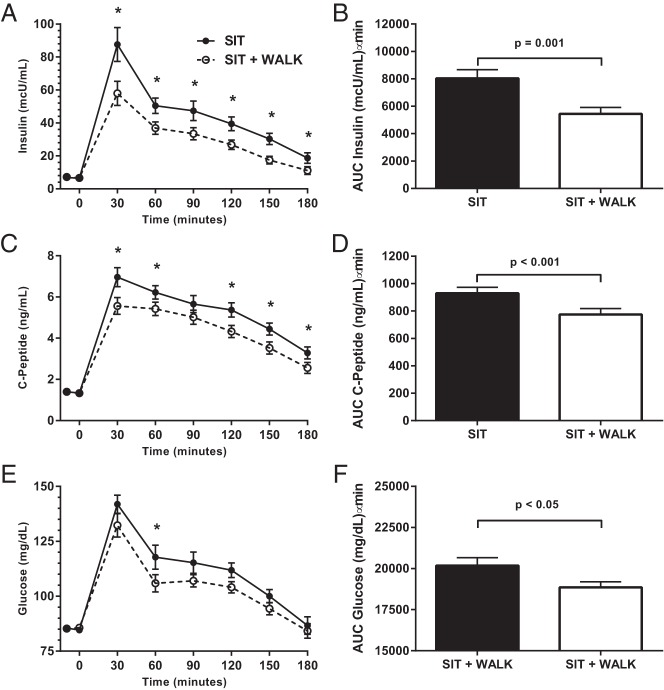
Figure 2 and Table 2 present the findings from the time course and AUC analyses. Results from mixed models found that insulin secretion over the 3-hour test was significantly lower in the SIT+WALK vs the SIT condition (Table 2; F = 2.28; P = .036). Postdrink mean insulin concentrations were significantly lower at all of the time points except 0 minutes (P < 0.02) in the SIT+WALK condition (Figure 2A). Mean insulin AUC was 32% lower in the SIT+WALK condition (Figure 2B; 5448 ± 2447 μU/mL · min) compared to the SIT condition (8036 ± 3346 μU/mL · min; P < .001). The Cohen's d for the insulin AUC differences between conditions was 0.88, yielding a moderate effect size (d = 0.40).
Figure 2.
The effect of sitting interrupted with 3 minutes of moderate-intensity walking every half hour (SIT+WALK) vs SIT on serum insulin concentrations (A); 3-hour insulin AUC (B); serum C-peptide concentrations (C); 3-hour C-peptide AUC (D); plasma glucose concentrations (E); and 3-hour glucose AUC (F). Unadjusted means ± SE are shown in A, C, and E. AUC results (B, D, and F) are mean ± SE adjusted for randomization order. *, Significantly different, SIT vs SIT+WALK; P < .05.
Table 2.
Results From Six Separate Mixed Models Predicting Change in Substrate/Hormone Concentrations Over Time by Visit Type: Sitting Interrupted With 3 Minutes of Moderate-Intensity Walking Every Half Hour (SIT + WALK) or Uninterrupted Sitting (SIT) (n = 28)
| Variable | Insulina |
C-Peptidea |
Glucosea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | F Valueb | P Value | Estimate | SE | F Valueb | P Value | Estimate | SE | F Valueb | P Value | |
| Intercept | 0.625 | 0.201 | 3.12 | .005 | 0.126 | 0.123 | 1.02 | .318 | 1.679 | 0.097 | 17.34 | <.001 |
| Age, y | 0.026 | 0.024 | 1.22 | .270 | 0.016 | 0.015 | 1.18 | .277 | 0.003 | 0.005 | 0.39 | .531 |
| Sex (ref = male) | 0.006 | 0.107 | 0.00 | .958 | −0.007 | 0.065 | 0.01 | .910 | −0.008 | 0.025 | 0.10 | .755 |
| Tanner stage 2 (ref = 1) | −0.041 | 0.107 | −0.39 | .699 | 0.033 | 0.066 | −0.51 | .610 | −0.028 | 0.025 | −1.14 | .257 |
| Tanner stage 3 (ref = 1) | 0.032 | 0.154 | 0.21 | .835 | 0.028 | 0.094 | 0.29 | .771 | 0.011 | 0.034 | 0.32 | .753 |
| Total fat mass, kg | 0.000 | 0.000 | 0.00 | .987 | 0.000 | 0.000 | 0.03 | .869 | 0.000 | 0.000 | 0.50 | .482 |
| Baseline variable | 0.036 | 0.007 | 28.34 | <.001 | 0.160 | 0.028 | 31.06 | <.001 | 0.003 | 0.001 | 9.55 | .002 |
| Randomization order (ref = SIT followed by SIT+WALK) | −0.020 | 0.053 | 0.14 | .705 | 0.001 | 0.033 | 0.00 | .983 | −0.005 | 0.013 | 0.17 | .676 |
| Time | 112.76 | <.001 | 206.40 | <.001 | 59.64 | <.001 | ||||||
| Visit type (ref = SIT) | −0.254 | 0.068 | 46.43 | <.001 | −0.119 | 0.033 | 42.10 | <.001 | −0.010 | 0.019 | 12.16 | <.001 |
| Time ∗ visit type | 2.28 | .036 | 1.60 | .146 | 0.77 | .594 | ||||||
| 0 min | −0.034 | 0.068 | −0.50 | .615 | 0.005 | 0.033 | 0.14 | .887 | −0.004 | 0.019 | −0.23 | .815 |
| 30 min | 0.197 | 0.068 | 2.89 | .004 | 0.107 | 0.033 | 3.22 | .001 | 0.036 | 0.019 | 1.90 | .058 |
| 60 min | 0.168 | 0.068 | 2.47 | .014 | 0.066 | 0.033 | 2.00 | .047 | 0.044 | 0.019 | 2.35 | .020 |
| 90 min | 0.164 | 0.068 | 2.41 | .017 | 0.055 | 0.033 | 1.65 | .100 | 0.029 | 0.019 | 1.57 | .118 |
| 120 min | 0.196 | 0.068 | 2.87 | .004 | 0.100 | 0.033 | 0.02 | .003 | 0.032 | 0.019 | 1.68 | .095 |
| 150 min | 0.286 | 0.068 | 4.20 | <.001 | 0.117 | 0.033 | 3.55 | <.001 | 0.027 | 0.019 | 1.45 | .149 |
| 180 min | 0.254 | 0.068 | 3.73 | <.001 | 0.119 | 0.033 | 3.60 | <.001 | 0.010 | 0.019 | 0.54 | .590 |
| Variable | Free Fatty Acidsa,c |
Triglyceridesa,c |
Cortisola |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | F Valueb | P Value | Estimate | SE | F Valueb | P Value | Estimate | SE | F Valueb | P Value | |
| Intercept | −1.166 | 0.166 | −7.02 | <.001 | 1.300 | 0.073 | 17.81 | <.001 | 0.680 | 0.080 | 8.47 | <.001 |
| Age, y | −0.008 | 0.019 | 0.02 | .658 | −0.009 | 0.009 | 10.8 | .299 | −0.009 | 0.009 | 0.92 | .337 |
| Sex (ref = male) | −0.095 | 0.084 | 1.28 | .259 | −0.042 | 0.039 | 1.16 | .283 | 0.063 | 0.041 | 2.37 | .124 |
| Tanner stage 2 (ref = 1) | −0.102 | 0.087 | −1.18 | .241 | −0.011 | 0.039 | −0.28 | .783 | 0.026 | 0.041 | 0.63 | .530 |
| Tanner stage 3 (ref = 1) | −0.212 | 0.119 | −1.78 | .075 | 0.009 | 0.054 | 0.17 | .864 | −0.085 | 0.058 | −1.48 | .140 |
| Total fat mass, kg | 0.00 | 0.00 | 0.45 | .501 | 0.00 | 0.00 | 0.00 | .957 | 0.00 | 0.00 | 0.06 | .812 |
| Baseline variable | 0.311 | 0.056 | 31.21 | <.001 | 0.007 | 0.000 | 767.45 | <.001 | 0.014 | 0.003 | 23.44 | <.001 |
| Randomization order (ref = SIT followed by SIT+WALK) | 0.019 | 0.044 | 0.18 | .673 | 0.000 | 0.020 | 0.00 | .988 | −0.017 | 0.021 | 0.70 | .402 |
| Time | 150.54 | <.001 | 52.68 | <.001 | 29.05 | <.001 | ||||||
| Visit type (ref = SIT) | 2.00 | .159 | 16.54 | <.001 | 0.09 | .766 | ||||||
| Time ∗ visit type | 2.90 | .009 | 0.75 | .610 | 0.05 | .999 | ||||||
| 0 min | 0.059 | 0.057 | 1.04 | .300 | 0.006 | 0.017 | 0.33 | .738 | 0.010 | 0.324 | 0.32 | .750 |
| 30 min | 0.144 | 0.057 | 2.25 | .012 | −0.040 | 0.017 | −2.31 | .022 | −0.000 | 0.324 | −0.01 | .999 |
| 60 min | −0.110 | 0.057 | −1.93 | .055 | −0.032 | 0.017 | −1.86 | .064 | −0.005 | 0.324 | −0.15 | .881 |
| 90 min | −0.049 | 0.057 | −0.87 | .386 | −0.034 | 0.017 | −1.97 | .050 | 0.014 | 0.324 | 0.042 | .675 |
| 120 min | −0.072 | 0.057 | −1.26 | .207 | −0.027 | 0.017 | −1.56 | .120 | 0.010 | 0.324 | 0.030 | .766 |
| 150 min | −0.060 | 0.057 | −1.06 | .292 | −0.028 | 0.017 | −1.62 | .105 | 0.001 | 0.324 | 0.02 | .983 |
| 180 min | −0.125 | 0.057 | −2.19 | .029 | −0.031 | 0.017 | −1.85 | .065 | −0.003 | 0.324 | −0.11 | .914 |
Log-transformed AUC.
t value for intercept and time∗visit type interactions (random effects).
n = 27 because of missing data.
The condition*time interaction for C-peptide (Figure 2C) was not significant in the mixed model (Table 2; F = 1.60; P = .146), indicating that there were no significant changes in C-peptide concentrations over the 3 hours between conditions related to time of measurement. However, there was a main effect of condition on C-peptide (P < .001), such that concentrations were lower 30, 60, 120, 150, and 180 minutes postdrink in the SIT+WALK condition (Figure 2C; P < .05). Mean C-peptide AUC was 17% lower in the SIT+WALK condition (Figure 2D; 774 ± 231 ng/mL · min) compared to the SIT condition (929 ± 233 ng/mL · min; P < .001). The Cohen's d for the C-peptide AUC difference between experimental conditions was 0.67, yielding a small effect size (d = 0.32).
For plasma glucose, the condition*time interaction was not significant in the mixed model (Table 2; F = 0.77; P = .594). However, there was a main effect (P < .001) of condition on glucose, such that concentrations were lower at 60 minutes postdrink in the SIT+WALK condition (Figure 2E). Mean glucose AUC was 7% lower in the SIT+WALK condition (Figure 2F; 18 855 ± 1822 mg/mL · min) compared to the SIT condition (20 181 ± 2548 mg/mL · min; P = .02). The Cohen's d for the glucose AUC differences between experimental conditions was 0.60, yielding a small effect size (d = 0.29).
There were no significant differences in FFA, triglycerides, and cortisol AUC. Table 2 presents findings from the mixed models. There was no significant main effect for FFA patterns; however, there was a statistically significant time*condition interaction (P = .009), such that concentrations were lower at 30 minutes in the SIT+WALK condition but higher at 60 and 180 minutes (P < .055). There was a main effect of condition for triglycerides (P < .001), such that concentrations were higher at 30 and 90 minutes postdrink in the SIT+WALK condition (P < .050). There were no statistically significant effects for cortisol. Total energy intake and the percentage of calories consumed from carbohydrates, fats, and proteins from the post-test ad libitum meal did not significantly differ by condition type (Supplemental Table 2).
Discussion
This study, to the best of our knowledge, provides the first evidence that interrupting sitting time with brief bouts of moderate-intensity walking can improve short-term metabolic function in healthy non-overweight children. Consistent with our hypothesis, OGTT-induced insulin, C-peptide, and glucose AUC were significantly lower in the SIT+WALK condition vs the SIT condition. Our findings are consistent with those of Dunstan et al (13), who reported that, in adults, interrupting sitting reduced insulin AUC by 23% compared to a continuous sitting condition. Similarly, Altenburg et al (15) reported lower postprandial C-peptide concentrations after moderate-intensity cycling bouts compared to continuous sitting over 8 hours. Taken together, these findings suggest that interrupting sitting with short bouts of activity has an effect on acute glucose homeostasis.
Glucose effectiveness (SG) is the ability of glucose per se to stimulate its own uptake and directly inhibit endogenous glucose production in the liver (22, 23). The hyperglycemia induced by the Glucola in our overnight fasted sample likely had an inhibitory effect on endogenous glucose production, independent of insulin and other hormones (24). However, peripheral tissues are responsible for most glucose uptake (23), and SG is improved with exercise, independent of insulin action (25). Brun et al (26) demonstrated that 15 minutes of submaximal exercise at 85% of predicted maximal heart rate increased SG via muscle contractions and enhanced glucose transporter4 on the skeletal muscle cell surface. The lower glucose AUC (when insulin concentrations were also lower) in the SIT+WALK condition is likely due to the influence of muscle-contracting activity of the moderate-intensity walking on SG.
The lower postprandial C-peptide and insulin AUC we observed in the SIT+WALK condition suggest lower endogenous insulin secretion causing lower circulating insulin concentrations. These acute responses may have long-term effects. Sustained sedentary behavior diminishes the use of skeletal muscle to assist with glucose uptake from the bloodstream after a meal, requiring the pancreas to secrete more insulin that may increase the risk for β-cell dysfunction (27). Also, lower insulin-mediated glucose uptake by skeletal muscle leads to a longer exposure to high circulating insulin. This hyperinsulinemia promotes lipogenesis and uptake of fatty acids into adipose tissue (28), resulting in increased adiposity and worsening insulin resistance (29). Additional research should explore how the duration and patterns of sedentary behaviors may produce negative health effects in children and whether these effects can be overcome by interruption with short periods of moderate-intensity walking.
Despite the positive results of our clinical study, there are conflicting findings for the effects of adding activity during sedentary behaviors on metabolic outcomes in children. Saunders et al (14) assessed the acute cardiometabolic effects of 8 hours of sitting compared to 2 minutes of light-intensity walking every 20 minutes and light-intensity walking (30% of VO2 peak) plus two 20-minute moderate-intensity physical activity breaks (60% VO2 peak) in youth. There were no significant differences in AUC for insulin, glucose, or triglycerides by condition. One explanation for these contrasting findings is that our sample consisted solely of normal-weight children, whereas their sample combined normal-weight and overweight youth that might have made the data considerably more variable. Another potential explanation is that their dose of physical activity precluded observing changes in metabolic parameters. Our moderate-intensity breaks were at 80% of the VT (below the anaerobic threshold), and participants were individually monitored to maintain activity at the specified level. It is possible that the higher level of activity studied by Saunders et al (14) may have resulted in changes in glucose concentrations to support working muscles during prolonged activity at or above the anaerobic threshold (30) and obscured their ability to detect acute changes in insulin and glucose.
One of the leading hypotheses linking sitting to increased cardiometabolic risk is decreased lipoprotein lipase activity (31) that reduces the uptake of postprandial fatty acids. This results in prolonged exposure of the arterial walls to plasma triglycerides, increasing cardiometabolic risk (32) via increased concentration of circulating cell adhesion molecules that play a role in the development of atherosclerosis and type 2 diabetes (33). Similar to previous clinical studies that gave participants a mixed meal (14, 15), we did not observe any consistent differences in triglyceride or FFA patterns between conditions. This may reflect the healthy nature of our study participants or indicate that a longer bout of continuous sitting is needed to see changes in these outcomes. There was some evidence that triglyceride concentrations were higher and FFA concentrations were lower in the SIT+WALK condition at 30 and 90 minutes. These findings may be explained by the suppressive effect that glucose and insulin have on lipolysis, which lowers plasma FFA (34). Alternatively, longer bouts of exercise (≥1 h) increase FFA uptake (35). Thus, the modest amount of walking may have slightly lowered FFA concentrations, although caution should be taken when interpreting these findings given the small differences in patterns of FFA over time, and that the amount of activity was much lower than that induced in the aforementioned exercise studies.
Sedentary behaviors, such as TV viewing, are associated with lower fruit and vegetable consumption and higher energy-dense snack and fast foods consumption (36). Fast food and snack consumption is also positively associated with sedentary time (37). The findings from these studies may suggest that sedentary behaviors could influence dietary choices toward less healthy foods but do not establish causality. We did not observe any differences in subsequent energy intake from the buffet meal by condition. This is consistent with results from Saunders et al (38). The dose of activity in the SIT+WALK condition was low and was not sufficient to produce increases in energy intake. If these effects can be demonstrated over longer time periods, the small increases in energy expenditure from interrupted sitting and improved metabolic responses may potentially lead to better energy balance and glucose homeostasis in healthy children. Longer-term data regarding the effects of interrupting sedentary behaviors on energy intake in children are needed.
There are some limitations to our study. First, we had a relatively small sample size that may have influenced our ability to detect significant associations for some outcomes. Indeed, the observed effect sizes for glucose and C-peptide AUC were small, indicating that a larger sample may be needed to determine whether postdrink patterns differ. However, we did have sufficient power to detect differences in insulin AUC, suggesting that our prestudy power calculations were accurate. Second, we did not limit participants' free-living activity before study visits. Research suggests that acute activity in the previous 24 hours influences insulin sensitivity, glucose control (39), and triglyceride responses (40). However, accelerometer-measured activity in our sample was not significantly different before each experimental condition. Finally, because we studied only non-overweight children, these results may not generalize to children who are overweight or obese.
This study has several strengths that also warrant discussion. We employed a rigorous randomized crossover study design, which limited the between-individual variation from test-related differences. The in-lab conditions were tightly controlled, leading to less potential confounding that may have influenced the results. Finally, the moderate-intensity walking was tailored to each participant's fitness level, and the free-living activity, sleep, and dietary assessments allowed us to determine whether any outside factors changed before the visits.
In conclusion, interrupting sitting with brief moderate-intensity walking improved short-term metabolic function in non-overweight children, without increasing subsequent energy intake. Recently, Larsen et al (41) assessed the 3-day effects of 2-minute light-intensity walking breaks every 20 minutes for 7 h/d compared to prolonged sitting for 7 h/d in overweight and obese adults. They reported that the walking breaks significantly reduced insulin and glucose AUC compared to the prolonged sitting condition. However, the walking breaks on subsequent days did not further improve the glycemic response. It is unclear whether similar effects would be observed in healthy children, and more research is needed to assess the longer-term duration of these acute effects. Should these findings be confirmed and extended in longer-term studies, interrupting sitting may be an effective prevention strategy to reduce cardiometabolic risk in children.
Acknowledgments
The authors thank the patients and their families for their participation, as well as the National Institutes of Health Clinical Center staff and the Metabolic Unit staff. B.R.B., D.B., and J.A.Y. thank Dr. Mai Chin A. Paw for her assistance with power calculations for the study.
This study was supported by the Intramural Research Programs of the National Cancer Institute (NCI) and National Institute of Child Health and Human Development (Z1A-HD-00641; to J.A.Y.) at NIH. B.R.B. was supported by a postdoctoral training award from the NCI Cancer Prevention Fellowship Program in the Division of Cancer Prevention. D.B. was supported by the NCI, Division of Cancer Control and Population Sciences. S.M.B., R.J.B., K.P.S., D.R.R., P.L.W., A.B.C., S.B.B., and K.Y.C. are federal employees supported by the Intramural Research Program of the NIH. I.L.T. was supported by the NIH Office of the Director. The funding agencies played no role in the decision to submit the manuscript.
J.A.Y. and B.E.D. are Commissioned Officers in the US Public Health Service. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the US Public Health Service.
Author Contributions: B.R.B., D.B., and J.A.Y. designed the study; oversaw the entire study including recruitment, data collection, and analysis; had full access to all the data in the study; take responsibility for the integrity of the data and the accuracy of the data analysis; drafted the manuscript; and take full responsibility for the work as a whole. A.B.C., B.E.D., D.R.R., P.L.W., and K.Y.C. provided feedback on the study design, supervised parts of the experimental paradigm, and were involved in the interpretation of study findings. A.P., S.M.B., I.L.T., S.B.B., R.J.B., J.B.H., and K.P.S. assisted in carrying out the study and were involved in the interpretation of study findings. All authors provided critical input to and review of the manuscript.
Trial Registration: Clinicaltrials.gov NCT01888939.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- FFA
- free fatty acid
- AUC
- area under the curve
- OGTT
- oral glucose tolerance test
- SG
- glucose effectiveness
- SIT
- uninterrupted sitting
- SIT+WALK
- sitting interrupted with walking
- VO2max
- maximum oxygen uptake
- VT
- ventilatory threshold.
References
- 1. Carson V, Janssen I. Volume, patterns, and types of sedentary behavior and cardio-metabolic health in children and adolescents: a cross-sectional study. BMC Public Health. 2011;11:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 3. Pate RR, O'Neill JR, Lobelo F. The evolving definition of “sedentary.” Exerc Sport Sci Rev. 2008;36:173–178. [DOI] [PubMed] [Google Scholar]
- 4. Belcher BR, Berrigan D, Dodd KW, Emken BA, Chou CP, Spruijt-Metz D. Physical activity in US youth: effect of race/ethnicity, age, gender, and weight status. Med Sci Sports Exerc. 2010;42:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vandewater EA, Shim MS, Caplovitz AG. Linking obesity and activity level with children's television and video game use. J Adolesc. 2004;27:71–85. [DOI] [PubMed] [Google Scholar]
- 6. Carson V, Stone M, Faulkner G. Patterns of sedentary behavior and weight status among children. Pediatr Exerc Sci. 2014;26:95–102. [DOI] [PubMed] [Google Scholar]
- 7. Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes (Lond). 2005;29:S84–S96. [DOI] [PubMed] [Google Scholar]
- 8. Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sardinha LB, Andersen LB, Anderssen SA, et al. Objectively measured time spent sedentary is associated with insulin resistance independent of overall and central body fat in 9- to 10-year-old Portuguese children. Diabetes Care. 2008;31:569–575. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell JA, Pate RR, Beets MW, Nader PR. Time spent in sedentary behavior and changes in childhood BMI: a longitudinal study from ages 9 to 15 years. Int J Obes (Lond). 2013;37:54–60. [DOI] [PubMed] [Google Scholar]
- 11. Biddle SJ, Pearson N, Ross GM, Braithwaite R. Tracking of sedentary behaviours of young people: a systematic review. Prev Med. 2010;51:345–351. [DOI] [PubMed] [Google Scholar]
- 12. Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. Am J Prev Med. 2011;41:207–215. [DOI] [PubMed] [Google Scholar]
- 13. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saunders TJ, Chaput JP, Goldfield GS, et al. Prolonged sitting and markers of cardiometabolic disease risk in children and youth: a randomized crossover study. Metabolism. 2013;62:1423–1428. [DOI] [PubMed] [Google Scholar]
- 15. Altenburg TM, Rotteveel J, Dunstan DW, Salmon J, Chinapaw MJ. The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J Appl Physiol. 2013;115:1751–1756. [DOI] [PubMed] [Google Scholar]
- 16. Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–E259. [DOI] [PubMed] [Google Scholar]
- 17. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data 2000;314:1–27. [PubMed] [Google Scholar]
- 18. NIH Toolbox Cognition Battery (CB). Monogr Soc Res Child Dev. 2013;78:1–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skinner JS. Exercise Testing and Exercise Prescription for Special Cases: Theoretical Basis and Clinical Application. 2nd ed Philadelphia, PA: Lea & Febiger; 1993. [Google Scholar]
- 20. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–2027. [DOI] [PubMed] [Google Scholar]
- 21. Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009;89:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ader M, Ni TC, Bergman RN. Glucose effectiveness assessed under dynamic and steady state conditions. Comparability of uptake versus production components. J Clin Invest. 1997;99:1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32:35–45. [DOI] [PubMed] [Google Scholar]
- 24. Tonelli J, Kishore P, Lee DE, Hawkins M. The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care. 2005;8:450–456. [DOI] [PubMed] [Google Scholar]
- 25. Hayashi Y, Nagasaka S, Takahashi N, et al. A single bout of exercise at higher intensity enhances glucose effectiveness in sedentary men. J Clin Endocrinol Metab. 2005;90:4035–4040. [DOI] [PubMed] [Google Scholar]
- 26. Brun JF, Guintrand-Hugret R, Boegner C, Bouix O, Orsetti A. Influence of short-term submaximal exercise on parameters of glucose assimilation analyzed with the minimal model. Metabolism. 1995;44:833–840. [DOI] [PubMed] [Google Scholar]
- 27. Gupta D, Krueger CB, Lastra G. Over-nutrition, obesity and insulin resistance in the development of β-cell dysfunction. Curr Diabetes Rev. 2012;8:76–83. [DOI] [PubMed] [Google Scholar]
- 28. Charansonney OL, Després JP. Disease prevention–should we target obesity or sedentary lifestyle? Nat Rev Cardiol. 2010;7:468–472. [DOI] [PubMed] [Google Scholar]
- 29. Maffeis C. Aetiology of overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159:S35–S44. [DOI] [PubMed] [Google Scholar]
- 30. Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology. 2005;20:260–270. [DOI] [PubMed] [Google Scholar]
- 31. Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev. 2004;32:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peddie MC, Rehrer NJ, Perry TL. Physical activity and postprandial lipidemia: are energy expenditure and lipoprotein lipase activity the real modulators of the positive effect? Prog Lipid Res. 2012;51:11–22. [DOI] [PubMed] [Google Scholar]
- 33. Martínez-Gómez D, Eisenmann JC, Gómez-Martínez S, Veses A, Marcos A, Veiga OL. Sedentary behavior, adiposity and cardiovascular risk factors in adolescents. The AFINOS study. Rev Esp Cardiol. 2010;63:277–285. [PubMed] [Google Scholar]
- 34. Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care. 1996;19:1018–1030. [DOI] [PubMed] [Google Scholar]
- 35. Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks GA. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol. 1999;276:E106–E117. [DOI] [PubMed] [Google Scholar]
- 36. Pearson N, Biddle SJ. Sedentary behavior and dietary intake in children, adolescents, and adults. A systematic review. Am J Prev Med. 2011;41:178–188. [DOI] [PubMed] [Google Scholar]
- 37. Mushtaq MU, Gull S, Mushtaq K, Shahid U, Shad MA, Akram J. Dietary behaviors, physical activity and sedentary lifestyle associated with overweight and obesity, and their socio-demographic correlates, among Pakistani primary school children. Int J Behav Nutr Phys Act. 2011;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saunders TJ, Chaput JP, Goldfield GS, et al. Children and youth do not compensate for an imposed bout of prolonged sitting by reducing subsequent food intake or increasing physical activity levels: a randomised cross-over study. Br J Nutr. 2014;111:747–754. [DOI] [PubMed] [Google Scholar]
- 39. Short KR, Pratt LV, Teague AM, Man CD, Cobelli C. Postprandial improvement in insulin sensitivity after a single exercise session in adolescents with low aerobic fitness and physical activity. Pediatr Diabetes. 2013;14:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacEneaney OJ, Harrison M, O'Gorman DJ, Pankratieva EV, O'Connor PL, Moyna NM. Effect of prior exercise on postprandial lipemia and markers of inflammation and endothelial activation in normal weight and overweight adolescent boys. Eur J Appl Physiol. 2009;106:721–729. [DOI] [PubMed] [Google Scholar]
- 41. Larsen RN, Kingwell BA, Robinson C, et al. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci. 2015;129:117–127. [DOI] [PubMed] [Google Scholar]