Abstract
Staphylococcus aureus is the most common bacteria associated with the development of osteomyelitis in pediatric patients. Osteomyelitis caused by methicillin-resistant Staphylococcus aureus (MRSA) can be difficult to safely and effectively treat. Vancomycin, linezolid, and clindamycin are commonly used to treat osteomyelitis caused by MRSA. While adult studies suggest intravenous (IV) daptomycin may by beneficial for the treatment of MRSA osteomyelitis, it is not Food and Drug Administration approved for use in pediatrics, and minimal data are available related to its use in this population. This case report describes the successful use of daptomycin (8 mg/kg/dose IV daily) combined with rifampin for 5 weeks, followed by 5 weeks of oral sulfamethoxazole/trimethoprim, for treatment of acute bilateral osteomyelitis caused by MRSA in an 8-year-old male. The patient did not initially respond to the combination of vancomycin plus rifampin and gentamicin, nor did he respond to ceftaroline treatment. After initiation of daptomycin, his fevers quickly subsided, his pain rapidly improved, and his inflammatory markers significantly decreased. While daptomycin was effective in this patient, additional research is needed to determine the true safety and efficacy of this drug for treatment of osteomyelitis caused by MRSA in pediatric patients.
INDEX TERMS: daptomycin, methicillin-resistant Staphylococcus aureus, osteomyelitis, pediatric
INTRODUCTION
Treatment of acute osteomyelitis continues to be challenging in the pediatric population. The severity of this infection, coupled with limited clinical studies, often lead to a trial-and-error type of treatment course. Staphylococcus aureus is the most common causative pathogen in children.1 Methicillin-resistant Staphylococcus aureus (MRSA) infections further complicate a patient's clinical picture and therapeutic regimen. Currently, the Food and Drug Administration (FDA) has approved vancomycin, linezolid, and clindamycin as appropriate treatment options for pediatric MRSA infections.2–4 These medications are also commonly used to treat MRSA osteomyelitis, given the coverage and bone penetration of these agents.5
Multiple studies6–8 have concluded that daptomycin is yet another plausible antibiotic available for treatment of MRSA infections in adults. This bactericidal agent has excellent activity against Gram-positive pathogens as well as a synergistic effect with rifampin in biofilms.9 Yet inadequate safety and efficacy data in pediatric patients limit its use in this population. Current Infectious Diseases Society of America (IDSA) guidelines suggest 6–10 mg/kg of intravenous (IV), once-daily daptomycin as an alternative agent for the management of MRSA infections, including pediatric osteomyelitis. Importantly, these recommendations are level CIII and have minimal pediatric evidence supporting the recommendation.10 To our knowledge there is currently 1 case report, albeit for methicillin-susceptible Staphylococcus aureus (MSSA), and 1 retrospective study including 2 pediatric patients receiving daptomycin for osteomyelitis that have been published.11,12
CASE SUMMARY
A 30-kg, 8-year-old male was admitted to a local community hospital because of bilateral leg pain, with difficulty walking, frequent fevers up to 39.7°C, nausea, and vomiting. At the onset of symptoms, the patient was traveling on a family vacation, and no history of trauma was noted. The patient's past medical history was significant for recurrent MRSA skin infections as well as one lesion requiring drainage 15 months prior to admission.
At the local community hospital, labs were drawn and antibiotic therapy was initiated. Pertinent labs included the following: C-reactive protein (CRP) ranging from 15.7 to 22.8 mg/dL, erythrocyte sedimentation rate (ESR) increasing from 64 to 140 mm/hr, creatinine phosphokinase (CPK) concentrations reaching 112 units/L, a white blood cell (WBC) count of 14.9 × 109 cells/L with a neutrophil predominance of 79%, and a right knee arthrocentesis, which demonstrated a WBC count of 34.0 × 109 cells/L with 86% polymorphonuclear leukocytes. The patient was febrile daily, tachycardic, intermittently hypotensive, and rated his leg pain subjectively as 10/10 throughout the weekend leading to his admission. Magnetic resonance imaging (MRI) and bone scan confirmed osteomyelitis (likely hematogenous) of the left fibula and right femoral epiphysis, as well as septic arthritis in the right knee. A Staphylococcus aureus infection was suspected (potentially MRSA), and 550 mg (18 mg/kg/dose) IV vancomycin every 6 hours was initiated. However, an initial trough concentration was reportedly found to be less than 15 mg/L while at the outside hospital. The appropriateness of this concentration was unable to be validated from the outside hospital. Morphine and ketorolac were given IV for pain management and the patient was transferred to our tertiary academic medical center for abscess drainage and further management of his infection.
Once admitted to our pediatric progressive care unit, as a result of hypotension as low as 89/45 mm Hg, infectious disease consultants recommended the addition of rifampin 300 mg (10 mg/kg/dose) IV every 12 hours to the patient's vancomycin treatment, which was increased to 600 mg (20 mg/kg/dose) every 6 hours. As his blood pressure dropped to 89/45, IV morphine and oral naproxen, 6 mg and 150 mg, respectively, were administered for pain control. Within 24 hours of admission, the patient was taken to the operating room for an incision and drainage of an identified left femoral subperiosteal abscess. Initial cultures were positive for Gram-positive cocci. A vancomycin trough concentration (30 minutes before the fourth dose) was then measured at 35.8 mg/L. Acute kidney injury was suspected (SCr increased from 0.4 to 0.7 mg/dL) due to multiple nonsteroidal anti-inflammatory drug administrations, hypotension, vancomycin, and volume depletion due to vomiting. Vancomycin was discontinued, and while waiting for pending blood and tissue cultures, ceftaroline 400 mg (13.3 mg/kg/dose) IV every 8 hours was started for the possibility of a MRSA infection, and rifampin was also continued. The patient's kidney function stabilized, but no clinical improvement was noted. Blood and subperiosteal abscess cultures returned significant for MRSA. The culture was noted to have susceptibility to clindamycin, linezolid, and vancomycin with a minimum inhibitory concentration (MIC) of <0.5 mg/L. As a result of this finding, normalization of renal function, and lack of improvement on ceftaroline, IV vancomycin therapy was reinitiated and rifampin was continued.
The patient remained on vancomycin for 8 days, with trough concentrations maintained between 13 and 22 mg/L. During this time, his clinical response was poor and the infection persisted. He continued to be febrile, with temperatures ranging from 36.5°C to 39.5°C (Figure 1); pain remained a significant factor, requiring hydromorphone 0.5 mg IV treatment every 2 hours; and the vancomycin MIC from repeated blood cultures increased from <0.5 to 1 mg/L. His WBC count declined to 5.0 × 109 cells/L, and his CRP declined to 12.5 mg/dL, but remained at that concentration for 3 days (Figure 2). He was constantly febrile (Figure 1) and still experienced severe pain. Seven days of gentamicin 30 mg (1 mg/kg/dose) IV every 8 hours was also added to his therapeutic regimen during this time for additional synergy. Although blood cultures drawn 2 days after reinitiating vancomycin treatment were preliminarily negative, the patient continued to decline physically and was still unable to ambulate. A repeat MRI of the abdomen and pelvis was performed showing no drainable abscess or fluid collection that could be contributing to the patient's fevers or could be drained. A second transthoracic echocardiogram was also performed and did not show evidence of endocarditis. Vancomycin was discontinued, and daptomycin 250 mg (8 mg/kg/dose) IV every 24 hours was initiated, while both gentamicin and rifampin were continued.
Figure 1.
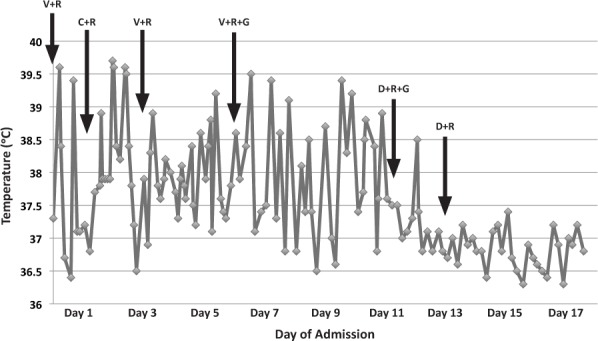
Temperature curve.
C, ceftaroline; D, daptomycin; G, gentamicin; R, rifampin; V, vancomycin
Figure 2.
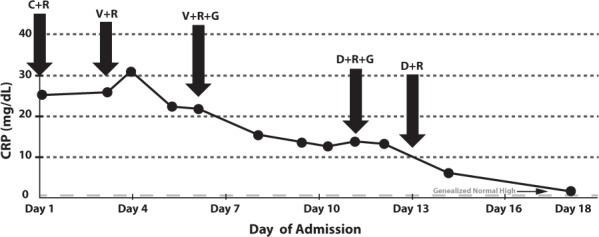
C-reactive protein measurements.
C, ceftaroline; D, daptomycin; G, gentamicin; R, rifampin; V, vancomycin
During the first day of daptomycin treatment, the patient's clinical condition improved. He became afebrile, except for a single 38.5°C reading (Figure 1), and his CRP started to decline again (Figure 2). He also began ambulating with assistance and working with physical therapy on day 2 of daptomycin therapy. His pain improved, and the use of hydromorphone was decreased. Repeat blood cultures were also negative. Creatinine phosphokinase concentrations were monitored throughout the daptomycin treatment and remained below 71 units/L (40–350 units/L is considered an acceptable range in our health system's laboratory). The patient received 8 days of daptomycin as an inpatient and was also transitioned from IV to oral rifampin during these 8 days. A peripherally inserted central catheter was placed, and he was discharged on dual therapy with the daptomycin provided by home health.
While at home and throughout his outpatient follow-up appointments, he continued to respond to his antibiotic regimen without reported side effects. The patient's CRP, ESR, CPK, and body temperature were monitored on a weekly basis. During this time, these values were consistently within normal limits. C-reactive protein concentrations were <0.5 mg/dL; ESR and CPK values remained below 38 mm/hr and 71 units/L, respectively. The patient was also afebrile during this time period. After nearly 5 weeks (roughly 1 week inpatient and 4 weeks outpatient), IV daptomycin and oral rifampin therapy were discontinued and switched to a 5-week course of oral sulfamethoxazole/trimethoprim suspension, 600/120 mg (4 mg/kg/dose of the trimethoprim component) twice a day. As an outpatient, oral rifampin 300 mg twice daily was taken for 6 weeks total. No adverse effects or limitations in daily activities were noted during the last follow-up appointment. Three months after the osteomyelitis diagnosis, all antibiotics were discontinued.
DISCUSSION
This case report describes the successful use of IV daptomycin for treatment of osteomyelitis caused by MRSA in an 8-year-old male who did not respond to the combination of vancomycin plus rifampin and gentamicin or ceftaroline treatment. On day 11 of our patient's hospital stay, IV daptomycin combined with rifampin and gentamicin became the backbone of his therapy. Within 24 hours of initiation, significant improvements were seen. While these improvements appeared to be related to daptomycin initiation, they may also be viewed as coincidental improvement based on the nature of the disease progression.
Although the IDSA guidelines recommend linezolid as an alternative option to vancomycin for acute hematogenous MRSA osteomyelitis, this antibiotic was not ideal in our patient for multiple reasons.10 Bone marrow suppression (particularly thrombocytopenia) has been documented with treatment duration lasting more than 2–3 weeks.13 The chance of this adverse event was high, as the expected length of treatment for osteomyelitis generally ranges from 4 to 6 weeks.5,10 Linezolid has also been linked to irreversible peripheral neuropathy.13 Lastly, linezolid's bacteriostatic activity was not thought to be ideal for our patient, who was persistently bacteremic and at risk for severe sepsis. Clindamycin was also not chosen, despite excellent bone penetration and FDA approval for pediatric Staphylococcus aureus infections, as a result of its bacteriostatic activity.10,14 While not an appropriate agent when used alone, another option in this case could have been to add clindamycin to vancomycin therapy (instead of gentamicin). However, the infectious disease consultants instead desired gentamicin treatment.
Ceftaroline was briefly initiated as a result of the patient's temporary acute kidney injury and its known MRSA coverage.15 Treatment with this antibiotic was deemed unsuccessful, leading to the discontinuation of ceftaroline and the reinitiation of IV vancomycin. Intravenous vancomycin continues to remain the gold standard for treating MRSA infections. As was done in the present case, the addition of gentamicin and/or rifampin for potential synergy may also be used with this agent; however, the use of these 2 antibiotics in combination with vancomycin for a patient with MRSA bacteremia remains controversial.10,16 The decision to discontinue vancomycin treatment was made as a result of the lack of patient response (e.g., 3-day plateau of inflammatory markers, persistent pain) and the potential for drug fever. Also, repeat cultures showed a potential increase in vancomycin's MIC for the MRSA infection, leading us to question the antibiotic's therapeutic effectiveness.
Daptomycin has rapid bactericidal activity, no required drug concentration monitoring, and is generally well tolerated.6,12 It is dosed once daily, and mild side effects, such as diarrhea, headache, and dizziness, can occur. Other adverse events include myopathy and elevations in CPK concentrations that require frequent laboratory monitoring.6 Our patient's CPK concentrations stayed within normal limits and allowed for continuous, successful treatment. It is also estimated that 9% and 54% of a standard 8-mg/kg dose of IV daptomycin penetrates into the bone and synovial fluid, respectively.17 Adequate bone penetration remains an important factor in the treatment of osteomyelitis.
There are currently limited data regarding daptomycin use in pediatric patients, and no dosing recommendations are given in the product label for this population. Current guidelines recommend 6–10 mg/kg of once-daily IV daptomycin as an alternative for managing acute hematogenous MRSA osteomyelitis in children.10 However, this recommendation is based mainly on expert opinion. This case report illustrates the potential value of daptomycin in osteomyelitis treatment and suggests this antibiotic could be considered as a treatment option for pediatric osteomyelitis due to MRSA.
There are currently 2 clinical trials18,19 examining the use of daptomycin in the pediatric population. A phase 4 interventional study18 is evaluating the safety and efficacy of daptomycin versus standard of care in the treatment of pediatric bacteremia caused by Staphylococcus aureus. A second trial19 is examining whether daptomycin is an effective and safe treatment option for pediatric subjects with acute hematogenous osteomyelitis when compared to vancomycin or nafcillin. This multicenter, randomized, double-blinded phase 3 comparative study is currently recruiting. Until such trials are concluded and published, case reports and retrospective studies are the main sources of literature related to pediatric osteomyelitis infections. However, this is an area that is also lacking comprehensive guidance.
A retrospective cohort study12 completed at the Children's Medical Center of Dallas from 2003 to 2007 included 16 children (median age 6.5 years) that received daptomycin for an invasive Gram-positive bacterial infection. Two of these patients had multifocal osteomyelitis, but it is unclear how the patients responded to daptomycin treatment and what doses were used. A case report11 published in 2012 was able to link successful daptomycin use in a 16-year-old male diagnosed with MSSA-induced osteomyelitis. This patient was refractory to 9 days of unsuccessful antibiotic therapy until 8 mg/kg daily of IV daptomycin was added. While there are some similarities between this case study and our patient, there are some important differences that should be noted. Our patient's infection was due to MRSA rather than MSSA, and his osteomyelitis presented bilaterally, not unilaterally, as in the 2012 case report. Nonetheless, these cases ultimately acknowledge the utility of daptomycin for the treatment of osteomyelitis in these 2 pediatric patients.
CONCLUSION
This patient case suggests daptomycin could be considered as a therapeutic option for treatment of MRSA osteomyelitis in the pediatric population. Further pediatric research and clinical studies must be completed in order to determine the safety and efficacy of daptomycin in this population, as well as the ideal role it will play in the treatment of pediatric osteomyelitis. Until additional data are obtained, case reports like this one may assist clinicians with choosing successful alternative treatment options if there is an unsatisfactory response to vancomycin.
ABBREVIATIONS
- CPK
creatinine phosphokinase
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FDA
Food and Drug Administration
- IDSA
Infectious Diseases Society of America
- IV
intravenous
- MIC
minimum inhibitory concentration
- MRI
magnetic resonance imaging
- MRSA
methicillin-resistant Staphylococcus aureus
- MSSA
methicillin-susceptible Staphylococcus aureus
- WBC
white blood cell
Footnotes
Disclosure During the preparation of this manuscript, Kelsey L. Billups was a PharmD student at the Virginia Commonwealth University School of Pharmacy. The authors declare no conflict or flnancial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Peltola H, Paakkonen M, Kallio P, Kallio MJ. Osteomyelitis-septic arthritis study group. Short-versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J. 2010;29(12):1123–1128. doi: 10.1097/INF.0b013e3181f55a89. [DOI] [PubMed] [Google Scholar]
- 2.Hospira Inc. Vancomycin Hydrochloride Package Information. Lake Forest, IL: Hospira Inc; 2011. [Google Scholar]
- 3.Pharmacia and Upjohn Co. Zyvox (Linezolid) Package Information. New York, NY: Pharmacia and Upjohn Co; 2013. [Google Scholar]
- 4.Pharmacia and Upjohn Co. Cleocin Phosphate (Clindamycin Phosphate) Package Information. New York, NY: Pharmacia and Upjohn Co; 2011. [Google Scholar]
- 5.Peltola H, Paakkonen M. Acute osteomyelitis in children. N Engl J Med. 2014;370(4):352–360. doi: 10.1056/NEJMra1213956. [DOI] [PubMed] [Google Scholar]
- 6.Durand C, Brueckner A, Sampadian C et al. Daptomycin use in pediatric patients. Am J Health Syst Pharm. 2014;71(14):1177–1182. doi: 10.2146/ajhp130601. [DOI] [PubMed] [Google Scholar]
- 7.Seaton RA, Malizos KN, Viale P et al. Daptomycin use in patients with osteomyelitis: a preliminary report from the EU-CORE(SM) database. J Antimicrob Chemother. 2013;68(7):1642–1649. doi: 10.1093/jac/dkt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher JC, Huntington JA, Culshaw D et al. Daptomycin therapy for osteomyelitis: a retrospective study. BMC Infect Dis. 2012;12:133. doi: 10.1186/1471-2334-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaPlante KL, Woodmansee S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother. 2009;53(9):3880–3886. doi: 10.1128/AAC.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 11.Erturan G, Holme H, Smith R et al. Successful use of daptomycin in Panton-Valentine leucocidin positive Staphylococcus aureus paediatric osteomyelitis. Int J Surg Case Rep. 2012;3(7):238–241. doi: 10.1016/j.ijscr.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardura MI, Mejias A, Katz KS et al. Daptomycin therapy for invasive Gram-positive bacterial infections in children. Pediatr Infect Dis J. 2007;26(12):1128–1132. doi: 10.1097/INF.0b013e31814523f8. [DOI] [PubMed] [Google Scholar]
- 13.Falagas ME, Siempos II, Papagelopoulos PJ, Vardaka KZ. Linezolid for the treatment of adults with bone and joint infections. Int J Antimicrob Agents. 2007;29(3):233–239. doi: 10.1016/j.ijantimicag.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Feigin RD, Pickering LK, Anderson D et al. Clindamycin treatment of osteomyelitis and septic arthritis in children. Pediatrics. 1975;55(2):213–223. [PubMed] [Google Scholar]
- 15.Amin AN, Cerceo EA, Deitelzweig SB et al. Hospitalist perspective on the treatment of skin and soft tissue infections. Mayo Clin Proc. 2014;89(10):1436–1451. doi: 10.1016/j.mayocp.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan SL. Osteomyelitis in children. Infect Dis Clin North Am. 2005;19(4):787–797. doi: 10.1016/S0891-5520(05)00084-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montange D, Berthier F, Leclerc G et al. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother. 2014;58(7):3991–3996. doi: 10.1128/AAC.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cubist Pharmaceuticals Holdings LLC. Clinicaltrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); A comparative evaluation of the safety and efficacy of daptomycin versus standard of care in pediatric subjects two-seventeen years of age with bacteremia caused by Staphylococcus aureus. 2000- [cited 2014 July 25]. Available from: http://clinicaltrials.gov/show/NCT01728376 NLM identifier: NCT 01728376. [Google Scholar]
- 19.Cubist Pharmaceuticals Holdings LLC. Clinicaltrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Safety and efficacy study of daptomycin when compared to active comparator in pediatric subjects with acute hematogenous osteomyelitis. 2000- [cited 2014 July 25]. Available from: http://clinicaltrials.gov/show/NCT01922011 NLM identifier: NCT01922011. [Google Scholar]


