Abstract
Magnetic resonance imaging (MRI) and ultrasound are the imaging modalities of choice to assess muscle injuries in athletes. Most authors consider MRI as the reference standard for evaluation of muscle injuries, since it superiorly depicts the extent of injuries independently of its temporal evolution, and due to the fact that MRI seems to be more sensitive for the detection of minimal injuries. Furthermore, MRI may potentially allow sports medicine physicians to more accurately estimate recovery times of athletes sustaining muscle injuries in the lower limbs, as well as the risk of re-injury. However, based on data available, the specific utility of imaging (including MRI) regarding its prognostic value remains limited and controversial. Although high-quality imaging is systematically performed in professional athletes and data extracted from it may potentially help to plan and guide management of muscle injuries, clinical (and functional) assessment is still the most valuable tool to guide return to competition decisions.
Keywords: Muscle injury, Sports medicine, MRI, Trauma injury
Introduction
Muscle injuries represent a major problem in elite sports, accounting for up to one third of all sports-related injuries [1–3, 4] and being responsible for a large proportion of time lost to competition [5, 6••, 7, 8]. In elite sports, the priority of sports medicine physicians is to optimize the return of the professional athlete to training and competition, without putting the athlete at risk for worsening of injuries or recurrent injury. To achieve such goal, it is of utmost importance to define prognosis based on the available clinical and imaging information. Initial clinical assessment with prompt conservative treatment (rest, ice, compression, and elevation (RICE)) is very important to improve prognosis and reduce recovery times. Although clinical and imaging (mainly represented by magnetic resonance imaging (MRI)) assessments have nearly the same performance in predicting the actual time required to return to competition [9], it has been shown that imaging assessment of the full morphological extent of muscle injuries plays a role in determining prognosis in profession athletes (e.g., return to play and risk of recurrent injury) [6••, 7, 9, 10, 11, 12, 13•, 14–16]. Imaging of sports-related muscle injury may potentially help to guide management, which directly impacts on prognosis, particularly in cases where there is uncertainty regarding the definite diagnosis or grade of injury, where the recovery time is longer than expected, or where surgical management may be necessary. Direct trauma (blunt trauma in the majority of cases) and indirect trauma (mainly represented by excessive eccentric force applied along the muscle-tendon-bone axis) represent the main mechanisms of muscle injuries in athletes. These are responsible for three distinct types of acute muscle injuries: contusion, strain (mainly represented by myotendinous strain), and tendon avulsion. Imaging assessment is pivotal for the detection and evaluation of the extent of these types of injuries, with different techniques widely and clinically available: Ultrasound and MRI are currently the ones most frequently applied in sports medicine. In this review, we will discuss the main imaging modalities for the assessment of sports-related muscle injury, focusing on the imaging findings of the most common muscle injuries in the lower limbs in sports (i.e., muscle strain) and focusing on the clinical relevance of such imaging features. We will also briefly discuss the potential of new advanced MRI techniques available to assess muscle injuries.
Mechanisms of injury and clinical features
In general, the most common mechanism of injury of muscles in the lower limbs is related to muscle strain (indirect muscle injury), with muscles being at risk of disruption during eccentric contraction as the force of active contraction is added to the passive stretching force applied to the myotendinous unit [17, 18]. Acute muscle strain in the lower limbs was demonstrated to be associated with both sprinting and stretching activities, mainly affecting the hamstring muscle complex, with more important initial function loss but faster recovery times for sprinting-related injuries and less important initial function loss but slower recovery times for stretching-related injuries [7, 8, 19]. Factors specifically related to muscles and activity increasing the risk for indirect muscle injury may include eccentric activity, muscles with fast twitch type 2 fibers, a sudden change in muscle function, muscles crossing multiple joints (biceps femoris, rectus femoris, gastrocnemius muscles), failure to absorb or counteract forces from other muscle groups or ground reaction, and muscle imbalance [20–22]. Indirect mechanisms are also responsible for acute avulsion injuries usually resulting from extreme, unbalanced, and often eccentric forces [23]. In muscle strain, athletes present with a sudden onset of pain usually localized in a specific muscle compartment of the limb during a period of eccentric muscle contraction, which prevent the athlete to continue the activity. Avulsion injuries are often associated with severe pain and loss of function. Clinically, muscle strain may be categorized in grade 1 injuries (no appreciable tissue tearing, with no loss of function or strength), grade 2 injuries (tissue damage with reduced strength of the myotendinous unit and some residual function), and grade 3 injuries (complete tear of the myotendinous unit with complete loss of function) [24].
Blunt trauma is the most common mechanism of direct muscle injury in sports, mainly affecting the lower limbs in modalities involving collision as in soccer, football, and rugby. Depending on the dissipation pattern of the blunt force directly applied to the recipient limb, different degrees of muscle contusion may occur from mild contusions to a massive rupture, usually occurring deep in the muscle belly with large intramuscular hematoma formation, which may also occur at the muscle-bone interface. High-grade injury is usually seen in cases where a massive blunt force is directed toward to the bone, with massive energy dissipated from the deep muscle to the bone. Penetrating trauma with muscle laceration may rarely occur in sports activity. Clinically, muscle contusions can be categorized in mild (range of motion loss less than one third with shorter recovery times), moderate (range of motion loss from one third to two thirds of normal, with moderate recovery times), and severe contusions (range of motion loss greater than two thirds with longer recovery times) [17]. Muscle contusions tend to be less symptomatic than muscle strain.
Magnetic resonance imaging (MRI)
MRI is considered the reference imaging method to assess the morphology of muscles in athletes due to its capability to visualize soft tissues with excellent contrast and provide high resolution and multiplanar assessment of muscles, especially in cases where traumatic lesions are clinically suspected [25, 26]. MRI is the method of choice to confirm and evaluate the extent and severity of muscle injuries [10]. Furthermore, some of the MRI morphologic features of acute muscle injury, such as the extent of injuries as well as the differentiation between edema and tears were proved to be related to important clinical features (time of recovery and risk of re-injury) in different athletes such as football (soccer) and rugby players, sprinters, and dancers [6••, 7–10, 11, 12, 13•, 14–16, 19, 27, 28••]. In chronic injuries, MRI may be useful in demonstrating scar tissue formation at the site of injury and involving important anatomic locations of muscles, as well as focal or diffuse fat atrophy of muscles affected, which may correlate with persistent clinical symptoms and loss of function.
Routine MRI technique
MRI is usually acquired unilaterally (limb affected only) using a dedicated surface coil, and acquisition of the contralateral limb will only be performed occasionally (e.g., bilateral injury). The coil selection should be based on the desired field of view. In order to correlate imaging and clinical findings, a skin marker should be ideally placed over the area of symptoms according to the athlete’s orientation (capsule filled with fish oil or vegetable oil). To accurately evaluate the morphology and extent of muscle injuries, multiplanar acquisitions are required in regard to the long and short axes of the involved muscle(s): Axial, coronal, and sagittal images are ideally acquired to achieve such assessment. Pulse sequences must always include fat-suppressed fluid-sensitive techniques which will allow for the detection of edematous changes around the myotendinous unit (eventually around the myofascial unit), as well as the delineation of intramuscular or perifascial fluid collections or hematomas. Fluid-sensitive techniques include fat-suppressed (fast or turbo) spin-echo T2-weighted (FS T2w), proton density-weighted (FS PDw), or intermediate-weighted (FS Iw) sequences, as well as the short tau inversion recovery (STIR) technique. T1-weighted spin-echo (T1w) sequences are less sensitive to detect edematous changes within the muscle in acute injury. However, its use may be useful in the assessment of subacute hemorrhage or hematoma associated with muscle injury. Furthermore, the T1w technique is useful to detect and evaluate the extent of atrophy and fatty infiltration, as well as to detect scar tissue in chronic injuries.
MRI features of muscle injury and clinical relevance
Regarding indirect muscle injury, most strains occur at the myotendinous junction since it usually represents the weakest link in the muscle complex. Interstitial edema and hemorrhage at the myotendinous junction may often extend along the adjacent muscle fibers and fascicles and is depicted on fluid-sensitive MRI coronal or sagittal sequences as an ill-defined focal or diffuse high signal intensity areas along the myotendinous junction with a classic feathery appearance [29, 30]. When only an edematous pattern is found within the muscle without significant disruption of muscle fibers or muscle architecture, these are referred as grade 1 strain (Fig. 1), and there is no function loss associated. The central tendon has usually normal signal and morphology, with regular contours and marked low signal intensity in all pulse sequences performed, but may also be mildly thickened with abnormal signal intensity, without disruption or laxity. Perifascial fluid may be present in grade 1 strains. In addition to the features above described for grade 1 strains, a partial disruption of muscle fibers with hematoma formation around the myotendinous junction will be classified as grade 2 strains (Fig. 1), with distortion of muscle architecture. Fiber disruption is usually depicted as a focal area of well-defined high signal intensity in fluid-sensitive MRI sequences. The central tendon may present features of laxity at the myotendinous junction, and partial disruption of the central tendon may be depicted as well. There is usually some function loss associated with grade 2 strains on MRI. Perifascial fluid is often present in grade 2 strains. Finally, grade 3 strains on MRI are represented by a complete disruption of the myotendinous unit with a local hematoma filling the gap created by the tear (Fig. 1). The diagnosis of grade 3 strains is usually made clinically, with complete loss of function, a palpable gap, and muscle fiber retraction. Complete avulsion injuries of the myotendinous unit from the bony attachment are also considered to represent grade 3 injury. The appearance of intramuscular hematomas, occurring not only with grades 2 and 3 strains but also with muscle contusions, may change in MRI according to the predictable pattern of blood degradation, which is not always true for intermuscular hematomas [17, 31]. Less frequently, strains may occur at the myofascial junction (around the epimysial interface), with the edema pattern (often represented by a feathery pattern) depicted at the periphery of muscles. These strains may or not be associated with focal fascial disruption, as well as with focal partial disruption of the adjacent muscle fibers.
Fig. 1.
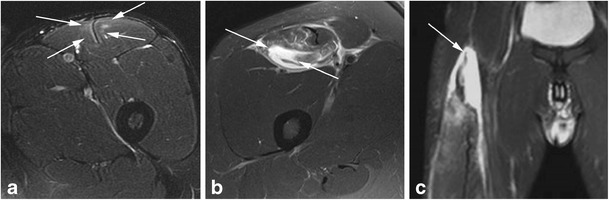
a, b, c Different grades of rectus femoris strain depicted on MRI. a Axial Iw FS MRI shows only ill-defined hyperintensity around the myotendinous unit corresponding to mild edema (arrows), without significant associated fiber disruption (grade 1 strain). b Axial Iw FS MRI demonstrates a large well-defined hyperintensity corresponding to an area of intramuscular fiber disruption (arrows) filled by fluid/blood, affecting less than 50 % of the cross-sectional area of the muscle (grade 2 strain). Note the adjacent edema of the remaining fibers of the muscle as well as perifascial fluid. c Coronal Iw FS MRI shows a complete discontinuity of the proximal myotendinous unit (arrow), with a moderate amount of fluid/blood filling the gap (grade 3 strain)
The clinical relevance of the MRI findings associated with muscle strain in the lower limbs was demonstrated in previous studies, the majority assessing both MRI and clinical features of acute hamstring injuries in different sports [6••, 7–10, 11, 12, 13•, 14–16, 19, 27, 28••]. Regarding the recovery time of athletes after sustaining muscle strains (outcome also referred in the literature as “return to play” or “return to competition”), several features and parameters depicted on MRI demonstrated some prognostic value. It was demonstrated that a negative (normal) MRI in players clinically sustained muscle injuries was associated with faster recovery times when compared to athletes for whom the MRI was positive (exhibited features of acute muscle strain) [6••, 32]. A large prospective cohort study including professional soccer players demonstrated a significant linear relationship between the MRI grades of acute hamstring strains and return to play, with higher grades associated with longer recovery times [6••]. The extent of injuries measured using MRI was extensively assessed in previous studies in regard to recovery times after injury, with different measurements’ methods applied (longitudinal length, cross-sectional area, volume, etc.). The majority of these studies showed a linear relationship between the extent of injuries and recovery times (the greater the extent of injury, the longer the recovery time) [7, 9, 10, 11, 13•, 33]. Recently, the importance of the integrity of the central tendon (myotendinous junction) in acute strains of the biceps femoris muscle in football and rugby players was demonstrated in a cohort study from Australia. This study showed that disruption of the central tendon was significantly associated with longer recovery times compared to strains that did not involve the central tendon [28••]. According to such finding, which needs to be confirmed in other muscles in the lower limbs, the integrity of the central tendon should be systematically assessed on MRI in athletes sustaining acute muscle strains. Regarding the location of injuries as assessed on MRI, few studies demonstrated longer recovery times in athletes with injuries around the myotendinous junction [7, 16], with injuries affecting the proximal aspect of hamstrings, the free proximal tendon [7], as well as with injuries affecting the middle third of the rectus femoris around the myotendinous junction [16].
Other studies further assessed MRI-based risk factors for recurrent hamstring injury [12, 14, 15], and a greater extent of injuries as measured on MR images (mainly cross-sectional area and volume) was found to be associated with a greater risk of re-injury. Regarding the assessment of the extent of injuries as measured on MRI, the main problem regarding most of the previous studies mentioned above is the lack of distinction between edema and fiber disruption (muscle tear) when assessing the extent, and most of these studies likely measured the limits of the edema pattern associated with muscle strain, without distinguishing if there was an associated fiber disruption within the area of edema. Also, one has to keep in mind that the relationships between the above-mentioned MRI features and relevant clinical features were demonstrated performing MRI in the acute phase of injury. Some previous reports showed that many MRI features may persist during follow-up of athletes after completion of rehabilitation, with complete clinical and functional resolution [7, 10, 34•]. Finally, there is no sufficient information regarding the relationships between direct (blunt) muscle injury in athletes and clinical features such as recovery time and risk of re-injury.
Advanced muscular MRI and sports medicine
Advanced MRI techniques for muscle assessment are available and have the potential to provide information on composition, microstructure, and function of muscles or groups of muscles. These techniques were mainly applied in a clinical research setting regarding other muscle pathology such as muscular dystrophy and other myopathies [35–43]. Some of these techniques are widely available on clinical scanners (T2 mapping, proton MR spectroscopy, fat-water separation techniques), whereas others require special software and hardware to be implemented and are not widely available in clinical practice (diffusion-tensor imaging (DTI), phosphorus MR spectroscopy, MR elastography). Such techniques have the potential to assess (1) muscle function, including assessment of recruitment of muscles for a given activity, as well as biological and metabolic function; (2) muscle composition, including assessment of early (microscopic) fat atrophy; (3) muscle microstructure, by evaluating the direction of muscle fibers as in tractography models after applying DTI; and (4) muscle elasticity (MR elastography).
T2 relaxation time mapping (T2 mapping, also known as “functional MRI” of muscles) measures the time constant of decay of the nuclear MR signal. A multiecho spin-echo technique is usually applied to measure T2 values. It is well demonstrated that the T2 relaxation time of skeletal muscles increases during and after exercise [44–49, 50•, 51, 52]. The underlying mechanism for such increase in muscle T2 is not fully understood, and probably, multiple factors are involved. Thus, for specific exercises applied in the upper and lower limbs, as well as in the trunk, it is possible to isolate which muscles or groups of muscles activate during and after exercising [44, 47–49, 50•]. Sometimes, even the degree of activation (function) observed may be estimated as well [46]. In sports medicine, quantitative T2 data has the potential to provide useful data about the capacity of a muscle (or a group of muscles) to be activated by a specific exercise for which we expect such muscle to work. This could be true in cases of chronic muscle strain/rupture in which the muscle affected still have its function impaired. Even without exercising the affected muscle, T2 mapping could be useful in demonstrating early fatty atrophy in cases where routine MRI does not exhibit unequivocal findings of atrophy, since an increase in the fat content of muscles would increase their T2.
Because of the highly anisotropic nature of muscle tissues, DTI may also be applied in skeletal muscles to assess the integrity and the orientation of muscle fibers [53–56]. The potential of DTI in assessing muscle injury on a microscopic level was shown in a study evaluating the correlations between DTI parameters and histologic changes (Z-band disruption) before and after 300 eccentric actions of the knee extensors performed on an isokinetic dynamometer [54]. Another recent study showed the ability of DTI in measuring maximum muscle power, which is an indirect measure of fiber type distribution [57]. In future sports medicine research, DTI for the assessment of muscle microstructure could be applied to understand why some athletes sustaining low-grade muscle strains in routine imaging (grade 1 strain) have recovery times longer than expected, as well as why some athletes with chronic muscle injuries have poor outcome in terms of muscle function after rehabilitation, with routine imaging unremarkable.
MR spectroscopy has the ability to provide information on the biochemical composition of tissues. A drawback is its inability to visualize the anatomic structure. Because most of the muscle metabolites relevant in energy transduction contain phosphorus, the 31P-MR spectroscopy is better suited than the widely available 1H-MR spectroscopy to assess their muscular concentrations in vivo and monitor changes over time [58], providing an indirect estimate of muscle function. By knowing the physiologic changes in muscle metabolites during rest, exercise, and recovery after exercise periods, it is possible then to detect metabolic abnormalities in athletes’ muscles where function and work is not optimal. Previous works could demonstrate significant differences in muscular force production, as well as in aerobic and anaerobic muscle metabolism when comparing different levels of physical activity [59, 60]. Thus, such technique has great potential to assess muscle function and work in athletes sustaining muscle injuries. Unfortunately, this technique is not widely available, and it is both costly and time-consuming, which makes it difficult to apply it in a large group of athletes. Other advanced MRI techniques available to assess the skeletal muscle lack data regarding their potential use in sports medicine.
Ultrasound
In experienced hands, ultrasound is a very useful tool in the assessment of muscle injuries in athletes [61]. Compared to MRI, ultrasound is inexpensive and widely available, is easily accessible (superior portability), has a greater spatial resolution allowing for assessment of superficial structures, and allows for dynamic imaging while mobilizing the injured limb, which is useful to increase the sensitivity in the detection of fiber disruption and to assess muscle injury healing. Furthermore, the use of Doppler imaging may help in the assessment of injuries. Finally, ultrasound is a useful tool to guide interventional procedures in athletes. Ultrasound is becoming increasingly popular in professional sports settings being performed by radiologists and sports medicine physicians in acute and hyperacute muscle injuries. Drawbacks of ultrasound when compared to MRI include operator dependency, limited field of view (evaluation of deep structures may be difficult), and reduced sensitivity in regard to morphologic injury. Furthermore, ultrasound may underestimate the degree of injury and may not identify areas within the muscle having only subtle edema. These factors may be responsible for decreasing the sensitivity of ultrasound in predicting the recovery time in professional athletes.
Ultrasound technique
Before directly assessing muscle injuries with ultrasound, the physician should interrogate the athlete for a brief history and perform physical examination, with palpation of the injured muscle (or group of muscles) with and without muscle contraction, which will allow the athlete to identify the location of maximum pain where ultrasound assessment should be focused. High-frequency linear probes (from 7 to 13 MHz) are usually sufficient to assess muscle injury. Ideally, not only the site of injury should be assessed but the whole muscle (or group of muscles) involved, in at least two orthogonal planes (transverse and longitudinal), from proximal to distal insertions. Depending of the athletes’ size and the depth of the muscle involved, the use of low-frequency probes may be required to accurately assess the site of injury. Power Doppler may be applied to identify hyperemia associated with acute injuries, especially for subtle injuries. Finally, dynamic ultrasound with passive and active movements is very useful in the assessment of fiber disruption (muscle tears), muscle hernias, and scars (healing). The application of concentric contractions seems to be the most useful maneuver when evaluating the presence of fiber disruption (Fig. 2).
Fig. 2.
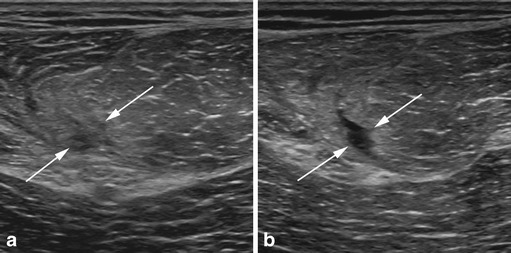
a, b Dynamic ultrasound assessment of a subacute biceps femoris muscle strain. a Assessment with the muscle at rest shows an ill-defined area of low echogenicity within the muscle (arrows), surrounded by ill-defined areas of increased echogenicity, and it is difficult to affirm the presence of focal fiber disruption. b After concentric contraction of the muscle, ultrasound assessment depicts a well-defined area of lower echogenicity in the same zone, consistent with focal fiber disruption (partial tear)
Ultrasound features of muscle injury and clinical relevance
The morphological changes depicted by ultrasound in different grades of muscle strain almost follows the patterns found when assessing injuries using MRI, with abnormalities frequently occurring around the myotendinous unit. The ultrasound features of muscle strain found in different grades of injury was previously described by Peetrons [62]. In clinically grade 1 strains, ultrasound may be either negative or exhibit focal or diffuse ill-defined areas of increased echogenicity within the muscle at the site of injury. Peetrons considered also as grade 1 strains those injuries exhibiting minimal focal elongated fiber disruption (small partial tears) occupying less than 5 % of the cross-sectional area of the muscle affected, represented by a well-defined focal hypoechoic or anechoic area within the muscle. Such definition is not a consensus among different sports medicine groups, with some authors considering any degree of partial fiber disruption as a grade 2 injury [17]. Also, perifascial fluid may be depicted by ultrasound in grade 1 strains, which may appear with an increased echogenicity due to the presence of extravascular blood. The presence of areas of partial fiber disruption (less than 100 % of the cross-sectional area of the muscle affected) seen at ultrasound represents grade 2 strains. Usually, there is discontinuity of the echogenic perimysial striae around either the myotendinous or the myofascial junction. An intramuscular hematoma may be depicted as well in grade 2 strains, and its echogenicity is highly dependent on the temporal evolution of injuries (increased echogenicity for acute injury and decreased echogenicity for chronic injury). Perifascial and intermuscular fluid collections are usually depicted on ultrasound with grade 2 strains. Finally, a complete discontinuity or disruption of the myotendinous unit with complete retraction depicted at ultrasound represents grade 3 injuries. As explained previously, these lesions are clinically evident with a palpable gap between the retracted ends of the muscle affected.
Compared to MRI, there is paucity of data regarding the clinical relevance of ultrasound findings in acute muscle injuries of the lower limbs. A previous longitudinal study comparing ultrasound and MRI for assessing hamstring injuries in professional football players in Australia showed that the location and extent of injuries as depicted using ultrasound were associated with increased recovery times [10]. Regarding the location of injuries, factors for increased recovery times included their presence in the biceps femoris muscle or outside of the myotendinous junction. Regarding the extent of injuries, factors for increased recovery times included the cross-sectional injury area (as a percentage score) and the length of injury. Furthermore, the presence of intermuscular hematomas depicted on ultrasound was also related to increased recovery times. With the exception of intermuscular hematomas, all factors above depicted on MRI were also associated with increased recovery times. Finally, the same study showed that the longitudinal length as assessed on MRI had the highest statistical correlation with recovery times, being the best radiologic predictor (superior than ultrasound) for return to competition [10].
Compliance with Ethics Guidelines
Conflict of Interest
Andre F. Yamada and Abdalla Y. Skaf declare that they have no conflict of interest.
Michel D. Crema has stock options in Boston Imaging Core Lab.
Ali Guermazi has received consultancy fees from MerckSerono, Genzyme, TissueGene, and OrthoTrophix and has stock options in Boston Imaging Core Lab.
Frank W. Roemer has stock options in Boston Imaging Core Lab.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Muscle Injuries
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Junge A, Engebretsen L, Mountjoy ML, et al. Sports injuries during the Summer Olympic Games 2008. Am J Sports Med. 2009;37:2165–72. doi: 10.1177/0363546509339357. [DOI] [PubMed] [Google Scholar]
- 2.Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997-2000. Br J Sports Med. 2002;36:39–44. doi: 10.1136/bjsm.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darrow CJ, Collins CL, Yard EE, Comstock RD. Epidemiology of severe injuries among United States high school athletes: 2005-2007. Am J Sports Med. 2009;37:1798–805. doi: 10.1177/0363546509333015. [DOI] [PubMed] [Google Scholar]
- 4.Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer) Am J Sports Med. 2011;39:1226–32. doi: 10.1177/0363546510395879. [DOI] [PubMed] [Google Scholar]
- 5.Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39:843–50. doi: 10.1177/0363546510394647. [DOI] [PubMed] [Google Scholar]
- 6.••.Ekstrand J, Healy JC, Walden M, Lee JC, English B, Hagglund M. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med. 2012;46:112–117. doi: 10.1136/bjsports-2011-090155. [DOI] [PubMed] [Google Scholar]
- 7.Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35:197–206. doi: 10.1177/0363546506294679. [DOI] [PubMed] [Google Scholar]
- 8.Askling CM, Tengvar M, Saartok T, Thorstensson A. Proximal hamstring strains of stretching type in different sports: injury situations, clinical and magnetic resonance imaging characteristics, and return to sport. Am J Sports Med. 2008;36:1799–804. doi: 10.1177/0363546508315892. [DOI] [PubMed] [Google Scholar]
- 9.Schneider-Kolsky ME, Hoving JL, Warren P, Connell DA. A comparison between clinical assessment and magnetic resonance imaging of acute hamstring injuries. Am J Sports Med. 2006;34:1008–15. doi: 10.1177/0363546505283835. [DOI] [PubMed] [Google Scholar]
- 10.Connell DA, Schneider-Kolsky ME, Hoving JL, et al. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am J Roentgenol. 2004;183:975–84. doi: 10.2214/ajr.183.4.1830975. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SB, Towers JD, Zoga A, et al. Hamstring injuries in professional football players: magnetic resonance imaging correlation with return to play. Sports Health. 2011;3:423–30. doi: 10.1177/1941738111403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs NJ, Cross TM, Cameron M, Houang MT. The accuracy of MRI in predicting recovery and recurrence of acute grade one hamstring muscle strains within the same season in Australian Rules football players. J Sci Med Sport. 2004;7:248–58. doi: 10.1016/S1440-2440(04)80016-1. [DOI] [PubMed] [Google Scholar]
- 13.•.Kerkhoffs GM, van Es N, Wieldraaijer T, Sierevelt IN, Ekstrand J, van Dijk CN. Diagnosis and prognosis of acute hamstring injuries in athletes. Knee Surg Sports Traumatol Arthrosc. 2013;21:500–9. doi: 10.1007/s00167-012-2055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koulouris G, Connell DA, Brukner P, Schneider-Kolsky M. Magnetic resonance imaging parameters for assessing risk of recurrent hamstring injuries in elite athletes. Am J Sports Med. 2007;35:1500–6. doi: 10.1177/0363546507301258. [DOI] [PubMed] [Google Scholar]
- 15.Verrall GM, Slavotinek JP, Barnes PG, Fon GT, Esterman A. Assessment of physical examination and magnetic resonance imaging findings of hamstring injury as predictors for recurrent injury. J Orthop Sports Phys Ther. 2006;36:215–24. doi: 10.2519/jospt.2006.36.4.215. [DOI] [PubMed] [Google Scholar]
- 16.Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32:710–9. doi: 10.1177/0363546503261734. [DOI] [PubMed] [Google Scholar]
- 17.Lee JC, Mitchell AW, Healy JC. Imaging of muscle injury in the elite athlete. Br J Radiol. 2012;85:1173–85. doi: 10.1259/bjr/84622172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrett WE., Jr Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc. 1990;22:436–43. doi: 10.1249/00005768-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during slow-speed stretching: clinical, magnetic resonance imaging, and recovery characteristics. Am J Sports Med. 2007;35:1716–24. doi: 10.1177/0363546507303563. [DOI] [PubMed] [Google Scholar]
- 20.Garrett WE, Jr, Califf JC, Bassett FH., 3rd Histochemical correlates of hamstring injuries. Am J Sports Med. 1984;12:98–103. doi: 10.1177/036354658401200202. [DOI] [PubMed] [Google Scholar]
- 21.Slocum DB, James SL. Biomechanics of running. JAMA. 1968;205:721–8. doi: 10.1001/jama.1968.03140370023006. [DOI] [PubMed] [Google Scholar]
- 22.Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36:1469–75. doi: 10.1177/0363546508316764. [DOI] [PubMed] [Google Scholar]
- 23.el-Khoury GY, Daniel WW, Kathol MH. Acute and chronic avulsive injuries. Radiol Clin North Am. 1997;35:747–66. [PubMed] [Google Scholar]
- 24.O’Donoghue DO. Treatment of injuries to athletes. Philadelphia: WB Saunders; 1962. [Google Scholar]
- 25.Hayashi D, Hamilton B, Guermazi A, de Villiers R, Crema MD, Roemer FW. Traumatic injuries of thigh and calf muscles in athletes: role and clinical relevance of MR imaging and ultrasound. Insights Imag. 2012;3:591–601. doi: 10.1007/s13244-012-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koulouris G, Connell D. Hamstring muscle complex: an imaging review. Radiographics. 2005;25:571–86. doi: 10.1148/rg.253045711. [DOI] [PubMed] [Google Scholar]
- 27.Slavotinek JP, Verrall GM, Fon GT. Hamstring injury in athletes: using MR imaging measurements to compare extent of muscle injury with amount of time lost from competition. AJR Am J Roentgenol. 2002;179:1621–8. doi: 10.2214/ajr.179.6.1791621. [DOI] [PubMed] [Google Scholar]
- 28.••.Comin J, Malliaras P, Baquie P, Barbour T, Connell D. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med. 2013;41:111–5. doi: 10.1177/0363546512463679. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch AL, Mink JH. Magnetic resonance imaging of musculoskeletal injuries. Radiol Clin North Am. 1989;27:983–1002. [PubMed] [Google Scholar]
- 30.Kneeland JP. MR imaging of muscle and tendon injury. Eur J Radiol. 1997;25:198–208. doi: 10.1016/S0720-048X(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 31.Douis H, Gillett M, James SL. Imaging in the diagnosis, prognostication, and management of lower limb muscle injury. Semin Musculoskelet Radiol. 2011;15:27–41. doi: 10.1055/s-0031-1271957. [DOI] [PubMed] [Google Scholar]
- 32.Verrall GM, Slavotinek JP, Barnes PG, Fon GT. Diagnostic and prognostic value of clinical findings in 83 athletes with posterior thigh injury: comparison of clinical findings with magnetic resonance imaging documentation of hamstring muscle strain. Am J Sports Med. 2003;31:969–73. doi: 10.1177/03635465030310063701. [DOI] [PubMed] [Google Scholar]
- 33.Pomeranz SJ, Heidt RS., Jr MR imaging in the prognostication of hamstring injury. work in progress. Radiology. 1993;189:897–900. doi: 10.1148/radiology.189.3.8234722. [DOI] [PubMed] [Google Scholar]
- 34.•.Silder A, Sherry MA, Sanfilippo J, Tuite MJ, Hetzel SJ, Heiderscheit BC. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43:284–99. doi: 10.2519/jospt.2013.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HK, Lindquist DM, Serai SD, et al. Magnetic resonance imaging of pediatric muscular disorders: recent advances and clinical applications. Radiol Clin North Am. 2013;51:721–42. doi: 10.1016/j.rcl.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forbes SC, Walter GA, Rooney WD, et al. Skeletal muscles of ambulant children with Duchenne muscular dystrophy: validation of multicenter study of evaluation with MR imaging and MR spectroscopy. Radiology. 2013;269:198–207. doi: 10.1148/radiol.13121948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maillard SM, Jones R, Owens C, et al. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology. 2004;43:603–8. doi: 10.1093/rheumatology/keh130. [DOI] [PubMed] [Google Scholar]
- 38.Arpan I, Forbes SC, Lott DJ, et al. T(2) mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5–15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26:320–8. doi: 10.1002/nbm.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh TJ, Jaw TS, Chuang HY, Jong YJ, Liu GC, Li CW. Muscle metabolism in Duchenne muscular dystrophy assessed by in vivo proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2009;33:150–4. doi: 10.1097/RCT.0b013e318168f735. [DOI] [PubMed] [Google Scholar]
- 40.Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255:899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- 41.Kim HK, Laor T, Horn PS, Wong B. Quantitative assessment of the T2 relaxation time of the gluteus muscles in children with Duchenne muscular dystrophy: a comparative study before and after steroid treatment. Korean J Radiol. 2010;11:304–11. doi: 10.3348/kjr.2010.11.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lodi R, Muntoni F, Taylor J, et al. Correlative MR imaging and 31P-MR spectroscopy study in sarcoglycan deficient limb girdle muscular dystrophy. Neuromuscul Disord. 1997;7:505–11. doi: 10.1016/S0960-8966(97)00108-9. [DOI] [PubMed] [Google Scholar]
- 43.Torriani M, Townsend E, Thomas BJ, Bredella MA, Ghomi RH, Tseng BS. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol. 2012;41:437–45. doi: 10.1007/s00256-011-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prior BM, Foley JM, Jayaraman RC, Meyer RA. Pixel T2 distribution in functional magnetic resonance images of muscle. J Appl Physiol. 1999;87:2107–14. doi: 10.1152/jappl.1999.87.6.2107. [DOI] [PubMed] [Google Scholar]
- 45.Shellock FG, Fleckenstein JL. Muscle physiology and pathophysiology: magnetic resonance imaging evaluation. Semin Musculoskelet Radiol. 2000;4:459–79. doi: 10.1055/s-2000-13171. [DOI] [PubMed] [Google Scholar]
- 46.Kinugasa R, Kawakami Y, Fukunaga T. Mapping activation levels of skeletal muscle in healthy volunteers: an MRI study. J Magn Reson Imaging. 2006;24:1420–5. doi: 10.1002/jmri.20772. [DOI] [PubMed] [Google Scholar]
- 47.Tawara N, Nitta O, Kuruma H, et al. Functional T(2) mapping of the trunkal muscle. Magn Reson Med Sci. 2009;8:81–3. doi: 10.2463/mrms.8.81. [DOI] [PubMed] [Google Scholar]
- 48.Tawara N, Nitta O, Kuruma H, Niitsu M, Itoh A. T2 mapping of muscle activity using ultrafast imaging. Magn Reson Med Sci. 2011;10:85–91. doi: 10.2463/mrms.10.85. [DOI] [PubMed] [Google Scholar]
- 49.Akima H, Kinugasa R, Kuno S. Recruitment of the thigh muscles during sprint cycling by muscle functional magnetic resonance imaging. Int J Sports Med. 2005;26:245–52. doi: 10.1055/s-2004-821000. [DOI] [PubMed] [Google Scholar]
- 50.•.Baffa AP, Felicio LR, Saad MC, Nogueira-Barbosa MH, Santos AC, Bevilaqua-Grossi D. Quantitative MRI of vastus medialis, vastus lateralis and gluteus medius muscle workload after squat exercise: comparison between squatting with hip adduction and hip abduction. J Hum Kinet. 2012;33:5–14. doi: 10.2478/v10078-012-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher MJ, Meyer RA, Adams GR, Foley JM, Potchen EJ. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest Radiol. 1990;25:480–5. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Yue G, Alexander AL, Laidlaw DH, Gmitro AF, Unger EC, Enoka RM. Sensitivity of muscle proton spin-spin relaxation time as an index of muscle activation. J Appl Physiol. 1994;77:84–92. doi: 10.1152/jappl.1994.77.1.84. [DOI] [PubMed] [Google Scholar]
- 53.Froeling M, Nederveen AJ, Heijtel DF, et al. Diffusion-tensor MRI reveals the complex muscle architecture of the human forearm. J Magn Reson Imaging. 2012;36:237–48. doi: 10.1002/jmri.23608. [DOI] [PubMed] [Google Scholar]
- 54.Cermak NM, Noseworthy MD, Bourgeois JM, Tarnopolsky MA, Gibala MJ. Diffusion tensor MRI to assess skeletal muscle disruption following eccentric exercise. Muscle Nerve. 2012;46:42–50. doi: 10.1002/mus.23276. [DOI] [PubMed] [Google Scholar]
- 55.Kan JH, Heemskerk AM, Ding Z, et al. DTI-based muscle fiber tracking of the quadriceps mechanism in lateral patellar dislocation. J Magn Reson Imaging. 2009;29:663–70. doi: 10.1002/jmri.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheel M, von Roth P, Winkler T, et al. Fiber type characterization in skeletal muscle by diffusion tensor imaging. NMR Biomed. 2013;26:1220–4. doi: 10.1002/nbm.2938. [DOI] [PubMed] [Google Scholar]
- 57.Scheel M, Prokscha T, von Roth P, et al. Diffusion tensor imaging of skeletal muscle - correlation of fractional anisotropy to muscle power. Röfo. 2013;185:857–61. doi: 10.1055/s-0033-1335911. [DOI] [PubMed] [Google Scholar]
- 58.Taylor DJ. Clinical utility of muscle MR spectroscopy. Semin Musculoskelet Radiol. 2000;4:481–502. doi: 10.1055/s-2000-13172. [DOI] [PubMed] [Google Scholar]
- 59.Johansen L, Quistorff B. 31P-MRS characterization of sprint and endurance trained athletes. Int J Sports Med. 2003;24:183–9. doi: 10.1055/s-2003-39085. [DOI] [PubMed] [Google Scholar]
- 60.Pesta D, Paschke V, Hoppel F, et al. Different metabolic responses during incremental exercise assessed by localized 31P MRS in sprint and endurance athletes and untrained individuals. Int J Sports Med. 2013;34:669–75. doi: 10.1055/s-0032-1327648. [DOI] [PubMed] [Google Scholar]
- 61.Koh ES, McNally EG. Ultrasound of skeletal muscle injury. Semin Musculoskelet Radiol. 2007;11:162–73. doi: 10.1055/s-2007-1001881. [DOI] [PubMed] [Google Scholar]
- 62.Peetrons P. Ultrasound of muscles. Eur Radiol. 2002;12:35–43. doi: 10.1007/s00330-001-1164-6. [DOI] [PubMed] [Google Scholar]


