Abstract
Background and Purpose
The World Health Organization (WHO) has classified thymic carcinoma and other thymomas (types A, AB, and B) as different neoplasms. Myasthenia gravis (MG) is an early sign of thymoma and theoretically does not accompany thymic carcinoma; however, cases of thymic carcinoma with MG have been reported. Whether thymic carcinoma can accompany MG has yet to be established.
Methods
The medical records of patients who underwent thymectomy for MG between 1990 and 2011 in a single hospital were reviewed. All cases with the diagnostic code of "thymic carcinoma" or "thymoma type C" (old terminology) were selected. A pathologist re-reviewed the pathologic specimens using the new WHO criteria. The rate of thymic carcinoma among these MG patients was then calculated.
Results
A total of 81 patients with MG had thymic tumors, 10 of whom had thymic carcinomas or thymoma type C. Seven cases of well-differentiated thymic carcinomas (type B3) were excluded, leaving three (3.7%) cases of thymic carcinoma with MG. All three of these cases were type B3 thymoma with a focal squamous cell carcinoma component that was very small and well demarcated. In addition, two out of the three tumors were found to be at an early clinical stage. All of the cases survived without recurrence over follow-up periods of at least 5 years.
Conclusions
Thymic carcinoma transformation from thymoma can occur during the early stages of thymoma. The association of this condition with MG is not as rare as was previously thought. Thymic carcinomas accompanying MG had a predominant B3 thymoma component with a focal thymic carcinoma area (squamous cell carcinoma).
Keywords: thymoma, thymic carcinoma, thymic squamous cell carcinoma, myasthenia gravis, thymectomy
INTRODUCTION
Early thymectomy is the standard treatment modality for myasthenia gravis (MG) patients with thymoma. MG patients have reduced pulmonary function, and surgery is often limited to using muscle relaxants and antibiotics. Therefore, minimally invasive techniques are more commonly used to treat MG.1,2,3 However, the basis for the use of these minimally invasive techniques is the absence of thymic carcinoma, because when it is present, complete resection is important for survival.4
Thymomas [World Health Organization (WHO) types A, AB, and B] are neoplasms arising from or exhibiting differentiation toward thymic epithelial cells; they also exhibit a nonneoplastic lymphoid structure. Their malignant potential ranges from absent to low. Collaboration between the dysfunctional neoplastic thymic structure and the nonneoplastic lymphoid structure induces autoimmune diseases such as MG. On the other hand, thymic carcinomas are malignant epithelial tumors with overt cytologic atypia, almost invariable invasiveness, and a lack of "organotypic" (thymus-like) features.5 MG has been most commonly associated with WHO type A, AB, or B thymoma, but not thymic carcinoma.6,7,8,9,10
While the WHO has classified thymoma and thymic carcinoma as different tumor types, it has been shown that thymic carcinomas (which have very poor prognoses) can actually arise after the development of the thymoma.5,11 Theoretically, thymic carcinoma can accompany MG after a sufficient period. MG is an early and easily detectable sign of a thymoma, and therefore thymoma with MG is generally detected at an early stage and has a better prognosis than thymoma without MG.9 Early thymectomy is the standard treatment of MG with thymoma. Whether or not thymic carcinoma can arise in thymoma with MG has yet to be established.
METHODS
The medical records of patients who underwent thymectomy between January 1990 and December 2011 at the Yonsei Medical Health System, Seoul, Korea were reviewed. All cases with a pathologic diagnostic code of "thymic carcinoma" or "WHO type C" were selected. From these, all cases with MG were selected. MG was diagnosed by a positive repetitive nerve stimulation study and/or the presence of anti-acetylcholine-receptor antibody. Each case was reviewed and reclassified according to the 2004 WHO classification.5
The study protocol was reviewed and approved by the Institutional Review Board (IRB No. 2014-08-114). Informed consent was waived by the board due to the retrospective nature of the study.
RESULTS
A total of 297 patients had undergone thymectomy due to thymic tumor during the test period, of whom 29 (9.8%) were classified as having thymic carcinoma. Eighty-one patients were found to have a thymic tumor with MG, 10 of which were classified as either thymic carcinoma or thymoma type C. After applying the new WHO criteria, seven of the ten cases were reclassified as having well-differentiated thymic carcinoma (WHO type B3). Three cases (3.7%) of thymic carcinoma were found (one man and two women; mean age, 47.3 years).
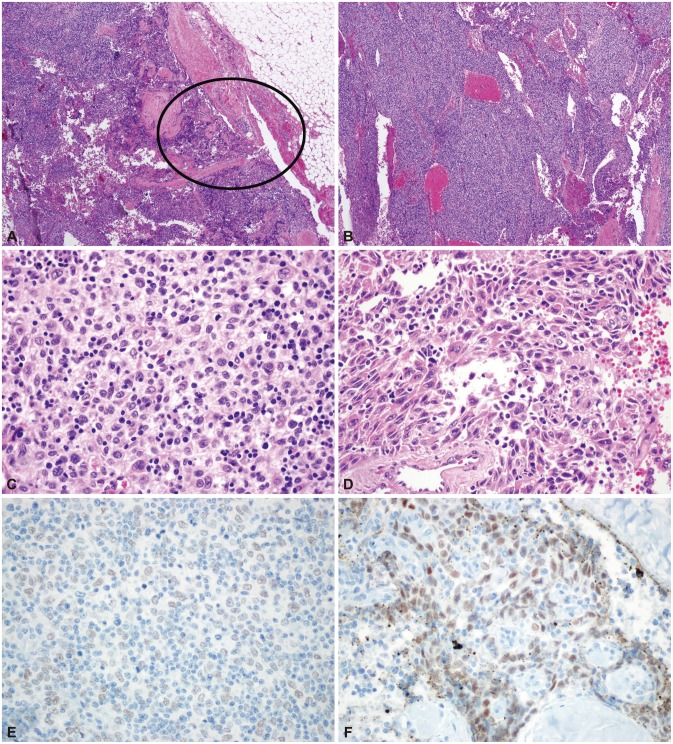
All three cases of thymic carcinoma had a predominant B3 or B2 thymoma area with a focal, well-demarcated squamous cell carcinoma area and overt cytologic atypia; two patients had a type B3 thymoma and the other patient had a type B2+B3 thymoma (a thymic carcinoma portion was seen in the B3 area). Thymic carcinoma portions were clearly identifiable from thymoma portions, and their areas were much smaller than that of the thymoma (Fig. 1A-D). The thymic carcinoma area exhibited strong immunoreactivity for p63, which is a squamous cell differentiation marker; the conventional thymoma B3 tissue was not p63 immunoreactive (Fig. 1E and F).
Fig. 1. Pathologic findings of case 2. Focal thymic carcinoma with an abundant thymoma (thymoma type B3) component. Low-power view showing a multinodular mass with extension to the peritumoral fat tissue [circle; hematoxylin-eosin stain (H-E); magnification, ×12] (A). The tumor is composed of abundant type B3 tissue (B: H-E; magnification, ×40; C: H-E, magnification, ×400) (B, C) and a focal thymic carcinoma component (squamous cell carcinoma) in the circled area (H-E; magnification, ×400) (D). Strong immunoreactivity for p63, a squamous cell differentiation marker, is found in the thymic carcinoma area (p63 immunostaining; magnification, ×400) (E). There is no p63 reactivity in the conventional thymoma B3 area (p63 immunostaining; magnification, ×400) (F).
All three patients are currently still alive and without recurrence, after follow-up periods of 5, 10, and 22 years. The pathologic findings are summarized in Table 1.
Table 1. Pathologic findings of myasthenia gravis patients with thymic carcinomas.
| Case | Sex/age | AntiAchR Ab titer | Time between diagnosis and thymectomy | Pathologic findings | Masaoka stage | Predominant thymoma portion |
|---|---|---|---|---|---|---|
| 1 | Female/67 years | 3.2 IU | 19 days | Thymoma, predominantly type B3 with a focal area of carcinoma of squamous cell type | 4 | B3 |
| 2 | Male/31 years | 11.3 IU | 10 days | Focal squamous cell carcinoma with type B3+B2 thymoma | 2B | B3 |
| 3 | Female/44 years | 2.6 IU | 17 days | Squamous cell carcinoma with type B3 thymoma | 2B | B3 |
AntiAchR Ab: anti-acetylcholine-receptor antibody.
DISCUSSION
While no de novo thymic carcinomas were found in the present study, a small (3.7%) portion of thymomas with MG had a mixed thymic carcinoma portion. Previous studies and other reports have clearly established that large thymic carcinomas with poor prognoses can arise from a preexisting thymoma.11,12
The point at which transformation to thymic carcinoma occurs is unknown. Two previously reported cases of thymic squamous cell carcinoma developed from thymomas after 10 and 14 years;12 it was unknown whether these thymic carcinomas were able to develop during the earlier stages of the thymoma. A recent study found that a large portion of a thymoma exhibited mixed histology findings.13 Moreover, additional research has shown that type B2 thymomas are genetically related to type B3 thymomas, in line with the frequent histologic observation of combinations of type B2 and B3 areas in the same tumor.14,15 It has further been suggested that some type B2 thymomas are precursor lesions of type B3 tumors.15 As a part of the thymic carcinoma, thymic squamous cell carcinomas frequently show gains of chromosomes 1q, 17q, and 18, and the loss of chromosomes 3p, 6, 16q, and 17p.16 Apart from the "thymic stemness" alteration on chromosome 6q25, thymic squamous cell carcinomas share other similarities with type B3 thymomas, namely gain of chromosome 1q and loss of chromosome 6. Apart from these similarities, they are genetically distinct from thymomas, justifying their listing as a separate entity in the WHO classification.15,17,18
A squamous cell carcinoma can theoretically develop inside a B3 thymoma. However, any type of thymic carcinoma can develop within all types of thymoma.11 We believe that this phenomenon is due to the long period before detection of malignant transformation. In addition, thymic carcinomas have been shown to develop in the necrotic portion of a thymoma.11 All of the thymic carcinomas in our case were squamous cell carcinomas and were accompanied by B3 thymomas.
The standard surgical technique for treating thymic carcinomas is median sternotomy with complete thymectomy and removal of all surrounding mediastinal fat.19 There are no published data on the use of minimally invasive surgery for thymic carcinoma.20
In conclusion, thymoma with MG had a thymic carcinoma component; this thymic carcinoma portion was small and localized within the thymoma in these patients.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Marulli G, Schiavon M, Perissinotto E, Bugana A, Di Chiara F, Rebusso A, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145:730–735. discussion 735-736. doi: 10.1016/j.jtcvs.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein SD, Yang SC. Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg. 2010;89:1080–1085. discussion 1085-1086. doi: 10.1016/j.athoracsur.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Seong YW, Kang CH, Choi JW, Kim HS, Jeon JH, Park IK, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg. 2014;45:e68–e73. discussion e73. doi: 10.1093/ejcts/ezt557. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS, Riely GJ, Akerley W, Borghaei H, Chang AC, Cheney RT, et al. Thymomas and thymic carcinomas: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2013;11:562–576. doi: 10.6004/jnccn.2013.0072. [DOI] [PubMed] [Google Scholar]
- 5.Müller-Hermelink HK, Engel P, Kuo TT, Ströbel P, Marx A, Harris NL, et al. Tumours of the thymus: introduction. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics: tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press; 2004. pp. 148–151. [Google Scholar]
- 6.Marx A, Willcox N, Leite MI, Chuang WY, Schalke B, Nix W, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity. 2010;43:413–427. doi: 10.3109/08916930903555935. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Zhang XJ, Ma S, Jing Y, Li F, Krasna MJ. Different characteristics of thymomas with and without myasthenia gravis. Ann Surg Oncol. 2012;19:94–98. doi: 10.1245/s10434-011-1896-8. [DOI] [PubMed] [Google Scholar]
- 8.Marx A, Ströbel P, Zettl A, Chan JK, Müller-Hermelink HK, Harris NL, et al. Thymomas. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics: tumours of the lung, pleura, thymus, and heart. Lyon: IARC Press; 2004. pp. 152–153. [Google Scholar]
- 9.Ruffini E, Filosso PL, Mossetti C, Bruna MC, Novero D, Lista P, et al. Thymoma: inter-relationships among World Health Organization histology, Masaoka staging and myasthenia gravis and their independent prognostic significance: a single-centre experience. Eur J Cardiothorac Surg. 2011;40:146–153. doi: 10.1016/j.ejcts.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa K, Asamura H, Matsuno Y, Suzuki K, Kondo H, Maeshima A, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg. 2003;126:1134–1140. doi: 10.1016/s0022-5223(03)00798-0. [DOI] [PubMed] [Google Scholar]
- 11.Kuo TT, Chan JK. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am J Surg Pathol. 1998;22:1474–1481. doi: 10.1097/00000478-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am J Surg Pathol. 1996;20:1469–1480. doi: 10.1097/00000478-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Moran CA, Weissferdt A, Kalhor N, Solis LM, Behrens C, Wistuba II, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol. 2012;137:444–450. doi: 10.1309/AJCP76KEGWQKWOKA. [DOI] [PubMed] [Google Scholar]
- 14.Kirchner T, Schalke B, Buchwald J, Ritter M, Marx A, Müller-Hermelink HK. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol. 1992;16:1153–1169. [PubMed] [Google Scholar]
- 15.Ströbel P, Marx A, Zettl A, Müller-Hermelink HK. Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today. 2005;35:805–811. doi: 10.1007/s00595-005-3047-y. [DOI] [PubMed] [Google Scholar]
- 16.Zettl A, Ströbel P, Wagner K, Katzenberger T, Ott G, Rosenwald A, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol. 2000;157:257–266. doi: 10.1016/S0002-9440(10)64536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ströbel P, Hohenberger P, Marx A. Thymoma and thymic carcinoma: molecular pathology and targeted therapy. J Thorac Oncol. 2010;5(10 Suppl 4):S286–S290. doi: 10.1097/JTO.0b013e3181f209a8. [DOI] [PubMed] [Google Scholar]
- 18.Badve S, Goswami C, Gökmen-Polar Y, Nelson RP, Jr, Henley J, Miller N, et al. Molecular analysis of thymoma. PLoS One. 2012;7:e42669. doi: 10.1371/journal.pone.0042669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syrios J, Diamantis N, Fergadis E, Katsaros L, Logothetis M, Iakovidou I, et al. Advances in thymic carcinoma diagnosis and treatment: a review of literature. Med Oncol. 2014;31:44. doi: 10.1007/s12032-014-0044-2. [DOI] [PubMed] [Google Scholar]
- 20.Ye B, Tantai JC, Ge XX, Li W, Feng J, Cheng M, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg. 2014;147:1599–1603. doi: 10.1016/j.jtcvs.2013.10.053. [DOI] [PubMed] [Google Scholar]