Abstract
Propionibacterium acnes (P. acnes) is a gram-positive anaerobic bacillus commonly isolated from the flora of the face, chest, and axilla region. It has emerged as a major pathogen responsible for postoperative shoulder infections after both arthroscopy and arthroplasty procedures. Patients with P. acnes shoulder infection typically present with normal laboratory values (white blood cells (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP)) making diagnosis difficult. Several intraoperative tissue cultures should be obtained and cultured in both agar plate and broth in aerobic and anaerobic conditions for a minimum of 13 days to optimize the sensitivity and specificity to detect P. acnes. The utilization of intraoperative frozen sections to detect P. acnes infection is not reliable. Risk factors include male, cloudy synovial fluid, lucencies around the implant, and periprosthetic membrane formation. Managements include irrigation and debridement, single or two-staged revision, and intravenous antibiotics. Open biopsy prior to the final implantation (two-staged revision) may help detect persistent P. acnes infection. Penicillin and cephalosporins are effective against clinical P. acnes infection and biofilm in vitro. Combination antibiotic therapy with rifampin and daptomycin may further increase the clinical efficacy of treatment.
Keywords: Propionibacterium acnes, Shoulder surgery, Arthroscopy, Arthroplasty, Infection, Management
Introduction
Shoulder pain and associated pathologies are among the most common complaints presenting to an orthopedic office [1]. The modern orthopedic practice can address many of these complaints with conservative management and surgical intervention depending on the nature of the pathology. The increasing prevalence of surgical treatment for shoulder conditions will likely increase in the future [2, 3]. Despite the innovation and success of modern arthroscopic and open shoulder surgery, postoperative infection remains a burden to both the patient and surgeon. The incidence of infection after rotator cuff repair and shoulder arthroplasty ranges from 0.27 to 1.9 % and 0 to 15 %, respectively [4–7]. Postoperative infections can be both difficult to diagnose, eradicate, and treat, with often devastating morbidity and possible mortality. Once considered to be an inconsequential skin flora, Propionibacterium acnes (P. acnes) has emerged as not only a major pathogen for postoperative shoulder infection but also a cause of shoulder pathology as well.
Organism and structure
P. acnes is a gram-positive anaerobic bacillus. Originally, it was described as “bacillus of acne” and classified in the genus Corynebacterium in 1923. In 1933, it was reclassified to the genus Propionibacterium secondary to its relationship with oxygen and ability to synthesize propionic acid [8]. Although the focus of P. acnes virulence is focused with skin infections, it has become an important contributor to postoperative infection after shoulder arthroplasty.
P. acnes is part of the natural skin and mucosal flora inhabiting the sebaceous follicles of the skin, conjunctiva, oral cavity, intestinal track, and external auditory canal. It has generally been thought to be a commensal organism and typically found in the skin around the face, chest, axilla, and thorax (Fig. 1) [9]. While P. acnes is an anaerobic organism, it can survive several hours in low-oxygen environments as well as in the low-oxygen tension and oxidation potential of human tissues [10]. The bacterium has adapted to resist the oxidative, bactericidal, and degradative abilities of macrophages. It is resistant to macrophagal lysozyme, chymotrypsin, hydrogen peroxide, and human serum factors [11]. The ability to form a biofilm, especially on orthopedic implants, is another approach P. acnes utilizes to evade and resist the host’s immune response. Genome mapping has identified several genes responsible for encoding adhesins and enzymes involved with the synthesis of the extracellular polysaccharides used to create biofilm [12].
Fig. 1.
Microscopic image of P. Acnes organism. Propionibacterium acnes by CDC-http://phil.cdc.gov ID#3083. Licensed under Public domain
P. acnes infection after shoulder surgery
The shoulder joint is prone to operative infection. Not only is it a synovial joint, with a nutrient-rich and immunologically diminished synovial fluid, but it also has close proximity to the axillary fossa, which is colonized by polymicrobial bacteria [13]. Surgical approaches to the shoulder near the axillary fold may increase the risk of infection with Staphylococcus aureus, Staphylococcus epidermidis, P. acnes, and Corynebacterium species [4, 5]. Patel et al. characterized the colonization of P. acnes in the flora of the shoulder (axilla), hip, and knee. Their findings demonstrated a greater rate of P. acnes colonization near the acromion and shoulder (axilla) compared to the knee and hip. Men also demonstrated higher rates of P. acnes colonization than women [6].
The rates of infection after arthroscopic surgery are fortunately low. Yeranosian et al. retrospectively reviewed a large insurance database of shoulder arthroscopic procedures and complications including postoperative infections. Out of 165,820 shoulder procedures, the rate of reoperation for infection was 450 (0.27 %) [14]. Shoulder infection after arthroplasty can range between 3 and 15 % [15•, 16–18], and the complications can be devastating in terms of patient morbidity and high economic burden to society (Fig. 1 and Table 1). P. acnes has been isolated as the infectious organism in 16 % of prosthetic shoulder infections and in 21 % of shoulder revision arthroplasties [18, 25]. Pottinger et al. reported 70 % of the positive intraoperative cultures in over 100 revision shoulder arthroplasty showed growth of P. acnes [19]. Grosso et al. found 83 % of patients that had a clinical suspicion of infection that underwent revision shoulder arthroplasty patients were P. acnes culture positive [21••]. The ability of P. acnes isolates to form biofilm may render it a more virulent pathogen than other isolates of skin flora P. acnes. Blood plasma helps prevent the formation of biofilm, which may explain why this skin flora may be more pathogenic around the plasma poor environment of an arthroplasty site [28].
Table 1.
Studies that show percentage of positive P. acnes found in shoulder surgery
| Study | Type of surgery | No. of patients | No. of infections | No. of P. acnes infection (%) |
|---|---|---|---|---|
| HereSingh et al. [15•] | Primary anatomic shoulder arthroplasty | 2588 | 32 | 6 (1.23) |
| Singh et al. [16] | Primary hemiarthroplasty | 1431 | 14 | 3 (0.21) |
| Athwal et al. [4] | Rotator cuff repair | 4886 | 39 | 20 (0.41) |
| Topolski et al. [5] | Revision shoulder arthroplasty | 439 | 85 | 45 (10.3) |
| Pottinger et al. [19] | Revision shoulder arthroplasty | 193 | 108 | 75 (38.7) |
| Kwon et al. [20] | Rotator cuff repair | 1904 | 14 | 7 (0.36) |
| Grosso et al. [21••] | Revision shoulder arthroplasty | 45 | 30 | 18 (40) |
| Coste et al. [22] | Arthroplasties | 2343 | 49 | 7 (0.29) |
| Herrera et al. [23] | Mini open rotator cuff repair | 360 | 7 | 6 (1.66) |
| Levy et al. [24] | Shoulder surgery | 276 | 16 | 9 (3.26) |
| Kelly et al. [25] | Revision shoulder arthroplasty | 28 | 8 | 6 (21.4) |
| Zheng et al. [26] | Shoulder arthroplasties | NA | 18 | 8 (44.4) |
| Mirzayan et al. [27] | Open rotator cuff infections | NA | 13 | 3 (23.1) |
Preoperative skin cleansing preparations may not fully address and diminish the bacterial load of P. acnes compared to other common skin flora. The three most commonly used perioperative skin preparations including ChloraPrep, DuraPrep, and povidone-iodine scrub and paint demonstrated no difference in the efficacy of decreasing P. acnes on the skin. However, in this study, ChloraPrep was significantly more effective than both DuraPrep and povidone-iodine at eliminating overall bacteria burden from the shoulder region [7]. Using cyanoacrylate microbial sealant may reduce the prevalence of positive cultures and P. acnes infection in revision shoulder arthroplasty compared to the traditional iodine-barrier drapes [29•].
Diagnosis of P. acnes infection
Acute pyogenic postoperative injections can be obvious with skin erythema, swelling, skin reaction, exudate production, and pain. Deeper infection with P. acnes are often subtle and difficult to diagnose with subacute infection being the common presentation. Pain, stiffness, and implant loosening can be presenting symptoms. Plain radiography and advanced imaging should be obtained in any patient with a concern for infection to try to identify sources of pain. Radiographic and operative humeral loosening are especially concerning for P. acnes specifically. Component loosening and radiographic osteolysis have been associated with a threefold to tenfold increase in the risk of a positive P. acnes culture [19].
Common laboratories, including white blood cells (WBC), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), are often a starting point for a postoperative infection. In both arthroscopic [20, 27] and arthroplasty [30, 31] infections, the WBC can be normal with a mildly elevated ESR and CRP. In their review of 11 patients with P. acnes infection after shoulder arthroplasty, Dodson et al. found no patient with an elevated preoperative leukocyte count of more than 10,000 cells/L, 5 patients (46 %) had an ESR of 30 mm/h or higher, and 8 patients (72 %) had a CRP of 1.0 mg/dL or higher [30]. Piper et al. found a lower rate with 42 % of infected patients having an elevated CRP and only 16 % with an elevated ESR [32]. It should be cautioned, however, that patients can have a postoperative infection with normal WBC, polymorphonuclear leukocyte (PMN) percentage distributions, ESR, and CRP values [5]. Piper et al. reported poor sensitivity and specificity of using CRP and ESR to diagnose shoulder infections. Using an ESR of 26 as the cutoff number, the sensitivity and specificity to detect true shoulder implant infection was 32 and 93 %, respectively. In terms of using CRP to detect shoulder implant infection, using 7 as the cutoff number, the sensitivity and specificity was 63 and 73 %, respectively [32]. Furthermore, Grosso et al. found serum IL-6 to have very low sensitivity (12 %) and specificity (7 %) for the detection of P. acnes in shoulder infection [33].
Preoperative aspiration and intraoperative cultures are also used to diagnose P. acnes infection. Foruria et al. reported a 15 % rate of unexpected positive cultures in a series of 107 patients who underwent revision shoulder arthroplasty [34]. Majority of the positive cultures were P. acnes (63 %) and S. epidermidis (20 %). In a review of the literature, Pottinger et al. also found that over half of subacute and chronic infections after shoulder arthroplasty surgery where culture positive for P. acnes [19]. It is well known that P. acnes cultures in several mediums take longer to be identified than other common shoulder pathogens. Operative cultures should be held for an extended period of time beyond that of the typical, ‘five days,’ incubation period as it may take up to 21 days for cultures to become positive for P. acnes [30, 31]. Microbiology laboratories may use differing media to culture P. acnes as there is no standard media. It is important to use both anaerobic and aerobic media in cases where P. acnes is suspected [35••]. Butler-Wu et al. evaluated the bacteria culture of 198 revision shoulder arthroplasty procedures and determined 13 days of incubation period is essential for the detection of P. acnes in this patient population [35••]. After the 13 days of incubation, 21 % of clinically unimportant P. acnes isolates were identified. However, a diagnosis of P. acnes would have been missed in 29 % of patients if the extended culture incubation had been applied only to the anaerobic culture media. Therefore, it is essential to hold the cultures for at least 13 days in both aerobic and anaerobic culture media. In contrast to the above study, Shannon et al. reported that a 7-day incubation period was sufficient when the intraoperative tissue cultures were placed in anaerobic thioglycolate broth solution transported in anaerobic tissue and fluid vials. In addition, tissue cultured in broth solution was significantly more likely to be positive by day 7 than in plate culture [36]. Both studies recommend taking multiple intraoperative tissue cultures from different locations to increase the likelihood of detecting P. acnes.
Caution should be taken in applying the literature on lower extremity frozen intraoperative histology sections to indolent shoulder infection by P. acnes. In revision hip and knee arthroplasties with culture-positive infection, frozen section demonstrates sensitivities of 77–95 % and a high specificity of 92–96 % [37]. Frozen sections with confirmed P. acnes infections are not as reliable. Recently, Grosso et al. described their results using five or more PMNs per high-powered field in each of three or more fields. Out of 18 patients with confirmed P. acnes infection of the shoulder, there was a 50 % sensitivity and 100 % specificity compared to 67 % for the non P. acnes infection group. The authors recommended using a total of ten PMNs in five high-power fields (400×) to increase the sensitivity to 72 % [21••].
The diagnosis of a shoulder infection can still be elusive with imaging, laboratory studies, and intraoperative cultures, which may all be normal in the presence of P. acnes infection. In addition to the risk factors that may increase infection in any surgical patient, such as diabetes, obesity, smoking, etc., there are additional risk factors that are associated with P. acnes infections. Pottinger et al. demonstrated that male sex, cloudy synovial fluid, humeral osteolysis and loosening, glenoid wear, and periprosthetic membrane formation are associated with an increased likelihood of culture-positive P. acnes infection [19].
P. acnes and arthrosis
P. acnes may be more than an opportunistic or cause of postoperative pain, infection, and implant loosening. It may also play a role in the etiology and pathophysiology of shoulder joint arthrosis. Although investigations into the pathology caused by postoperative P. acnes infection has increased due to the increased prevalence of shoulder arthroplasty, animal studies were conducted as early as the 1980s to correlate P. acnes with both radiographic arthrosis and clinical arthritis. Trimble et al. serially infected rat joints with P. acnes and assessed both radiographic and histologic outcomes. With repeat infections, they found radiographic arthrosis and erosive synovitis in sacrificed specimens [38]. Case reports also noted postinfectious arthritis in the setting of P. acnes inoculation and contamination after arthrocentesis [39, 40].
More recently, Levy et al. obtained aspirates and biopsy tissue from 55 shoulders undergoing shoulder arthroplasty for arthritis without any evidence of infection. They discovered that 41.8 % of samples were positive for P. acnes infection prior to the implantation of the arthroplasty components. The authors note that contamination may be a confounder, but their protocol attempted to obtain samples under sterile conditions [41••]. It is unclear if P. acnes is a causative agent for shoulder arthritis. This may be comparable to Helicobacter pylori as the causative agent for gastritis and duodenal ulcers [41••]. Further research needs to investigate any causal link and what treatment options there may potentially be to not only prevent shoulder arthritis with possible antibiotic therapy but how to also treat patients undergoing arthroplasty with preoperative culture-positive P. acnes infection.
Management of P. acnes infection
Treatment options for infection include irrigation and debridement, antibiotic therapy and suppression, and single and two-stage exchange revision. However, most data regarding the management of P. acnes infections are case series that are retrospective and observational in nature. As a result, clinicians have limited high-level evidence-based guidance in making management decisions. Following arthroscopic shoulder surgery, the common treatment of infection consists of multiple surgical debridements as well as intravenous antibiotic therapy for 4 to 6 weeks [38]. However, it is unclear whether or not during the debridement surgery of foreign materials such as sutures should be left in place [38, 39]. The optimal treatment of P. acnes infection following shoulder arthroplasty consists of systemic antibiotics, irrigation and debridement, removal of foreign bodies, and staged revision if clinically appropriate [28]. As a result of the simplification of surgical treatment and better joint function restoration, some authors prefer one-stage exchange arthroplasty, with two-stage arthroplasty reserved for more difficult cases [39, 40, 41••]. If reimplantation is not possible, long-term oral suppressive antibiotic therapy may serve as an alternative to surgical intervention [28]. However, there are no clear data on prosthesis retention and long-term outcomes.
Although P. acnes is usually susceptible to the broad range of antibiotics, resistance to metronidazole, tetracyclines, clindamycin, and erythromycin has begun to emerge with regional variability [28, 42]. As a result, several authors recommend the use of two antibiotics in order to prevent emergence of resistant strains as well as for the treatment of polymicrobial infections [29•]. Studies have shown various combinations of antibiotics, including cephalexin and rifampin, clindamycin and ofloxacin, and amoxicillin and rifampin, all with good to excellent clinical effect [29•, 40, 41••].
Bayston et al. looked at the antibiotic susceptibility of mature P. acnes biofilms in vitro. They found that penicillin alone was effective in the eradication of P. acnes biofilm without regrowth [43]. Crane et al. also supported this finding and found that penicillins (penicillin G and amoxicillin) and cephalosporins (cephalothin and ceftriaxone) both showed strong activities against P. acnes (lowest MIC values) [42]. However, both vancomycin and clindamycin demonstrated fair activity against P. acnes. In vitro biofilm infection studies have reported an excellent eradication rate with a combination of rifampin and daptomycin [43]. The clinical practice guidelines for the treatment of prosthetic joint infections published by the Infectious Disease Society of America recommends 4 to 6 weeks of intravenous penicillin G or intravenous ceftriaxone. Intravenous or oral clindamycin can be given as an alternative treatment, and vancomycin can be used only in the case of medication allergies [22]. Additional data from prospective clinical studies are needed in order to better ascertain the ideal combination of antibiotic and surgical treatment for P. acnes infections.
Outcomes of P. acnes infection
Most published literature on the outcome of postoperative shoulder infections are small heterogenous series of patients with infections caused by different organisms other than P. acnes. Coste et al. retrospectively reviewed the results and outcomes of 49 infected shoulders status post arthroplasty in 42 patients. Overall 71 % of patients were asymptomatic and free of infection. Five patients were treated with only antibiotic suppression therapy and 3 of these patients still had persistent symptomatic painful shoulders [22]. There is no specific literature regarding long-term chronic suppression of P. acnes infection and outcomes.
Two-stage exchange revision with antibiotic spacer is an option to attempt infection eradication. Strickland et al. reviewed 19 infected arthroplasties in 17 patients that underwent two-stage revision. At a minimum of 2 years of follow-up, only 63 % of patients were considered to have had their infections eradicated. However, only 1 patient in their series had a confirmed diagnosis of P. acnes [44]. Coste et al. found a similar percentage of eradication in their series of 10 patients after a two-stage revision as well [22]. Zhang et al. recommended an open biopsy prior to the second stage of reimplantation. In their series of 18 patients, P. acnes (44 %) and S. epidermidis (39 %) infections were the most common organisms. Biopsy results before reimplantation demonstrated that 3 out of 4 patients with positive cultures grew P. acnes. These 4 patients underwent another I&D with spacer exchange, antibiotics, and repeat open biopsy. Reimplantation was performed only when the cultures were negative from the open biopsy. This protocol resulted in 100 % eradication of the shoulder prosthetic infection on final follow-up [26]. Alternatively, an arthroscopic biopsy technique with a dry scope has been done by the senior author (XL) prior to reimplantation of the final component for P. acnes infection (Fig. 2).
Fig. 2.
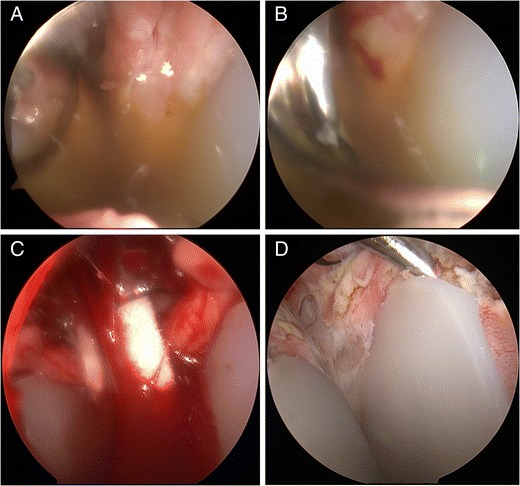
Arthroscopic evaluation and culture with biopsy for shoulder infection workup in a patient who presented with pain after total shoulder arthroplasty. a Diagnostic arthroscopy performed first with a dry scope technique. Cloudy synovial fluid was seen after the introduction of a 30° scope. b Spinal needle (18 gauge) was place anteriorly into the glenohumeral joint to aspirate the synovial fluid. c Multiple swab cultures were done through a 6-mm cannula placed anteriorly. d Glenoid component seen on the right and the humeral head on the left. A trochar was used to evaluate for glenoid loosening. Multiple tissue biopsies were taken using arthroscopic technique. Cultures were held for 14 days for P. Acnes
One-stage revision has also been proposed in the literature. O’Klatte et al. reviewed their experience with one-stage revision in Germany in patients with mostly P. acnes and/or S. epidermidis [45•]. Their published results of successful eradication at 94 % are similar to Coste et al. review of single-stage revision successful infection eradication [22]. However, the follow-up ranged from 1 to 13 years, with five patients lost to final follow-up and additional four other patients that underwent revision surgery for noninfection reasons. Thus, the reader should be cautious when interpreting these above results regarding single-stage revision.
More research needs to be done to assess the optimal treatment modality for P. acnes infection as well as the functional outcomes after treatment. The literature has heterogenetic outcome results in small series of patients for bacterial species, treatment modalities, single vs. two-staged exchange, and types of arthroplasties utilized. Patient age, comorbidities, bone stock, and chronicity of infection may all confound treatment outcomes and must be taken into account to select the best method of infection eradication and maximization of functional outcomes.
Conclusion
P. acnes is a gram-positive bacillus that has emerged as not only a major pathogen for postoperative infection but also as a possible cause of pathoanatomy of the glenohumeral joint arthrosis. Common anti-bacterial preps used in the operating room may not provide sufficient efficacy for skin eradication compared to other common skin flora [7]. P. acnes infection provides challenges for a treating surgeon as diagnosis is often elusive and treatment algorithms are not fully elucidated in the literature. Inflammatory makers including CRP, ESR, white blood cell count, and even IL-6 have all shown poor sensitivity and specificity for the detection of infection [27, 30, 32]. A low index of suspicion for P. acnes infection must be considered in a postoperative patient with persistent shoulder pain and radiographic implant loosening in the setting of negative inflammatory markers and cultures. It is crucial that institutional microbiology labs are notified to hold aspiration and operative cultures for an extended period of time as it may take up to 21 days for a culture to become positive with P. acnes [30, 31, 35••].
The management of infection includes one or two-stage exchange arthroplasty depending on surgeon preference and clinical scenario [41••, 46]. Traditionally, the penicillin family of antibiotics have been utilized to assist in infection eradication [42]; however, some advocate coverage with two differing agents to help prevent resistance in addition to covering polymicrobial infection [31]. There is a paucity in the literature regarding long-term functional outcomes and success of the eradication of P. acnes infection. More research is needed to elucidate optimal antibiotic coverage and duration, one vs. two-stage exchange arthroplasty, host metabolism and nutrition, and prevention of infection.
Compliance with Ethics Guidelines
Conflict of Interest
David Saper, Nina Capiro, and Richard Ma declare that they have no conflict of interest.
Xinning Li reports compensation from Tornier and Mitek Sports Medicine, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Shoulder Surgery: Complications
Contributor Information
David Saper, Email: dave.saper@gmail.com.
Nina Capiro, Email: nmcapiro@gmail.com.
Richard Ma, Email: richardmamd@gmail.com.
Xinning Li, Phone: (508) 816-3939, Email: xinning.li@gmail.com.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Department of Research & Scientific Affairs, American Academy of Orthopaedic Surgeon. <br />Annual incidence of common musculoskeletal procedures and treatment. American Academy of Orthopaedic Surgeons. 2014(March).
- 2.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249–54. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 3.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Should Elbow Surg. 2010;19(8):1115–20. doi: 10.1016/j.jse.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Deep infection after rotator cuff repair. J Should Elbow Surg. 2007;16(3):306–11. doi: 10.1016/j.jse.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Should Elbow Surg. 2006;15(4):402–6. doi: 10.1016/j.jse.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Should Elbow Surg. 2009;18(6):897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009;91(8):1949–53. doi: 10.2106/JBJS.H.00768. [DOI] [PubMed] [Google Scholar]
- 8.DOUGLAS HC, GUNTER SE. The taxonomic position of Corynebacterium acnes. J Bacteriol. 1946;52:15–23. doi: 10.1128/JB.52.1.15-23.1946. [DOI] [PubMed] [Google Scholar]
- 9.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–53. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csukas Z, Banizs B, Rozgonyi F. Studies on the cytotoxic effects of Propionibacterium acnes strains isolated from cornea. Microb Pathog. 2004;36(3):171–4. doi: 10.1016/j.micpath.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Webster GF, Leyden JJ, Musson RA, Douglas SD. Susceptibility of Propionibacterium acnes to killing and degradation by human neutrophils and monocytes in vitro. Infect Immun. 1985;49(1):116–21. doi: 10.1128/iai.49.1.116-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruggemann H. Insights in the pathogenic potential of Propionibacterium acnes from its complete genome. Semin Cutan Med Surg. 2005;24(2):67–72. doi: 10.1016/j.sder.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Perry A, Lambert P. Propionibacterium acnes: infection beyond the skin. Expert Rev Anti Infect Ther. 2011;9(12):1149–56. doi: 10.1586/eri.11.137. [DOI] [PubMed] [Google Scholar]
- 14.Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437–41. doi: 10.1177/0363546513510686. [DOI] [PubMed] [Google Scholar]
- 15.•.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Should Elbow Surg. 2012;21(11):1534–41. doi: 10.1016/j.jse.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh JA, Sperling JW, Schleck C, Harmsen W, Cofield RH. Periprosthetic infections after shoulder hemiarthroplasty. J Should Elbow Surg. 2012;21(10):1304–9. doi: 10.1016/j.jse.2011.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–85. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 18.Sperling JW, Kozak TK, Hanssen AD, Cofield RH. Infection after shoulder arthroplasty. Clin Orthop Relat Res. 2001;382:206–16. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 19.Pottinger P, Butler-Wu S, Neradilek MB, et al. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94(22):2075–83. doi: 10.2106/JBJS.K.00861. [DOI] [PubMed] [Google Scholar]
- 20.Kwon YW, Kalainov DM, Rose HA, Bisson LJ, Weiland AJ. Management of early deep infection after rotator cuff repair surgery. J Should Elbow Surg. 2005;14(1):1–5. doi: 10.1016/j.jse.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.••.Grosso MJ, Frangiamore SJ, Ricchetti ET, Bauer TW, Iannotti JP. Sensitivity of frozen section histology for identifying Propionibacterium acnes infections in revision shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(6):442–7. doi: 10.2106/JBJS.M.00258. [DOI] [PubMed] [Google Scholar]
- 22.Coste JS, Reig S, Trojani C, Berg M, Walch G, Boileau P. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg (Br) 2004;86(1):65–9. [PubMed] [Google Scholar]
- 23.Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. J Should Elbow Surg. 2002;11(6):605–8. doi: 10.1067/mse.2002.127302. [DOI] [PubMed] [Google Scholar]
- 24.Levy PY, Fenollar F, Stein A, et al. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis. 2008;46(12):1884–6. doi: 10.1086/588477. [DOI] [PubMed] [Google Scholar]
- 25.Kelly JD, 2nd, Hobgood ER. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467(9):2343–8. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang AL, Feeley BT, Schwartz BS, Chung TT, Ma CB. Management of deep postoperative shoulder infections: is there a role for open biopsy during staged treatment? J Shoulder Elbow Surg. 2014. [DOI] [PubMed]
- 27.Mirzayan R, Itamura JM, Vangsness CT, Jr, Holtom PD, Sherman R, Patzakis MJ. Management of chronic deep infection following rotator cuff repair. J Bone Joint Surg Am. 2000;82-A(8):1115–21. doi: 10.2106/00004623-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Holmberg A, Lood R, Morgelin M, et al. Biofilm formation by Propionibacterium acnes is a characteristic of invasive isolates. Clin Microbiol Infect. 2009;15(8):787–95. doi: 10.1111/j.1469-0691.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 29.•.Lorenzetti AJ, Wongworawat MD, Jobe CM, Phipatanakul WP. Cyanoacrylate microbial sealant may reduce the prevalence of positive cultures in revision shoulder arthroplasty. Clin Orthop Relat Res. 2013;471(10):3225–9. doi: 10.1007/s11999-013-2854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodson CC, Craig EV, Cordasco FA, et al. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Should Elbow Surg. 2010;19(2):303–7. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 31.Lutz MF, Berthelot P, Fresard A, et al. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: a retrospective study of 52 cases, 1995–2002. Eur J Clin Microbiol Infect Dis. 2005;24(11):739–44. doi: 10.1007/s10096-005-0040-8. [DOI] [PubMed] [Google Scholar]
- 32.Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One. 2010;5(2):e9358. doi: 10.1371/journal.pone.0009358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grosso MJ, Frangiamore SJ, Saleh A, et al. Poor utility of serum interleukin-6 levels to predict indolent periprosthetic shoulder infections. J Should Elbow Surg. 2014;23(9):1277–81. doi: 10.1016/j.jse.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 34.Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Should Elbow Surg. 2013;22(5):620–7. doi: 10.1016/j.jse.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 35.••.Butler-Wu SM, Burns EM, Pottinger PS, et al. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49(7):2490–5. doi: 10.1128/JCM.00450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon SK, Mandrekar J, Gustafson DR, et al. Anaerobic thioglycolate broth culture for recovery of Propionibacterium acnes from shoulder tissue and fluid specimens. J Clin Microbiol. 2013;51(2):731–2. doi: 10.1128/JCM.02695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berbari E, Mabry T, Tsaras G, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92(11):2102–9. doi: 10.2106/JBJS.I.01199. [DOI] [PubMed] [Google Scholar]
- 38.Trimble BS, Evers CJ, Ballaron SA, Young JM. Intraarticular injection of Propionibacterium acnes causes an erosive arthritis in rats. Agents Actions. 1987;21(3–4):281–3. doi: 10.1007/BF01966491. [DOI] [PubMed] [Google Scholar]
- 39.Kooijmans-Coutinho MF, Markusse HM, Dijkmans BA. Infectious arthritis caused by Propionibacterium acnes: a report of two cases. Ann Rheum Dis. 1989;48(10):851–2. doi: 10.1136/ard.48.10.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeverbeke T, Lequen L, de Barbeyrac B, et al. Propionibacterium acnes isolated from synovial tissue and fluid in a patient with oligoarthritis associated with acne and pustulosis. Arthritis Rheum. 1998;41(10):1889–93. doi: 10.1002/1529-0131(199810)41:10<1889::AID-ART23>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 41.••.Levy O, Iyer S, Atoun E, et al. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Should Elbow Surg. 2013;22(4):505–11. doi: 10.1016/j.jse.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Crane JK, Hohman DW, Nodzo SR, Duquin TR. Antimicrobial susceptibility of Propionibacterium acnes isolates from shoulder surgery. Antimicrob Agents Chemother. 2013;57(7):3424–6. doi: 10.1128/AAC.00463-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furustrand Tafin U, Corvec S, Betrisey B, Zimmerli W, Trampuz A. Role of rifampin against Propionibacterium acnes biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2012;56(4):1885–91. doi: 10.1128/AAC.05552-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strickland JP, Sperling JW, Cofield RH. The results of two-stage re-implantation for infected shoulder replacement. J Bone Joint Surg (Br) 2008;90(4):460–5. doi: 10.1302/0301-620X.90B4.20002. [DOI] [PubMed] [Google Scholar]
- 45.•.Klatte TO, Junghans K, Al-Khateeb H, et al. Single-stage revision for peri-prosthetic shoulder infection: outcomes and results. Bone Joint J. 2013;95-B(3):391–5. doi: 10.1302/0301-620X.95B3.30134. [DOI] [PubMed] [Google Scholar]
- 46.Millett PJ, Yen YM, Price CS, Horan MP, van der Meijden OA, Elser F. Propionibacterium acnes infection as an occult cause of postoperative shoulder pain: a case series. Clin Orthop Relat Res. 2011;469(10):2824–30. doi: 10.1007/s11999-011-1767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


