Abstract
Osteonecrosis of femoral head is a rare but disabling condition that usually results in progressive femoral head collapse and secondary arthritis necessitating total hip arthroplasty if not treated appropriately in early stages. However, early diagnosis is challenging as the onset of disease is insidious and the symptoms and signs are usually minimal and nonspecific until it becomes advanced. Of several diagnostic modalities, magnetic resonance imaging (MRI) is considered the imaging method of choice with the highest sensitivity and specificity, while detection of potential risk factors is very important as well. Many investigators have developed several different classification systems; however, there still is controversy regarding the optimal classification system. Diagnostic methods and the evolution of different classification systems will be reviewed in this paper.
Keywords: Osteonecrosis of femoral head, Diagnosis, Risk factors, Classification system
Introduction
Osteonecrosis (ON) of the femoral head is an uncommon disease primarily affecting a younger, active population as opposed to degenerative joint disease, which affects older individuals. It is estimated that 20,000 to 30,000 new patients are diagnosed with osteonecrosis annually, and 5 to 12 % of total hip arthroplasties (THAs) are performed based on this diagnosis in the USA [1, 2•, 3]. The etiology and pathogenesis are not fully understood; however, several risk factors have been demonstrated and various etiologic theories proposed.
Osteonecrosis is a disease of the bone as in the early stages of disease, and the necrotic zone is visible on magnetic resonance imaging (MRI), yet there is no subchondral fracture present and the adjacent hyaline cartilage is normal. In the later stages of disease, a subchondral fracture may occur leading to eventual femoral head collapse with consequent instability and buckling of the overlying articular cartilage, thereby leading to end-stage secondary joint arthritis. Treatment is based on the disease stage. The goal of management is to accurately diagnose the condition at an early stage before subchondral fracture develops as treatments can be performed to minimize the risk of femoral head collapse. Once collapse occurs, salvage of the native hip joint is often impossible or associated with suboptimal pain and function with continued progression to arthritis. For this reason, after collapse occurs, arthroplasty is often the preferred treatment to relieve pain and improve joint function.
In this paper, the diagnosis and staging system of ON will be addressed.
Diagnosis
History and physical examination
The diagnosis of ON is primarily based upon imaging findings. Nevertheless, a careful history should be taken to screen for potential risk and/or prognostic factors, to determine if other joints are symptomatic, to look for other conditions that might present similarly, and to optimize management. In many patients, predisposing factors and conditions can be identified. However, the onset of disease is insidious and the symptoms and signs are usually minimal and nonspecific until it becomes advanced. Therefore, a high index of suspicion may contribute to an early diagnosis.
Associated factors
Factors associated with ON are trauma or prior surgery of the hip joint, alcohol consumption, and corticosteroid administration. Several other medical conditions are known to contribute to the development of ON including hyperlipidemia, hemoglobinopathy, dysbarism, and coagulation abnormalities (Table 1).
Table 1.
Etiologic factors associated with osteonecrosis
| Trauma |
| Hip dislocation |
| Femoral neck fracture |
| Corticosteroid use |
| Solid organ transplantation |
| Bone marrow transplantation |
| Acute lymphoblastic leukemia |
| Alcohol consumption |
| Systemic lupus erythematosus |
| Coagulation disorders |
| Antithrombin III deficiency |
| Protein C deficiency |
| Protein S deficiency |
| Thrombocytosis |
| Disseminated intravascular coagulation |
| Human immunodeficiency virus (HIV) infection |
| Hemoglobinopathy |
| Sickle cell disease |
| Thalassemia |
| Polycythemia |
| Metabolic disease |
| Gaucher’s disease |
| Gout |
| Other rare disorders |
| Hyperlipidemia |
| Liver disease |
| Dysbaric phenomenon |
| Miscellaneous factors |
| Smoking |
| Pregnancy |
| Chemotherapy |
| Radiation |
Trauma
Trauma is one of the most common causes of ON. A displaced femoral neck fracture or dislocation can jeopardize the local blood supply to the femoral head, resulting in ON. The reported incidence after a femoral neck fracture varies widely ranging from 11 to 86 %, depending upon many factors such as age, type, displacement of the fracture, quality and timing of reduction, and method of treatment [4–8]. Surgical trauma may also put a patient at risk for developing ON. Both intramedullary nailing and surgical dislocation of the hip may disrupt the medial femoral circumflex artery-based vascular leash resulting in ON [9, 10].
Alcohol
Alcohol has been shown to be associated with ON in patients and in animal models [11]. Studies have demonstrated that alcohol contributes to abnormal lipid metabolism in the stromal cells of bone marrow, decreasing osteogenesis while enhancing adipogenesis. This abnormal metabolism produces intracellular lipid deposits resulting in the death of osteocytes, which may be associated with the development of ON [12]. The adipogenesis may also pack the marrow, thereby increasing the intraosseous pressure and negatively affecting the bone microcirculation.
The incidence of ON in persons who have had medical treatment for excessive consumption of alcohol was reported to be 5.3 % (62/1157) by Orlic et al. [13]. They found that patients with alcohol-associated ON had femoral head involvement in 89.1 % and multiple foci in 6.1 %.
Evaluation of the quantity and quality of alcohol consumption necessary to develop ON is scant, and therefore the threshold for acquiring the disease is unknown. It appears that a greater alcohol ingestion is related to a higher risk of ON. Jacobs [14] reported the average duration of alcohol abuse was 9.5 years in their analysis of 164 patients with alcohol-induced ON. However, the reason why many individuals who have longstanding histories of ethanol exposure never develop ON remains unknown.
Corticosteroids
Corticosteroid exposure is a well-recognized risk factor for ON. Both oral intake and intravenous injection of corticosteroids have shown a strong relationship with the onset of ON; however, there is no definitive evidence linking these inhalers or local injections to ON. The incidence associated with corticosteroid use varies depending on the medical condition, dose and duration of therapy, as well as age and gender of the patients [15]. It has been demonstrated that cumulative intravenous methylprednisolone at doses of >2 g for >3 months significantly increased the risk for ON [16]. The frequent inclusion of corticosteroids in treatment protocols for various medical conditions, such as acute lymphoblastic leukemia (ALL), various lymphomas, and either solid organ or bone marrow transplantation, clearly put these patients at an increased risk for ON [17–24].
In a prospective MRI study, the incidence of ON associated with corticosteroid therapy was significantly higher in systemic lupus erythematosus (SLE) patients than in non-SLE patients (37 versus 21 %, P = 0.001) [25•]. Risk factors for ON were age (adolescents and adults compared to pediatric patients), high daily corticosteroid dosage (>40 mg/day), SLE patient compared to non-SLE patient, and male gender. In solid organ transplantation patients, Marston and Cheng [20] prospectively analyzed 52 patients (103 hips) and reported the prevalence of osteonecrosis of the femoral head as 11 % at 1 year after the transplantation. In patients with acute lymphoblastic leukemia treated with multiple, prolonged courses of corticosteroid, the Children’s Cancer Group reported that 111 of 1409 patients had ON with a 3-year life-table estimated incidence of 9.3 % [21]. In children who received an allogeneic bone marrow transplantation, nearly 30 % of patients were found to have MR-documented ON of the hips or knees, with more severe involvement in the hip rather than the knee [22].
Marrow packing disorders
Other rare conditions such as sickle cell disease [26], Gaucher’s disease [27], or mastocytosis are known to be associated with ON. The pathophysiology is likely related to the obstruction of normal blood flow within the intraosseous marrow space due to the packing of a structure of fixed volume and obliteration of normal marrow vasculature.
Others
Other miscellaneous factors associated with ON include human immunodeficiency virus (HIV) infection treatment with highly active antiretroviral therapy, coagulopathy, decompression sickness, and genetic factors.
Highly active antiretroviral therapy for HIV-infected patients is associated with a higher incidence of ON [28]. Many studies [29–31] have shown an association of ON in HIV-positive individuals undergoing antiretroviral therapy. It is unknown whether or not the association is due to protease inhibitors alone or is multifactorial in combination with other risk factors such as the HIV infection itself, a history of systemic corticosteroid use, or hyperlipidemia [32–35].
Coagulation abnormalities such as low protein C, low protein S, high lipoprotein, or high von Willebrand factor levels have been associated with a significantly higher incidence of ON [36, 37]. A genetic basis for thrombophilic traits leading to ON is postulated [38, 39].
The incidence of ON is considerably higher in certain parts of the world such as Asia (China, Korea, Japan, and Taiwan). This could be explained by many factors. Reports of a number of genetic factors have recently been identified [2•]. These studies have shown associations between ON and the type II collagen gene in a Taiwanese familial cohort [40] and genetic polymorphisms in the endothelial nitric oxide synthase gene in Korean and Polish patients [41, 42]. The association between the genetic polymorphism of P-glycoprotein gene and susceptibility of corticosteroid-associated ON was also suggested [43].
Imaging
Magnetic resonance imaging
MRI is considered the imaging method of choice with the highest sensitivity and specificity compared to plain radiographs, computed tomography, or scintigraphy [44–47] (Fig. 1). It is the most useful screening tool for early diagnosis, quantitative evaluation of the disease extent within the femoral head, and staging of the disease [48, 26]. A single-density “band-like” lesion with low signal intensity rim surrounding the necrosis on T1-weighted images, and a “double-line” sign consisting of a low signal intensity outer rim and a high signal intensity inner rim on a T2-weighted image are considered diagnostic of the disease [49, 46] (Fig. 2).
Fig. 1.
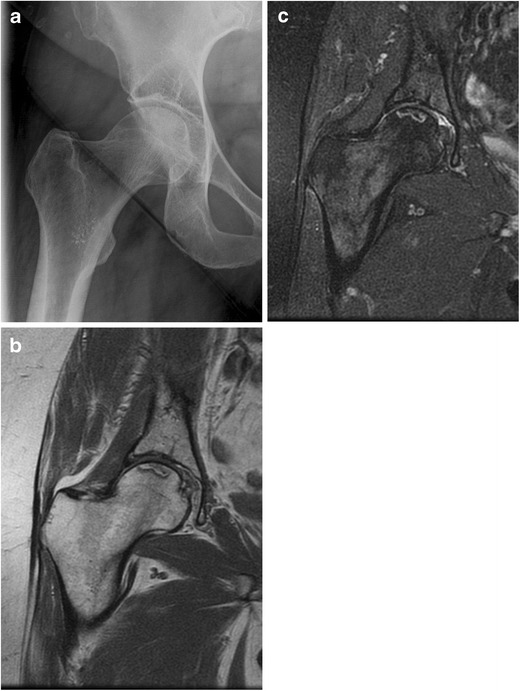
Plain radiograph shows no significant abnormality (a); however, MRI exam shows typical band-like pattern of ON lesion in T1W (b) and T2W (c) images
Fig. 2.
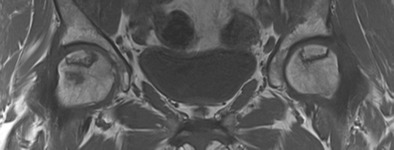
MRI shows typical band-like pattern of ON in both femoral heads
Computerized tomography
Computerized tomography (CT) is considered the most sensitive test for detecting subchondral fracture of the femoral head [50]. While radiographs and MR are useful, a CT delineates the outline of the subchondral bone most clearly (Fig. 3). The best plane of view to see a subchondral fracture depends upon the orientation of the necrotic segment. Most necrotic zones are in the anterior superior segment of the femoral head. Typically, a subchondral fracture is first seen along the superior lateral border. CT also is useful to visualize small areas of collapse which are suspected but not seen on plain films or MRI. The disadvantage of CT is that this test does subject the patient to radiation.
Fig. 3.
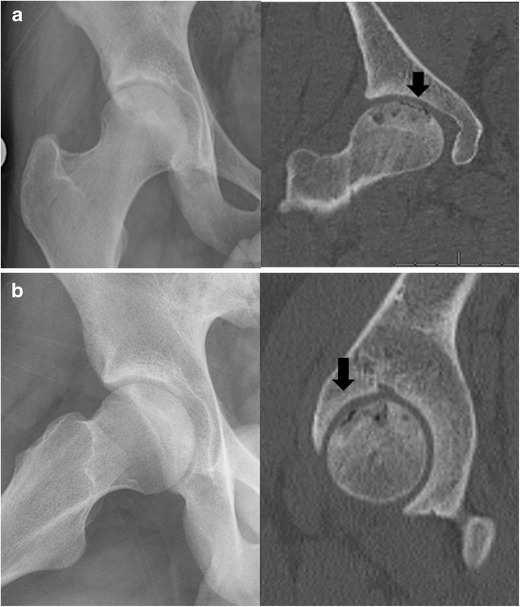
Although subchondral fracture is not clearly seen in plain radiographs, it is readily demonstrated in both coronal (a) and sagittal (b) reconstruction of the CT (arrow)
Simple X-rays
Plain radiographs are the most appropriate initial test in the management of hip pain given their low cost, simplicity, and ready availability. In ON, a subchondral fracture, also known as a “crescent sign,” heralds the presence of an advanced stage that will progress to degenerative joint disease (Fig. 4). This is usually seen best on the frog leg lateral view as it shows profile of the most common location for a subchondral fracture to occur, i.e., the superior lateral portion of the femoral head’s anterior segment. The disadvantage of radiographs is its insensitivity for detecting ON in its early stages. Therefore, radiographs cannot exclude the presence of ON. In early stage disease, sclerosis surrounding an osteopenic area may be seen but it can be subtle. Subsequent sclerosis, cystic change, and a crescent-shaped lucent lesion in a subchondral location are characteristic (Fig. 5). In late stage disease, the femoral head loses its sphericity with a subchondral collapse and degenerative arthritis ensues eventually involving arthritic changes on the acetabular side (Fig. 6).
Fig. 4.
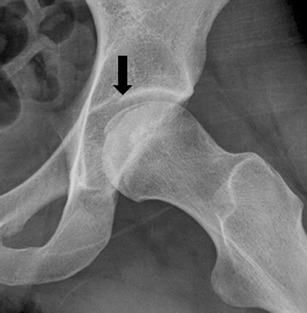
Left hip lateral plain X-ray shows subchondral fracture line (“crescent sign,” arrow)
Fig. 5.
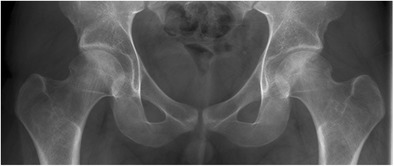
Pelvis X-ray shows radiolucency and surrounding sclerosis of both femoral heads suggesting stage 2 osteonecrosis lesion
Fig 6.
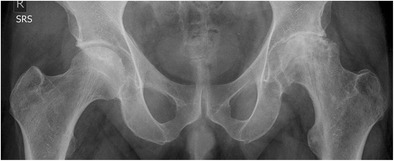
Pelvis AP X-ray shows collapsed femoral head and advanced osteoarthritic change involving acetabulum of left hip
Bone scan
The necrotic region of bone does not take up the technetium-99 isotope and therefore appears “cold” on the scan. The surrounding rim of reactive bone remodeling appears “hot” on the scan. Therefore, in the early stage of disease, this results in a bone scan showing a “cold within hot” area. Later, after subchondral fracture, the viable areas of bone surrounding the region of necrosis are engaged in attempts at repair and often show a “hot lesion” that obscures the original cold area. Although a bone scan is a useful tool for detecting multifocal lesions, limitations are poor spatial resolution, low specificity to differentiate other disorders, and inability to quantify the lesion [49]. For these reasons, it is not usually performed routinely when managing ON.
Differential diagnosis
Transient bone marrow edema syndrome (transient osteoporosis)
This is a rare disease characterized by the sudden onset of acute disabling hip pain without any history of trauma or unusual physical activities. The unique clinical feature is a self-limited, spontaneous regression of the pain over several months (6 to 12 months) without any surgical intervention. The characteristic MRI finding is widespread marrow edema throughout the proximal femur including femoral head, neck, and trochanteric region, whereas ON is usually located focally in the femoral head or subchondral region. Plain radiographs are frequently unremarkable except relative osteopenia [51–53]. A bone scan shows marked, diffuse increased uptake throughout the entire femoral head and neck region. On rare occasion, reports have shown that bone marrow edema syndrome (BMES) may coexist with osteonecrosis. This finding has generated some controversy as to whether the two conditions actually coexist or if BMES is a precursor to osteonecrosis.
Subchondral insufficiency fracture
Subchondral insufficiency fracture of the femoral head presents with an acute onset of hip pain, occurring in older or elderly adults as a result of minor injury, such as hip joint twisting, bending forward, or long walks. It involves bone fragility, usually secondary to osteoporosis or osteopenia in elderly women or transplant recipients without any evidence of predisposing ON [54, 55]. The radiographic differential diagnosis between ON and an insufficiency fracture can be somewhat confusing. The MRI shows the most characteristic findings differentiating these disorders. A band-like low signal intensity in subchondral insufficiency fracture is usually irregular, disconnected, parallel, and convex to the cartilage surface. However, the band in ON usually shows a smooth, well-delineated line that is concave, curving away from the cartilage surface resembling a mirror image. At times, however, the low signal intensity band may appear similarly in both diagnoses making differentiation challenging. While the history and patient age may help differentiate ON from a subchondral insufficiency fracture, from a practical standpoint, both conditions are likely to progress to degenerative arthritis best managed by a hip arthroplasty. An insufficiency fracture is seldom amenable to nonsurgical treatment, and patients with joint space narrowing are at higher risk of failing nonoperative treatment [56].
Neoplasm
It is important to consider the possibility of a tumor which always should be differentiated from ON. Clear cell chondrosarcoma (CCSA) and chondroblastoma are two neoplasms that may occur in the femoral head. CCSA is a rare subtype of chondrosarcoma that usually involves the epiphyseal portion of long bones. Plain radiographs show a predominantly lytic lesion in the femoral head with a poorly defined sclerotic margin. On MRI, the lesion is typically heterogeneous and of low-intermediate signal on T1-weighted sequence without reactive medullary edema. A chondroblastoma also appears as a well-defined radiolucent lesion in epiphysis and should be included in differential. Clinically, however, CCSA occurs in older adults while chondroblastoma most commonly occurs in adolescents and young adults.
Classification
Several classification systems have been developed to stage ON and provide information on prognosis, treatment decision, and outcome comparison. However, there still is controversy regarding the classification of ON, and this lack of a universally accepted classification system makes it difficult to compare and analyze the data from different centers.
In a systematic review of the literature, Mont et al. [57] identified 16 major classification systems used to classify and describe ON. Of these, four classification systems accounted for greater than 85.4 % of the reported studies: the Ficat Classification [58] was the most frequently used system (63 %), followed by the University of Pennsylvania system (20 %) [47, 26], the Association Research Circulation Osseous (ARCO) system (12 %) [59], and the Japanese Orthopaedic Association system (5 %) [60, 61] (Table 2).
Table 2.
Evolution and comparison of different classification and staging systems for ON
| Marcus and Enneking (Florida) | Ficat and Arlet (French) | Modified Ficat and Arlet (French) | Steinberg (Philadelphia) | ARCO (International) | |
|---|---|---|---|---|---|
| Year of introduction | 1973 | 1977 | 1985 | 1984 | 1993 |
| Pathology | |||||
| 0 (asymptomatic, XR−) | |||||
| Clinically at risk and symptomatic | I (symptomatic, XR−, biopsy+) | I (symptomatic, XR−, biopsy+) | 0 (normal or nondiagnostic XR, bone scan, and MRI) | 0 (all imaging studies negative) | |
| Infarct/hyperemic marrow border | I (XR+/−) | II | II | I (XR−, bone scan+, MRI+), [A: mild <15 %, B: moderate 15–30 %, C: severe >30 %] | I (XR−, CT−, scintigraph+, MRI+), {a: medial, b: central, c: lateral}, [area involvement: minimal A: <15 %, moderate B: 15–30 %, extensive C: >30 %] |
| Granulation tissue repair, lucent, sclerosis, cysts, calcified marrow | II (XR+) | II (XR+), [A,B,C] | II (XR+, CT+, scintigraph+, MRI+), {a,b,c}, [A,B,C] | ||
| Subchondral fracture (crescent sign) | III | Transitional stage | III (XR+: crescent sign without collapse), [A,B,C] | Early III, (XR+, CT+), {a,b,c}, [A,B,C] | |
| Collapse | IV | III | III | IV (XR+: flattening), [A,B,C] | Late III, (XR+, CT+), {a,b,c}, [A,B,C] |
| Early arthritis | V | IV | IV | V | IV (XR+) |
| Advanced arthritis | VI | VI | IV (XR+) | ||
| Contribution | Initial staging system | Acknowledged the need for a biopsy to confirm functional changes in bone | Acknowledged presence of ONFH with negative XR | Added MRI criteria and lesion size and category for subchondral fracture with no collapse | Added location of lesion |
Modified from Cheng EY, from Oxford Textbook of Orthopaedics and Trauma, Volume 2, edited by Bulstrode, et al. (2002), by permission of Oxford University Press
− negative, + positive
Prognostic factors
While there is no universal agreement in classification systems, it is well established that the prognosis is directly related to several factors: the extent of the osteonecrotic lesion [48, 62–65], the presence of a subchondral fracture [66, 67], and the location of the lesion [68, 69]. Most classification systems are based upon these prognostic factors.
A number of methods indicating lesion size have been introduced by investigators, and the importance of indicating the size and extent of involvement in addition to the stage is well recognized. The prognosis for large lesions is worse than that for small lesions, and most methods for measuring or estimating lesion size show a relationship to outcome [48, 62, 63, 65]. Kerboul et al. [62] reported a method measuring the arc of the femoral articular surface involved by necrosis on both AP and lateral radiographs. They reported that the outcome of proximal femoral osteotomy was related to location and extent of necrosis. However, this method may not be accurate in measuring the true size of a three-dimensional lesion and shifts most hips into the “large” category. In 1995, this was adapted by Koo for using MRI to measure the necrotic arc [63] and subsequently modified by Cheng in 2003 [70] and Ha in 2006 [48], by using either the coronal and sagittal slices showing the maximal area of involvement or the midcoronal and midsagittal MRI slices. They both demonstrated that there was a strong correlation between the combined necrotic angle and the risk of future collapse.
A subchondral fracture is clearly a poor prognostic sign, and nearly all patients with this finding eventually have disabling hip pain due to premature arthritis. Most of these patients will be salvaged with an eventual hip arthroplasty. While a subchondral fracture has been incorporated into the various staging systems, none have specified the usage of CT to screen for a fracture. This likely has resulted in some patients being misclassified as not having a subchondral fracture and may in part explain the widely varying outcomes of various interventions in patients with supposedly pre-collapse disease.
The lesion location was felt to be of prognostic value by the Japanese Investigation Committee, and they reported that prevalence of collapse was higher when the lesion was involved more weight-bearing portion and lateral extension [71]. For this reason, the lesion’s location was incorporated into the Japanese staging systems.
Evolution of staging systems
The first classification system for ON was described by Arlet and Ficat in the early 1960s in French, which had only three stages [72]. It has been revised with fourth and 0 stages afterwards [58]. Initially, this system included clinical symptoms and functional evaluation of bone including bone marrow pressure, intramedullary venography, and core biopsy. This system is based upon radiographic findings only. The drawback of this system is that it was developed before the MRI was available, and there is no assessment of the size or extent of the lesion. However, it still is widely utilized because it is simple and easy to use.
Since the development of MRI, it has been considered as the method of choice for detecting and staging ON and has been widely adopted in classification system. The University of Pennsylvania classification was developed by Steinberg and presented in the early 1980s [47, 26]. The important features of this system was that it included measurement of lesion size and extent of joint involvement using MRI [47, 26, 2•].
The ARCO classification system [59] was developed at the meeting of the Association Research Circulation Osseous in 1991 to establish simplified, internationally accepted one uniform classification with uniform definition and terminology [73]. The classification system initially was divided into six stages with a subdivision of size of the necrotic lesion, extent of femoral head, and joint involvement [59]. The location was included in 1992 at which time the Japanese Investigation Committee description was added. The 1993 version of ARCO also combined stages III and IV, so the distinction between a crescent sign without femoral head flattening and a hip with flattening was eliminated. This distinction was later restored, now indicating an “Early 3” and a “Late 3” instead of the original stages 3 and 4 [74].
In 1987, the Japanese Investigation Committee for Avascular Necrosis described Japanese Orthopaedic Association system [60, 61]. It has been modified in 2001 emphasizing the location of lesion as an important factor to predict impending collapse [71]. This system indicates that lesions progress from medial to lateral as they become larger and cannot categorize a small central lesion, which is often present. Conversely, the small medial lesion they do include is rarely present. Most lesions are in the anterosuperior aspect of the head and indicating lesion size also indicates location. It is seldom used outside Japan.
Future staging considerations
The most appropriate staging classification depends upon the purpose of the staging system. In most cases, staging systems are used to group together patients that have a similar prognosis so that they may guide treatment decisions. Ideally, the staging is accurate (i.e., based upon known independently prognostic factors), and simple to use with a minimum of testing data, and compatible with prior staging systems. In some situations, staging systems are used for research purposes and therefore may include potential prognostic factors that have yet to be independently verified and may involve the usage of more complicated data measurements. Given the evolution of our understanding of osteonecrosis and the ongoing development of new imaging and measuring techniques, designing an ideal staging system is challenging as it cannot completely satisfy all the prerequisites stated.
The most important prognostic factors in osteonecrosis of the femoral head are the presence of a subchondral collapse, the size or quantification of the lesion, and the lesion location within the femoral head. An example of a straightforward staging system that would include these factors (fracture, lesion extent, location, acetabular involvement) in order of worsening prognosis would be:
-
Stage 1
No collapse—small, location (A: non articular, B: medial, C: central, D: lateral)
-
Stage 2
No collapse—medium, location (A: non articular, B: medial, C: central, D: lateral)
-
Stage 3
No collapse—large, location (A: non articular, B: medial, C: central, D: lateral)
-
Stage 4
Subchondral fracture (A: Collapse, 0–2 mm , B: Collapse, >2 mm)
-
Stage 5
Acetabular DJD
Definitions:
Subchondral fracture. This should be based upon CT with sagittal and coronal reconstructions as radiographs are less sensitive to detecting non-displaced fractures [50].
Lesion extent. This can be assessed by MRI and categorized as <15, 15–30, or >30 % of the femoral head [26, 2•].
Location. This is assessed on radiographs or MRIs using the acetabular subchondral bone (sourcil) as a reference point (divided into three, i.e., medial, central, lateral) for the most lateral border of the lesion [60, 61].
While this example of a staging system is both predictive prognostically and relatively straightforward, it does not completely satisfy the needs of researchers and is not compatible with prior staging systems developed that are in current usage.
At the present time, we believe it is advisable to focus less on a particular staging system and instead focus more on the most important prognostic factors (subchondral fracture determined by CT/extent/location), regardless of how they are incorporated into a staging system. Recording these factors will likely ensure compatibility with prior and future staging systems.
Conclusions
Most patients with ON have lesions that progress to femoral head collapse and degenerative arthritis, resulting in an eventual requirement for joint replacement surgery. Aside from minimizing the exposure to established risk factors, there are no interventions that can reliably prevent ON. Therefore, awareness of risk factors associated with the disease and making an early diagnosis is essential for the success of any joint preserving procedures and thereby avoiding joint replacement surgery. The diagnosis should be started with careful history and confirmed with imaging studies including MRI, CT, and plain radiographs to make a correct diagnosis and stage accurately. Detecting prognostic factors, such as subchondral fracture, extent of involvement, and location of the lesion, and understanding the treatment options based on the stage would be an essential part of management.
Compliance with Ethics Guidelines
Conflict of Interest
Ho-Rim Choi, Marvin E. Steinberg, and Edward Cheng declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Modern Surgical Treatment of Hip Avascular Necrosis
Contributor Information
Ho-Rim Choi, Phone: 612-273-7951, Email: choinagoya@gmail.com.
Marvin E. Steinberg, Phone: 215-294-9102, Email: Marvin.Steinberg@uphs.upenn.edu
Edward Y. Cheng, Phone: 612-273-7951, Email: cheng002@umn.edu.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- 2.•.Steinberg ME, Steinberg DR. Osteonecrosis: Historical perspective. In: Koo KH, Mont MA, Jones LC, editors. Osteonecrosis. Heidelberg: Springer; 2014. p. 3–15. The study describes historical perspective of diagnosis, classification, and treatment of osteonecrosis.
- 3.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22(7):455–64. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 4.Lu-Yao GL, Keller RB, Littenberg B, Wennberg JE. Outcomes after displaced fractures of the femoral neck. A meta-analysis of one hundred and six published reports. J Bone Joint Surg Am. 1994;76(1):15–25. doi: 10.2106/00004623-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Min BW, Kim SJ. Avascular necrosis of the femoral head after osteosynthesis of femoral neck fracture. Orthopedics. 2011;34(5):349. doi: 10.3928/01477447-20110317-13. [DOI] [PubMed] [Google Scholar]
- 6.Naranje SM, Cheng EY. Epidemiology of osteonecrosis in the USA. In: Koo KH, Mont MA, Jones LC, editors. Osteonecrosis. Heidelberg: Springer; 2014. p. 39–45.
- 7.Protzman RR, Burkhalter WE. Femoral-neck fractures in young adults. J Bone Joint Surg Am. 1976;58(5):689–95. [PubMed] [Google Scholar]
- 8.Tooke SM, Favero KJ. Femoral neck fractures in skeletally mature patients, fifty years old or less. J Bone Joint Surg Am. 1985;67(8):1255–60. [PubMed] [Google Scholar]
- 9.Dora C, Leunig M, Beck M, Rothenfluh D, Ganz R. Entry point soft tissue damage in antegrade femoral nailing: a cadaver study. J Orthop Trauma. 2001;15(7):488–93. doi: 10.1097/00005131-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Orler R, Hersche O, Helfet DL, Mayo KA, Ward T, Ganz R. Avascular femur head necrosis as severe complication after femoral intramedullary nailing in children and adolescents. Unfallchirurg. 1998;101(6):495–9. doi: 10.1007/s001130050301. [DOI] [PubMed] [Google Scholar]
- 11.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88(Suppl 3):148–54. doi: 10.2106/JBJS.F.00534. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–24. doi: 10.1097/01.blo.0000063602.67412.83. [DOI] [PubMed] [Google Scholar]
- 13.Orlic D, Jovanovic S, Anticevic D, Zecevic J. Frequency of idiopathic aseptic necrosis in medically treated alcoholics. Int Orthop. 1990;14(4):383–6. doi: 10.1007/BF00182650. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs B. Alcoholism-induced bone necrosis. N Y State J Med. 1992;92(8):334–8. [PubMed] [Google Scholar]
- 15.Nakamura J, Saisu T, Yamashita K, Suzuki C, Kamegaya M, Takahashi K. Age at time of corticosteroid administration is a risk factor for osteonecrosis in pediatric patients with systemic lupus erythematosus: a prospective magnetic resonance imaging study. Arthritis Rheum. 2010;62(2):609–15. doi: 10.1002/art.27236. [DOI] [PubMed] [Google Scholar]
- 16.Saisu T, Sakamoto K, Yamada K, Kashiwabara H, Yokoyama T, Iida S, et al. High incidence of osteonecrosis of femoral head in patients receiving more than 2 g of intravenous methylprednisolone after renal transplantation. Transplant Proc. 1996;28(3):1559–60. [PubMed] [Google Scholar]
- 17.Hedri H, Cherif M, Zouaghi K, Abderrahim E, Goucha R, Ben Hamida F, et al. Avascular osteonecrosis after renal transplantation. Transplant Proc. 2007;39(4):1036–8. doi: 10.1016/j.transproceed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Zhang J, He JW, Wang K, Wang GS, Jiang N, et al. Symptomatic osteonecrosis of the femoral head after adult orthotopic liver transplantation. Chin Med J. 2012;125(14):2422–6. [PubMed] [Google Scholar]
- 19.Lieberman JR, Roth KM, Elsissy P, Dorey FJ, Kobashigawa JA. Symptomatic osteonecrosis of the hip and knee after cardiac transplantation. J Arthroplast. 2008;23(1):90–6. doi: 10.1016/j.arth.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Marston SB, Gillingham K, Bailey RF, Cheng EY. Osteonecrosis of the femoral head after solid organ transplantation: a prospective study. J Bone Joint Surg Am. 2002;84-A(12):2145–51. doi: 10.2106/00004623-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18(18):3262–72. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Leung WH, Deqing P, Yang J, Rochester R, Britton L, et al. Osteonecrosis in children after allogeneic hematopoietic cell transplantation: study of prevalence, risk factors and longitudinal changes using MR imaging. Bone Marrow Transplant. 2012;47(8):1067–74. doi: 10.1038/bmt.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauchmanova L, De Rosa G, Serio B, Fazioli F, Mainolfi C, Lombardi G, et al. Avascular necrosis in long-term survivors after allogeneic or autologous stem cell transplantation: a single center experience and a review. Cancer. 2003;97(10):2453–61. doi: 10.1002/cncr.11373. [DOI] [PubMed] [Google Scholar]
- 24.Torii Y, Hasegawa Y, Kubo T, Kodera Y, Minami S, Morishita Y, et al. Osteonecrosis of the femoral head after allogeneic bone marrow transplantation. Clin Orthop Relat Res. 2001;382:124–32. doi: 10.1097/00003086-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 25.•.Shigemura T, Nakamura J, Kishida S, Harada Y, Ohtori S, Kamikawa K, et al. Incidence of osteonecrosis associated with corticosteroid therapy among different underlying diseases: prospective MRI study. Rheumatology. 2011;50(11):2023–8. doi: 10.1093/rheumatology/ker277. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br Vol. 1995;77:34–41. [PubMed] [Google Scholar]
- 27.Rodrigue SW, Rosenthal DI, Barton NW, Zurakowski D, Mankin HJ. Risk factors for osteonecrosis in patients with type 1 Gaucher’s disease. Clin Orthop Relat Res. 1999;362:201–7. [PubMed] [Google Scholar]
- 28.Morse CG, Mican JM, Jones EC, Joe GO, Rick ME, Formentini E, et al. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;44(5):739–48. doi: 10.1086/511683. [DOI] [PubMed] [Google Scholar]
- 29.Martin K, Lawson-Ayayi S, Miremont-Salame G, Blaizeau MJ, Balestre E, Lacoste D, et al. Symptomatic bone disorders in HIV-infected patients: incidence in the Aquitaine cohort (1999-2002) HIV Med. 2004;5(6):421–6. doi: 10.1111/j.1468-1293.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 30.Molia AC, Strady C, Rouger C, Beguinot IM, Berger JL, Trenque TC. Osteonecrosis in six HIV-infected patients receiving highly active antiretroviral therapy. Ann Pharmacother. 2004;38(12):2050–4. doi: 10.1345/aph.1E154. [DOI] [PubMed] [Google Scholar]
- 31.Reddy R, Daftary MN, Delapenha R, Dutta A, Oliver J, Frederick W. Avascular necrosis and protease inhibitors. J Natl Med Assoc. 2005;97(11):1543–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Keruly JC, Chaisson RE, Moore RD. Increasing incidence of avascular necrosis of the hip in HIV-infected patients. J Acquir Immune Defic Syndr. 2001;28(1):101–2. doi: 10.1097/00042560-200109010-00017. [DOI] [PubMed] [Google Scholar]
- 33.Matos MA, Alencar RW, Matos SS. Avascular necrosis of the femoral head in HIV infected patients. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2007;11(1):31–4. doi: 10.1590/s1413-86702007000100009. [DOI] [PubMed] [Google Scholar]
- 34.Mazzotta E, Agostinone A, Rosso R, Di Biagio A, De Socio GV, Cappelletti A, et al. Osteonecrosis in human immunodeficiency virus (HIV)-infected patients: a multicentric case-control study. J Bone Miner Metab. 2011;29(3):383–8. doi: 10.1007/s00774-010-0245-5. [DOI] [PubMed] [Google Scholar]
- 35.Whitlock GG, Herbert S, Copas A, Gilson R, Ainsworth JG. Avascular necrosis in HIV patients: a case-control study. Int J STD AIDS. 2013;24(10):799–803. doi: 10.1177/0956462413482814. [DOI] [PubMed] [Google Scholar]
- 36.Jones JP., Jr Coagulopathies and osteonecrosis. Acta Orthop Belg. 1999;65(Suppl 1):5–8. [PubMed] [Google Scholar]
- 37.Zalavras C, Dailiana Z, Elisaf M, Bairaktari E, Vlachogiannopoulos P, Katsaraki A, et al. Potential aetiological factors concerning the development of osteonecrosis of the femoral head. Eur J Clin Investig. 2000;30(3):215–21. doi: 10.1046/j.1365-2362.2000.00621.x. [DOI] [PubMed] [Google Scholar]
- 38.Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Factor V Leiden and prothrombin gene mutations in femoral head osteonecrosis. Thromb Haemost. 2002;87(6):1079–80. [PubMed] [Google Scholar]
- 39.Zalavras CG, Vartholomatos G, Dokou E, Malizos KN. Genetic background of osteonecrosis: associated with thrombophilic mutations? Clin Orthop Relat Res. 2004;422:251–5. doi: 10.1097/01.blo.0000127921.13253.e3. [DOI] [PubMed] [Google Scholar]
- 40.Liu YF, Chen WM, Lin YF, Yang RC, Lin MW, Li LH, et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med. 2005;352(22):2294–301. doi: 10.1056/NEJMoa042480. [DOI] [PubMed] [Google Scholar]
- 41.Gagala J, Buraczynska M, Mazurkiewicz T, Ksiazek A. Endothelial nitric oxide synthase gene intron 4 polymorphism in non-traumatic osteonecrosis of the femoral head. Int Orthop. 2013;37(7):1381–5. doi: 10.1007/s00264-013-1892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo KH, Lee JS, Lee YJ, Kim KJ, Yoo JJ, Kim HJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res Off Publ Orthop Res Soc. 2006;24(8):1722–8. doi: 10.1002/jor.20164. [DOI] [PubMed] [Google Scholar]
- 43.He W, Li K. Incidence of genetic polymorphisms involved in lipid metabolism among Chinese patients with osteonecrosis of the femoral head. Acta Orthop. 2009;80(3):325–9. doi: 10.3109/17453670903025378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hauzeur JP, Pasteels JL, Schoutens A, Hinsenkamp M, Appelboom T, Chochrad I, et al. The diagnostic value of magnetic resonance imaging in non-traumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1989;71(5):641–9. [PubMed] [Google Scholar]
- 45.Markisz JA, Knowles RJ, Altchek DW, Schneider R, Whalen JP, Cahill PT. Segmental patterns of avascular necrosis of the femoral heads: early detection with MR imaging. Radiology. 1987;162(3):717–20. doi: 10.1148/radiology.162.3.3809485. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell DG, Rao VM, Dalinka MK, Spritzer CE, Alavi A, Steinberg ME, et al. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162(3):709–15. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 47.Steinberg DR, Steinberg ME. The University of Pennsylvania classification of osteonecrosis. In: Koo KH, Mont MA, Jones LC, editors. Osteonecrosis. Heidelberg: Springer; 2014. p. 201–6.
- 48.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(Suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 49.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 50.Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, et al. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. Am J Roentgenol. 2003;180(2):363–8. doi: 10.2214/ajr.180.2.1800363. [DOI] [PubMed] [Google Scholar]
- 51.Korompilias AV, Karantanas AH, Lykissas MG, Beris AE. Transient osteoporosis. J Am Acad Orthop Surg. 2008;16(8):480–9. doi: 10.5435/00124635-200808000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Patel S. Primary bone marrow oedema syndromes. Rheumatology. 2014;53(5):785–92. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
- 53.Szwedowski D, Nitek Z, Walecki J. Evaluation of transient osteoporosis of the hip in magnetic resonance imaging. Pol J of Radiol Pol Med Soc Radiol. 2014;79:36–8. doi: 10.12659/PJR.889827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ikemura S, Yamamoto T, Motomura G, Nakashima Y, Mawatari T, Iwamoto Y. The utility of clinical features for distinguishing subchondral insufficiency fracture from osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133(12):1623–7. doi: 10.1007/s00402-013-1847-x. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T. Subchondral insufficiency fractures of the femoral head. Clin Orthop Surg. 2012;4(3):173–80. doi: 10.4055/cios.2012.4.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoon PW, Kwak HS, Yoo JJ, Yoon KS, Kim HJ. Subchondral insufficiency fracture of the femoral head in elderly people. J Korean Med Sci. 2014;29(4):593–8. doi: 10.3346/jkms.2014.29.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mont MA, Marulanda GA, Jones LC, Saleh KJ, Gordon N, Hungerford DS, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 58.Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br Vol. 1985;67(1):3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 59.Gardeniers J. A new international classification of osteonecrosis of the ARCO committee on terminology and classification. ARCO Newsl. 1992;4(4):41–6. [Google Scholar]
- 60.Ono K. Diagnostic criteria, staging system and roentgenographic classification of avascular necrosis of the femoral head (steroid induced, alcohol associated or idiopathic nature) (in Japanese) Annual report of Japanese investigation committee for intractable disease, avascular necrosis of the femoral head. Tokyo: Ministry of health and Welfare; 1987.
- 61.Sugano N, Atsumi T, Ohzono K, Kubo T, Hotokebuchi T, Takaoka K. The 2001 revised criteria for diagnosis, classification, and staging of idiopathic osteonecrosis of the femoral head. J Orthop Sci Off J Jpn Orthop Assoc. 2002;7(5):601–5. doi: 10.1007/s007760200108. [DOI] [PubMed] [Google Scholar]
- 62.Kerboul M, Thomine J, Postel M, Merle d'Aubigne R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br Vol. 1974;56(2):291–6. [PubMed] [Google Scholar]
- 63.Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br Vol. 1995;77(6):875–80. [PubMed] [Google Scholar]
- 64.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90(3):477–84. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 65.Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–71. [PubMed] [Google Scholar]
- 66.Banerjee S, Kapadia BH, Jauregui JJ, Cherian JJ, Mont MA. Natural history of osteonecrosis. In: Koo KH, Mont MA, Jones LC, editors. Osteonecrosis. Heidelberg: Springer; 2014. p. 161–4.
- 67.Cheng EY. Osteonecrosis of the femoral head. The North American perspective. In: Bulstrode D, Buckwalter J, Carr A, Marsh L, Fairbank J, Wilson-MacDonald J, editors. Oxford textbook of orthopaedics and trauma. Oxford: Oxford University Press; 2002. pp. 981–91. [Google Scholar]
- 68.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92(12):2165–70. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 69.Sugano N, Takaoka K, Ohzono K, Matsui M, Masuhara K, Ono K. Prognostication of nontraumatic avascular necrosis of the femoral head. Significance of location and size of the necrotic lesion. Clin Orthop Relat Res. 1994;303:155–64. [PubMed] [Google Scholar]
- 70.Cherian SF, Laorr A, Saleh KJ, Kuskowski MA, Bailey RF, Cheng EY. Quantifying the extent of femoral head involvement in osteonecrosis. J Bone Joint Surg Am. 2003;85-A(2):309–15. doi: 10.2106/00004623-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 71.Sugano N, Ohzono K. Natural Course and the JIC Classification of Osteonecrosis of the Femoral Head. In: Koo KH, Mont MA, Jones LC, editors. Osteonecrosis. Heidelberg: Springer; 2014. p. 207–10.
- 72.Arlet J, Ficat RP. Forage-biopsie de la tete femorale dans I'osteonecrose primitive. Observations histopathologiques portant sur huit forances. Rev Rheum. 1964;31(31):257–64. [Google Scholar]
- 73.Gardeniers J. ARCO committee on terminology and staging. A new proposition of terminology and an international classification of osteonecrosis. ARCO Newsl. 1991;3(3):153–9. [Google Scholar]
- 74.Gardeniers J. ARCO Committee on Terminology and Staging. Report on the committee meeting at Santiago de Compostella. ARCO Newsl. 1993;5(5):79–82. [Google Scholar]


