Abstract
Background and Purpose
It has been suggested that oxidative stress is one of the pathomechanisms underlying amyotrophic lateral sclerosis (ALS), and thus antioxidants such as uric acid (UA) that could reduce oxidative stress might be beneficial in the prevention or treatment of this disease. The objective of this study was to prospectively investigate serum UA levels in Korean ALS patients and to relate them to disease progression.
Methods
ALS patients and healthy controls who were individually well-matched for sex, age, and body mass index (BMI) underwent blood testing for serum UA levels, and analyzed whether UA levels were correlated with the disease status of the patients, as defined by the ALS Functional Rating Scale-Revised (ALSFRS-R).
Results
The study included 136 ALS patients and 136 matched controls. The UA level was lower in the ALS patients (4.50±1.17 mg/dL, mean±SD) than in the controls (5.51±1.22 mg/dL; p<0.001). Among the ALS patients, the level of UA acid was inversely correlated with the rate of disease progression (decrease in ALSFRS-R score). Kaplan-Meier analysis revealed that a better survival rate was more strongly correlated with top-tertile levels of serum UA than with bottom-tertile levels (log-rank test: p=0.035).
Conclusions
ALS patients had lower serum UA levels than did healthy individuals. UA levels in ALS were negatively correlated with the rate of disease progression and positively associated with survival, suggesting that UA levels contribute to the progression of ALS. UA levels could be considered a biomarker of disease progression in the early phase in ALS patients.
Keywords: amyotrophic lateral sclerosis, uric acid, survival, oxidative stress, prognosis
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a devastating, adult-onset neurodegenerative disorder that is characterized by wasting and weakness of the limbs and bulbar and respiratory muscles due to degeneration of the upper and lower motor neurons. ALS usually leads to death within 3-5 years of symptom onset.1,2
Uric acid (UA) is the main end product of purine metabolism in humans and circulates at high concentrations due to inactivation of the enzyme urate oxidase as a result of a genetic defect that arose during human evolution.3 Serum UA is a natural antioxidant that can scavenge superoxides, thus helping to prevent the reaction of superoxide with nitric oxide, which leads to the formation of the strong oxidant peroxynitrite. The antioxidant effects of high concentrations of UA may protect against the development of neurodegenerative diseases and modulate the progression of these diseases.4,5 Elevated levels of serum UA have been associated with a reduced risk of Parkinson's disease.6 Serum UA may also affect the progression of cognitive impairment, in that higher levels of UA are associated with better cognitive function and a decreased risk of dementia.4,7
Studies of the association between serum UA levels and ALS have yielded conflicting results.5,8,9 Recent studies have found serum UA concentrations to be lower in subjects with ALS than in healthy controls4,10,11,12 and that lower concentrations of UA are associated with a more rapid progression of ALS, compared with those who have higher levels of UA and ALS. These results were reported for Japanese and Chinese patients;10,13 however, it is unknown whether UA affects disease progression in Korean ALS patients. The aim of this study was to determine serum levels of UA in Korean patients with sporadic ALS and to search for a correlation between UA levels and disease progression.
METHODS
ALS patients and healthy controls
ALS patients from the MND Clinic at the Neurology Department of Hanyang University Hospital in Seoul, Korea were prospectively enrolled from May 2009 to November 2012. All subjects were selected according to the revised El Escorial criteria, and fulfilled the criteria for possible, probable, probable-laboratory-supported or definite ALS.14 None of the patients had a family history of ALS or mutations in the gene encoding superoxide dismutase 1 (SOD1), so their diagnosis was seemingly sporadic ALS without SOD1 mutations. The ALS Functional Rating Scale-Revised (ALSFRS-R) was used to assess the patients' functional status.15 Patients in the early stages of ALS were recruited, as defined by a disease duration of ≤3 years. This study was approved by the Institutional Review Board of Hanyang University Hospital. The controls were recruited from outpatients who visited the Health Promotion Center of Hanyang University Hospital during the same period. The availability of more than 3,000 healthy individuals enabled the identification of well-matched controls for each patient in terms of sex, age, and body mass index (BMI). Thus, 136 healthy matched controls were included in the study.
Subjects who had been diagnosed with any condition associated with changes in serum UA concentrations were excluded, such as 1) stroke, angina, or myocardial infarction; 2) renal dysfunction; 3) history of alcohol abuse; 4) acute inflammatory state or medical disease (e.g., pneumonia or gastroenteritis); 5) administration of UA-lowering or UA-increasing medications (thiazide or allopurinol); 6) history of gout; or 7) history of percutaneous gastrostomy.
Clinical evaluation
Relevant demographic and clinical data were collected at enrollment, including age, sex, age at symptom onset and ALS examination, region of symptom onset, disease duration (interval between symptom onset and examination), forced vital capacity (FVC), medical history, alcohol consumption, and medications and treatment provided.
The progression rate of the disease (ΔFS) from symptom onset to the time of examination was calculated as follows: ΔFS=(48-ALSFRS-R score at time of examination)/[duration between symptom onset and time of examination (months)].16
Blood chemistry examination
Baseline serological data were obtained from serum samples obtained at the first visit to our clinic for the referral diagnosis of ALS. Patients who fulfilled the inclusion criteria after at least 6 months had elapsed since they entered the study had a second blood examination to obtain a longitudinal follow-up of serum UA level. Nonfasting blood was collected and centrifuged, and UA was assayed using the kinetic method (SICDIA L UA reagent, Shin Yang Pharm., Seoul, Korea). Within 30 minutes of collection, samples of blood were centrifuged at 3,000 rpm for 10 minutes. The serum levels of blood urea nitrogen, creatinine, and UA were measured using standard methods with the aid of an automatic chemistry analyzer (Hitachi 7600-210, Hitachi, Tokyo, Japan).
Statistical analysis
Continuous variables (e.g., age, BMI, and serum UA level) are presented as mean±SD values. Comparisons between ALS patients and control subjects regarding demographic and laboratory characteristics were performed using Student's t-test and chi-square tests. A paired t-test was used to compare serum UA levels at the times of the first and second examinations. The correlations between serum UA levels and the variables were calculated using Pearson's correlation. Multiple regression analyses were used to examine the association between serum levels of UA and the other variables (age, sex, BMI, and ALSFRS-R score). The cutoff for statistical significance was set at p<0.05 for all of the data analyses. Statistical analyses were carried out using SPSS (version 18; SPSS Inc., Chicago, IL, USA).
The survival analysis was conducted after stratifying UA levels according to sex-specific tertiles; survival was analyzed using the Kaplan-Meier method with the log-rank test. Survival was defined as the duration from the time of examination to death or tracheostomy.
RESULTS
Patients and healthy controls
During the study period, 206 patients visited the MND Clinic of the Neurology Department at Hanyang University Hospital. Of these, 19 were excluded due to refusal to participate in the study, and 16 were excluded due to a disease duration of longer than 36 months. Patients were further excluded due to a family history of motor neuron disease (n=11), presence of a concurrent respiratory tract infection (n=3), history of gout (n=3), cancer (n=10), cardiovascular disease (myocardial ischemia, angina, or stroke; n=6), a renal disease (n=4), and gastrostomy at the time of the first examination (n=1). After these patients were excluded, 136 patients were finally enrolled in the study group. Males constituted 69 (50.7%) of the study participants.
The ALS patients were 55.0±11.4 years old at the time of examination, had a disease duration of 13.6±7.4 months and a BMI of 22.6±3.4 kg/m2. The region of symptom onset was bulbar in 37 patients, limb in 95, and axial muscles in the remaining 4. Thirty-seven patients (27.2%) were being treated with riluzole at the time of the first examination (Table 1). There were no significant differences in age at symptom onset, age at examination, BMI, ALSFRS-R score, disease duration, or FVC between male and female patients.
Table 1. Demographic and clinical characteristics of the patients and healthy controls.
| Variable | Patients (n =136) | Healthy controls (n =136) | p | ||
|---|---|---|---|---|---|
| Median | Mean±SD | Median | Mean±SD | ||
| Age at examination (years) | 54.8 | 55.0±11.4 | 54.0 | 54.7±10.9 | 0.848 |
| Age at symptom onset (years) | 53.9 | 53.8±11.3 | |||
| Sex (male/female) | 69/67 | 69/67 | 1 | ||
| Bulbar onset | 37 (27.2%) | ||||
| Total ALSFRS-R score | 41.0 | 40.0±4.9 | |||
| Disease duration (months) | 11.9 | 13.6±7.4 | |||
| BMI (kg/m2) | 22.5 | 22.6±3.4 | 23.1 | 22.9±2.8 | 0.331 |
| Males (n=69) | 22.9 | 22.7±3.5 | 22.8 | 22.7±3.0 | 0.998 |
| Females (n=67) | 22.4 | 22.4±3.3 | 23.1 | 23.1±2.5 | 0.146 |
| Serum UA (mg/dL) | 4.60 | 4.50±1.17 | 5.40 | 5.51±1.22 | <0.001 |
| Males (n=69) | 5.00 | 4.98±1.24 | 6.20 | 6.13±1.10 | <0.001 |
| Females (n=67) | 3.90 | 4.01±0.85 | 4.80 | 4.87±0.98 | <0.001 |
ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised, BMI: body mass index, UA: uric acid.
The age (54.7±10.9 years) and BMI (22.9±2.8 kg/m2) of the matched healthy controls did not differ significantly from those of the ALS patients (p=0.848 and 0.331, respectively) (Table 1).
Serum UA levels and correlations between the clinical variables and UA
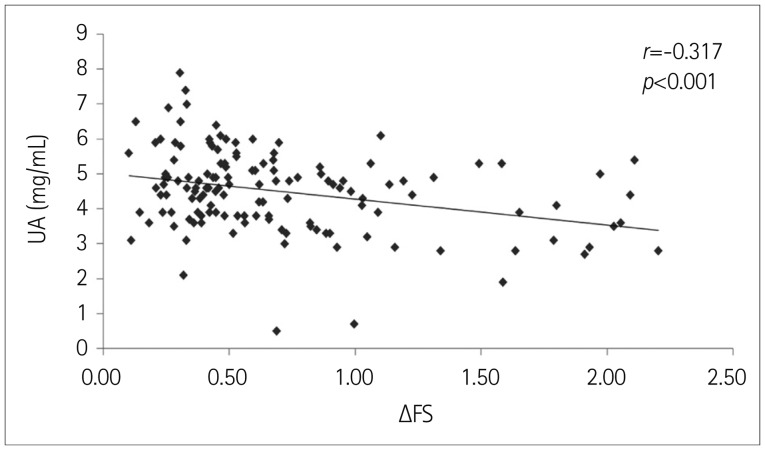
The serum UA level was significantly lower in the ALS patients (4.50±1.17 mg/dL) than in the healthy controls (5.51±1.22 mg/dL, p<0.001) for both males and females (Table 1). UA levels were lower among female patients and controls at the time of the first examination. The serum UA level was positively correlated with BMI, ALSFRS-R score, and creatinine, and was negatively correlated with ΔFS (r=0.280, p=0.001; r=0.218, p=0.011; r=0.381, p<0.001; and r=-0.317, p<0.001, respectively) (Fig. 1). Age at examination, age at symptom onset, disease duration, and FVC were not correlated with the serum UA level (r=-0.060, p=0.485; r=-0.066, p=0.448; r=0.090, p=0.295; and r=0.029, p=0.744, respectively).
Fig. 1. Correlation between serum uric acid (UA) level and disease progression rate (ΔFS). Slower progression of amyotrophic lateral sclerosis was correlated with a higher level of serum UA.
Simple and multiple regression analyses were used to control for confounders. Possible correlations between the serum UA level and the demographic and clinical parameters of the ALS patients were evaluated, such as age at examination, sex, region of onset, administration of riluzole, BMI, ALSFRS-R score, disease duration, ΔFS, FVC, and creatinine. Multiple linear backward regression revealed that sex, BMI, bulbar onset, and ΔFS contributed significantly to serum UA levels (R2=0.331, p<0.001 for the model) (Table 2). This indicates that lower serum UA level is associated with faster disease progression (as expressed by the decrease in ALSFRS-R score per month).
Table 2. Linear regression analysis of variables associated with serum UA levels in amyotrophic lateral sclerosis.
| Variable | Simple regression | Multiple regression | ||
|---|---|---|---|---|
| β (SE) | p | β (SE) | p | |
| Age at examination (years) | -0.006 (0.009) | 0.485 | ||
| Sex | 0.975 (0.183) | <0.001 | 1.026 (0.172) | <0.001 |
| Bulbar onset | 0.158 (0.226) | 0.485 | 0.494 (0.195) | 0.013 |
| BMI (kg/m2) | 0.097 (0.029) | 0.001 | 0.067 (0.025) | 0.010 |
| Riluzole | -0.050 (0.226) | 0.827 | ||
| ALSFRS-R | 0.052 (0.020) | 0.011 | ||
| Disease duration (months) | 0.014 (0.014) | 0.295 | ||
| ΔFS | -0.750 (0.194) | <0.001 | -0.499 (0.180) | 0.007 |
| FVC (%) | 0.001 (0.004) | 0.744 | ||
| Creatinine (mg/dL) | 3.254 (0.683) | <0.001 | ||
Factors included in the multiple linear backward regression analyses were age, sex, bulbar onset, BMI, administration of riluzole, ALSFRS-R score, disease duration, ΔFS, FVC, and creatinine.
ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised, BMI: body mass index, FVC: forced vital capacity, SE: standard error, UA: uric acid, ΔFS: disease progression rate.
Survival analysis of ALS patients according to serum UA level
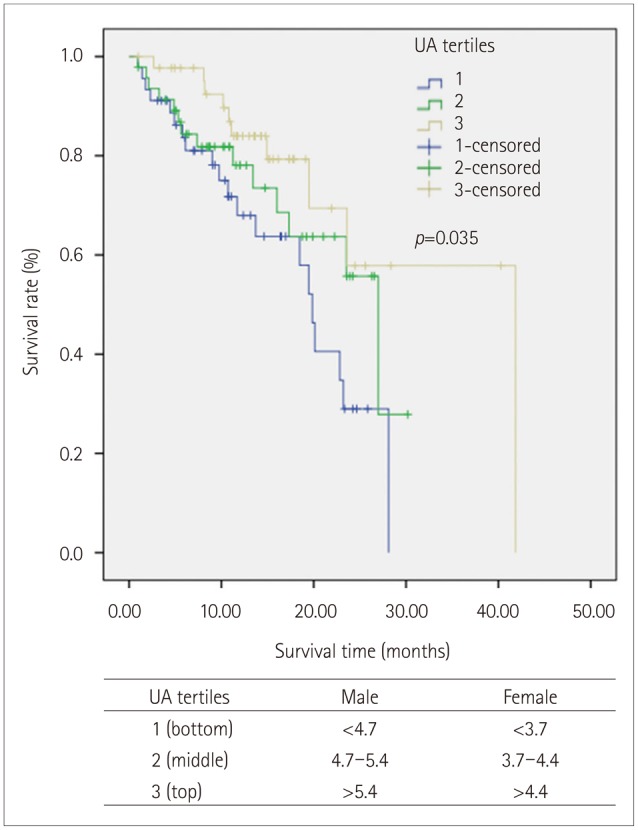
Serum UA levels were higher among males than females, and the tertile ranges of serum UA levels differed with sex. The tertile ranges were classified into bottom, middle, and top tertiles separately for males and females, and the survival rate was analyzed in the ALS patients using the sex-specific tertiles (for males: <4.7, 4.7-5.4, and >5.4 mg/dL; for females: <3.7, 3.7-4.4, and >4.4 mg/dL).
According to our survival analysis of males and females combined in a single model, there was a dose-dependent survival advantage for top-tertile serum UA levels compared to bottom-tertile serum UA levels (log-rank test: p=0.035) (Fig. 2). The mean (SE) survival times in the bottom, middle, and top tertiles were 17.9 (1.6), 21.2 (1.8), and 31.0 (3.4) months, respectively. After separation into male and female groups, the mean survival time did not significantly differ between the top and bottom tertiles among the males [31.4 (5.2) vs. 17.0 (2.3) months, p=0.057] or the females [26.7 (3.2) vs. 17.2 (1.9) months, p=0.218].
Fig. 2. Kaplan-Meier survival curves using stratified serum UA levels. The tertile ranges of serum UA levels differed with sex. The tertile ranges were classified as <4.7, 4.7-5.4, and >5.4 mg/dL in male subjects, and as <3.7, 3.7-4.4, and >4.4 mg/dL in female subjects. The survival curves demonstrate a relationship between the serum UA level and survival rate in the total population. UA: uric acid.
Utility of change in the significance of serum UA between the early and late phases of ALS as a biomarker
The temporal change in serum UA levels and the relationship between disease severity and serum UA level were investigated through follow-up examinations in 58 patients. Similar serum UA results were found in the 58 patients who had a second blood test at least 6 months after the first one (11.1±5.5 months, range 6.0-31.1 months): the serum UA level of the 58 patients who were followed up was 4.55±1.02 mg/dL (Table 3). The ΔFS from symptom onset to the time of the first or second examination (ΔFS1st or ΔFS2nd, respectively) was calculated as follows: ΔFS=(48-ALSFRS-R score at time of examination)/[duration between symptom onset and time of first or second examination (months)]. The sequential changes in the correlations between the serum UA level and the other variables, including age at examination, sex, bulbar onset, ALSFRS-R score, BMI, disease duration and the progression rate of the disease, were analyzed using multiple linear regression analysis. Multiple linear backward regression analysis for the time of the first examination revealed that sex and ΔFS1st were correlated with the serum UA level (R2=0.431, p<0.001 for the model), whereas age at the time of the first examination, bulbar onset, ALSFRS-R score, disease duration, and BMI were not significantly correlated. At the time of the second examination, only sex was correlated with the serum UA level (R2=0.381, p<0.001 for the model), while age at the time of the second examination, bulbar onset, ALSFRS-R score, disease duration, BMI, and ΔFS2nd were not significantly correlated.
Table 3. Comparison between characteristics at the first and second examinations.
| Variable | First examination (n=58) | Second examination (n=58) | p | ||
|---|---|---|---|---|---|
| Median | Mean±SD | Median | Mean±SD | ||
| Age at symptom onset (years) | 49.8 | 51.3±9.6 | |||
| Sex (male/female) | 26/32 | 26/32 | |||
| Age at examination (years) | 51.1 | 52.3±9.8 | 52.4 | 53.3±9.6 | <0.001 |
| Total ALSFRS-R score | 43.0 | 42.3±2.9 | 35.0 | 35.2±5.4 | <0.001 |
| Disease duration (months) | 12.0 | 12.7±6.4 | 21.3 | 24.1±9.4 | <0.001 |
| BMI (kg/m2) | 23.4 | 23.5±3.3 | 22.7 | 22.5±3.7 | <0.001 |
| Serum UA (mg/dL) | 4.60 | 4.55±1.01 | 4.40 | 4.54±1.17 | 0.885 |
| Males (n=26) | 5.05 | 5.21±0.95 | 5.35 | 5.30±1.06 | 0.682 |
| Females (n=32) | 3.90 | 4.02±0.73 | 4.10 | 3.92±0.85 | 0.351 |
| Serum Cr (mg/dL) | 0.80 | 0.78±0.12 | 0.68 | 0.68±0.13 | <0.001 |
| Males (n=26) | 0.80 | 0.83±0.08 | 0.71 | 0.71±0.10 | <0.001 |
| Females (n=32) | 0.70 | 0.73±0.13 | 0.64 | 0.66±0.14 | <0.001 |
| FVC, % (n=52) | 84.5 | 84.6±16.9 | 74.0 | 72.6±19.5 | <0.001 |
| Males (n=23) | 82.0 | 82.7±10.3 | 69.0 | 69.3±15.7 | <0.001 |
| Females (n=29) | 87.0 | 86.1±19.7 | 77.0 | 75.1±22.0 | 0.001 |
| ΔFS | 0.43 | 0.56±0.42 | 0.51 | 0.59±0.30 | 0.468 |
ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised, BMI: body mass index, Cr: creatinine, FVC: forced vital capacity, UA: uric acid, ΔFS: disease progression rate.
DISCUSSION
The presence of decreased serum UA levels was identified in a large cohort of patients with ALS and compared with the serum UA levels in a large group of healthy, well-matched controls. It was found that among the ALS patients, lower levels of UA were associated with lower ALSFRS-R scores and faster disease progression (i.e., faster ΔFS), suggesting that such patients are in a more debilitated state and that their disease progresses more rapidly. These data support the previously suggested role of UA in the pathogenesis of ALS (i.e., inhibition of or protection against motor neuron death).
The proposed pathogenic mechanisms underlying ALS emphasize the involvement of oxidative stress, glutamate toxicity, calcium-mediated toxicity, neurotrophic factor withdrawal, genetic defects, immune inflammation, and the accumulation of abnormal proteins.17 Among these various factors, neuronal cell death induced by oxidative stress appears to be one of the most important factors in the motor neuron degeneration that leads to ALS. UA is an important antioxidant and scavenger of free radicals,18 and serum UA has been reported to protect spinal cord neurons against glutamate neurotoxicity in rat embryo cultures.19 The protective effect of UA on neurodegeneration has been widely studied, which has revealed elevated serum levels of UA to be associated with slower disease progression in patients with other neurodegenerative diseases, including Parkinson's disease and Alzheimer's disease.4,6,7
However, the association between the serum UA level and ALS has not been clear, and the results of previous studies of ALS patients were limited and conflicting. A recent Italian study found that patients with ALS with longer disease durations had lower serum UA levels. Patients with bulbar-onset ALS had lower serum UA levels than patients with limb-onset ALS. Malnutrition induced by ALS might also reduce UA levels. One Japanese study found no difference in UA levels between ALS patients and healthy controls,9 but a more recent Japanese study found lower levels of UA,10,20 and a recent Chinese study produced similar results.13 In the present study, above-baseline serum UA levels were an independent risk factor for the rapid deterioration of ALSFRS-R score between the onset of symptoms and the time of the first examination. In addition, higher levels of serum UA were associated with higher survival rates.
The findings of this study should be considered in the light of certain limitations. First, although it was well defined, the study population was relatively small (in particular, few patients were followed up), and so the results must be interpreted cautiously. The possibility that the patients who were deteriorating more rapidly were less likely to visit the clinic for the follow-up examination or were more likely to have died might explain the lack of a correlation between this set of data and ΔFS. In addition, because a wide range of ALS patients was included, such as those with possible ALS according to the revised El Escorial criteria,14 some of the ALS patients in this study had slower ΔFS than those included in previously reported cohorts.16,21 However, the present study was designed to make the most of an opportunity and to enable prospective observation during patient enrollment in this cohort, and so the benefits of this design minimized these limitations.
Second, adjustments were made for known confounding factors of UA levels, including sex and BMI, but there are other possible confounding factors that may be associated with serum UA levels that could not be accounted for, including dietary preferences (e.g., the consumption of vitamin supplements, alcohol, dairy products, and an Asian or Western diet) and other environmental factors. Although the regression analysis did not reveal a correlation between the administration of riluzole and serum UA levels, the effect of riluzole on serum UA levels might be unclear.
Third, the associations between unmeasured genetic effects and UA have not been explained. Many studies have found an association between UA level and genetic background with respect to the UA transporter, kidney-function-related genes, apolipoprotein E, and susceptibility genes.22,23 Although genetic associations with the level of UA have been reported in East Asians,24,25 they have not been studied in ALS patients. Lower levels of UA in ALS patients are associated with secondary malnutrition or the disease itself, but genetic differences may also affect UA metabolism. Our previous work has suggested that the genetic makeup of ALS patients differs from that of normal Western populations26,27; therefore, further study is needed into the associations between UA and genetic makeup in patients with ALS.
Interestingly, the correlation between ΔFS and serum UA changed over time in the present study, with the regression analysis revealing a correlation between ΔFS and serum UA in the early phase but not in the late phase. These results suggest that serum UA could be used as a biomarker of disease progression in the early phase but not in the late phase. The lack of correlation in the late phase could be explained by dietary changes, muscle wasting, and the use of various drugs including riluzole and multivitamins, which could affect serum UA levels in the late phase.
An increase in serum free-radical-scavenging capacity was observed after systemic administration of UA in healthy volunteers.18,28,29 Although a method of UA administration for increasing UA in patients with ALS was not examined in the present study, UA is a putative indicator of decreased oxidative stress and free-radical scavenging.
In conclusion, in agreement with studies, the level of serum UA were lower in the ALS patients than in the well-matched controls in the present study, and UA level was inversely correlated with both ΔFS and survival rate. These results support the hypothesis that oxidative stress is an important mechanism in ALS and that UA protects neurons from oxidative stress and inhibits disease activity. The administration of UA or other agents to increase serum UA levels could be a potential therapeutic or modulatory agent for ALS patients affected by oxidative damage.
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI12C0135).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Bae JS, Hong YH, Baek W, Sohn EH, Cho JY, Kim BJ, et al. Current status of the diagnosis and management of amyotrophic lateral sclerosis in Korea: a multi-center cross-sectional study. J Clin Neurol. 2012;8:293–300. doi: 10.3988/jcn.2012.8.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 3.Oda M, Satta Y, Takenaka O, Takahata N. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 4.Abraham A, Drory VE. Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: a meta-analysis. J Neurol. 2014;261:1133–1138. doi: 10.1007/s00415-014-7331-x. [DOI] [PubMed] [Google Scholar]
- 5.Keizman D, Ish-Shalom M, Berliner S, Maimon N, Vered Y, Artamonov I, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–99. doi: 10.1016/j.jns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132(Pt 2):377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- 8.Kokić AN, Stević Z, Stojanović S, Blagojević DP, Jones DR, Pavlović S, et al. Biotransformation of nitric oxide in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Redox Rep. 2005;10:265–270. doi: 10.1179/135100005X70242. [DOI] [PubMed] [Google Scholar]
- 9.Sohmiya M, Tanaka M, Suzuki Y, Tanino Y, Okamoto K, Yamamoto Y. An increase of oxidized coenzyme Q-10 occurs in the plasma of sporadic ALS patients. J Neurol Sci. 2005;228:49–53. doi: 10.1016/j.jns.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51:1501–1508. doi: 10.2169/internalmedicine.51.7465. [DOI] [PubMed] [Google Scholar]
- 11.Paganoni S, Zhang M, Quiroz Zárate A, Jaffa M, Yu H, Cudkowicz ME, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012;259:1923–1928. doi: 10.1007/s00415-012-6440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoccolella S, Simone IL, Capozzo R, Tortelli R, Leo A, D'Errico E, et al. An exploratory study of serum urate levels in patients with amyotrophic lateral sclerosis. J Neurol. 2011;258:238–243. doi: 10.1007/s00415-010-5735-9. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Z, Guo X, Wei Q, Song W, Cao B, Huang R, et al. Serum uric acid level is associated with the prevalence but not with survival of amyotrophic lateral sclerosis in a Chinese population. Metab Brain Dis. 2014;29:771–775. doi: 10.1007/s11011-014-9510-y. [DOI] [PubMed] [Google Scholar]
- 14.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 15.Gordon PH, Miller RG, Moore DH. ALSFRS-R. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(Suppl 1):90–93. doi: 10.1080/17434470410019906. [DOI] [PubMed] [Google Scholar]
- 16.Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–267. doi: 10.1212/01.wnl.0000194316.91908.8a. [DOI] [PubMed] [Google Scholar]
- 17.Goodall EF, Morrison KE. Amyotrophic lateral sclerosis (motor neuron disease): proposed mechanisms and pathways to treatment. Expert Rev Mol Med. 2006;8:1–22. doi: 10.1017/S1462399406010854. [DOI] [PubMed] [Google Scholar]
- 18.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 19.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Kawabe K, Iwasaki Y. Do serum uric acid levels reflect oxidative stress in the progression of ALS? J Neurol Sci. 2009;287:294, author reply 295. doi: 10.1016/j.jns.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Baek W, Park A, Kim HY, Kim SH. Amyotrophic lateral sclerosis in Korea: clinical characteristics and prognostic factors. J Korean Neurol Assoc. 2011;29:16–24. [Google Scholar]
- 22.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nath SD, Voruganti VS, Arar NH, Thameem F, Lopez-Alvarenga JC, Bauer R, et al. Genome scan for determinants of serum uric acid variability. J Am Soc Nephrol. 2007;18:3156–3163. doi: 10.1681/ASN.2007040426. [DOI] [PubMed] [Google Scholar]
- 24.Okada Y, Sim X, Go MJ, Wu JY, Gu D, Takeuchi F, et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat Genet. 2012;44:904–909. doi: 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sull JW, Park EJ, Lee M, Jee SH. Effects of SLC2A9 variants on uric acid levels in a Korean population. Rheumatol Int. 2013;33:19–23. doi: 10.1007/s00296-011-2303-2. [DOI] [PubMed] [Google Scholar]
- 26.Jang JH, Kwon MJ, Choi WJ, Oh KW, Koh SH, Ki CS, et al. Analysis of the C9orf72 hexanucleotide repeat expansion in Korean patients with familial and sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2013;34:1311.e7–1311.e9. doi: 10.1016/j.neurobiolaging.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Kwon MJ, Baek W, Ki CS, Kim HY, Koh SH, Kim JW, et al. Screening of the SOD1, FUS, TARDBP, ANG, and OPTN mutations in Korean patients with familial and sporadic ALS. Neurobiol Aging. 2012;33:1017.e17–1311.e23. doi: 10.1016/j.neurobiolaging.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin Sci (Lond) 2003;105:425–430. doi: 10.1042/CS20030149. [DOI] [PubMed] [Google Scholar]
- 29.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–371. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]