Abstract
The management of blast-related soft tissue wounds requires a comprehensive surgical approach that acknowledges extensive zones of injury and the likelihood of massive contamination. The experiences of military surgeons during the last decade of war have significantly enhanced current understandings of the optimal means of mitigating infectious complications, the timing of soft tissue coverage attempts, and the reconstructive options available for definitive wound management. Early administration of antibiotics in the setting of soft tissue wounds and associated open fractures is the single most important aspect of open fracture care. Both civilian and military reports have elucidated the incidence of invasive fungal infection in the setting of high-energy injuries with significant wound burdens, and novel treatment protocols have emerged. The type of reconstruction is predicated upon the zone of injury and location of the soft tissue defect. Multiple reports of military cohorts have suggested the equivalency of various techniques and types of soft tissue coverage. Longer-term follow-up will inform future perspectives on the durability of these surgical approaches.
Keywords: Blast, Review, Soft tissue, Infection control, Flap reconstruction
Introduction
Blast-associated soft tissue injuries present the surgeon with a challenging array of treatment considerations for patients with extensive zones of injury. The proximity to the epicenter of the blast dictates the nature of the pattern of injury sustained from explosive munitions [1]. The effects of blast exposure stem from the propagation of the wave of energy through the victim, and categories of injury—primary, secondary, tertiary, quaternary, and quinary—contribute to the unique nature of explosion [2, 3]. Injuries related to fragments (secondary blast injury) and exposure to the by-products of explosions (quinary blast injury), such as radiation, metals, and bacteria, contribute to the unique circumstances under which many of these injuries are sustained. While the standard principles of wound management remain applicable, blast injuries necessitate a specialized approach that may require the application of novel strategies for soft tissue reconstruction and limb salvage. Moreover, the systemic effects of blasts (i.e., pulmonary, neurologic, and gastrointestinal) play an important role in the patients’ underlying physiology. Frequently, associated injuries dictate the timing of subsequent reconstructive or soft tissue coverage procedures.
The past decade of military contingency operations has presented military surgeons with high volume of blast injuries requiring vascular repair and soft tissue coverage [4, 5]. It is widely accepted that restoration of a soft tissue envelope in the setting of open fractures plays a pivotal role in optimizing osseous healing and mitigating the likelihood of complications [6, 7, 8••]. In spite of this consensus and the wide acknowledgement of the importance of early and frequent debridement in the setting of highly contaminated wounds, controversy persists over the type and timing of soft tissue reconstruction of the blast-injured extremity [8••, 9••, 10]. While the combat-related blast injury creates a unique set of circumstances, the volume of data accumulated from a decade of war has the potential to enhance current understandings of these injuries. The purposes of this review are to offer a concise description of the state of the art in blast-related soft tissue management and describe areas in need of further investigation.
Initial management
The gross contamination, marginal viability of residual soft tissues, and underlying osseous trauma characteristic of explosion-related injuries necessitate staged protocols that begin with provisional skeletal fixation, reperfusion of dysvascular extremities, and, thorough, serial debridement. A low threshold should be used to determine the indication for fasciotomies, as patients are often critically ill and an accurate neurovascular assessment is difficult to obtain. Extensive zones of injury are the rule, as the blast energy frequently dissects fascial planes and advances contamination far beyond the visible primary soft tissue wound. Radical, serial debridements with longitudinal extension of wounds should be employed to fully evaluate the extent of evolving tissue necrosis and remove the burden of significant, gross contamination.
Infection control
The prevalence of infection among casualties of explosions, particularly in the setting of combat-related blasts, must be acknowledged [11••]. Broad-spectrum antibiotics should be administered upon presentation, as these wounds tend to be colonized by multiple pathogens. The expeditious administration of antibiotics appears to be the key treatment principle in the minimization of infection in the setting of open fracture treatment. Multiple reports have emphasized that the timing of administration is the key factor in decreasing rates of infection associated with open fractures [12, 13••, 14–16]. Although previous series had suggested that antibiotic dosing accomplished within 3 h of injury was optimal (decreasing infection rates from 7.4 to 4.7 %), a recent retrospective review by Lack et al. of 137 patients with type III open tibia fractures showed that time to antibiotic dosing greater than 66 min was an independent predictor of infection (P < 0.001) [13••].
Complex soft tissue wounds sustained in the setting of high-energy mechanisms are particularly susceptible to invasive fungal infections (IFI), most likely due to the high likelihood of penetrating trauma and aerosolized environmental matter. Neblett Fanfair et al. recently described a cohort of 13 subjects injured in the 2011 tornado in Joplin, Missouri [17•]. Among this group, mortality was particularly high (5 of 13, 39 %), and all IFI occurred within the most severe zones of injury. Military cohorts of patients with blast-related wounds have been shown to be particularly susceptible to IFI [11••, 18, 19, 20••]. Warkentien et al. characterized all IFI attributable to casualties of combat operations in Afghanistan. During an 18-month period, a mean of 324 patients (range, 95–509 patients) per quarter was evacuated from Afghanistan with a mean of 5 patients (range, 0–12 patients) being diagnosed with an IFI, all of which were casualties of blasts. Common findings of blast-associated, culture-proven IFI (37 patients) included 78 % with traumatic amputation (n = 29), 68 % with multiple extremity amputations (n = 25), 68 % with significant genital and perineal wounds (n = 25), and massive transfusion (mean transfusion 30 units of packed red blood cells and plasma) of blood product requirements within the first 24 h after injury. The most common pathogen isolated was Mucormycosis spp. (9 of 69 mold cultures, 13 %). On average, 11 procedures (range, 7–16 debridements and/or amputation revisions) were performed prior to wounds being deemed “clean,” as determined at the discretion of the treating surgeon, at a median of 21 days (range, 18–35 days from the point of injury). The overall mortality rate in this series was 14 % (5 of 37 patients), with 3 (8 %) patients’ death at least partially attributed to the IFI. Perhaps most notably, the median time from injury to diagnosis of IFI was 10 days (range, 6–14 days), underscoring the importance of a high index for suspecting IFI in the setting of blast [20••].
In 2012, the Department of Defense’s (DoD) Joint Theater Trauma System published a clinical practice guideline (CPG), reiterating blast injury characteristics (Table 1) that should raise awareness for IFI and the salient features of an appropriate management approach [21••]. In addition, one should maintain a high index of suspicion in the setting of significant, progressive tissue necrosis observed over the course of two consecutive debridements. Under these circumstances, systemic antifungal therapy should also be initiated with either voriconazole or liposomal amphotericin B in conjunction with infectious disease consultation. Topical anti-fungal solutions should be used and are prepared in several different ways (Table 2). In the setting of culture-proven or strongly suspected IFI, antibacterial and antifungal bead application is recommended. Beads can be made with liposomal amphotericin B 500 mg, voriconazole 200 mg, tobramycin 1.2 g, and vancomycin 1 g [21••].
Table 1.
Risk factors for invasive fungal infection (IFI)
| 1. Dismounted blast injury |
| 2. Traumatic, above-knee amputation OR progressive, proximal amputation transition |
| 3. Extensive perineal, genitourinary, and/or rectal injury |
| 4. Transfusion >25 units packed red blood cells and/or whole blood |
Treatment of suspected invasive fungal infection in war wounds. Joint Theater Trauma System Clinical Practice Guideline (2012 Nov)
Table 2.
Topical antifungal solution (0.0025 % Dakin’s solution) preparations
| 1. Five-milliliter 0.5 % Dakin’s solution in 995 ml sterile water |
| 2. Ten-milliliter 0.25 % Dakin’s solution in 990 ml sterile water |
Treatment of suspected invasive fungal infection in war wounds. Joint Theater Trauma System Clinical Practice Guideline (2012 Nov)
Negative-pressure wound therapy
Provisional wound management with negative-pressure wound therapy (NPWT) has emerged as an effective tool in the treatment of complex extremity wounds by providing a durable means to obtain temporary coverage. Wound management is facilitated by NPWT capacity to remove edema, promote granulation and angiogenesis, and decrease wound surface area, thereby enhancing the likelihood of delayed primary closure or decreasing the amount of tissue coverage needed [22, 23] NPWT has also been proven to reduce infection rates in the setting of complex, open fractures when compared to standard dressings [24]. Various adjuncts, including the addition of deep drains and the addition of silver to the NPWT dressing, have been advocated to increase the effectiveness of diminishing bacterial loads [25, 26]. The usage of local antibiotic delivery to surrounding tissues by the placement of polymethylmethacrylate (PMMA) cement beads also presents an attractive option for decreasing infection rates by increasing local concentrations of antibiotics within the surrounding wound bed, especially within the first 24–48 h after placement [27, 28]. However, the ramifications of applying NPWT in the proximity to PMMA beads have raised some concerns regarding the local effects of adding a negative-pressure environment to the antibiotic depot of the wound bed. While Stinner et al. showed that the addition of NPWT to antibiotic-impregnated PMMA bead pouches reduces the levels of antibiotic in the wound effluent, resulting in higher levels of persistent bacterial growth compared to PMMA beads alone, there are no studies to date comparing the effectiveness of NPWT versus NPWT plus PMMA beads at reducing overall rates of wound infection [29•]. Ultimately, the potential for NPWT to diminish local antibiotic concentrations must be weighed against the aforementioned benefits to managing soft tissue wounds in which definitive closure is likely to remain an issue.
Timing of soft tissue coverage
The principle of early coverage (within 72 h from injury) of critical defects, especially in the case of those associated with fractures, has long been advocated by a number of authors based upon the pivotal work of Godina [10, 30, 31•]. However, the practice of applying the success of “fix and flap” technique deserves some scrutiny as many of these results supporting this approach considered outcomes following blunt trauma. The recent data derived from the experiences of military surgeons treating blast injuries suggests that the massive soft tissue loss and significant, gross contamination preclude early closure/coverage and that delayed reconstructions can be approached with a reasonable expectation of success [9••, 16, 32, 33•, 34]. Although early soft tissue coverage remains a priority in the treatment of combat-related blast injuries, the underlying wound bed must be clean and viable prior to any definitive soft tissue procedures.
Reconstructive considerations
When addressing blast wounds, the reconstructive ladder serves as a guide to decision making in the management of soft tissue defects by proposing a sequence of coverage techniques that progress in the complexity of the closure method. Local wound care, primary closures, split-thickness skin grafts (STSGs), local skin flaps, pedicled flaps, and free flaps are all important techniques (or rungs) suitable for addressing circumstances involving soft tissue coverage. A stepwise progression up the ladder is predicated by the severity of the underlying wound [35]. However, in the setting of the blast injuries, a rapid escalation of the complexity of a coverage approach practice may be warranted. In this sense, the reconstructive ladder may be better thought of as an elevator. The extensive zones of injury with a high burden of devitalized tissues requiring debridement frequently obviate the utility of the simpler, lower rungs of the reconstructive ladder.
Dermal substitutes
Although dermal substitutes are not integral to the traditional reconstructive ladder, they provide great value in addressing full-thickness extremity wounds with a nonviable dermis. Adherence to the principles of its effective application is vital to the durability of definitive coverage [34]. Integra bilayer wound matrix (Integra LifeSciences, Plainsboro, NJ, and Graftjacket, KCI, San Antonio, TX) is one example of a dermal substitute. This material is composed of two layers: a bovine tendon collagen layer cross-linked with glycosaminoglycans and a layer of either silicon or polysiloxane. The collagen layer is applied to the wound bed and is incorporated by way of native angiogenesis and granulation. During this process, the silicon layer maintains moisture, while enhancing the strength and resistance to shear of the underlying regenerate. The silicone layer is eventually removed and either accommodates epidermal proliferation from adjacent tissue or provides a foundation upon which STSGs can be applied to larger wounds; the latter is typically performed approximately 14–21 days from initial application (Fig. 1) [32]. Graftjacket (KCI, San Antonio, TX), which is fabricated from donated human skin that has undergone histochemical preparation to remove all cellular components but retains the scaffolding of the donor tissue, is another example of a dermal substitute that may prove useful in complex soft tissue reconstructions. It exists as a single layer, which, much in the same way as Integra, offers a foundation upon which dermal growth and vascular invasion can occur [33•].
Fig. 1.
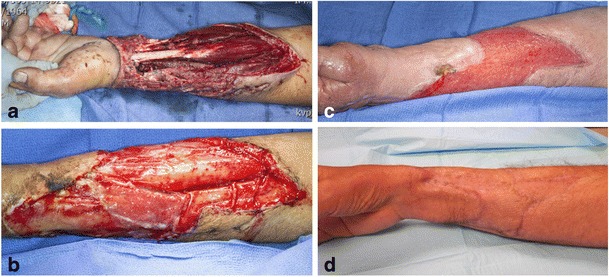
Case example of a 49-year-old gentleman who sustained a mangled left upper extremity with soft tissue loss. Initial injury (a), 2 weeks (b) and 3 weeks (c) after Integra bilayer matrix application, and 6 months after split-thickness skin graft application (d)
A recent, systematic review of the literature identified 13 reports published between 1950 and 2011 pertaining to acellular dermal substitutes used in the treatment of complex soft tissue wounds, and several authors have reported on its utility in the treatment of combat-related extremity blast wounds [32, 33•, 36]. Foong et al. described the usage of Integra in a series of seven (11 wounds) patients who had sustained IED-related extremity injuries necessitating amputation. Prior to application, these authors described the practice of soaking the Integra in a solution composed of amphotericin and ciprofloxacin. All subjects underwent an average of 6.1 procedures (range 4–10) prior to Integra application to wounds of 5 % TBSA (range 1–11.5 %). The average time to Integra application was 11.6 days (range 6–24 days) after the index injury. “Good take” was described for eight wounds, “partial take” (defined as 65–85 % take) in two wounds, and one complete failure. All wounds eventually went on to satisfactory healing, although no specific description of a “satisfactory” outcome was offered by the authors. No serious complications were noted, and all wounds were eventually covered with STSGs and healed satisfactorily. Helgeson et al. presented data on 16, blast-related wounds ranging from 15 to 275 cm2 with exposed tendon and/or bone that underwent application of Integra in conjunction with delayed STSGs. The STSG was performed an average of 19 days (range, 14–28 days) after Integra placement. Thirteen (83 %) reconstructions were deemed “successful,” defined as durable, cosmetic coverage. All three failures (17 %) involved attempts at coverage of wounds with exposed bone without periosteum. For those wounds successfully treated, the average time to Integra application from index injury was 46 days (range 20 to 180) with patients undergoing on average 8.5 debridements prior to definitive Integra application [37]. In comparison, there is a paucity of data on the application of Graftjacket in the setting of blast-related, soft tissue injuries. Moreover, to date, there is no report directly comparing outcomes of these two adjuncts in the treatment of blast-related, soft tissue injuries. Regardless of the material that is ultimately chosen, it is imperative to apply a dermal substitute in a clean wound bed after serial debridements; otherwise, infection and subsequent STSG will fail.
Soft tissue coverage options: review of the literature
Flap coverage remains a vital tool in dealing with complex soft tissue wounds and, not surprisingly, over a decade of war, has provided an abundance of data on the topic [9••, 16, 32, 33•, 34]. Tintle et al. performed a retrospective review of the flap treatment of combat blast-related soft tissue defects at a tertiary referral center from 2004 to 2009 [9••]. Overall, 75 reconstructions employed flaps, 59 of which were pedicled flaps and 16 were free flaps. The average number of debridements prior to definitive reconstruction was 5, and the average time to reconstruction was 21 days. Forty percent of wounds (30 of 75) were culture positive, the most common isolated pathogen being Acinetobacter species. Overall, these authors claim 97 and 93 % rates of flap success and limb salvage, respectively [9••]. Kumar et al. reported similar data although confining their analysis to 32 upper extremity soft tissue reconstructions, of which 84 % (20 of 23 patients) were injured in explosions. Fascial flaps (fasciocutaneous and adipofascial) were used to address soft tissue wounds of the forearm, while larger muscle flaps (type not specified) were used to treat elbow and upper arm wounds. The average number of debridements prior to definitive coverage was 6 with an average time to definitive soft tissue reconstruction of 31 days. These authors reported an overall success rate of 96 %. Positive wound cultures were reported in 46 % (15 patients) of patients, with Acinetobacter species isolated in 75 % (24 patients) of these cases [37].
The type of flap used to cover complex soft tissue defects is predicated upon the location of the zone of injury and the viability of potential donor sites (Figs. 2 and 3), and there remains controversy over which flap (free versus pedicled and muscle versus fasciocutaneous) offers the best chance of a successful reconstructive approach [9••]. Recently, the notion of the superiority of free flaps over rotational flaps espoused by Pollack and associates has been challenged by data on combat-related open tibia fractures [38]. Using experience of military surgeons treating 67 type IIIB open tibia fractures, 80 % (54 of 67 fractures) of which were secondary to explosions, there were no significant differences in time to osseous union or infection rates (P = 0.99 and P = 0.49, respectively) between groups treated with a free or rotational flap. Moreover, rotational flaps were shown to have significantly lower rates of reoperation and subsequent amputation (P = 0.05 and P = 0.03, respectively) [39••].
Fig. 2.
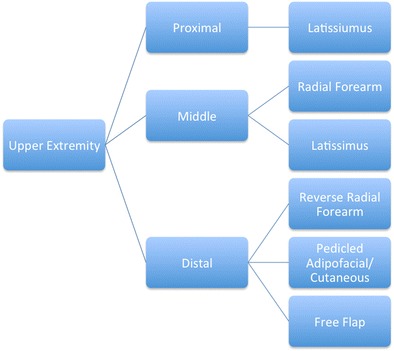
Type of upper extremity flap reconstruction based upon location of injury (reproduced from [9••])
Fig. 3.
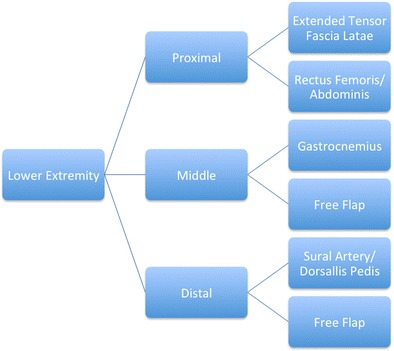
Type of lower extremity flap reconstruction based upon location of injury (reproduced from [9••])
In a large retrospective series examining the results of 359 flap (216 pedicle and 143 free flaps) procedures (197 muscle, 152 fasciocutaneous/perforator flaps) performed at a tertiary referral center between 2003 and 2012, Sabino et al. showed significantly higher rates of muscle flap failure compared to fasciocutaneous/perforator flaps (13 % versus 6 %, P = 0.030) [8••]. There were no significant differences among groups in terms of infection, osteomyelitis, and amputation rates. The average number of debridements prior to definitive reconstruction in this series was 5, and the average time to flap coverage was 19 days. Complications occurred at a rate of 30 % (99 complications) with partial necrosis or flap infection being most common. Four patients (less than 1 %) underwent amputation secondary to flap failure. The authors attributed the relatively high complication rate in this series to the nature of combat-related blast injuries frequently involving massive zones of injury with gross contamination. Overall, these results allowed the authors to confirm several key points. First, fasciocutaneous/perforator flaps perform just as well, if not better, than muscle flaps in the setting of complex soft tissue reconstruction. Second, these flaps may be preferential in the setting of the ongoing necessity for secondary procedures such as tendon repairs, nerve, and bone grafting, as the authors suggest that these flaps may be less likely to adhere to these underlying tissues. Third, fasciocutaneous/perforator flaps may better accommodate postoperative rehabilitation by preserving muscles, which, though remote to the zone of injury, are essential to mobilization and core stabilization.
Conclusion
The preponderance of data on blast-related soft tissue injuries published within the last 10 years is derived from combat-injured cohorts who, for a number of reasons, may demonstrate results that are of limited generalizability to civilian cohorts but may prove useful in the management of civilian mass casualties in the future. In general, active duty service members tend to be younger, healthier groups of patients with theoretically higher degrees of physiologic reserve capable of enduring the dramatic physiologic insult associated with a blast exposure. Furthermore, these patients, because of both logistical considerations unique to the aeromedical evacuation chain and systemic illnesses that preclude immediate, definitive reconstructive procedures, are frequently delayed. In spite of these delays, efforts to reconstruct devastating soft tissue wounds have produced durable results incorporating a number of lessons learned from treating blast-related casualties.
Compliance with Ethics Guidelines
Conflict of Interest
Andrew J. Sheean, Scott M. Tintle, and Peter C. Rhee declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Gunshot Wounds and Blast Injuries
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Emergency War Surgery, Fourth Revision. U.S. Department of Defense, U.S. Army, Office of the Surgeon General, 2014. Print.
- 2.Centers for Disease Control. Explosions and blast injuries: a primer for clinicians. Centers for Disease Control; 2007, Atlanta, GA.
- 3.Taxonomy of injuries from explosive devices. Medical Research for Prevention, Mitigation, and Treatment of Blast Injuries. Washington, DC: Department of Defense. Enclosure, 2, 9, July 5, 2006.
- 4.Fleming M, Waterman S, Dunne J, D’Alleyrand JC, Andersen RC. Dismounted complex blast injuries: patterns of injuries and resource utilization associated with the multiple extremity amputee. J Surg Orthop Adv. 2012;212(1):32–7. [PubMed] [Google Scholar]
- 5.Ritenour AE, Blackbourne LH, Kelly JF, McLaughlin DF, Pearse LA, Holcomb JB, et al. Incidence of primary blast injury in US military overseas contingency operations: a retrospective study. Ann Surg. 2010;251(6):1140–4. doi: 10.1097/SLA.0b013e3181e01270. [DOI] [PubMed] [Google Scholar]
- 6.Byrd HS, Cierny G, Tebbetts JB. The management of open tibial fractures with associated soft-tissue loss: external pin fixation with early flap coverage. Plast Reconstr Surg. 1981;68(1):73–82. doi: 10.1097/00006534-198107000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss: limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431–41. doi: 10.2106/00004623-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 8.••.Sabino J, Polfer E, Tintle S, Jessie E, Fleming M, Martin B, Shashikant M, Valerio I. A decade of conflict: flap coverage options and outcomes in traumatic war-related extremity reconstruction. Plast Reconstr Surg, 2014. This series describes the outcomes of 359 flap reconstructions between 2003 and 2012. Of these procedures, 197 were muscle and 152 were fasciocutaneous flaps. There were no significant differences between the groups in terms of infection, osteomyelitis, or amputation. Moreover, the majority of reconstructions occurred within the subacute period underscoring the feasibility of a delayed approach to reconstructing blast-related soft tissue wounds.
- 9.••.Tintle SM, Gwinn DE, Andersen RC, Kumar AR. Soft tissue coverage of combat wounds. J Surg Orthop Adv Spring. 2010;19(1):29–34. [PubMed] [Google Scholar]
- 10.Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstruc Surg. 1987;80(1):1–14. doi: 10.1097/00006534-198707000-00001. [DOI] [PubMed] [Google Scholar]
- 11.••.Murray CK, Obremskey WT, Hsu JR, Andersen RC, Calhoun JH, Clasper JC, Whitman TJ, Curry TK, Fleming ME, Wenke JC, Ficke JR; Prevention of Combat-Related Infections Guidelines Panel. Prevention of infections associated with combat-related extremity injuries. J Trauma. Aug. 7 (2 Suppl): S235-57. This report provides an exhaustive, evidence-based review of civilian and military data to offer recommendations for management strategies to mitigate infection in the setting of combat-related extremity injuries. The authors describe the incidence of novel pathogen colonization and provide guidance for antibiosis, the role of negative pressure dressing therapy, oxygen supplementation, and wound effluent characterization.
- 12.Hoff WS, Bonadies JA, Cachecho R, Dorlac WC. East practice management guidelines work group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70(30):751–4. doi: 10.1097/TA.0b013e31820930e5. [DOI] [PubMed] [Google Scholar]
- 13.••.Lack WD, Karunakar MA, Angerame MR, Seymour RB, Sims S, Kellam JF, et al. Type III open tibia fractures: immediate antibiotic prophylaxis minimizes infection. J Orthop Trauma. 2015;29(1):1–6. doi: 10.1097/BOT.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 14.Melvin S, Dombroski D, Torbert J, Kovach SJ, Esterhai JL, Mehta S. Open tibia shaft fractures I: evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10–9. doi: 10.5435/00124635-201001000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Patzakis MJ, Bains RS, Lee J, Shepherd L, Singer G, REssler R, et al. Prospective, randomized, double-blind study comparing single-agent antibiotic therapy, ciprofloxacin, to combination antibiotic therapy in open fracture wounds. J Orthop Trauma. 2000;14:529–33. doi: 10.1097/00005131-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;246:36–40. [PubMed] [Google Scholar]
- 17.•.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):2214–25. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 18.Paolino KM, Henry JA, Hospenthal DR, Wortmann GW, Hartzell JD. Invasive fungal infections following combat-related injury. Mil Med. 2012;177(6):681–5. doi: 10.7205/MILMED-D-11-00364. [DOI] [PubMed] [Google Scholar]
- 19.Tribble DR, Rodriguez CJ. Combat-related invasive fungal wound infections. Curr Fungal Infect Rep. 2014;8(4):277–86. doi: 10.1007/s12281-014-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.••.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne JR, et al. Infectious disease clinical research program trauma infectious disease outcomes study group. invasive mold infections following combat-related injuries. Clin Infect Dis. 2012;55(11):1441–9. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.••.Treatment of suspected invasive fungal infection in war wounds. Joint Theater Trauma System Clinical Practice Guideline. 2012 Nov. This resource provides a concise description of injury characteristics commonly associated with IFI and clinical practice guidelines for the initial management of these injuries.
- 22.Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117(Suppl):121S. doi: 10.1097/01.prs.0000225450.12593.12. [DOI] [PubMed] [Google Scholar]
- 23.Streubel PN, Stinner DJ, Obremskey WT. Use of negative-pressure wound therapy in orthopaedic trauma. J Am Acad Orthop Surg. 2012;20(9):564–74. doi: 10.5435/JAAOS-20-09-564. [DOI] [PubMed] [Google Scholar]
- 24.Stannard JP, Volgas DA, Stewart R, McGwin G, Jr, Alonso JE. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23(8):552–7. doi: 10.1097/BOT.0b013e3181a2e2b6. [DOI] [PubMed] [Google Scholar]
- 25.Rispoli DM, Horne BR, Kryzak TJ, Richardson MW. Description of a technique for vacuum-assisted deep drains in the management of cavitary defects and deep infections in devastating military and civilian trauma. J Trauma. 2010;68(5):1247–52. doi: 10.1097/TA.0b013e3181d3cc3c. [DOI] [PubMed] [Google Scholar]
- 26.Stinner DJ, Waterman SM, Masini BD, Wenke JC. Silver dressings augment the ability of negative pressure wound therapy to reduce bacterial in a contaminated open fracture model. J Trauma. 2011;71(1 Suppl):S147–50. doi: 10.1097/TA.0b013e318221944a. [DOI] [PubMed] [Google Scholar]
- 27.Eckman JB, Jr, Henry SL, Mangino PD, Seligson D. Wound and serum levels of tobramycin with the prophylactic use of tobramycin-impregnated polymethylmethacrylate beads in compound fractures. Clin Orthop Relat Res. 1988;237:213–5. [PubMed] [Google Scholar]
- 28.Wahlig H, Dingeldein E, Bergmann R, Reuss K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmokinetic study. J Bone Joint Surg Br. 1978; 60-B(2): 270–5. [DOI] [PubMed]
- 29.•.Stinner DJ, Hsu JR, Wenke JC. Negative pressure wound therapy reduces the effectiveness of traditional antibiotic depot in a large complex musculoskeletal wound animal model. J Orthop Trauma. 2012;26(9):512–8. doi: 10.1097/BOT.0b013e318251291b. [DOI] [PubMed] [Google Scholar]
- 30.Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78:285–96. doi: 10.1097/00006534-198609000-00001. [DOI] [PubMed] [Google Scholar]
- 31.•.Gopal S, Majumder S, Batchelor AGB, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg (Br) 2000;82(7):959–66. doi: 10.1302/0301-620X.82B7.10482. [DOI] [PubMed] [Google Scholar]
- 32.Foong DP, Evriviades D, Jeffery SL. Integra permits early durable coverage of improvised amputation device (IED) amputation stumps. J Plast Reconstr Aesthet Surg. 2013;66(12):1717–24. doi: 10.1016/j.bjps.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 33.•.Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast. Reconstr. Surg. 2012;130(5 Suppl 2):232–41S. doi: 10.1097/PRS.0b013e3182615703. [DOI] [PubMed] [Google Scholar]
- 34.Rizzo M. The use of Integra in hand and upper extremity surgery. J Hand Surg. 2012;37(3):583–6. doi: 10.1016/j.jhsa.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich JB, Katolik LI, Hanel DP. Reconstruction of soft-tissue injury associated with lower extremity fracture. J Am Acad Orthop Surg. 2011;19(2):81–90. doi: 10.5435/00124635-201102000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Helgeson MD, Potter BK, Evans KN, Shawen SB. Bioartificial dermal substitute: a preliminary report on its use for the management of complex combat-related soft tissue wounds. J Orthop Trauma. 2007;21(6):349–9. doi: 10.1097/BOT.0b013e318070c028. [DOI] [PubMed] [Google Scholar]
- 37.Kumar AR, Grewal NS, Chung TL, Bradley JP. Lessons from the modern battlefield: successful upper extremity injury reconstruction in the subacute period. J Trauma. 2009;67(4):752–7. doi: 10.1097/TA.0b013e3181808115. [DOI] [PubMed] [Google Scholar]
- 38.Pollack AN, McCarthy ML, Burgesss AR. Short-term wound complications after application of flaps for coverage of traumatic soft-tissue defects about the tibia. The Lower Extremity Assessment Project (LEAP) Study Group. J Bone Joint Surg Am. 2000;82-A(12):1681–91. [PubMed] [Google Scholar]
- 39.••.Burns TC, Stinner DJ, Possley DR, Mack AW, Eckel TT, Potter BK, et al. Skeletal Trauma Research Consortium (STReC). Does the zone of injury in combat-related type III open tibia fractures preclude the use of local soft tissue coverage? J Orthop Trauma. 2010;24(11):697–703. doi: 10.1097/BOT.0b013e3181d048b8. [DOI] [PubMed] [Google Scholar]


