Abstract
Kentucky has among the highest rates of diabetes and obesity in the United States. The Kentucky Diabetes and Obesity Collaborative (KDOC) was designed to develop a novel research infrastructure that can be used by researchers focusing on obesity and diabetes among patients cared for by Federally Qualified Health Centers (FQHC) serving rural Kentucky. Focus groups were carried out to develop an understanding of the needs and interests of FQHC practitioners and staff regarding participation in KDOC. Focus groups were conducted with 6 FQHCs and included a total of 41 individuals including health care providers, administrative staff and clinical staff. The discussions ranged in time from 30 to 70 minutes and averaged 45 minutes. Analysis of the transcripts of the focus groups revealed 4 themes: 1) contextual factors, 2) infrastructure, 3) interpersonal relationships, and 4) clinical features. The participants also noted four requirements that should be met for a research project to be successful in rural primary care settings: 1) there must be a shared understanding of health priorities of rural communities between the researcher and the practices/providers; 2) the proposed research must be relevant to clinics and their communities; 3) research and recommendations for evidence-based interventions need to reflect the day-to-day challenges of rural primary care providers; and 4) there needs to be an understanding of community norms and resources. Although research-clinic partnerships were viewed favourably overall, challenges in data integration to support both research and clinical outcomes were identified.
Keywords: Diabetes, Health Information Technology, Rural Health, Primary Care, Qualitative
I. INTRODUCTION
Kentucky ranks among the worst in the nation for diabetes (38th) and obesity (42nd) [1]. These conditions and co-occurring risk factors contribute to the overall low health status of Kentucky residents, particularly those who live in medically underserved areas [1, 2]. Kentuckians diagnosed with diabetes nearly tripled from 3.6% in 1995 to 9.3% in 2010, and obesity grew from 16.5% to 31.4%. In the United States, diagnosed diabetes rose from 4.5% to 8.2% over the same years [3]. An estimated 7 million people in the US are undiagnosed and, alarmingly, an estimated 79 million are pre-diabetic [4, 5]. The total direct and indirect costs of diabetes in the US tops $174 million annually, and it is a major risk factor for serious health complications including kidney disease, heart disease, stroke, peripheral neuropathy, amputations, periodontal disease and pregnancy complications [1, 2]. From public health and clinical treatment perspectives, the rates of diabetes in Kentucky are concerning. Understanding the rapid growth in diabetes and obesity in these medically underserved areas is critical to develop appropriate primary care interventions to curb the associated financial and human costs.
Unlike efficacy research, in which potential external validity factors are tightly controlled, effectiveness research occurs in settings where variability in patient outcomes and experiences are influenced by the context in which the research takes place [6]. Rising health care costs and the national emphasis on quality improvement science, coordinated care, patient-centered medical homes, and evidence-based practice, has led to greater accountability and reporting along with growing interest in data-driven quality improvements in practice [7]. Federally-Qualified Community Health Centers (FQHCs), who provide care to medically underserved people in urban and rural communities, including Appalachian Kentucky are emerging as leaders among in these endeavors.
A growing body of providers and researchers acknowledge the critical role of practice-based research as a component of the translational research spectrum for accomplishing translation of research into practice. Community-based participatory research (CBPR) methods have been identified as an effective strategy for scientists interested in practice-based research to engage healthcare delivery systems, communities, and clinical staff [8]. CBPR principles include creating research partnerships within which research questions and study approach are jointly developed and implemented, and interpretation of findings and dissemination of results are equally shared among researchers and community partners (in this case, rural primary care clinics) [9]. This method ensures that research questions are pertinent to the day to day operations and experiences of primary care clinics and improves research endeavor success. Rural FQHCs can provide an ideal platform for such clinical and health services research and dissemination of new knowledge into health-disparity communities via research applying community-based participatory research methods [10]. Nearly ubiquitous healthcare payments using electronic claims and the rapidly growing use of Electronic Medical Records (EMRs) have created new opportunities for applied research to address rural health disparities.
The Kentucky Diabetes and Obesity Collaborative (KDOC) project was designed to accomplish three main objectives: a) develop a novel research infrastructure, b) facilitate its use by researchers and clinicians to mitigate the public health burdens of obesity and diabetes in a rural medically underserved population through research, and c) generate new knowledge applicable to similar populations. KDOC aimed to create and maintain a new infrastructure for using electronic clinical data and healthcare claims data to facilitate bidirectional collaborations between researchers at the University of Kentucky Academic Health Center (UK AHC) and rural primary healthcare providers to accomplish these objectives. FQHCs serving rural Kentucky were selected to be practitioner partners for KDOC because they serve health disparity populations with high rates of diabetes and, as a federal requirement, they use and report electronic healthcare data for quality assurance and quality improvement.
KDOC began with formative research, focus groups, to understand FQHC practitioners ’ and staffs’ interest in participation, to probe their necessary assets, and the barriers that would have to be overcome to accomplish KDOC goals. Qualitative data collection, such as this study, is an important step in health services research in order to provide depth and context to complex issues in health care settings [11]. In this report, researchers present findings from the focus groups and implications for development of a novel resource for research on diabetes among low income, rural populations.
II. METHODS
A. Setting
This study was carried out among FQHCs in rural Appalachian Kentucky. As in most rural areas of the US, the population of rural Kentucky is medically underserved, and FQHCs and county health departments are often the only ‘safety net ’ providers. Specialist care is sparse and access is often through providers in urban and suburban areas. In 2012, among the 21 FQHC’s in Kentucky, 12.7% of patients were diagnosed with diabetes (higher than US and KY rates overall) [12].
B. Recruitment
The goal was to recruit participants who could provide input on all aspects of FQHCs relative to diabetes care, medical records, and EMR, and included FQHC leadership, administrative staff, clinic staff, and health care providers. In 2012–2013, focus group participants were recruited in collaboration with FQHC leadership. Investigators contacted the FQHCs by email, described KDOC and the purpose of the focus groups, after which the FQHC leadership invited clinic staff, IT staff, and providers to participate. After establishing initial email communication, investigators followed up by telephone to discuss the focus group project, identify a key contact person at the FQHC to work with investigators, and determine strategies for recruiting participants. The investigators explained the criteria for participation and, in collaboration with the FQHC contact, identified potential focus group participants. The FQHC then reached out to the individuals and invited them to participate in the focus group. The focus groups were scheduled over lunch to accommodate the clinic needs, and food was provided by the investigators. No other incentives were provided.
C. Data Collection
The focus groups were conducted in meeting rooms at the FQHCs. Investigators greeted focus group participants and began by distributing and explaining informed consent. Only individuals providing informed consent were included in the focus groups. The focus group moderator provided a brief explanation of the focus group process and then led the group through discussion of topics related to obesity and diabetes, research, and experience with EMRs. The discussions lasted 45 minutes on average. All protocols and procedures were approved by the University of Kentucky Institutional Review Board.
D. Data Processing and Analysis
All focus groups were recorded and transcribed. The moderator took notes during the discussion. A qualitatively trained investigator who was not previously involved with the project reviewed the transcripts using directed content analysis in 2013 [13]. Directed content analysis allows for a structured analysis to explore specific facets of a theory or research question, utilizing open-ended questions to probe pre-determined categories. Categories are coded based on the phenomenon being described by the participants, and any data that could not be coded using this scheme given a new code [13]. Transcripts were independently coded and reviewed by the authors until agreement was reached. This integrated method of inductive, grounded-theory approach allowing for unexpected themes to emerge, combined with pre-determined categories based on a theory or guided by the research questions to focus on specific aspects of health services research is recommended to improve our understanding of complex issues within real-world settings [14].
III. RESULTS
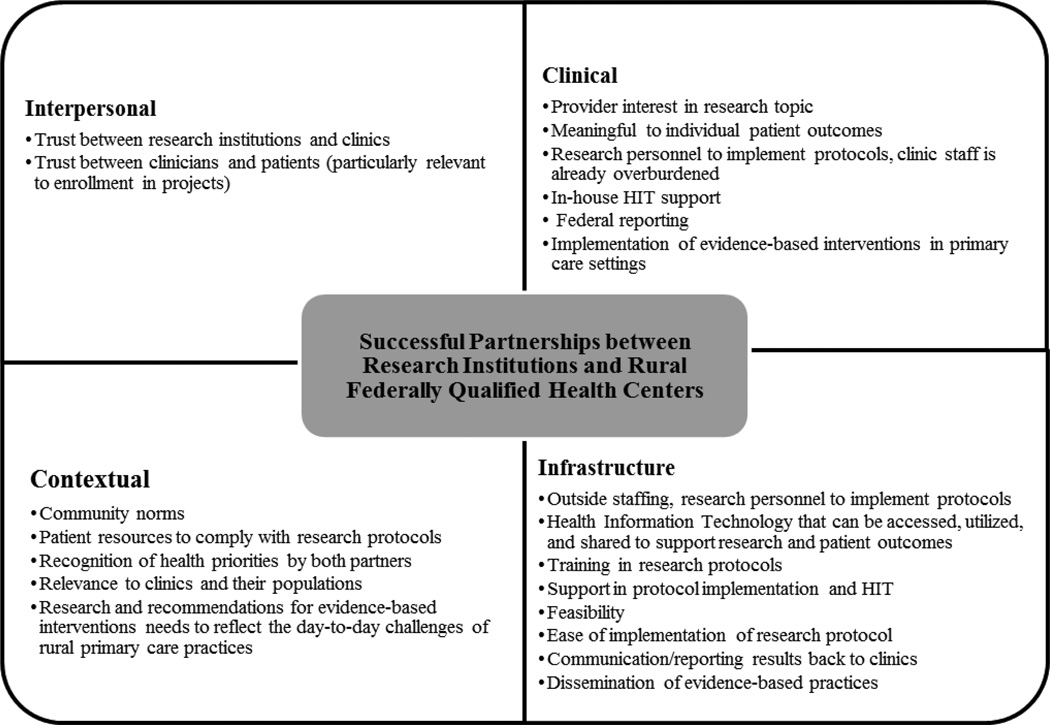
Focus groups were carried out with 6 FQHCs and 41 individuals participated. Participants included health care providers, administrative staff and clinical staff. Overall, the groups agreed that research, specifically, collaborations with research institutions, could improve patient outcomes and inform clinical practices. In order to understand how to best conduct research in these rural practices, a key function of KDOC, the authors explored general perceptions of research partnerships, experience with research, perceived barriers to research and suggestions for successful collaborations. As illustrated in Fig. 1, perceived elements of successful partnerships emerged from the data in four general categories: 1) contextual factors, 2) infrastructure, 3) interpersonal relationships, and 4) clinical features. The participants also noted four components related to the community/clinical context for a research project in rural primary care settings to be successful: 1) there must be a shared understanding of health priorities of rural communities between the researcher and the practices/providers; 2) the proposed research must be relevant to clinics and their communities; 3) research and recommendations for evidence-based interventions need to reflect the day-to-day challenges of rural primary care providers; and 4) there needs to be an understanding of community norms and resources.
Fig. 1.
Categories Related to Successful Rural Research Institution and Community Health Center Partnerships
A. Contextual Factors
There is a growing understanding that the context within which applied research is conducted is related to clinical acceptance and implementation of research protocols, dissemination and adoption of best practices, and ultimately, patient outcomes. For this study, it was important for researchers understand of the scope of the problem (diabetes and obesity) in their communities and the perceived cultural barriers and facilitators to research partnerships within a rural health center setting. When asked about the elevated rates of obesity and diabetes in rural Kentucky, all of the participants agreed that their clinical experience supported the high rates that are published in various reports, and commented that increased numbers of obese children and young adults is particularly troubling. Participants attributed increased rates to complex issues including access to preventive measures such as nutritious foods, opportunities for physical activity, built environment limitations, and appropriate education around health risks associated with obesity and diabetes the individual and community levels. Specifically, they spoke of the increase in the penetration of fast food outlets in rural Kentucky that led to increased consumption of calorie dense foods and less consumption of food prepared at home, decreases in exercise and, particularly for children, and an increase in screen time. As described by one participant, “With the kids, the video games, the electronics….they never go out and play. [I] got her (my daughter) one (a bicycle) for her birthday, and she’s like, oh, really, a bike? It’s hot outside; I don’t want to go out there. She’d rather watch her movies…”.
They also cited challenges with self-management following a diagnosis, financial burden of treatment, and pervasive cultural normalization of expectations around obesity and diabetes. Given these challenges, participants suggested ways to improve outcomes including providing education to counter the notion that ‘everyone’ has diabetes and there is little that can be done to prevent it, and to try to find ways to improve dietary practices. As noted by one participant “[We] had one woman diagnosed with diabetes who didn’t have a clue what to eat, doctors don't have time. She scheduled an appointment with a dietician… [but before that] she was getting tidbits of information on what to eat from a friend.”
Several participants cited potential challenges associated with diabetes interventions such as low compliance, transportation issues, and multiple required visits to the clinic in order to participate. They also noted that prevention must start early and focus on children, include personalized treatment and monitoring for those who are already diagnosed, and improve educational opportunities for those who have literacy issues.
B. Clinical-Research Partnership Infrastructure
Most of the discussion in each focus group was spent on issues related to the infrastructure needed to sustain research partnerships. Notably, almost every group commented on the need for additional staff and support to implement research protocols in their practices. The participants indicated that their rural clinics had small, overburdened staff and research partners should consider using outside staff specific to the research protocol. Additional staff would be needed to provide specialized support in the form of supervision, training, assistance with health information technology, and enacting research protocols. As stated by one participant “My thing is we…have small staff; I love to participate but I don’t want to put anything more on these people…the EMR is a wonderful tool and if we could use the data without burdening them too much.” Research partnerships were viewed as more successful when they included support, training, and were viewed as easy and feasible to conduct within the practice.
Health information technology (HIT), specifically, the use of an EMR, were viewed as an important non-invasive method to conduct research related to diabetes outcomes in rural clinics. However, participants identified variation in access to information that would address their EMR Meaningful Use goals [15] including comfort of individual clinics in pulling reports, and time and costs related to requests for vendor support. Most did not have in-house HIT personnel to address issues as they arose, to collaborate with potential research partners when planning protocols, or to analyze data. They also identified challenges in integration of data across clinics because there is no uniformity in EMR systems. One group spoke to the need to train providers to enter data in the EMR in ways that would be appropriate for analyses. There were various levels of comfort using EMR and data reports for research across clinics, and one participant highlighted the need to integrate any data collection into the current flow of practice.
C. Interpersonal Relationships
Research partnerships rely on two levels of trust. First, trust between clinics/clinicians and patients would enhance enrollment in research projects. Second, the providers must trust researchers/research institutions to conduct ethical and meaningful research for practices and patients. Although clinics support research is relevant to their communities, it is more likely to be successful when initiated by trusted research partners than from within the clinic.
Clinics reported that they were not hesitant to share data as long as patient privacy was protected, especially when using EMR data. Clinic experience with EMR and government oversight has been mixed; the ability to better manage practice is a plus, but there is concern about monitoring by those outside the practice. However, there was consensus among the focus groups in favor of participating in and promoting research, particularly research that would support FQHC federal reporting requirements. Finally, the following conversation in one of the groups illustrates how trust is interpreted to enhance research partnership success.
“Participant 1: …I don’t know that our patients fully understand it [EMR]. We tell them we’ve went EMRs but I really don’t know that they fully understand what that means.
Participant 2: …I would agree…I know in the beginning when we implemented, they were more concerned with us giving them eye to eye…attention…..
Participant 3: I think they’ve been with us long enough that they know they can trust us”.
D. Clinical Features
Although all of the clinics recognized diabetes as a top issue for their patients and communities, they noted that planning research projects in primary care clinics, specifically rural FQHCs, must take into consideration clinic practices and patient flow. As stated above, clinic staff is already over-burdened, has little time to implement protocols, and additional outside staff was viewed as a condition for success by participants. In addition, clinics that had in-house HIT personnel saw them as a potential way to enhance research opportunities. Providers in the focus groups were clear that diabetes research must be directly meaningful to individual patient outcomes to get support within the clinic. There was also concern for the implementation of evidence-based practices in diabetes care. As summarized by one provider “…using evidence-based practices is a good idea, but it often slows down patient flow- how to implement evidence-based practices without hurting the practice?” Finally, all clinics who participated had federal reporting responsibilities; research that could help them to meet those needs was viewed most favorably among providers in the groups.
IV. CONCLUSIONS
Qualitative data collection has inherent limitations, including limited generalizability. All participants were from clinics in Kentucky who volunteered to take part in the focus groups. However, the results from this study can provide guidance on developing research partnerships with rural FQHCs around diabetes and obesity in primary care clinics.
A major goal of KDOC was to develop a secure data warehouse that would allow clinics and researchers to monitor and use clinical and claims data at a patient level, across healthcare organizations, as a tool for Quality Improvement and research. Before this goal could be achieved, it was necessary to explore perceptions of research partnerships and potential barriers within practices and research institutions for implementation. Results from the focus groups provide important insights into how to implement diabetes research and evidence-based interventions in rural primary care clinics, FQHCs. Overall, participants viewed research favourably and all participated in some research prior to the focus groups, which was related to increased trust in research institutions to respectfully conduct research in their practices. All clinics viewed diabetes as a growing issue in their communities and clinics, and were open to participating in research to contribute to meaningful evidence-based interventions. Research was viewed as most successful when initiated by research partners and those clinics sharing the research focus as a health priority opting to participate, given clinic features, and additional support by the research institution to implement the protocols.
Most concerns reported by clinics related to research involved data collection and reporting. Numerous previous studies documented challenges associated with adoption and implementation of the EMR in small rural practices [16], but this is among the first to qualitatively investigate the real-world implications for research of jointly identified health priorities: diabetes and obesity. Infrastructure to support training in documentation, data collection, patient privacy, and data reporting across and among various providers and EMRs were identified as key HIT issues in diabetes research. Although a recent national survey indicated HIT adoption in rural clinics is no longer significantly different from urban settings, barriers described by study participants is consistent with previous studies citing technical issues, organizational and financial burden, and lack of a unified application and reporting system for coordinated care as primary concerns of HIT in clinical settings overall [16–19]. As noted by Bradley, et al., infrastructure to support data linkages such as disease registries, clinical data from EMRs, and claims files could significantly benefit translational research and patient outcomes, but there are significant gaps in capacity across both research and medical settings [11]. Additionally, participants in this study indicated the data warehouse, such as the one proposed in KDOC, would need to incorporate user-friendly interfaces to allow clinics to easily meet their own federal reporting needs. Although some of the study clinics had access to in-house HIT support for maintaining the internal system and connectivity, such as registering patients, recording the encounter, and billing the patient/insurance, most did not have the analytical training to address research questions, making partnerships with research institutions mutually beneficial. What is clear from prior research and the rural primary care clinic participants in this study, is that an integrated data reporting and management system that meets the dual needs of research and practice is a primary step in building a successful research partnership. Research and clinical partners can use the results from this study as a tool to discuss research design, implementation and interpretation in order to enhance clinical relevance.
ACKNOWLEDGMENTS
Funding for this project was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (RC4 DK089866) and supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Kevin A. Pearce, Email: kpearce@uky.edu.
Traci D. Jarrett, Email: tjarrett@hsc.wvu.edu.
F. Douglas Scutchfield, Email: scutch@uky.edu.
Jeffery C. Talbert, Email: jeff.talbert@uky.edu.
W. David Bolt, Email: dbolt122148@gmail.com.
Mary A. Barron, Email: mabarr2@email.uky.edu.
Jessica M. Houlihan, Email: jaberr0@email.uky.edu.
Mark B. Dignan, Email: mbdign2@uky.edu.
REFERENCES
- 1.America’s Health Rankings 2013: Kentucky. [Accessed December 30, 2013];United Health Foundation. 2013 www.americashealthrankings.org/KY. [Google Scholar]
- 2.County Health Rankings: Kentucky. [Accessed December 30, 2013];University of Wisconsin Population Health Institute. 2013 http://www.countyhealthrankings.org/app/kentucky/2013/rankings/outcomes/overall/by-rank. [Google Scholar]
- 3.Diabetes Interactive Atlas Data. [Accessed December 30, 2013];Centers for Disease Control and Prevention. 2013 http://www.cdc.gov/diabetes/atlas/obesityrisk/atlas.html.
- 4.National Diabetes Fact Sheet. [Accessed December 30, 2013];Centers for Disease Control and Prevention. 2011 http://www.cdc.gov/diabetes/pubs/estimates11.htm#2. Updated October 25, 2013.
- 5.Diabetes Basics: Diabetes Statistics. [Accessed December 30, 2013];American Diabetes Association. 2013 http://www.diabetes.org/diabetes-basics/statistics/ [Google Scholar]
- 6.Green LW. Public health asks of systems science: to advance our evidence-based practice, can you help us get more practice-based evidence? Am J Public Health. 2006 Mar;96(3):406–409. doi: 10.2105/AJPH.2005.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devers KJ. The state of Quality Improvement Science in health: what do we know about how to provide better care? The Urban Institute; 2011. Nov, [Accessed December 11, 2013]. Available at: http://www.urban.org/uploadedpdf/412454-State-of-Quality-Improvement-Science-in-Health.pdf. [Google Scholar]
- 8.Schmittdeil JA, Grumbach K, Selby JV. System-based participatory research in health care: an approach for sustainable translational research in quality improvement. Ann. Fam. Med. 2010 May;8(3):256–259. doi: 10.1370/afm.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minkler M, Wallerstein N. Community-based Participatory Research for Health. San Fransisco: Josey Bass; 2003. [Google Scholar]
- 10.Green LW. Making research relevant: if it is an evidence-based practice, where’s the practice-based evidence? Family Pract. 2008 Dec;25(Suppl 1):i20–i24. doi: 10.1093/fampra/cmn055. [DOI] [PubMed] [Google Scholar]
- 11.Bradley CJ, Penberthy L, Devers KJ, Holden DJ. Health services research and data linkages: issues, methods, and directions for the future. Health Serv. Res. 2010 Oct;45(5 Pt 2):1468–1488. doi: 10.1111/j.1475-6773.2010.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Health Center Data: Kentucky Program Grantee. 2012. [Accessed December 30, 2013];Health Resources and Services Administration. 2012 http://bphc.hrsa.gov/uds/datacenter.aspx?year=2012&state=KY.
- 13.Hsieh H, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005 Nov;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 14.Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv. Res. 2007 Aug;42(4):1758–1772. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.How to implement EHRs: Step 5 Achieve Meaningful Use. HealthIT.gov.; [Accessed January 7, 2014]. http://www.healthit.gov/providers-professionals/ehr-implementation-steps/step-5-achieve-meaningful-use. [Google Scholar]
- 16.Bahensk JA, Jaana M, Ward MM. Health care information technology in rural America: Electronic Medical Record adoption status in meeting the National agenda. J. Rural Health. 2008;24(2):101–105. doi: 10.1111/j.1748-0361.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Carayon P, Alyousef B, Hoonakker P, et al. Challenges to care coordination posed by the use of multiple health IT applications. Work. 2012;41(Suppl 1):4468–4473. doi: 10.3233/WOR-2012-0746-4468. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Lichter MI, Danzo A, Taylor J, Rosenthal T. The adoption and use of Health Information Technology in rural areas: results of a National survey. J Rural Health. 2012 Jan;28(1):16–27. doi: 10.1111/j.1748-0361.2011.00370.x. [DOI] [PubMed] [Google Scholar]
- 19.Ajami S, Bagheri-Tadi T. Barriers for adopting Electronic Health Records (EHRs) by Physicians. Acta Inform Med. 2013;21(2):129–134. doi: 10.5455/aim.2013.21.129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]