Abstract
OBJECTIVES: Multidrug-resistant Gram-negative bacteria, including extended-spectrum beta-lactamase (ESBL)–producing organisms, are a growing problem. The primary objective of this study was to describe the proportion of children with ESBL-producing urinary isolates at a tertiary medical center as well as these organisms' susceptibility patterns. The secondary objective was to identify the risk factors for acquiring ESBL urinary pathogens.
METHODS: This retrospective study evaluated a cohort of children with ESBL urinary isolates, admitted to a tertiary children's hospital during a 6-year period. The proportion of patients with an ESBL-producing urinary isolate among all patients who grew a Gram-negative isolate is described together with the organism's susceptibility pattern. Patients with non-ESBL Gram-negative urinary organisms were used as a control group for identifying patient risk factors for ESBL.
RESULTS: A total of 7.8% (29 of 370) of patients in our cohort grew Gram-negative urinary isolates with an ESBL strain. Most of the ESBL organisms isolated were sensitive to carbapenems (100% of ESBL organisms susceptible to ertapenem and 93.8% susceptible to meropenem) and amikacin (92.3% of ESBL organisms susceptible). Patients with longer hospitalization, recent antibiotic use, and recent intensive care unit admission were found to be at increased risk for ESBL organisms in the urine.
CONCLUSIONS: When selecting empiric antibiotic therapy for suspected urinary tract infection in children, it may be prudent to consider the risk factors identified for acquiring an ESBL urinary pathogen.
INDEX TERMS: extended-spectrum beta-lactamase, pediatric, urinary
INTRODUCTION
Multidrug-resistant Gram-negative bacteria are a growing problem worldwide. Higher mortality has been observed in patients with multidrug-resistant Gram-negative bloodstream infections and urinary tract infections.1,2 Extended-spectrum beta-lactamases (ESBLs) are plasmid-mediated groups of enzymes that hydrolyze penicillins, extended-spectrum cephalosporins, and aztreonam.3 Carbapenems often serve as effective therapy.3 Understanding patient risk factors for acquiring resistant pathogens is important in selecting empiric therapy.
In the neonatal intensive care unit (ICU), risk factors for ESBL infection include lower gestational age, prematurity, prolonged rupture of membranes, and antibiotic exposure, especially to third-generation cephalosporins.4,5 In other pediatric populations, factors associated with ESBL bacteremia include central venous catheters, previous use of antibiotics (vancomycin, third-generation and extended-spectrum cephalosporins), and number of antibiotics used, female sex, corticosteroid use within 30 days before infection, prior hospitalization, and admission to an ICU within the previous month.1,6–8 Higher mortality has been observed in patients with multidrug-resistant Gram-negative bloodstream infections and urinary tract infections.1,2
In this study, we describe the proportion of patients with an ESBL-producing urinary isolate among patients with Gram-negative urine isolates in children hospitalized at a tertiary care medical center, characterize the susceptibility patterns of these organisms, and identify risk factors for acquiring ESBL urinary pathogens.
MATERIALS AND METHODS
A retrospective cohort study was conducted of all hospitalized pediatric patients at The Johns Hopkins Hospital with urine cultures growing Gram-negative bacteria from January 2002 to January 2008. The proportion of patients with ESBL-producing isolates was calculated from the total number of patients with any Gram-negative isolate. The method for assessment of ESBL production at this institution has changed over time. From 2000 to 2006, the method was agar dilution confirmed with E-test. Since 2006, the laboratory has switched to the Phoenix instrument with E-test for confirmation. The Phoenix has a built-in algorithm that flags isolates as potential ESBL producers and this is confirmed phenotypically by using E-test strips that contain ceftazidime and cefotaxime with and without clavulanic acid. Antibiotic susceptibility testing was conducted by using the Clinical and Laboratory Standards Institute cutoff method. Susceptibilities of the ESBL organisms obtained were assessed.
A control group was selected from patients with non–ESBL-producing Gram-negative urinary cultures in a 2:1 ratio of controls to cases. Given the low proportion of patients with ESBL, a ratio of 2:1 for controls versus cases was chosen on the basis of feasibility and availability of controls. Case patients were not eligible to be controls, and no patient in either group was used more than once. Cases and controls were compared to identify potential risk factors for acquiring ESBL-producing isolates. Patient characteristics analyzed include demographics, duration of hospital stay before Gram-negative culture, previous systemic antibiotic administration within 30 days of positive culture, underlying comorbidities, urinary hardware devices, use of steroids or immunosuppressive medications within 30 days of positive culture, and history of ICU admission within 30 days of positive culture.
All study patients were identified by the institution's microbiology laboratory electronic database, and antibiotic susceptibilities of ESBL organisms were collected from electronic patient records. The aforementioned patient characteristics evaluated for ESBL risk were also extracted from the institution's electronic patient records.
Differences between the case and control groups with respect to continuous variables were evaluated with the Wilcoxon rank sum test, and categorical variables were compared by using the Fisher exact test. The institution's investigational review board approved this study.
RESULTS
From January 2002 through January 2008, a total of 370 patients had 1 or more urine cultures grow a Gram-negative organism. Of these, 29 patients grew an ESBL-producing Gram-negative organism (7.8% of patients with a Gram-negative organism). These organisms included Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca. The proportion of E coli isolates producing ESBL was 9.3%. Approximately one-quarter (24.7%) of K pneumoniae isolates produced ESBL, and 35.3% of K oxytoca isolates were ESBL producers.
Most ESBL organisms (Table 1) were susceptible to the tested carbapenem antibiotics of ertapenem (100%) and meropenem (93.8%), with only 1 isolate, a K pneumoniae isolate, having intermediate resistance to meropenem. Some isolates were susceptible to piperacillin/tazobactam (38.9%), and most were susceptible to amikacin (92.3%) with only 2 isolates, 1 K pneumoniae isolate and 1 E. coli isolate, having intermediate resistance. Fewer than half of the ESBL-producing organisms tested were susceptible to tobramycin (23.1%) and gentamicin (25.8%), but more than half were susceptible to fluoroquinolones, including ciprofloxacin (68.8%) and gatifloxacin (87.5%). All organisms tested were resistant to sulfonamide. Susceptibility to trimethoprim/sulfamethoxazole and tetracycline was low at 22.6% and 32.3%, respectively. More than half the organisms tested were susceptible to nitrofurantoin (58.6%).
Table 1.
Antibiotic Susceptibilities of ESBL-Producing Isolates

In the control group, the isolates collected from these 58 patients included E. coli from 24 patients, Pseudomonas aeruginosa from 10 patients, Proteus mirabilis from 7 patients, Enterobacter cloacae from 6 patients, K pneumoniae from 4 patients, Stenotrophomonas maltophilia from 2 patients, and Acinetobacter baumannii, K oxytoca, Serratia marcescens, Enterobacter aerogenes, and Citrobacter amalonaticus from 1 patient each.
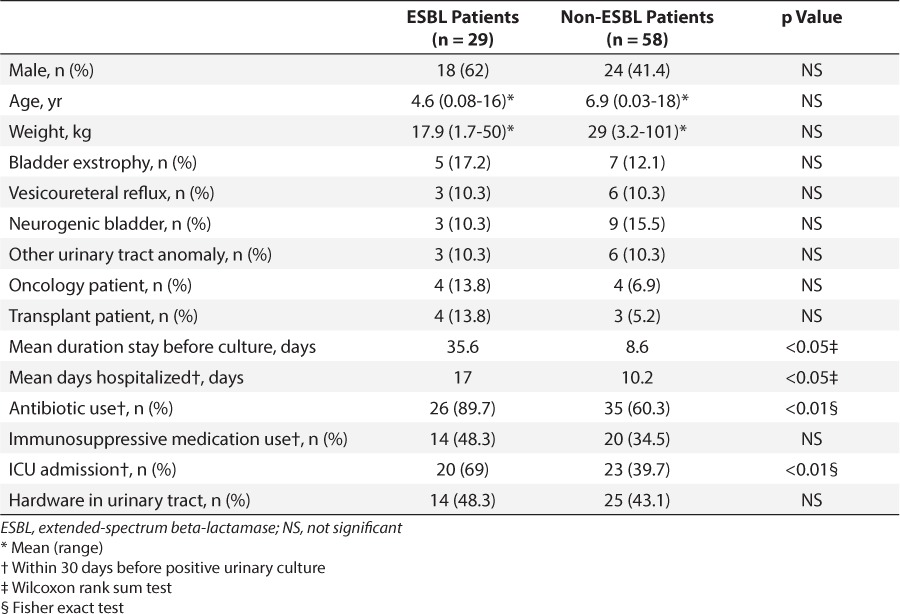
Clinical characteristics were compared between the 29 cases and 58 controls (Table 2). Case and control groups were similar in terms of sex. Case patients tended to be younger and weigh less than control patients, but these differences did not reach statistical significance. There was no difference between groups in pertinent comorbidities, which included urinary tract abnormalities, malignancy, or transplant history. Case patients were in the hospital for significantly longer before their positive urine culture when compared to the control group (35.6 versus 8.6 days, p < 0.05). Case patients also were hospitalized for a greater percentage of the 30 days before culture as compared with control patients (17 versus 10.2 days, p < 0.05, respectively). More patients in the ESBL group had received antibiotics within 30 days before the positive culture (89.7% versus 60.3%, p < 0.01). Antibiotics used before positive urinary culture varied widely and included amikacin, ampicillin, amoxilillin/clavulanic acid, cefazolin, cefepime, cefotaxime, ceftriaxone, ciprofloxacin, clindamycin, gentamicin, meropenem, metronidazole, oxacillin, penicillin G, piperacillin/tazobactam, sulfamethazole/trimethoprim, tobramycin, and vancomycin. Patients in the ESBL group were also more likely to have been in an ICU within 30 days before the positive urinary culture (69% versus 39.7%, p < 0.01). There was no significant difference in immunosuppressive medication use within 30 days or presence of hardware device in the urinary tract at the time of the culture.
Table 2.
Clinical Characteristics and Risk Factors for ESBL Case and Control Patients
DISCUSSION
Of all pediatric inpatients with urine cultures positive for Gram-negative organisms at our institution from January 2002 to January 2008, 7.8% grew an ESBL organism. These ESBL-producing organisms demonstrated the least resistance to carbapenems and amikacin, based on susceptibility results.
In comparison to our study, a 2004 report from Poland described 11.5% of urine isolates as ESBL producers in children.9 We had a higher percentage of ESBL-producing K oxytoca (35.5% vs. 25%) but a similar percentage of E coli (13.2%) and K pneumoniae (20%) ESBL producers. Of note, ESBL-producing P mirabilis was found in 10% of their isolates; however, our institution did not test for ESBL-producing P mirabilis. Additionally, a recent study in a pediatric ICU population by Benner and colleagues8 describes a similar percentage of ESBL-producing E coli (10.3% vs. 9.3%) and lower percentage of K pneumoniae ESBL producers (13.5% vs. 24.7%). This study reported 110 isolates from multiple sources, which include blood (7.3%), urine (32.7%), tracheal aspirate (50.9%), cerebral spinal fluid (1.8%), and wound/abscess (7.3%).8 For a direct comparison of urinary isolates, a detailed number of non–ESBL-producing E coli and K pneumoniae urinary isolates was not provided in this report.
Our data suggest that risk factors for having ESBL-producing urinary pathogens include longer hospitalization time before positive culture, previous antibiotic use, and prior ICU admission. We measured hospitalizations in the previous 30 days to identify bounce-back admissions because those patients were recently hospitalized, perhaps exposing them to resistant organisms. In contrast to the ESBL risk factors identified in children with bacteremia,1,6,7 sex, presence of hardware device, and use of immunosuppressive agents do not seem to increase the risk of having a urinary ESBL-producing organism. The presence of urinary hardware was not found to be a risk factor for acquiring ESBL-producing organisms; however, this may be underreported owing to inconsistent documentation of the presence of catheters in the medical record.
Limitations of this study include reliance on retrospective data and small sample size. Also, the patients in this study had urine cultures positive for Gram-negative organisms, but they did not necessarily have true urinary tract infections; however, it is important to be aware of risk factors for colonization of resistant bacteria as well, since colonization with ESBL-producing K pneumoniae has been suggested to lead to infection in the neonatal and neurosurgical ICUs.10,11 We did not evaluate cases of infection in this study. Therefore, we are unable to assess associations of risk factors between colonization and infection.
In conclusion, 7.8% of all pediatric inpatients with urine cultures positive for Gram-negative organisms at a tertiary care children's hospital from January 2002 to January 2008 grew an ESBL organism. ESBL-producing organisms in the urine cultures of patients in this study demonstrated the least resistance to the carbapenems and amikacin. Potential risk factors for acquiring ESBL urinary pathogens, identified in this study, include longer hospitalization time before positive culture, previous antibiotic use within 30 days, and prior ICU admission within 30 days. Antibiotics are often empirically selected. It may be prudent to consider the risk factors identified for acquiring an ESBL urinary pathogen when selecting empiric antibiotic therapy for suspected urinary tract infection in pediatric patients.
ACKNOWLEDGMENT
Dr Degnan was a PGY-2 Pediatric Pharmacy Resident at The Johns Hopkins Hospital at the time of the study. This research was presented at the 48th Annual Meeting of the American Society for Microbiology and the Infectious Diseases Society of America in Washington, DC, and supported by the Maryland Society of Health-System Pharmacist Research Grant. Work was conducted at The Johns Hopkins Hospital. Karen C. Carroll, MD, Professor of Pathology and Medicine, Director, Division of Medical Microbiology, at The Johns Hopkins Hospital is recognized for her assistance with this project.
ABBREVIATIONS
- ESBL
extended-spectrum beta-lactamase
- ICU
intensive care unit
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Arnoni M, Berezin E, Martino M. Risk factors for nosocomial bloodstream infection caused by multi-drug resistant gram-negative bacilli in pediatrics. Braz J Infect Dis. 2007;11(2):267–271. doi: 10.1590/s1413-86702007000200020. [DOI] [PubMed] [Google Scholar]
- 2.Melzer M, Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J Infect. 2007;55(3):254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Ang JY, Ezike E, Asmar BI. Antibacterial resistance. Indian J Pediatr. 2004;71(3):229–239. doi: 10.1007/BF02724275. [DOI] [PubMed] [Google Scholar]
- 4.Linkin DR, Fishman NO, Patel JB et al. Risk factors for extended-spectrum beta-lactamase-producing enterobacteriaceae in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2004;25(9):781–783. doi: 10.1086/502477. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal R, Gaind R, Chellani H et al. Extended-spectrum B lactamase-producing gram-negative bacteria: clinical profile and outcome in a neonatal intensive care unit. Ann Trop Pediatr. 2007;27(1):45–54. doi: 10.1179/146532807X170501. [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Goyal M, Chu J et al. Risk factors for and outcomes of bloodstream infection caused by extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella species in children. Pediatrics. 2005;115(4):942–949. doi: 10.1542/peds.2004-1289. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Pai H, Lee HJ et al. Bloodstream infections by extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Chemother. 2002;46(5):1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benner KW, Parbhakaran P, Lowros AS. Epidemiology of infections due to extended-spectrum beta-lactamase-producing bacteria in a pediatric intensive care unit. J Pediatr Pharmacol Ther. 2014;19(2):83–90. doi: 10.5863/1551-6776-19.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delinska-Galinska A, Kurlenda J, Kozielska E et al. Prevalence of ESBL strains in urinary tract infections in children in 1996 and 2004. Przeglad Epidemiologiczny. 2006;60(1):59–64. [PubMed] [Google Scholar]
- 10.Tamma PD, Savard P, Pal T et al. An outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2012;33(6):631–634. doi: 10.1086/665715. [DOI] [PubMed] [Google Scholar]
- 11.Arpin C, Rogues AM, Kabouche S et al. Prospective survey of colonization and the infection caused by SHV-4 producing Klebsiella pneumoniae in a neurosurgical intensive care unit. Epidemiol Infect. 2000;124(3):401–408. doi: 10.1017/s0950268899003908. [DOI] [PMC free article] [PubMed] [Google Scholar]


