Abstract
Rotator cuff repair (RCR) is a common procedure performed by orthopedic surgeons via arthroscopic, open, or mini-open techniques. While this surgery is considered to be of low morbidity, several potential complications can arise either intraoperatively or during the postoperative time period. Some of these complications are related to the surgical approach (arthroscopic or open), while others are patient dependent. Many of these complications can be managed through nonoperative means; however, early recognition and timely treatment is essential in limiting the long-term sequela and improving patient outcome. There are several different ways to classify complications after RCR repair: timing, severity, preventability, whether or not the pathology is intra- or extra-articular, and the type of treatment necessary. It is essential that the surgeon is cognizant of the etiology contributing to the failed RCR surgery in order to provide timely and proper management.
Keywords: Rotator cuff tear, Complications, Management, Failed surgery
Introduction
Rotator cuff repairs (RCRs) were first described over 100 years ago by Codman in 1911 [1]. Since then, advanced arthroscopic techniques have revolutionized this procedure. Despite this, there still remains much controversy not only on which patients should be treated operatively, but also on the operative approach (arthroscopic vs open) and the technique (single- vs. double-row repair) that should be utilized [2]. Nonoperative treatment is appropriate for the majority of patients with partial thickness tears and even for many patients with full thickness tears [3], especially those who are older, have lower demand, and are asymptomatic. Although RCR surgery is of low morbidity compared to many other orthopedic procedures, complications still occur and can have considerable long-term sequela for patients. These surgeries are commonly performed arthroscopically, open and also through mini-open techniques. They are performed with patients in either a beach chair or the lateral decubitus position. There are specific complications that are related to positioning patients in either a beach chair or a lateral decubitus positioning, and they will not be discussed here as they are inherent to the positioning and are not specific to a RCR.
Complications following RCR can be classified based on many factors such as timing, severity, preventability, whether or not the pathology is intra- or extra-articular, or the type of treatment necessary. Of these classifications, the most important aspect of managing failed RCR surgery is recognizing the factors responsible for the failure and provide timely treatment which is what this manuscript will focus on. Treatments consist of nonoperative management and revision surgery and the subset of patients who are initially treated nonoperatively may eventually require surgical intervention (Tables 1 and 2). Overall, complications after RCR are not common and are equivalent between arthroscopic and open techniques. Complications after arthroscopic and open RCR have been reported at 10.5 % [4] and 10.6 %, respectively [5].
Table 1.
Complications after rotator cuff repair commonly treated by nonoperative means
| Complication | Usual signs/symptoms | Description of treatment |
|---|---|---|
| Complex regional pain syndrome | Severe pain, hypersensitivity, edema, coloration changes, dystonia | Multidisciplinary approach with medications, physical and occupational therapy, counseling, possible sympathetic blockade |
| Fracture | Localized pain | Immobilization, rarely becomes an operative issue |
| Nerve injury | Numbness, paresthesias | Monitoring, usually resolves without intervention |
| Superficial infection | Erythema | Antibiotic treatment |
| Stiffness | Loss of active and passive range of motion | Physical therapy, self-therapy, surgery can be indicated in cases that do not respond to conservative treatment |
| Venous thromboembolism | Edema, vague soreness | Possible systemic thrombolysis followed by chronic anticoagulation |
Table 2.
Complications after rotator cuff repair commonly treated by operative means
| Complication | Usual signs/symptoms | Description of treatment |
|---|---|---|
| Chondrolysis | Pain, crepitus, loss of motion | May have a role for arthroscopic debridement or require arthroplasty |
| Deep infection | Pain, erythema, drainage | Irrigation, debridement, and removal of all hardware and foreign material able to be removed. May require open excision of sinus tract. Culture-specific intravenous antibiotics, transitioned to oral antibiotics |
| Deltoid injury | Pain, loss of active abduction, fluid collection at superior shoulder (AC joint) | Open direct repair or reconstruction with rotational deltoidplasty |
| Implant failure | Pain, crepitus, loss of strength | Loose body removal with possible revision rotator cuff repair |
| Osteonecrosis | Pain, loss of active and passive motion, crepitus | Reverse shoulder arthroplasty |
| Re-tear | Pain, loss of active motion, or decreased strength | Possible initial treatment with physical therapy in select case or revision rotator cuff repair in physiologically young, active patients. Muscle transfer is an option as well. In the elderly low demand patient, reverse shoulder arthroplasty is an excellent revision option |
Complications treated by nonoperative means
Complex regional pain syndrome
Complex regional pain syndrome (CRPS) is defined as severe pain with associated hypersensitivity, edema, skin changes, and/or dystonia [6]. There is surprisingly little published evidence regarding the relationship between rotator cuff repair surgery and CRPS [7]. In a prospective randomized study comparing immediate passive motion to 6 weeks of immobilization, Arndt et al. [8] found the incidence of complex regional pain syndrome to be 20.9 % in the immobilization group versus 8.2 % in the immediate passive motion groups. However, the difference was not found to be statistically significant, and the incidence of CRPS found in this study is much greater than most other reports [9, 10]. In contrast, Bishop et al. [9] reviewed 512 patients that had undergone shoulder surgery with interscalene regional anesthesia. There was one case of CRPS identified in a patient that had undergone shoulder arthroplasty, but no cases of CRPS was reported with arthroscopic or open rotator cuff repair. Borgeat et al. found a 1 % incidence (5/520 patients) of CRPS in a prospective study of interscalene regional anesthesia for shoulder surgery [10]. Based on these studies, it is difficult to determine if CRPS is a complication of rotator cuff repair surgery or a complication of regional anesthesia. Regardless, shoulder surgeons should discuss CRPS as a risk of shoulder surgery (arthroscopic or open) with their patients preoperatively and should be prepared to refer the patient to a physician experienced in CRPS management. Treatment usually consists of a multidisciplinary approach that involves a variety of medications, physical therapy, occupational therapy, counseling, and possible sympathetic blocks [6].
Fracture
Fracture after RCR is a rare entity that requires severe trauma to the shoulder. Acromial fracture has been noted after acromioplasty which is usually considered to be a technical error from over-resecting of the acromion [11]. Mansat et al. reviewed 40 series published between 1982 and 1995 representing almost 3000 patients with RCR and reported two fractures, one in the greater tuberosity and the other with the acromion [5]. These complications should be evaluated with radiography in patients who sustain a trauma after their RCR and complain of pain and/or limited motion. Acromial fractures are best identified on the scapular-Y or axillary film and may not be evident on a routine anteroposterior or Grashey view. CT scans will also help to delineate the pattern and displacement of the fracture which will help dictate management. Acromial and proximal humerus fractures may be treated nonoperatively initially in most cases. Patients that failed conservative management will need open reduction and internal fixation.
Nerve injury
Iatrogenic nerve injuries during RCR are likely an underappreciated complication due to failure in obtaining a thorough postoperative examination. Estimates of 1–2 % [12–15, 16•] of nerve injuries occur after RCR. Nerve injuries can occur during patient positioning for shoulder surgery [17], but as this is not as relevant as to the surgery itself being performed, it will not be discussed in detail here.
Warner et al. studied mobilization of large retracted rotator cuff tears and the effect on the suprascapular nerve (SSN). They determined that mobilization of over 3 cm places the SSN at risk for injury [18]. They also found that medially directed dissection of over 3 cm from the biceps origin into the supraspinatus fossa or more than 2 cm from the posterior glenoid rim into the infraspinatus fossa could also place the nerve at risk for injury. In contrast to the above findings, Zanotti et al. [19] evaluated 10 patients after massive rotator cuff repair that required mobilization and reported one patient (10 %) with injury to the suprascapular nerve on EMG testing. Furthermore, 2 of the 10 repairs (20 %) failed with ultrasound evaluation. Hoellrich et al. [20] also performed a similar study and documented no suprascapular nerve injury in nine patients after massive RCR surgery with a mean of 2.5 cm advancement (range 2–3.5 cm). Thus, it is controversial whether cuff mobilization and advancement will result in suprascapular nerve injury.
The deltoid-split approach to open or mini-open rotator cuff repair can also place the axillary nerve at risk of damage as the distance from the nerve to the lateral edge of the acromion is between 5 and 7 cm [21]. Some surgeons recommend placing a suture 5 cm from the edge of the acromion in the inferiormost aspect of the deltoid when performing a deltoid-split to prevent distal dissection.
The majority of these injuries are neuropraxias and can resolve over time. Electromyleography (EMG) is generally recommended at the 3-month mark if normalization of the deficit has not occurred. Consultation with a surgeon specializing in peripheral nerve surgery is an option, although surgery is rarely indicated in this setting. Most nerve injury will resolve with time, unless the nerve is transected. A brachial plexus specialty clinic reviewed all cases over a 10-year period and found 26 patient with iatrogenic nerve injuries from previous shoulder surgery [22]. These included nine structural injuries (suture entrapment or laceration). These occurred from both open and arthroscopic procedures. Over half of these patients did not recover with observation and went on to surgical management. Early referral and evaluation by a specialist is recommended by the authors.
Superficial infection
Despite antibiotic prophylaxis, surgical site preparation, and various draping techniques, infections can occur after RCR. The rate of superficial infection, suture granuloma, or suture “abscess” is not well defined and treatment options vary regarding these entities. If there is concern for deep contamination, then a formal surgical irrigation and debridement maybe warranted. There are isolated cases of postoperative cellulitis or localized infection around a suture that is superficial. In these cases, removal of the suture if possible, combined with appropriate antibiotics and monitoring for full resolution of symptoms, is the best treatment protocol. Antibiotics should be directed at the most commonly isolated bacteria which are Staphylococcus aureus, Staphylococcus epidermidis, Propionibacterium acnes, and Corynebacterium species [23, 24•, 25]. Surgical preparation solutions have been tested against these common bacteria and although no significant difference was found in the ability to eliminate P. acnes, ChloraPrep (2 % chlorhexidine gluconate and 70 % isopropyl alcohol) was found to be more effective than DuraPrep (0.7 % iodophor and 74 % isopropyl alcohol) or povidone-iodine at eliminating coagulase-negative Staphylococcus [26]. In another study, 4 % chlorhexidine gluconate was also shown to have significantly lower bacterial colonization and postoperative wound infections compared to povidone-iodine, although no mention of P. acnes was made in this study [27]. Failure of symptoms to fully resolve after a course of oral antibiotics should be considered to be indicative of a deep infection, and at that point, operative treatment should be performed.
Stiffness
One of the most common complications of RCR, whether performed by arthroscopic or open means, is postoperative stiffness. Stiffness has been found to be present in 4.8–8.7 % of arthroscopic repairs in some series [4, 28, 29] while seemingly less common (0.5–4.8 %) in large reviews of open cases [5, 29]. Definition of stiffness varies among clinicians as does the semantics of whether or not decreased motion is labeled “stiffness” or “frozen shoulder” after RCR. In the series by Brislin et al. [4], the authors defined stiffness as passive external rotation (ER) at the side of less than 10°, total passive ER with the arm in 90° of abduction of less than 30° or total passive forward flexion (FF) of less than 100° which persists after 90 days postoperatively. These authors did not identify any differences in tear size associated with stiffness; however, they did report that 22/23 patients had their stiffness improve with physical therapy alone and did not necessitate a further surgery. The majority (67 %) of these patients had their stiffness resolve within the first 5 months postoperatively, and the remaining patients had some residual motion deficits, but not enough for them to wish to undergo any surgical intervention.
Tauro performed a retrospective review of the total range of motion deficits in preoperative examination in 72 patients with RCRs. He found that bursal and capsular abnormalities were a common finding in the stiffer subgroup of patients, but that evidence of adhesive capsulitis was found only in three patients with the highest preoperative stiffness (total range of motion deficit of greater than 70°). These three patients required a subsequent surgery even after an initial capsular release performed at the time of their RCR [30].
In another series by Mormino et al., patients that did require a subsequent surgery returned to the operating room at an average of 37 weeks and postoperatively had increased in both ROM as well as objective outcome scores. In the 4.9 % of patients that underwent a secondary procedure for stiffness after RCR reported by Huberty et al., an association was found between development of postoperative stiffness and patients with worker’s compensation insurance, patients <50 years, and those with a diagnosis of calcific tendonitis, adhesive capsulitis, requiring additional postoperative therapy, PASTA tear, or concomitant labral repair [28]. They found that patients with no risk factors had a risk of postoperative stiffness of 2.3 %; however, for those with at least one risk factor, the risk of stiffness was 7.8 %. In the 24 patients that required secondary surgery, 23 or 96 % showed complete healing of the RCR at the time of arthroscopy.
Timing of when to initiate physical therapy has come into question related to the development of postoperative stiffness. Current reports however, have shown that early motion is not beneficial; however, it also does not cause increased rate of failure after cuff repair [31]. Some studies have even demonstrated a higher failure rate in patients with early, aggressive motion [32]. In a systematic review, Denard et al. [33] reported a 1.5 % postoperative stiffness in patients with passive range of motion, 4.5 % in a 6-week sling immobilization protocol, and 0 % with a modified protocol. Arthroscopic capsular release did improve the motion in the patients with resistant postoperative stiffness after RCR surgery.
Despite the type of approach or the method of postoperative rehabilitation, stiffness after RCR occurs not uncommonly [34, 35] and should be treated initially with physical therapy, although patients should be counseled for the possibility of a need for a secondary surgery if conservative management fails. With arthroscopic capsular release, most patients will have a lasting improvement of their range of motion and pain, although these patients do not perform as well as those who have a release for idiopathic frozen shoulder [36].
Venous thromboembolism
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are potentially serious complications following any type of surgery. While incidence rates and prophylactic strategies are well defined in the hip and knee arthroplasty literature [37], there is significantly less published data regarding shoulder surgery. There are several studies investigating the incidence of venous thromboembolism (VTE) following either shoulder surgery in general or arthroscopic shoulder surgery, but there is insufficient data to determine an accurate postoperative VTE rate following rotator cuff repair surgery specifically. Regardless, the incidence of VTE following an arthroscopic rotator cuff repair may be similar to the rate following shoulder arthroscopy in general.
The majority of the published data on VTE after rotator cuff repair consist of individual patients in a larger series or case reports [4, 38]. Similar evidence exists in regard to VTE after shoulder arthroscopy in general [39–42]. However, investigations into larger cohorts have recently been published that provide more insight into the relationship between shoulder arthroscopy and VTE. Imberti et al. determined the incidence of symptomatic venous thromboembolism within 90 days following any type of shoulder surgery was 0.66 % in a registry of over 1300 patients. Duration of surgery greater than 60 minutes was a risk factor for the development of VTE. For shoulder arthroscopy specifically, the rate of symptomatic VTE was 0.3 %. The incidence of lower extremity DVT in these patients was 0.2 % and none of the 982 arthroscopic patients developed an upper extremity DVT. A symptomatic pulmonary embolism was identified in 0.1 % of these patients and there were no patient deaths [43]. Takashashi et al. found a 5.7 % incidence of DVT in a cohort of 175 patients within 90 days after arthroscopic shoulder surgery. All DVTs that were identified were asymptomatic, and all were identified within 2 days of surgery. One pulmonary embolus was found in 175 patients, which was also asymptomatic [44]. Keremsky et al. found a 0.31 % incidence of symptomatic VTE in 1908 patients that had undergone arthroscopic surgery. Out of the six patients identified, there were three upper extremity DVTs, two lower extremity DVTs, and four pulmonary emboli. Four of these patients had undergone an arthroscopic rotator cuff repair [45].
Anakwe et al. synthesized the available evidence to develop recommendations for the prevention of VTE in upper extremity surgery. The authors found that the current guidelines by various specialty societies were often unclear and the quality of evidence was often poor. However, they were able to articulate several recommendations for evidence base practice in patients presenting with VTE. Primarily, all patients should be screened for risk factors for VTE, and chemical prophylaxis should be considered in high risk patients. However, the authors state that chemoprophylaxis is likely unnecessary for outpatient surgery if patients mobilize quickly. In addition, the authors recommend mechanical prophylactic devices in all upper extremity surgery patients even though the evidence is poor because there is minimal risk using these devices [46]. We routinely use pneumatic compression devices for our rotator cuff repair patients for these same reasons and risk stratify each patient to determine if they would benefit from more aggressive VTE prophylactic treatment.
Once diagnosed, postoperative VTEs are generally treated with anticoagulation for at least 3 months. There are a variety of oral and intravenous pharmacologic agents used to anticoagulate, and there is currently no compelling evidence to recommend any single medication. Other modalities such as inferior vena cava filters and surgical thrombectomy are options for a limited number of patients for whom pharmacologic therapy is not appropriate, such as those with significantly increased bleeding risks [47]. Any patient suspected of having an acute, postoperative DVT or pulmonary embolism should be evaluated with the appropriate imaging modality such as duplex ultrasound for a DVT or a chest computed tomography angiogram for a pulmonary embolism. Once diagnosed, consultation with a physician experienced in the management of VTE should be obtained to direct further treatment. Generally, more distal DVTs in the lower extremity do not require treatment. If the thrombus extends or the patient experiences any changes in symptoms, then it should be treated appropriately [48]. Upper extremity DVTs encompass only 1–4 % of all DVTs diagnosed in the general population. The incidence of subsequent pulmonary embolism is variable, ranging between 2 and 35 %. Because of this risk, upper extremity DVTs are generally successfully treated with anticoagulation in the majority of patients [49].
Complications treated by operative means
Chondrolysis
Postsurgical glenohumeral chondrolysis has become a well-recognized complication of arthroscopic shoulder surgery. Glenohumeral chondrolysis was originally described as a complication of open rotator cuff repair surgery with gentian violet dye that was used to identify full thickness rotator cuff defects [50, 51]. As the popularity of the arthroscopic treatment of shoulder pathology has risen over the last 15 years, glenohumeral chondrolysis has been better recognized as a complication of arthroscopic shoulder surgery [52]. Specifically, it has been commonly associated with arthroscopic thermal devices, intra-articular local anesthetic, and proud intra-articular anchor placement [53, 54]. Solomon et al. [54] identified 88 cases of postarthroscopic glenohumeral chondrolysis (PAGCL) in the literature in order to determine risk factors. While the majority of the diagnoses in this series were instability (32 %) or superior labrum anterior to posterior (SLAP) tears (23 %), the authors found 10 % (9/91) of these shoulders involved a partial or full thickness rotator cuff tear. Scheffel et al. [53] reviewed 100 cases of glenohumeral chondrolysis and found 13 % of the cases involved rotator cuff surgery. Eight cases were rotator cuff debridements, four were open rotator cuff repairs, and only one case involved an arthroscopic rotator cuff tear. All four open rotator cuff repairs were associated with the use of gentian violet dye that was used to identify full thickness rotator cuff tears [50, 51]. Glenohumeral chondrolysis clearly demonstrates a greater association with instability and/or labral surgery than rotator cuff surgery. While there are several documented cases of chondrolysis following arthroscopic debridement of a partial thickness rotator cuff [55–58], there is currently minimal evidence in the literature that suggests glenohumeral chondrolysis is a relevant complication in rotator cuff repair surgery. The most reliable treatments of chondrolysis involve shoulder arthroplasty [59], as previous attempts at biologic resurfacing have been associated with a high failure rate [60].
Deep infection
While superficial infections can often be treated with nonsurgical means, the presence of a deep infection implies that surgical treatment is necessary. A deep infection may present after RCR performed through arthroscopic, mini-open, or open approach [25, 61–63]. Patients with this complication can present weeks or even months after surgery with pain, erythema, or drainage, although they typically do not have systemic signs and symptom of a deep infection [62]. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels can be elevated, although these are known to be elevated after surgery even when infection is not present. A high clinical suspicion is necessary to initiate prompt treatment. Treatment consists of irrigation and debridement as well as sampling of tissues for cultures. Broad spectrum antibiotics should be initiated after cultures have been taken and then tailored based on the results of the cultures. Consultation with an infectious disease specialist can be beneficial in determining the final antibiotic regimen including the route and length of duration of treatment. Reports of final culture results vary; however, some series have shown as high as 51–86 % of infections after RCR to be caused by P. acnes [25, 61].
Patients should be counseled that most series demonstrating multiple surgical debridements are necessary to eradicate the infection. Most patients have poor overhead function following even successful treatment of this complication [23]. Historically, the incidence of deep infection after open repair was reported to be between 0.27 and 1.7 % [5, 64, 65]. Incidence after arthroscopically assisted, mini-open RCR has been found to be similarly at 1.9 % [25]. Based on this finding, these surgeons recommend that a second skin preparation be performed with Betadine paint and that all surgeons change their gloves after the arthroscopic portion and before making the incision. Infection after all-arthroscopic RCRs is rare, with one survey of Italian surgeons reporting a rate of 0.0016 or 1.6/1000 [6]. Other current reports list the rate of deep infection following any type of arthroscopic shoulder surgery as being between 0.04 and 0.23 % [4, 13, 66]. Infection following revision arthroscopic RCR has been found to be much higher than after primary surgery [67].
The composition of suture anchors and whether or not they are retained during surgical debridements does not appear to affect the success of clearance of the infection [62]. Ultimately, this complication must be treated by operative means and augmented with appropriate antibiotic therapy specific to the organism to ensure eradication of the infection.
Deltoid injury
With the advent of advanced instrumentation and the continuing development of arthroscopic techniques, there are many rotator cuff tears that are repaired completely arthroscopically. Open surgeries through a formal take-down of the anterior deltoid and “mini-open” techniques involving deltoid splits continue to be performed for RCRs. However, with the open technique, there is the possibility of injury to the deltoid muscle, through atrophic changes to complete rupture of the origin. Damage to the deltoid muscle has been reported historically after this procedure [68–70]. Although more recent reviews rarely list this as a complication, it can lead to significantly decreased outcomes and may affect the possibility of salvage surgery with a reverse shoulder arthroplasty. A deltoid-splitting, “mini-open” approach was compared to a historical control of patients who had a formal open approach for RCR with respect to postoperative changes in the deltoid musculature [70]. Magnetic resonance imaging (MRI) was used to evaluate both groups of patients at 6 and 12 months, and the width of the deltoid was measured and compared to the preoperative MRI. There were no cases of deltoid dehiscence, and no cases of atrophy in the mini-open group; however, 60 % of the patients with a formal approach had measurable atrophy of the deltoid.
Rupture or defect in the deltoid in conjunction with a failed rotator cuff repair may present as mimicking the clinical signs of patients with an acromioclavicular cyst or “geyser sign” with a large fluid collection on the superior shoulder [71]. Reconstruction through a rotational deltoidplasty has been described both in patients after RCR and after other open surgeries without RCR [72]. The majority of these patients had an unsatisfactory outcome and glenohumeral fusion was eventually performed in 2/24 (8 %) patients.
Implant failure
There is a multitude of different types of implants available for RCR and they vary in terms of size, shape, material, and method of fixation. Most RCR implants are composed of an anchor with attached suture for fixation, although there are others that do not fit these criteria. Regardless of the implant material or its dimensions and use, there are several different modes of failure that can exist due to technique error or the intrinsic quality of the anchors or sutures.
A cadaveric study was performed to evaluate the effect of the angle of insertion of suture anchors on a simulated RCR of the supraspinatus [73]. Anchors were inserted either at the typical “deadman’s angle” of 45° to the articular surface of the humeral head at the greater tuberosity or 90° or perpendicular to the articular surface. Matched cadavers were then cyclically loaded until failure. The 90° anchors were found to have a statistically significant increase in number of cycles until failure compared to those inserted at the historical “deadman’s angle” of 45°.
Patients can present with this complication either after an acute trauma or with just an increased amount of pain that has happened without any inciting event. They may describe loose body sensations or may just have vague pain that is poorly described. Often, advanced imaging such as MR arthrogram or CT arthrogram is needed to visualize the implant if it is not metal.
There are many possible methods of failure [74–77], but the ones that lead to a need for revision surgery are usually fracture of the implant or pull-out of the implant [78, 79]. This rare event should only warrant further investigation once more common reasons for postoperative pain are excluded. Due to the risk of chondral injury and pain, many authors recommend surgical removal of broken hardware even if the rotator cuff itself has healed (Fig. 1).
Fig. 1.
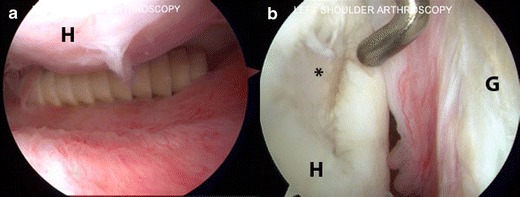
Arthroscopic pictures taken of a left shoulder in the beach chair position demonstrates (a) a loose rotator cuff anchor in the axillary pouch below the humeral head (H). b A large chondral defect (asterisk) in the humeral head (H) due to the loose anchor. The glenoid (G) is shown as well
Osteonecrosis
Humeral head osteonecrosis following arthroscopic rotator cuff surgery is a rare complication that has been recently described in the literature. Although there have only been five cases reported in the literature, all cases occurred following either an arthroscopic rotator cuff repair or an arthroscopic rotator cuff debridement [80–82]. While several authors hypothesize that postarthroscopic humeral head osteonecrosis is due to aberrant anchor placement into the ascending branch of the anterior humeral circumflex artery [80, 82], there is insufficient data to definitively ascribe causality. Humeral head osteonecrosis following rotator cuff surgery is generally treated with reverse shoulder arthroplasty with satisfactory results [80, 81].
Re-tear/failure of rotator cuff healing
Failure to heal and re-tear after RCR may be two distinct entities, but these terms are used interchangeably in much of the literature. Several factors may help distinguish a re-tear from a failure to heal, such as the timing of symptoms, whether or not the patient sustained a trauma and comparison of imaging studies pre- and post-RCR. Ultimately, whether or not a re-tear occurred or the primary RCR did not heal leads to similar outcomes and, thus, will be grouped together for the purpose of this review.
There is debate on which technique and approach leads to the highest healing rates after RCR; however, failure to heal or re-tear is a common occurrence after RCR, regardless of the specific technique utilized. One recent review found that the double-row technique was associated with a lower re-tear rate than single row for tears greater than 1 cm in size, but that the approach used did not affect the re-tear rate [83]. This review found that re-tear rates were found between 7 and 17 % in tears <1 cm and 41–69 % in tears greater than 5 cm; other studies corroborate this finding in re-tears based on the size of the tear [84]. Systematic literature reviews have not found a significant difference in percentage of re-tears based on the technique used for cuff repair, nor have more recent articles comparing newer techniques of fixation such as the suture-bridge technique [85, 86]. There has also been recent focus to determine whether or not clinical results are associated with the ultimate integrity of the repair; however, patients are found to do well clinically even when their repairs have failed radiographically [87–91]. Further stratification of these findings has been difficult to interpret as many studies have utilized different outcome scores and different imaging modalities when providing their results. Review studies have found only slight differences when attempting to pool results in clinical results of patients based on whether or not they had re-tears [92]. Imaging studies can be mixed even in the same study utilizing ultrasound (U/S), MRI, CT, or even MR arthrogram (MRA) or CT arthrogram (CTA).
Attempts have been made to identify factors that are associated with re-tear after RCR. Tear size, muscle atrophy, fatty infiltration, age, and level of work have all been recognized as potential reasons that some RCRs end in re-tear [88, 93]. Many of these available studies are retrospective reviews that were conducted to determine if the rate of re-tear was acceptable in determining whether or not to perform a RCR based on the size of the original tear. Most of these studies recommend initial fixation of even massive RCRs based on the indeterminate data correlating the presence of re-tears to functional outcomes. Iannotti et al. [94] determined that most re-tears after RCR occur between 6 and 26 weeks after surgery seen in 19 out of 113 patients (17 %) in their cohort. Miller et al. [95] also supported the above findings and reported a 41 % re-tear rate (9/22 patients) in patients with large rotator cuff tears >3 cm in size with majority of the re-tears occurring during the early postoperative time frame (within 3 months of surgery). Cho et al. [93] found a 33 % re-tear rate in their series and determined the risk factors as older age, larger size of tear, and presence of fatty infiltration. However, despite the re-tears seen in the postoperative time period, majority of patients had excellent pain relief and improvement in their activities of daily living.
When a re-tear is present after a RCR, the question the surgeon must ask is whether it is prudent to proceed with a revision repair. It is possible to detect a re-tear through imaging studies as part of a review or routine protocol, even when a patient is asymptomatic. Certainly in these cases, there is not a clear indication for a revision surgery. In younger patients who are not pleased with their outcomes after RCR and are found to have a re-tear documented on subsequent imaging, a revision repair can be a valid option. Reviews demonstrate that up to 50 % of revision RCRs remain intact at 1 year postoperatively and improvements in pain and outcome scores are observed [96–98]. Revision RCR does have a different complication profile than primary RCR and complications are twice as common with revisions compared to primary RCR [67]. As expected, a history of more than one previous attempt at RCR is related to a worse prognosis after revision RCR [67, 98]. Ultimately, the decision to undertake a revision RCR must be made jointly between the surgeon and the patient after all factors are taken under consideration. These include patient factors such as symptoms, functional losses, medical history, and previous surgeries as well as anatomic factors such as acromiohumeral interval, presence of fatty infiltration, size and characteristic of tear, quality of tendon, and concomitant pathology.
Management of re-tear after rotator cuff repair
When a RCR fails and both the surgeon and the patient agree to surgical management, there are several options of treatment available besides a revision repair including scaffold augmentation, tendon transfer, or reverse shoulder arthroplasty (RSA).
Scaffold augmentation
There has been significant interest and advancement in the use of biologic augmentation in orthopedic surgery. This field is especially relevant in rotator cuff surgery. RCR tears are considered “irreparable” if the native tendon cannot be mobilized and repaired to the bone or the muscle is no longer functional due to fatty infiltration, as is often the case in failed rotator cuff repairs. The patient’s own tissue therefore makes a repair prohibitive, so biologics that can augment or restore native tissue are an attractive solution. One example of this is scaffolds that are designed to augment or bridge the muscle-tendon-bone unit and eventually incorporate into the host tissue. There are a variety of recent reports of utilizing synthetic scaffolds, biologic scaffolds, treated allograft/xenograft tissue, or autograft tissue to augment rotator cuff repairs [99–107]. However, many of these reports consist of technique articles or have short-term follow-up with no control group. They report varying rotator cuff tear sizes and varying techniques to augment versus bridge the tendon-bone interface. In addition, there are several reports of negative outcomes or adverse reactions to scaffolds [108, 109]. Currently, there is not enough evidence to analyze the results of scaffold augmentation to failed rotator cuff repairs specifically. In light of the paucity of high-level data on this topic, the authors currently do not have a routine role for scaffold augmentation in the management of failed rotator cuff repairs. We feel that it may serve a role in the future as a short-term mechanical augment to “splint” a tenuous repair when muscle quality is preserved.
Tendon transfer
Tendon transfers can be a good solution for patients with irreparable damage to the muscle-tendon unit. The success of RSA to restore function in rotator cuff-deficient shoulders has diminished the role of tendon transfers in this patient population because of improved reliability and less sophisticated postoperative rehabilitation. However, there is still a role for tendon transfers in younger and more active patients that are not willing to comply with the life-long activity restrictions of constrained shoulder arthroplasty. The most commonly utilized tendon transfer for failed rotator cuff repairs is the latissimus dorsi transfer. It can reliably and durably restore function, active range of motion, and strength and achieve pain relief in patients with irreparable posterosuperior rotator cuff tears [110–113]. However, poor results are often seen in patients with subscapularis insufficiency and teres minor fatty infiltration [110, 111]. Arthroscopic assisted latissimus transfer has also been reported with comparable short-term results to formal open transfer [114]. The main benefit of the procedure is avoiding the detachment of the deltoid from the acromion and potential postoperative deltoid complications that have been associated with trans-deltoid approaches to the shoulder. In addition, trapezius transfer is an option to restore external rotation [115, 116]. However, further research is required to determine the procedure’s efficacy in the rotator cuff-deficient population. Irreparable superior rotator cuff tears in patients that also have subscapularis insufficiency remain a difficult problem in patients that are not candidates for RSA. Because of the work of Gerber et al. [110, 111], we consider subscapularis insufficiency a contraindication to traditional latissimus transfer. A ventral latissimus transfer has been proposed and may be useful in this patient population [117], but there are currently no clinical outcomes reported in the literature to support the use of the procedure.
Reverse shoulder arthroplasty
Since the development of the modern RSA in Europe in the early 1990s and in the USA in the early 2000s, RSA has become the shoulder surgeon’s workhorse for the treatment of painful and dysfunctional rotator cuff-deficient shoulders. Prior attempts using unconstrained arthroplasty designs were met with limited success in this patient population [118]. While acceptable outcomes are possible with rotator cuff repair and concomitant anatomic shoulder arthroplasty [119], these patients are high risk for complications, and RSA is a more reliable treatment to restore function and eliminate pain in rotator cuff-deficient shoulder both with and without arthritis [120–122]. RSA is a reliable treatment for rotator cuff failure after a prior rotator cuff repair, and the essentially equivalent outcomes have been reported in patients that have undergone a previous rotator cuff repair that has failed [123, 124]. However, Boileau et al. [125] demonstrated that surgeons should be cautious when performing RSA in patients that have failed a previous rotator cuff surgery. RSA can achieve excellent pain relief and restoration of function specifically in patients with pseudoparalysis (less than 90° of active forward elevation with maintenance of full passive elevation). However, modest results can be expected if patients present with pain as their predominant complaint after a previous failed rotator cuff repair with persevered active elevation greater than 90°. In these patients, the authors found less postoperative pain relief, a mean decrease in subjective mobility, and a 24° decrease in active forward elevation compared with the preoperative state [125]. The authors consider maintenance of greater than 90° of active forward elevation in a painful shoulder after a rotator cuff repair, a contraindication to RSA. We use RSA as our primary treatment of failed rotator cuff repair in older patients. However, in light of the report of Boileau et al., we avoid RSA in painful postrotator cuff repair shoulders with maintained forward elevation without arthritis.
Conclusions
Rotator cuff repair is a commonly performed and generally successful surgery; however, complications can arise even when the surgery is performed technically well. When evaluating a patient with less than optimal outcome after RCR, it is essential to listen to the history and complaints, obtain appropriate imaging or ancillary testing, and determine the etiology responsible for the failed repair. Timely management of complications will result in better patient outcomes. Certain complications are prudently treated nonoperatively, while others require operative intervention. Revision rotator cuff surgery carries a higher risk of failure. Therefore, it is necessary to have the surgeon educate the patient about their condition and the expectations for surgery so that they can exercise shared decision making on the best treatment plan.
General Compliance with Ethics Guidelines
Conflict of Interest
Matthew F Dilisio, Colin D. Kennedy, and Stephen A. Parada declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Shoulder Surgery: Complications
Contributor Information
Stephen A. Parada, Email: Stephen.a.parada@gmail.com
Matthew F. Dilisio, Email: dilisiom@gmail.com
Colin D. Kennedy, Email: colink2@uw.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Codman EA. Rupture of the supraspinatus tendon and other lesions in or about the subacromial bursa. The shoulder. Boston: Thomas Todd; 1934. [Google Scholar]
- 2.Pedowitz RA, Yamaguchi K, Ahmad CS, Burks RT, Flatow EL, Green A, et al. Optimizing the management of rotator cuff problems. J Am Acad Orthop Surg. 2011;19(6):368–79. doi: 10.5435/00124635-201106000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wirth MA, Basamania C, Rockwood CA., Jr Nonoperative management of full-thickness tears of the rotator cuff. Orthop Clin N Am. 1997;28(1):59–67. doi: 10.1016/s0030-5898(05)70264-9. [DOI] [PubMed] [Google Scholar]
- 4.Brislin KJ, Field LD, Savoie FH., 3rd Complications after arthroscopic rotator cuff repair. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2007;23(2):124–8. doi: 10.1016/j.arthro.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Mansat P, Cofield RH, Kersten TE, Rowland CM. Complications of rotator cuff repair. Orthop Clin N Am. 1997;28(2):205–13. doi: 10.1016/s0030-5898(05)70280-7. [DOI] [PubMed] [Google Scholar]
- 6.Freedman M, Greis AC, Marino L, Sinha AN, Henstenburg J. Complex regional pain syndrome: diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25(2):291–303. doi: 10.1016/j.pmr.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Koike Y, Sano H, Imamura I, Goto M, Ooyama M, Kita A. Changes with time in skin temperature of the shoulders in healthy controls and a patient with shoulder-hand syndrome. Ups J Med Sci. 2010;115(4):260–5. doi: 10.3109/03009734.2010.503354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arndt J, Clavert P, Mielcarek P, Bouchaib J, Meyer N, Kempf JF, et al. Immediate passive motion versus immobilization after endoscopic supraspinatus tendon repair: a prospective randomized study. Orthop Traumatol Surg Res: OTSR. 2012;98(6 Suppl):S131–8. doi: 10.1016/j.otsr.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JY, Sprague M, Gelber J, Krol M, Rosenblatt MA, Gladstone J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am Vol. 2005;87(5):974–9. doi: 10.2106/JBJS.D.02003. [DOI] [PubMed] [Google Scholar]
- 10.Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology. 2001;95(4):875–80. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Weber SC, Abrams JS, Nottage WM. Complications associated with arthroscopic shoulder surgery. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2002;18(2 Suppl 1):88–95. doi: 10.1053/jars.2002.31801. [DOI] [PubMed] [Google Scholar]
- 12.Boardman ND, 3rd, Cofield RH. Neurologic complications of shoulder surgery. Clin Orthop Relat Res. 1999;368:44–53. [PubMed] [Google Scholar]
- 13.McFarland EG, O’Neill OR, Hsu CY. Complications of shoulder arthroscopy. J South Orthop Assoc. 1997;6(3):190–6. [PubMed] [Google Scholar]
- 14.McIlveen SJ, Duralde XA, D’Alessandro DF, Bigliani LU. Isolated nerve injuries about the shoulder. Clin Orthop Relat Res. 1994;306:54–63. [PubMed] [Google Scholar]
- 15.Stanish WD, Peterson DC. Shoulder arthroscopy and nerve injury: pitfalls and prevention. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 1995;11(4):458–66. doi: 10.1016/0749-8063(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 16.•.Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717–26. doi: 10.5435/JAAOS-21-12-717. [DOI] [PubMed] [Google Scholar]
- 17.Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2011;27(4):532–41. doi: 10.1016/j.arthro.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Warner JP, Krushell RJ, Masquelet A, Gerber C. Anatomy and relationships of the suprascapular nerve: anatomical constraints to mobilization of the supraspinatus and infraspinatus muscles in the management of massive rotator-cuff tears. J Bone Joint Surg Am Vol. 1992;74(1):36–45. [PubMed] [Google Scholar]
- 19.Zanotti RM, Carpenter JE, Blasier RB, Greenfield ML, Adler RS, Bromberg MB, et al. The low incidence of suprascapular nerve injury after primary repair of massive rotator cuff tears. J Should Elb Surg / Am Should Elb Surg. 1997;6(3):258–64. doi: 10.1016/s1058-2746(97)90014-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoellrich RG, Gasser SI, Morrison DS, Kurzweil PR. Electromyographic evaluation after primary repair of massive rotator cuff tears. J Should Elb Surg / Am Should Elb Surg. 2005;14(3):269–72. doi: 10.1016/j.jse.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Apaydin N, Tubbs RS, Loukas M, Duparc F. Review of the surgical anatomy of the axillary nerve and the anatomic basis of its iatrogenic and traumatic injury. Surg Radiol Anat: SRA. 2010;32(3):193–201. doi: 10.1007/s00276-009-0594-8. [DOI] [PubMed] [Google Scholar]
- 22.Carofino BC, Brogan DM, Kircher MF, Elhassan BT, Spinner RJ, Bishop AT, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am Vol. 2013;95(18):1667–74. doi: 10.2106/JBJS.L.00238. [DOI] [PubMed] [Google Scholar]
- 23.Mirzayan R, Itamura JM, Vangsness CT, Jr, Holtom PD, Sherman R, Patzakis MJ. Management of chronic deep infection following rotator cuff repair. J Bone Joint Surg Am Vol. 2000;82-A(8):1115–21. doi: 10.2106/00004623-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 24.•.Saltzman MD, Marecek GS, Edwards SL, Kalainov DM. Infection after shoulder surgery. J Am Acad Orthop Surg. 2011;19(4):208–18. doi: 10.5435/00124635-201104000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. J Should Elb Surg / Am Should Elb Surg. 2002;11(6):605–8. doi: 10.1067/mse.2002.127302. [DOI] [PubMed] [Google Scholar]
- 26.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am Vol. 2009;91(8):1949–53. doi: 10.2106/JBJS.H.00768. [DOI] [PubMed] [Google Scholar]
- 27.Paocharoen V, Mingmalairak C, Apisarnthanarak A. Comparison of surgical wound infection after preoperative skin preparation with 4 % chlorhexidine [correction of chlohexidine] and povidone iodine: a prospective randomized trial. J Med Assoc Thailand =Chotmaihet Thangphaet. 2009;92(7):898–902. [PubMed] [Google Scholar]
- 28.Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2009;25(8):880–90. doi: 10.1016/j.arthro.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Mormino MA, Gross RM, McCarthy JA. Captured shoulder: a complication of rotator cuff surgery. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 1996;12(4):457–61. doi: 10.1016/s0749-8063(96)90040-7. [DOI] [PubMed] [Google Scholar]
- 30.Tauro JC. Stiffness and rotator cuff tears: incidence, arthroscopic findings, and treatment results. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2006;22(6):581–6. doi: 10.1016/j.arthro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Chung SW, Kim JY, Ok JH, Park I, Oh JH. Is early passive motion exercise necessary after arthroscopic rotator cuff repair? Am J Sports Med. 2012;40(4):815–21. doi: 10.1177/0363546511434287. [DOI] [PubMed] [Google Scholar]
- 32.Lee BG, Cho NS, Rhee YG. Effect of two rehabilitation protocols on range of motion and healing rates after arthroscopic rotator cuff repair: aggressive versus limited early passive exercises. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2012;28(1):34–42. doi: 10.1016/j.arthro.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Denard PJ, Ladermann A, Burkhart SS. Prevention and management of stiffness after arthroscopic rotator cuff repair: systematic review and implications for rotator cuff healing. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2011;27(6):842–8. doi: 10.1016/j.arthro.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson GP. Arthroscopic capsular release for stiff shoulders: effect of etiology on outcomes. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2003;19(1):40–9. doi: 10.1053/jars.2003.50010. [DOI] [PubMed] [Google Scholar]
- 35.Warner JJ, Greis PE. The treatment of stiffness of the shoulder after repair of the rotator cuff. Instr Course Lect. 1998;47:67–75. [PubMed] [Google Scholar]
- 36.Elhassan B, Ozbaydar M, Massimini D, Higgins L, Warner JJ. Arthroscopic capsular release for refractory shoulder stiffness: a critical analysis of effectiveness in specific etiologies. J Should Elb Surg / Am Should Elbow Surg. 2010;19(4):580–7. doi: 10.1016/j.jse.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am Vol. 2013;95(19):1801–11. doi: 10.2106/JBJS.L.01328. [DOI] [PubMed] [Google Scholar]
- 38.Amarasekera SS, van Dalen J, Thompson TJ, Osman M. Pulmonary embolism after acromioplasty and rotator cuff repair. J Should Elb Surg / Am Should Elb Surg. 2008;17(5):e13–4. doi: 10.1016/j.jse.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Cortes ZE, Hammerman SM, Gartsman GM. Pulmonary embolism after shoulder arthroscopy: could patient positioning and traction make a difference? J Should Elb Surg / Am Should Elb Surg. 2007;16(2):e16–7. doi: 10.1016/j.jse.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Creighton RA, Cole BJ. Upper extremity deep venous thrombosis after shoulder arthroscopy: a case report. J Should Elb Surg / Am Should Elb Surg. 2007;16(1):e20–2. doi: 10.1016/j.jse.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Durant TJ, Swanson BT, Cote MP, Allen DA, Arciero RA, Mazzocca AD. Upper extremity deep venous thromboembolism following arthroscopic labral repair of the shoulder and biceps tenodesis: a case report. Int J Sports Phys Ther. 2014;9(3):377–82. [PMC free article] [PubMed] [Google Scholar]
- 42.Manaqibwala MI, Ghobrial IE, Curtis AS. Upper extremity thrombosis presenting as medial elbow pain after shoulder arthroscopy. Case Rep Orthop. 2014;2014:653146. doi: 10.1155/2014/653146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imberti D, Ivaldo N, Murena L, Paladini P, Castagna A, Barillari G, et al. Venous thromboembolism in patients undergoing shoulder surgery: findings from the RECOS Registry. Thromb Res. 2014;134(2):273–7. doi: 10.1016/j.thromres.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi H, Yamamoto N, Nagamoto H, Sano H, Tanaka M, Itoi E, et al. Venous thromboembolism after elective shoulder surgery: a prospective cohort study of 175 patients. J Should Elb Surg / Am Should Elb Surg. 2014;23(5):605–12. doi: 10.1016/j.jse.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 45.Kuremsky MA, Cain EL, Jr, Fleischli JE. Thromboembolic phenomena after arthroscopic shoulder surgery. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2011;27(12):1614–9. doi: 10.1016/j.arthro.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Anakwe RE, Middleton SD, Beresford-Cleary N, McEachan JE, Talwalkar SC, et al. Preventing venous thromboembolism in elective upper limb surgery. J Should Elb Surg / Am Should Elb Surg. 2013;22(3):432–8. doi: 10.1016/j.jse.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. JAMA, J Am Med Assoc. 2014;311(7):717–28. doi: 10.1001/jama.2014.65. [DOI] [PubMed] [Google Scholar]
- 48.Sule AA, Chin TJ, Handa P, Earnest A. Should symptomatic, isolated distal deep vein thrombosis be treated with anticoagulation? Int J Angiol: Off Publ Int Coll Angiol Inc. 2009;18(2):83–7. doi: 10.1055/s-0031-1278332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajid MS, Ahmed N, Desai M, Baker D, Hamilton G. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118(1):10–8. doi: 10.1159/000101700. [DOI] [PubMed] [Google Scholar]
- 50.Shibata Y, Midorikawa K, Koga T, Honjo N, Naito M. Chondrolysis of the glenohumeral joint following a color test using gentian violet. Int Orthop. 2001;25(6):401–3. doi: 10.1007/s002640100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamai K, Higashi A, Cho S, Yamaguchi T. Chondrolysis of the shoulder following a “color test”-assisted rotator cuff repair—a report of 2 cases. Acta Orthop Scand. 1997;68(4):401–2. doi: 10.3109/17453679708996186. [DOI] [PubMed] [Google Scholar]
- 52.Levine WN, Clark AM, Jr, D’Alessandro DF, Yamaguchi K. Chondrolysis following arthroscopic thermal capsulorrhaphy to treat shoulder instability. A report of two cases. J Bone Joint Surg Am Vol. 2005;87(3):616–21. doi: 10.2106/JBJS.D.02158. [DOI] [PubMed] [Google Scholar]
- 53.Scheffel PT, Clinton J, Lynch JR, Warme WJ, Bertelsen AL, Matsen FA, 3rd, et al. Glenohumeral chondrolysis: a systematic review of 100 cases from the English language literature. J Should Elb Surg / Am Should Elb Surg. 2010;19(6):944–9. doi: 10.1016/j.jse.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 54.Solomon DJ, Navaie M, Stedje-Larsen ET, Smith JC, Provencher MT. Glenohumeral chondrolysis after arthroscopy: a systematic review of potential contributors and causal pathways. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2009;25(11):1329–42. doi: 10.1016/j.arthro.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Athwal GS, Shridharani SM, O’Driscoll SW. Osteolysis and arthropathy of the shoulder after use of bioabsorbable knotless suture anchors. A report of four cases. J Bone Joint Surg Am Vol. 2006;88(8):1840–5. doi: 10.2106/JBJS.E.00721. [DOI] [PubMed] [Google Scholar]
- 56.Bailie DS, Ellenbecker TS, et al. Severe chondrolysis after shoulder arthroscopy: a case series. J Should Elb Surg / Am Should Elb Surg. 2009;18(5):742–7. doi: 10.1016/j.jse.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Petty DH, Jazrawi LM, Estrada LS, Andrews JR. Glenohumeral chondrolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32(2):509–15. doi: 10.1177/0363546503262176. [DOI] [PubMed] [Google Scholar]
- 58.Saltzman M, Mercer D, Bertelsen A, Warme W, Matsen F. Postsurgical chondrolysis of the shoulder. Orthopedics. 2009;32(3):215. [PubMed] [Google Scholar]
- 59.Denard PJ, Wirth MA, Orfaly RM. Management of glenohumeral arthritis in the young adult. Jo Bone Joint Surg Am Vol. 2011;93(9):885–92. doi: 10.2106/JBJS.J.00960. [DOI] [PubMed] [Google Scholar]
- 60.Elhassan B, Ozbaydar M, Diller D, Higgins LD, Warner JJ. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am Vol. 2009;91(2):419–24. doi: 10.2106/JBJS.H.00318. [DOI] [PubMed] [Google Scholar]
- 61.Athwal GS, Sperling JW, Rispoli DM, Cofield RH, et al. Deep infection after rotator cuff repair. J Should Elb Surg / Am Should Elb Surg. 2007;16(3):306–11. doi: 10.1016/j.jse.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Kwon YW, Kalainov DM, Rose HA, Bisson LJ, Weiland AJ, et al. Management of early deep infection after rotator cuff repair surgery. J Should Elb Surg / Am Should Elb Surg. 2005;14(1):1–5. doi: 10.1016/j.jse.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M, et al. Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Should Elb Surg / Am Should Elb Surg. 2010;19(1):97–101. doi: 10.1016/j.jse.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Post M. Complications of rotator cuff surgery. Clin Orthop Rel Res. 1990;254:97–104. [PubMed] [Google Scholar]
- 65.Settecerri JJ, Pitner MA, Rock MG, Hanssen AD, Cofield RH, et al. Infection after rotator cuff repair. J Should Elb Surg / Am Should Elb Surg. 1999;8(1):1–5. doi: 10.1016/s1058-2746(99)90045-9. [DOI] [PubMed] [Google Scholar]
- 66.Johnson LL, Schneider DA, Austin MD, Goodman FG, Bullock JM, DeBruin JA. Two-percent glutaraldehyde: a disinfectant in arthroscopy and arthroscopic surgery. J Bone Joint Surg Am. 1982;64(2):237–9. [PubMed] [Google Scholar]
- 67.Parnes N, DeFranco M, Wells JH, Higgins LD, Warner JJ. Complications after arthroscopic revision rotator cuff repair. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2013;29(9):1479–86. doi: 10.1016/j.arthro.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Bigliani LU, Cordasco FA, McIlveen SJ, Musso ES. Operative treatment of failed repairs of the rotator cuff. J Bone Joint Surg Am Vol. 1992;74(10):1505–15. [PubMed] [Google Scholar]
- 69.Groh GI, Simoni M, Rolla P, Rockwood CA, et al. Loss of the deltoid after shoulder operations: an operative disaster. J Should Elb Surg / Am Should Elb Surg. 1994;3(4):243–53. doi: 10.1016/S1058-2746(09)80042-6. [DOI] [PubMed] [Google Scholar]
- 70.Hata Y, Saitoh S, Murakami N, Kobayashi H, Takaoka K. Atrophy of the deltoid muscle following rotator cuff surgery. J Bone Joint Surg Am Vol. 2004;86-A(7):1414–9. doi: 10.2106/00004623-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Cooper HJ, Milillo R, Klein DA, DiFelice GS. The MRI geyser sign: acromioclavicular joint cysts in the setting of a chronic rotator cuff tear. Am J Orthop. 2011;40(6):E118–21. [PubMed] [Google Scholar]
- 72.Sher JS, Iannotti JP, Warner JJ, Groff Y, Williams GR. Surgical treatment of postoperative deltoid origin disruption. Clin Orthopa Relat Res. 1997;343:93–8. [PubMed] [Google Scholar]
- 73.Strauss E, Frank D, Kubiak E, Kummer F, Rokito A. The effect of the angle of suture anchor insertion on fixation failure at the tendon-suture interface after rotator cuff repair: deadman’s angle revisited. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2009;25(6):597–602. doi: 10.1016/j.arthro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 74.Cobaleda Aristizabal AF, Sanders EJ, Barber FA. Adverse events associated with biodegradable lactide-containing suture anchors. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2014;30(5):555–60. doi: 10.1016/j.arthro.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 75.De Carli A, Lanzetti RM, Monaco E, Labianca L, Mossa L, Ferretti A. The failure mode of two reabsorbable fixation systems: Swivelock with Fibertape versus Bio-Corkscrew with Fiberwire in bovine rotator cuff. J Orthop Sci: Off J Jpn Orthop Assoc. 2012;17(6):789–95. doi: 10.1007/s00776-012-0275-z. [DOI] [PubMed] [Google Scholar]
- 76.Kummer F, Hergan DJ, Thut DC, Pahk B, Jazrawi LM. Suture loosening and its effect on tendon fixation in knotless double-row rotator cuff repairs. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2011;27(11):1478–84. doi: 10.1016/j.arthro.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 77.Wieser K, Farshad M, Vlachopoulos L, Ruffieux K, Gerber C, Meyer DC. Suture slippage in knotless suture anchors as a potential failure mechanism in rotator cuff repair. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2012;28(11):1622–7. doi: 10.1016/j.arthro.2012.04.150. [DOI] [PubMed] [Google Scholar]
- 78.Dunn JC, Friedman DJ, Higgins LD, et al. Anchor fracture leading to supraspinatus failure. J Should Elb Surg / Am Should Elb Surg. 2010;19(1):e24–6. doi: 10.1016/j.jse.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 79.McCarty LP, 3rd, Buss DD, Datta MW, Freehill MQ, Giveans MR. Complications observed following labral or rotator cuff repair with use of poly-L-lactic acid implants. J Bone Joint Surg Am Vol. 2013;95(6):507–11. doi: 10.2106/JBJS.L.00314. [DOI] [PubMed] [Google Scholar]
- 80.Beauthier V, Sanghavi S, Roulot E, Hardy P. Humeral head osteonecrosis following arthroscopic rotator cuff repair. Knee Surg Sports Traumatol, Arthroscop: Off J ESSKA. 2010;18(10):1432–4. doi: 10.1007/s00167-009-1016-5. [DOI] [PubMed] [Google Scholar]
- 81.Dilisio MF, Noble JS, Bell RH, Noel CR. Postarthroscopic humeral head osteonecrosis treated with reverse total shoulder arthroplasty. Orthopedics. 2013;36(3):e377–80. doi: 10.3928/01477447-20130222-30. [DOI] [PubMed] [Google Scholar]
- 82.Goto M, Gotoh M, Mitsui Y, Okawa T, Higuchi F, Nagata K. Rapid collapse of the humeral head after arthroscopic rotator cuff repair. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013. [DOI] [PubMed]
- 83.Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38(4):835–41. doi: 10.1177/0363546509359679. [DOI] [PubMed] [Google Scholar]
- 84.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL, et al. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Should Elb Surg / Am Should Elb Surg. 2006;15(3):290–9. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 85.Kim KC, Shin HD, Lee WY, Han SC. Repair integrity and functional outcome after arthroscopic rotator cuff repair: double-row versus suture-bridge technique. Am J Sports Med. 2012;40(2):294–9. doi: 10.1177/0363546511425657. [DOI] [PubMed] [Google Scholar]
- 86.Saridakis P, Jones G. Outcomes of single-row and double-row arthroscopic rotator cuff repair: a systematic review. J Bone Joint Surg Am Vol. 2010;92(3):732–42. doi: 10.2106/JBJS.I.01295. [DOI] [PubMed] [Google Scholar]
- 87.Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96–104. doi: 10.4055/cios.2009.1.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi S, Kim MK, Kim GM, Roh YH, Hwang IK, Kang H. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons [et al]. 2014.\ [DOI] [PubMed]
- 89.Chung SW, Kim JY, Kim MH, Kim SH, Oh JH. Arthroscopic repair of massive rotator cuff tears: outcome and analysis of factors associated with healing failure or poor postoperative function. Am J Sports Med. 2013;41(7):1674–83. doi: 10.1177/0363546513485719. [DOI] [PubMed] [Google Scholar]
- 90.Kim KC, Shin HD, Lee WY. Repair integrity and functional outcomes after arthroscopic suture-bridge rotator cuff repair. J Bone Joint Surg Am Vol. 2012;94(8):e48. doi: 10.2106/JBJS.K.00158. [DOI] [PubMed] [Google Scholar]
- 91.Lee KW, Seo DW, Bae KW, Choy WS. Clinical and radiological evaluation after arthroscopic rotator cuff repair using suture bridge technique. Clin Orthop Surg. 2013;5(4):306–13. doi: 10.4055/cios.2013.5.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slabaugh MA, Nho SJ, Grumet RC, Wilson JB, Seroyer ST, Frank RM, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2010;26(3):393–403. doi: 10.1016/j.arthro.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 93.Cho NS, Lee BG, Rhee YG. Arthroscopic rotator cuff repair using a suture bridge technique: is the repair integrity actually maintained? Am J Sports Med. 2011;39(10):2108–16. doi: 10.1177/0363546510397171. [DOI] [PubMed] [Google Scholar]
- 94.Iannotti JP, Deutsch A, Green A, Rudicel S, Christensen J, Marraffino S, et al. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am Vol. 2013;95(11):965–71. doi: 10.2106/JBJS.L.00708. [DOI] [PubMed] [Google Scholar]
- 95.Miller BS, Downie BK, Kohen RB, Kijek T, Lesniak B, Jacobson JA, et al. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med. 2011;39(10):2064–70. doi: 10.1177/0363546511413372. [DOI] [PubMed] [Google Scholar]
- 96.Keener JD, Wei AS, Kim HM, Paxton ES, Teefey SA, Galatz LM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am Vol. 2010;92(3):590–8. doi: 10.2106/JBJS.I.00267. [DOI] [PubMed] [Google Scholar]
- 97.Ladermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2011;27(12):1620–7. doi: 10.1016/j.arthro.2011.08.290. [DOI] [PubMed] [Google Scholar]
- 98.Piasecki DP, Verma NN, Nho SJ, Bhatia S, Boniquit N, Cole BJ, et al. Outcomes after arthroscopic revision rotator cuff repair. Am J Sport Med. 2010;38(1):40–6. doi: 10.1177/0363546509346401. [DOI] [PubMed] [Google Scholar]
- 99.Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 100.Ciampi P, Scotti C, Nonis A, Vitali M, Di Serio C, Peretti GM, et al. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears: a 3-year follow-up study. Am J Sport Med. 2014;42(5):1169–75. doi: 10.1177/0363546514525592. [DOI] [PubMed] [Google Scholar]
- 101.Encalada-Diaz I, Cole BJ, Macgillivray JD, Ruiz-Suarez M, Kercher JS, Friel NA, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. J Should Elb Surg / Am Should Elb Surg. 2011;20(5):788–94. doi: 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mihara S, Ono T, Inoue H, Kisimoto T. A new technique for patch augmentation of rotator cuff repairs. Arthrosc Tech. 2014;3(3):e367–71. doi: 10.1016/j.eats.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthrosc: J Arthroscop Relat Surg: Off Publ Arthroscop Assoc N Am Int Arthroscop Assoc. 2013;29(12):1911–21. doi: 10.1016/j.arthro.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 104.Proctor CS, et al. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Should Elb Surg / Am Should Elb Surg. 2014;23(10):1508–13. doi: 10.1016/j.jse.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Russo R, Lombardi LV, Visconti V, Della RG. Massive rotator cuff tear treated with a synthetic patch: a case report 16 years after surgery. Musculoskelet Surg. 2011;95(Suppl 1):S83–7. doi: 10.1007/s12306-011-0124-9. [DOI] [PubMed] [Google Scholar]
- 106.Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci: Off J Jpn Orthop Assoc. 2010;15(3):310–6. doi: 10.1007/s00776-010-1453-5. [DOI] [PubMed] [Google Scholar]
- 107.Wong I, Burns J, Snyder S, et al. Arthroscopic GraftJacket repair of rotator cuff tears. J Should Elb Surg / Am Should Elb Surg. 2010;19(2 Suppl):104–9. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 108.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am Vol. 2006;88(6):1238–44. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 109.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am Vol. 2007;89(4):786–91. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- 110.Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Amer Vol. 2006;88(1):113–20. doi: 10.2106/JBJS.E.00282. [DOI] [PubMed] [Google Scholar]
- 111.Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am Vol. 2013;95(21):1920–6. doi: 10.2106/JBJS.M.00122. [DOI] [PubMed] [Google Scholar]
- 112.Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res. 1988;232:51–61. [PubMed] [Google Scholar]
- 113.Miniaci A, MacLeod M. Transfer of the latissimus dorsi muscle after failed repair of a massive tear of the rotator cuff. A two to five-year review. J Bone Joint Surg Am Vol. 1999;81(8):1120–7. doi: 10.2106/00004623-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 114.Castricini R, Longo UG, De Benedetto M, Loppini M, Zini R, Maffulli N, et al. Arthroscopic-assisted latissimus dorsi transfer for the management of irreparable rotator cuff tears: short-term results. J Bone Joint Surg Am Vol. 2014;96(14):e119. doi: 10.2106/JBJS.L.01091. [DOI] [PubMed] [Google Scholar]
- 115.Elhassan B, Bishop A, Shin A, Spinner R. Shoulder tendon transfer options for adult patients with brachial plexus injury. J Hand Surg. 2010;35(7):1211–9. doi: 10.1016/j.jhsa.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 116.Elhassan B, Bishop AT, Hartzler RU, Shin AY, Spinner RJ. Tendon transfer options about the shoulder in patients with brachial plexus injury. J Bone Joint Surg Am Vol. 2012;94(15):1391–8. doi: 10.2106/JBJS.J.01913. [DOI] [PubMed] [Google Scholar]
- 117.Elhassan B, Christensen TJ, Wagner ER, et al. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Should Elb Surg / Am Should Elb Surg. 2014;23(4):492–9. doi: 10.1016/j.jse.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 118.Walch G, Boileau P, Noel E. Shoulder arthroplasty: evolving techniques and indications. Joint Bone Spine Rev Rhum. 2010;77(6):501–5. doi: 10.1016/j.jbspin.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Simone JP, Streubel PH, Sperling JW, Schleck CD, Cofield RH, Athwal GS. Anatomical total shoulder replacement with rotator cuff repair for osteoarthritis of the shoulder. Bone Joint J. 2014;96-B(2):224–8. doi: 10.1302/0301-620X.96B.32890. [DOI] [PubMed] [Google Scholar]
- 120.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am Vol. 2005;87(8):1697–705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 121.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am Vol. 2010;92(15):2544–56. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 122.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am Vol. 2007;89(7):1476–85. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 123.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am Vol. 2008;90(6):1244–51. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 124.Sadoghi P, Vavken P, Leithner A, Hochreiter J, Weber G, Pietschmann MF, et al. Impact of previous rotator cuff repair on the outcome of reverse shoulder arthroplasty. J Should Elb Surg / Am Should Elb Surg. 2011;20(7):1138–46. doi: 10.1016/j.jse.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 125.Boileau P, Gonzalez JF, Chuinard C, Bicknell R, Walch G, et al. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Should Elb Surg / Am Shoul Elb Surg. 2009;18(4):600–6. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]


