Abstract
Osteonecrosis, also known as avascular necrosis or AVN, is characterized by a stereotypical pattern of cell death and a complex repair process of bone resorption and formation. It is not the necrosis itself but rather the resorptive component of the repair process that results in loss of structural integrity and subchondral fracture. Most likely, a common pathophysiological pathway exists involving compromised subchondral microcirculation. Decreased femoral head blood flow can occur through three mechanisms: vascular interruption by fractures or dislocation, intravascular occlusion from thrombi or embolic fat, or intraosseous extravascular compression from lipocyte hypertrophy or Gaucher cells. In this review, we emphasize etiologic relationships derived mostly from longitudinal cohort studies or meta-analyses whose causal relationships to osteonecrosis can be estimated with confidence. Understanding risk factors and pathophysiology has therapeutic implications since several treatment regimens are available to optimize femoral head circulation, interrupt bone resorption, and preserve the subchondral bone.
Keywords: Intraosseous extravascular compression, Intravascular occlusion, Microcirculation, Subchondral bone resorption, Vascular interruption, Osteonecrosis
Introduction
There is a general consensus on the histopathology and pathomechanics of osteonecrosis (ON) of bone, but there is no such consensus with regard to etiological associations that may constitute risk factors for this disease. The methodological approach to defining risk factors accounts for much of the uncertainty of what constitutes a risk factor and the strength of that association. Historically, the retrospective case-control approach has been utilized to examine populations of patients with ON the presence of comorbidities that might be associated as risk factors. In general, this approach has resulted in (1) a large number of putative risk factors of low prevalence and failure to establish causation between etiologic factors and ON and (2) an overestimation of the prevalence of etiologies that do constitute risk factors. A more contemporary approach is to examine cohorts of patients with assumed risk factors (e.g., alcohol, corticosteroids, trauma) and look prospectively for the development of ON and estimate the true prevalence.
This review focuses on the structural consequences of bone ischemia and suggests a common pathway via compromised circulation leading to ischemia and consequent marrow and osteocyte necrosis. Etiologies that are established risk factors for ON are presented.
Pathophysiology
Cell and tissue necrosis
Osteonecrosis is characterized by a typical pattern of cell death and a complex process of bone resorption and formation (Fig. 1) [1]. The earliest pathologic characteristics of ON are necrosis of hematopoietic cells and adipocytes followed by interstitial marrow edema (Fig. 1a). Osteocyte necrosis occurs after approximately 2 to 3 h of anoxia, but histological signs of osteocyte death do not appear until approximately 24 to 72 h after oxygen deprivation [2–4]. Osteocyte necrosis is reflected initially by pyknosis of nuclei and subsequently by empty osteocyte lacunae. Reactive hyperemia and capillary revascularization occur to a degree in the periphery of the necrotic zone, and with the entry of blood vessels, a repair process begins consisting of both bone resorption and production that incompletely replaces dead with living bone. New living bone is laminated onto dead trabeculae with partial resorption of dead bone (Fig. 1b). In the subchondral trabeculae, bone resorption exceeds formation leading to the net removal of bone, loss of structural integrity of trabeculae, subchondral fracture, and joint incongruity (Fig. 1c). It is not the necrosis itself but rather the repair process and in particular the resorptive component that results in loss of structural integrity and subchondral fracture [5–7]. Finite element modeling has demonstrated that it is the loss of structural integrity of subchondral trabeculae rather than the subchondral plate that is responsible for the subchondral fracture [8].
Fig. 1.
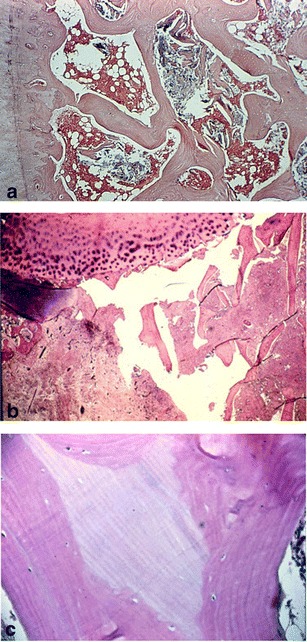
Histopathological hallmarks of osteonecrosis. a Necrotic marrow with interstitial edema and empty lacunae indicating necrotic osteocytes. b Subchondral fracture through partially resorbed trabeculae. c Lamination of loving on dead bone. Osteocytes are present in lacunae of living but not dead bone (reprinted with permission from Wolters Kluwer/LWW)
These histopathologic changes are reflected in the radiographic appearance of the femoral head producing the appearance of sclerosis and lucency. Areas of lucency reflect bone resorption, while areas of sclerosis are comprised of both living reparative bone and dead trabeculae (Fig. 2).
Fig. 2.
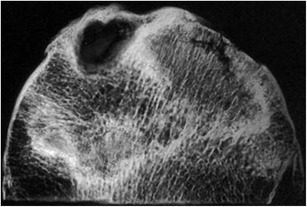
Specimen radiograph exhibiting both sclerotic and lucent areas. Sclerotic areas represent both dead and repair bone; lucent areas reflect bone resorption. A subchondral fracture is also present (reprinted with permission from Wolters Kluwer/LWW)
Compromised circulation
Understanding the pathophysiology and risk factors of ON is limited by the unavailability of longitudinal studies in humans and the lack of a bipedal mammalian model. Therefore, descriptions of pathophysiology are synthesized from unrelated observations of human disease or are extrapolated from other animal models [1]. Nevertheless, the best evidence suggests a common pathophysiological pathway involving compromised subchondral microcirculation. The vascular anatomy of the femoral head and neck has been well described [9•]. Examination of the microcirculation of the femoral head has demonstrated the vulnerability of the blood supply to the subchondral bone to vascular interruption, intravascular occlusion, and extravascular compression [1]. Compromise of perfusion to the ascending retinacular vessels results in decreased circulation of the femoral head subchondral bone [10–12]. Other studies have shown that extensive areas of ON are accompanied by involvement of the superficial and inferior metaphyseal arteries [13]. Using direct measurements and computer simulations, a decrease in blood flow of 1.6 would be expected to reduce the intraosseous pO2 from 75 to 50 mmHg, assuming a constant oxygen consumption rate, resulting in marked ischemia [14]. A unifying concept of the pathogenesis of ON has been presented that emphasizes the central role of vascular pathology and ischemia leading to osteocyte necrosis (Fig. 3) [1]. Decreased femoral head blood flow can occur through three pathogenic mechanisms: vascular interruption by fractures or dislocation, intravascular occlusion from thrombi or embolic fat, or intraosseous extravascular compression from lipocyte hypertrophy or Gaucher cells. A fourth mechanism, extraosseous venous obstruction, has been shown experimentally but probably has minimal clinical significance [15].
Fig. 3.
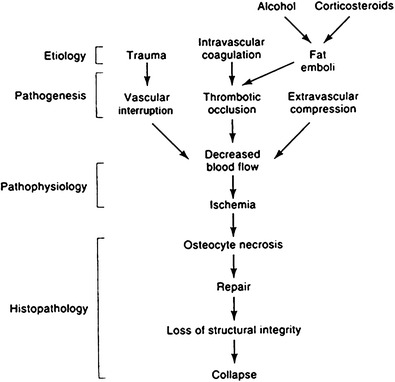
Unified concept of circulatory pathophysiology. Diverse etiologies contribute to ischemia and necrosis (reprinted with permission from Wolters Kluwer/LWW)
Intraosseous hypertension
Attention has focused on elevated intraosseous pressure as a pathogenic mechanism in ON and has recently been reviewed [1]. Elevated intraosseous pressures have been measured within ON femoral heads associated with venous outflow obstruction and venous stasis [16–18]. Normal bone marrow pressures have been reported to be about 15 mmHg with recordings above 30 mmHg considered abnormal. The infusion of 5 mL of saline provides a stress to the volume of the venous system within bone [17]. Infusions into normal femoral heads result in transient pressure elevations of about 10 mmHg, which should return to baseline within a few seconds. In ON, this stress test results in sustained elevations in intraosseous pressure of greater than 30 mmHg. However, other observers have not found elevated intraosseous pressure measurements to be reproducible, specific, or sensitive [19–21]. One study suggested that the diagnostic sensitivity of the test in ON is low with 17 % of hips with ON having intraosseous pressures below 30 mmHg [14]. Another study found pressures to be below 30 mmHg in 32 % of hips with ON and concluded that this stress test lacks sufficient precision as a clinical diagnostic tool [21]. Other observations also cast doubt on the precision of intraosseous pressure measurements. The experimental increase of intraosseous pressure to greater than 30 mmHg has been observed to increase endosteal, periosteal, and cancellous new bone formation but not to produce ON [22]. Elevated intraosseous pressures can be found in osteoarthritis as well as ON and can also be produced by elevations in intra-articular pressure [23–25]. These observations have led to the conclusion that elevated intraosseous pressure, although often observed in ON, is not causally related to the pathogenesis of the clinical syndrome [26, 27]. In this view, elevated intraosseous pressure is a nonspecific and secondary, but potentially contributory, factor in the pathogenesis of ON [1]. The clinical usefulness of intraosseous pressure measurements appears to vary widely because of differing perceptions of precision. It should be noted however that space-occupying tissue within the intraosseous extravascular space, such as adipocytes or Gaucher cells, may not be detectable consistently by measurements of intraosseous pressure, yet still constitute etiologic factors for ON.
Risk factors
Most reports concerning associating risk factors for ON are observational studies. Observational studies can, (1) start with potentially related factors and follow a cohort forward in time, determining the prevalence of ON (longitudinal cohort studies), (2) measure etiological factors and ON at the same time (cross-sectional studies), or (3) identify a cohort of ON patients and look backward in time for presumptive associations (case-control studies). An example of a cohort study is to ask what fraction of patients with femoral neck fractures develop ON, while a case-control study asks what percentage of patients with ON have had a fracture. Similarly, the question of what percentage of patients treated with corticosteroids develop ON is a cohort study while determining the percentage of ON patients who have been treated with corticosteroids is a case-control study. The methodologically strongest observational design is the cohort study; the weakest is the case-control study [28]. Unfortunately, many studies of risk factors for ON have been anecdotal or have used case-control methods resulting in relationships of assumed risk factors to ON whose causality is questionable. In this review, we will emphasize relationships derived mostly from longitudinal cohort studies whose causal relationships to ON can be estimated with the greatest confidence.
Several pathogenic mechanisms may result in ischemia and ON including vascular interruption, intravascular occlusion, and intraosseous extravascular compression.
Vascular interruption
The femoral head is supplied by branches of the medial and lateral femoral circumflex arteries (MFCA/LFCA). These branches enter the metaphyseal region and the nonarticular portion of the epiphysis of the femoral head [29]. The deep branch of the MFCA has been cited as the most important blood supply to the femoral head [30, 31]. The location of these vessels makes them susceptible to direct injury in the setting of trauma [1, 32].
Intracapsular fractures of the femoral neck may cause direct trauma to vessels that supply the subchondral bone, and relatively high incidences of ON of the femoral head have been reported in patients with these fractures. A recent meta-analysis reported the incidence of ON to be 14.3 % (range 10 to 25 %) in this population [1, 33]. Liu et al. showed through digital subtraction angiography that the rate of ON after a femoral neck fracture was directly linked to the number of vessels crossing the fracture line [34•]. Other studies have attempted to relate fracture type, accuracy of reduction, and time to reduction to the occurrence of ON [1]. A study by Garden revealed that malreduction based on radiographic criteria led to ON and segmental collapse in 65.4 % of patients, compared to 6.6 % of patients with acceptable alignment after open reduction, and internal fixation of femoral neck fractures [35]. Acceptable alignment in this study was defined as the angle between the medial cortex of the femoral shaft and the center of the neck on an antero-posterior radiograph and through the center of the femoral shaft and neck on a lateral radiograph to be between 155 to 180°. In a prospective study, Barnes and Garden reported on a large series of 1108 patients with femoral neck fracture, most of whom underwent an open reduction and internal fixation [36]. They found segmental collapse of the femoral head in 16 % of patients with stage I nondisplaced fracture and 27.6 % of patients with stage III and IV displaced fractures. Similar to the study noted above, the authors found significantly higher rates of nonunion and ON in those patients who were malreduced at the time of their operative intervention. Their results demonstrated that patients fixed in varus alignment had a significantly lower rate of union when compared to those in valgus alignment (50 vs. 71 %). However, those in valgus were also at the highest risk for ON of the femoral head [36]. Similarly, Nikolopoulos et al. also demonstrated that nondisplaced fractures of the femoral neck (Garden classification I, II) led to lower rates of ON when compared to displaced fractures of the femoral neck (Garden III, IV) when treated with internal screw fixation (19.5 vs. 39.5 %) [37]. Tao et al. also found the fracture type, as defined by the Garden classification, as well as poor reduction to be significant risk factors for femoral head ON in their retrospective review [38]. As for the time to treatment, a meta-analysis performed by Papakostidis et al. failed to establish a relationship between the incidence of ON and the interval between injury and definitive internal fixation [39].
Decompressing the hip joint following an intra-articular femoral neck fracture has been proposed to reduce the risk of ON by theoretically minimizing extraosseous compression of the vessels supplying the femoral head. The literature, however, lacks strong support for or against this theory [40]. Shrader et al. did not find any relationship between capsular decompression and the subsequent development of osteonecrosis of the femoral head [41]. However, in a retrospective analysis, Ng et al. found that hip decompression significantly reduced the risk of ON of the femoral head type II and III fractures of the femoral neck [42].
Extracapsular fractures of the intertrochanteric region that are distal to the entry of the arterial branches that supply the femoral head have a significantly lower incidence of ON [43].
Hip dislocations may also interrupt the vascular supply to the femoral head [30, 44–46]. The deep branch of the MFCA can be injured during a posterior dislocation as it traverses posterior to the obturator externus and anterior to the quadratus femoris muscles [30, 47]. Associated with posterior dislocations, the incidence of ON has been reported to be between 5 and 60 % depending on the time to reduction and severity of the associated fractures and other injuries [44–46]. Hougaard et al. found the rate of ON to be 4.8 and 52.9 % in their case-control study that analyzed patients with a posterior dislocation reduced before and after 6 h following the injury [46]. There is a paucity of data on long-term outcomes of anterior hip dislocations, but some studies suggest the occurrence of ON of the femoral head in about 10 % of patients [48, 49].
Intravascular occlusion
Interruption of the vascular flow to the femoral head may be secondary to intravascular obstruction. A variety of etiologies may cause this obstruction, namely, sickle cell aggregations or clots or lipid thrombi [1].
Sickle cell disease is caused by a genetic mutation in the beta chain of hemoglobin producing an abnormal hemoglobin which polymerizes under physiologic stresses. The polymerization of multiple hemoglobin molecules leads to red blood cells that assume the characteristic sickled appearance. The incidence of ON in patients with sickle cell anemia has been reported as high as 11 to 37 % in prospective and cross-sectional studies [50, 51•, 52]. It has been hypothesized that low oxygen tension environments trigger hemoglobin precipitation in these patients leading to erythrocyte sickling. The sickled cells are more prone to adhere to each other and cause intravascular obstruction, especially in the low flow areas of osseous vasculature, particularly the femoral head [53]. As such, the rate of ON of the femoral head was noted to increase with the patient’s age. Presumably, this was due to the repeated vascular insults to which these patients are prone over the course of their life. Matos et al. reported ON in 11.1 % of their series of patients less than 21 years of age, and Mukisi-Mukaza et al. found ON in either one or both hips in 37.2 % of their patients greater than 18 years of age [50, 51•]. Additionally, a high likelihood of progression of ON was seen in sickle cell patients who have radiographic evidence of its presence. Hernigou et al. prospectively followed a group of 123 patients with sickle cell disease with symptomatic ON on one side and asymptomatic ON evident on imaging of the contralateral side. On follow-up, 91 % of these patients had pain on the previously asymptomatic side and 77 % had collapse of that femoral head [54].
Coagulation factor abnormalities have also been implicated in ON of the femoral head. Genetic defects resulting in hypofibrinolysis or thrombophilia may lead to increased thrombi formation and impaired blood flow in the osseous circulation [1, 32, 55–57]. Hypofibrinolysis secondary to high levels of plasminogen activator inhibitor (PAI) was noted in 31 % of patients with ON compared to 3 % of controls in a prospective cohort study of patients with known ON of the femoral head [55]. With respect to thrombophilia, Zalavras et al. reported in a retrospective case-control study that a significantly higher percent of patients with osteonecrosis had Factor V Leiden mutation than did controls (18 vs. 4.6 %) [56].
In those without known genetic defects, elevated coagulation factor levels have been reported in patients with ON using case-control methodology. Jones et al. reported that 82.2 % of patient ON had at least one abnormal coagulation factor compared to 30 % of unaffected controls; 50 % of the affected patients had two or more abnormalities compared to 2.5 % of controls [58]. Similarly, higher levels of lipoprotein A, von Willebrand factor, and lower levels of protein C and S were reported in patients with idiopathic ON and secondary ON compared to healthy controls [59]. Decreased levels of protein C and S and genetic resistance to activated protein C both decrease the physiologic regulation of prothrombotic factors V and VIII, allowing intravascular insults to occur in the femoral head [1]. Lipoprotein A has been implicated in several studies [1, 58, 59]. However, it must be noted that there exists some controversy regarding the role of hypercoagulability and ON of the femoral head. It is not always observed in ON of the femoral head and is often seen in other conditions affecting the femoral head, namely, osteoarthritis of the hip and bone marrow edema syndrome [60, 61]. Similarly, abnormalities of protein C and S, antithrombin III, or resistance to activated protein C have not been found in two studies that retrospectively examined patients with Perthes disease [62, 63]. Information emerging in longitudinal cohort studies may show a lower incidence of coagulopathies in ON.
Intraosseous extravascular compression
The concept of the Starling resistor can be used to conceptualize this subsection of etiologies that lead to ON. This resistor consists of a rigid-walled chamber through which pass compressible tubes. Fluid flow through these tubes is related to the amount of pressure exerted on them by the extratubular contents of the chamber. The femoral head can be conceptualized as analogous to a Starling resistor with the osseous vasculature representing the compressible tubes and the intraosseous extravascular space representing the extratubular space of the chamber (Fig. 4a, b). Elevations in pressure within the intraosseous extravascular space, even if not consistently recordable, could decrease blood flow in the small vessels passing through it.
Fig. 4.
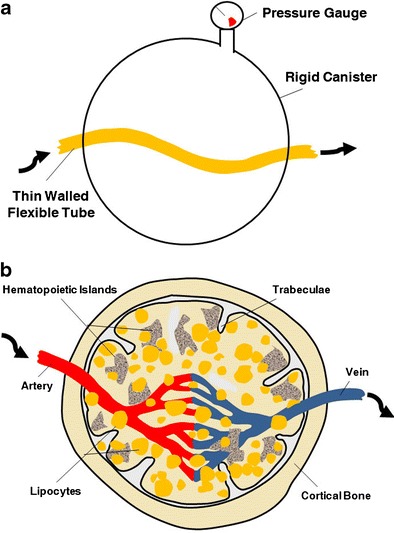
Concept of the Starling resistor as applied to bone microcirculation. a Raising the pressure in a rigid-walled chamber can decrease fluid flow in a flexible-walled tube passing through the chamber, b In the case of bone, the intraosseous extravascular compartment may function like a rigid-walled chamber. Intraosseous hypertension or space-occupying tissue may sufficiently restrict microcirculatory blood flow to produce ischemia (reprinted with permission from Elsevier)
Lipid deposition and adipocyte hypertrophy in the marrow space are the two predominant clinical conditions by which intraosseous extravascular pressure could reduce circulation [1]. Often associated with corticosteroid or alcohol intake, the increase in extravascular pressure has been hypothesized to create an obstruction for arterial inflow or venous outflow leading to ischemia of the marrow elements and osteocytes in the femoral head [64–66]. Both alcohol and corticosteroid use have been shown to increase adipocyte size, alter lipid metabolism, and shunt cells from an osteocytic to an adipocytic lineage [1, 64–67].
Osteonecrosis associated with corticosteroid use has been implicated in 10 to 30 % of cases in retrospective studies [1]. Corticosteroids are used in a variety of disease conditions including systemic lupus erythmatosus, rheumatoid arthritis, asthma, organ transplantation, and vasculitis. Though it has been difficult to separate the contribution to the development of ON of corticosteroid use from the underlying condition, a large meta-analysis of 22 studies found no association between the underlying disease and ON [68]. An important effort has been made to establish which component of dose—the mean daily dose, the cumulative dose, or duration—is associated with development of ON [69•, 70–73]. Prospective cohort studies have suggested that a mean daily dose of >20 mg/day was associated with a significant risk for ON. A meta-analysis by Felson et al. determined a 4.6-fold increase in ON for every 10 mg/day increase in oral corticosteroid intake [68].
Clinical impressions of the relationships between corticosteroid intake and ON have been formed largely from retrospective and cross-sectional studies that establish the prevalence of corticosteroid use in patients with ON. These studies do not establish the incidence of ON in patients who use corticosteroids and do not establish the causal risk of corticosteroids but rather inform on the fraction of patients with ON that also use corticosteroids [69•, 74]. The studies that have looked with prospective cohort studies at the incidence of ON in patients using corticosteroids report a rate of 4 to 7 % [68, 72, 75].
Excessive alcohol use has also been associated with ON of the femoral head in 10 to 40 % of cases [73, 76, 77]. Matsuo et al. found that individuals consuming more than 400 mL of alcohol per week were at a 9.8-fold greater risk of developing ON than those who consumed none. This risk rose to 17.9-fold in individuals who consumed greater than 1000 mL of alcohol per week [78].
Gaucher disease has also been implicated in the development of ON of the femoral head due to its role in decreasing capillary blood flow, possibly by increasing the pressures in the intraosseous extravascular space [74, 79]. Due to the deficiency of beta-glucocerebrosidase, patients with Gaucher disease accumulate large amounts of glucocerebrosides in the lysosomes of their histiocytes, aptly named Gaucher cells. With time, there is a substantial extravascular, medullary collection of Gaucher cells resulting in increased intraosseous pressures. This increased pressure can, in turn, compress the vascular elements leading to ischemia [1]. A recent study by Poll et al. reported the incidence of ON seen on MRI to be 46 % in these patients [80].
Other etiologies
Many other etiologies have been associated with ON of the femoral head in case reports and retrospective case-control series. Hyperlipidemia, hyperuricemia, pancreatitis, leukemia or lymphoma, and hypertriglyceridemia have all been implicated as potential causes of ON of the femoral head [1, 58, 81–87]. Osteonecrosis has also been reported in pregnant patients, those undergoing radiation, bone marrow transplantation, and those with metastatic or disseminated malignancies [1, 88]. Dysbaric ON is well-characterized but is largely of historical interest since safe working compressed air pressures and diving decompression schedules have been established. Other presumptive etiologies have not yet to be studied in prospective studies, and their relative risks have not been established.
Conclusions
Many conditions have been associated as risk factors for ON. However, it is difficult to assign true etiologic status when anecdotal or retrospective methods are used, implicating etiologies of low prevalence, especially when many studies report idiopathic ON constituting 10 to 15 % of cases. When possible, we have relied on reports using a prospective, longitudinal cohort method, or analyses, to identify robust associations between risk factors and ON. This is not to say that we exclude other possible conditions as risk factors for ON, but substantial proof of causation is required.
Risk factors that are strongly implicated as causal for ON seem to converge through mechanisms of vascular impairment to produce subchondral or segmental ischemia and marrow and osteocyte death. A repair process follows that usually results in resorption of subchondral bone that exceeds formation, leading to structural compromise and fracture. Understanding risk factors and pathophysiology has therapeutic implications since several treatment regimens are available to optimize femoral head circulation, to prevent bone resorption and to preserve the subchondral bone. In traumatic ON, acceptable fracture position and time, technique, and approach to fixation are important. In nontraumatic ON, specific prophylaxis is available to prevent lipocyte hypertophy (by limiting alcohol intake and corticosteroid dose), using statins, and treating Gaucher disease with enzyme replacement. Osteonecrosis associated with hypercoagulable syndromes have been prophylaxed with warfarin and enoxaparin [89].Bisphosphonates have been used to rebalance the rates of subchondral bone resorption and formation with the expectation of preserving the mechanical integrity of subchondral bone and to minimize the risk of fracture and joint incongruity.
Acknowledgments
Compliance with ethics guidelines
ᅟ
Conflict of interest
Kalpit N. Shah, Jennifer Racine, and Roy K. Aaron declare that they have no conflict of interest.
Lynne C. Jones is President of ARCO International. This is a medical society that promotes research and education regarding bone circulation and related diseases (including osteonecrosis). There is no financial reimbursement or pay associated with this position.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Modern Surgical Treatment of Hip Avascular Necrosis
Contributor Information
Kalpit N. Shah, Email: kalpit_shah@brown.edu
Jennifer Racine, Email: Jracine@lifespan.org.
Lynne C. Jones, Email: ljones3@jhmi.edu
Roy K. Aaron, Email: Roy_Aaron@Brown.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Aaron RK, Gray R. Osteonecrosis: etiology, natural history, pathophysiology, and diagnosis. In: Callaghan JJ, Rosenberg AG, Rubash HE, editors. The adult hip. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 465–76. [Google Scholar]
- 2.James J, Steijn-Myagkaya GL. Death of osteocytes. Electron microscopy after in vitro ischaemia. J Bone Joint Surg (Br) 1986;68(4):620–4. doi: 10.1302/0301-620X.68B4.3733842. [DOI] [PubMed] [Google Scholar]
- 3.Bauer TW, Stulberg BN, et al. The histology of osteonecrosis and its distinction from histologic artifacts. In: Schoutens A, et al., editors. Bone circulation and vascularization in normal and pathological conditions. New York: Plenum Press; 1993. pp. 283–92. [Google Scholar]
- 4.Kenzora JE GM, et al. Osteonecrosis. In: Kelly WN HE, Ruddy S, et al., editors. Textbook of rheumatology. Philadelphia: WB Saunders; 1981. pp. 1755–82. [Google Scholar]
- 5.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: I. Tissue biology. Clin Orthop Relat Res. 1979;138:284–309. [PubMed] [Google Scholar]
- 6.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;139:283–312. [PubMed] [Google Scholar]
- 7.Glimcher MJ, Kenzora JE. The biology of osteonecrosis of the human femoral head and its clinical implications: III Discussion of the etiology and genesis of the pathological sequelae; comments on treatment. Clin Orthop Relat Res. 1979;140:273–312. [PubMed] [Google Scholar]
- 8.Brown TD, Baker KJ, Brand RA. Structural consequences of subchondral bone involvement in segmental osteonecrosis of the femoral head. J Orthop Res. 1992;10(1):79–87. doi: 10.1002/jor.1100100110. [DOI] [PubMed] [Google Scholar]
- 9.•.Lorich D, Lazaro L. Fractures and bone repair. In: Aaron RK, editor. Skeletal circulation in clinical practice. Singapore: World Scientific; 2015. [Google Scholar]
- 10.Atsumi T, et al. Bone arteriography of the femoral head of humans in normal and pathological conditions. In: Schoutens A, et al., editors. Bone circulation and vascularization in normal and pathological conditions. US: Springer; 1993. pp. 293–9. [Google Scholar]
- 11.Atsumi T, Kuroki Y. Role of impairment of blood supply of the femoral head in the pathogenesis of idiopathic osteonecrosis. Clin Orthop Relat Res. 1992;277:22–30. [PubMed] [Google Scholar]
- 12.Atsumi T, Kuroki Y, Yamano K. A microangiographic study of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1989;246:186–94. [PubMed] [Google Scholar]
- 13.Ohzono K, et al. Intraosseous arterial architecture in nontraumatic avascular necrosis of the femoral head. Microangiographic and histologic study. Clin Orthop Relat Res. 1992;277:79–88. [PubMed] [Google Scholar]
- 14.Kiaer T, et al. Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. J Bone Joint Surg (Br) 1990;72(6):1023–30. doi: 10.1302/0301-620X.72B6.2246284. [DOI] [PubMed] [Google Scholar]
- 15.Tsukamoto H, et al. Evaluation of marrow perfusion in the femoral head by dynamic magnetic resonance imaging. Effect of venous occlusion in a dog model. Invest Radiol. 1992;27(4):275–81. doi: 10.1097/00004424-199204000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg. 1985;67B:3–9. doi: 10.1302/0301-620X.67B1.3155745. [DOI] [PubMed] [Google Scholar]
- 17.Hungerford DS. Early diagnosis and treatment of ischemic necrosis of the femoral head. In: Weil UH, editor. Progress in orthopaedic surgery. Berlin: Springer-Verlag; 1981. p. 29. [Google Scholar]
- 18.Hungerford DS, Lennox DW. The importance of increased intraosseous pressure in the development of osteonecrosis of the femoral head: implications for treatment. In Orthopaedic clinics of North America1985. p. 635. [PubMed]
- 19.Camp JF, Colwell CW. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg. 1986;68A:1313. [PubMed] [Google Scholar]
- 20.Hauzeur JPH, Pasteels JL, Orloff S. Bilateral non-traumatic aseptic osteonecrosis in the femoral head. J Bone Joint Surg. 1987;69A:1221–5. [PubMed] [Google Scholar]
- 21.Learmonth ID, Maloon S, Dall G. Core decompression for early atraumatic osteonecrosis of the femoral head. J Bone Joint Surg. 1990;72B:387. doi: 10.1302/0301-620X.72B3.2341433. [DOI] [PubMed] [Google Scholar]
- 22.Welch RD, et al. Bone changes associated with intraosseous hypertension in the caprine tibia. J Bone Joint Surg Am. 1993;75(1):53–60. doi: 10.2106/00004623-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Arnoldi CC. The relationship between intraosseous and intra-articular pressure. In: Arlet J, Ficat RP, Hungerford DS, editors. Bone circulation. Baltimore: Williams & Wilkins; 1984. pp. 213–221. [Google Scholar]
- 24.Downey DJ, Simkin PA, Taggart R. The effect of compressive loading on intraosseous pressure in the femoral head in vitro. J Bone Joint Surg. 1988;70A:871. [PubMed] [Google Scholar]
- 25.Kiaer T, Gronlund J, Sorensen KH. Subchondral pO2, pCO2, pressure, pH, and lactate in human osteoarthritis of the hip. Clin Orthop Relat Res. 1988;229:149–55. [PubMed] [Google Scholar]
- 26.Jones JP., Jr Concepts of etiology and early pathogenesis of osteonecrosis. Instr Course Lect. 1994;43:499–512. [PubMed] [Google Scholar]
- 27.Jones JP., Jr Etiology and pathogenesis of osteonecrosis. Semin Arthroplast. 1991;2:160–8. [Google Scholar]
- 28.Spitzer WO, Horwitz SM, et al. Selected nonexperimental methods: an orientation. In: Troidl H, et al., editors. Principles and practice of research. US: Springer; 1991. pp. 104–13. [Google Scholar]
- 29.Johnson EO, Soultanis K, Soucacos PN. Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthop Clin N Am. 2004;35(3):285–91. doi: 10.1016/j.ocl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Zlotorowicz M, et al. The blood supply to the femoral head after posterior fracture/dislocation of the hip, assessed by CT angiography. Bone Joint J. 2013;95-B(11):1453–7. doi: 10.1302/0301-620X.95B11.32383. [DOI] [PubMed] [Google Scholar]
- 31.Zlotorowicz M, et al. Anatomy of the medial femoral circumflex artery with respect to the vascularity of the femoral head. J Bone Joint Surg Br. 2011;93(11):1471–4. doi: 10.1302/0301-620X.93B11.26993. [DOI] [PubMed] [Google Scholar]
- 32.Assouline-Dayan Y, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32(2):94–124. doi: 10.1053/sarh.2002.33724b. [DOI] [PubMed] [Google Scholar]
- 33.Slobogean GP, et al. Complications following young femoral neck fractures. Injury. 2014. [DOI] [PubMed]
- 34.•.Liu Y, et al. Femoral neck fractures: prognosis based on a new classification after superselective angiography. J Orthop Sci Off J Japan Orthop Assoc. 2013;18(3):443–50. doi: 10.1007/s00776-013-0367-4. [DOI] [PubMed] [Google Scholar]
- 35.Garden RS. Malreduction and avascular necrosis in subcapital fractures of the femur. J Bone Joint Surg. 1971;53B:183–90. [PubMed] [Google Scholar]
- 36.Barnes R, et al. Subcapital fractures of the femur. A prospective review. J Bone Joint Surg (Br) 1976;58(1):2–24. doi: 10.1302/0301-620X.58B1.1270491. [DOI] [PubMed] [Google Scholar]
- 37.Nikolopoulos KE, et al. Long-term outcome of patients with avascular necrosis, after internal fixation of femoral neck fractures. Injury. 2003;34(7):525–8. doi: 10.1016/S0020-1383(02)00367-4. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, et al. Analysis of risk factors for femoral head necrosis after internal fixation in femoral neck fractures. Orthopedics. 2014;37(12):e1117–23. doi: 10.3928/01477447-20141124-60. [DOI] [PubMed] [Google Scholar]
- 39.Papakostidis C, et al. Timing of internal fixation of femoral neck fractures. A systematic review and meta-analysis of the final outcome. Injury. 2015. [DOI] [PubMed]
- 40.Doak J, Schiller J, Eberson C. Circulation of the pediatric and adolescent hip. In: Aaron RK, editor. Skeletal circulation in clinical practice. Singapore: World Scientific; 2015. [Google Scholar]
- 41.Shrader MW, et al. Femoral neck fractures in pediatric patients: 30 years experience at a level 1 trauma center. Clin Orthop Relat Res. 2007;454:169–73. doi: 10.1097/01.blo.0000238794.82466.3d. [DOI] [PubMed] [Google Scholar]
- 42.Ng GP, Cole WG. Effect of early hip decompression on the frequency of avascular necrosis in children with fractures of the neck of the femur. Injury. 1996;27(6):419–21. doi: 10.1016/0020-1383(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 43.Barquet A, et al. Avascular necrosis of the femoral head following trochanteric fractures in adults: a systematic review. Injury. 2014;45(12):1848–58. doi: 10.1016/j.injury.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 44.Dwyer AJ, et al. Complications after posterior dislocation of the hip. Int Orthop. 2006;30(4):224–7. doi: 10.1007/s00264-005-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKee MD, et al. Irreducible fracture-dislocation of the hip: a severe injury with a poor prognosis. J Orthop Trauma. 1998;12(4):223–9. doi: 10.1097/00005131-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Hougaard K, Thomsen PB. Traumatic posterior dislocation of the hip—prognostic factors influencing the incidence of avascular necrosis of the femoral head. Arch Orthop Trauma Surg Archiv fur orthopadische und Unfall-Chirurgie. 1986;106(1):32–5. doi: 10.1007/BF00435649. [DOI] [PubMed] [Google Scholar]
- 47.Gautier E, et al. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br. 2000;82(5):679–83. doi: 10.1302/0301-620X.82B5.10426. [DOI] [PubMed] [Google Scholar]
- 48.Bastian JD, et al. Long-term outcome after traumatic anterior dislocation of the hip. Arch Orthop Trauma Surg. 2011;131(9):1273–8. doi: 10.1007/s00402-011-1299-0. [DOI] [PubMed] [Google Scholar]
- 49.Dreinhofer KE, et al. Isolated traumatic dislocation of the hip. Long-term results in 50 patients. J Bone Joint Surg Br. 1994;76(1):6–12. [PubMed] [Google Scholar]
- 50.Mukisi-Mukaza M, et al. Prevalence, clinical features, and risk factors of osteonecrosis of the femoral head among adults with sickle cell disease. Orthopedics. 2000;23(4):357–63. doi: 10.3928/0147-7447-20000401-17. [DOI] [PubMed] [Google Scholar]
- 51.•.Matos MA, et al. Avascular necrosis of the femoral head in sickle cell disease patients. Ortop Traumatol Rehabil. 2012;14(2):155–60. doi: 10.5604/15093492.992286. [DOI] [PubMed] [Google Scholar]
- 52.Axelrod AR, Clifford GO, Tanaka KR. Sickle cell anemia (homozygous S) with aseptic necrosis of femoral head. Blood. 1956;11(11):998–1008. [PubMed] [Google Scholar]
- 53.Colin Y, Le Van Kim C, El Nemer W. Red cell adhesion in human diseases. Curr Opin Hematol. 2014;21(3):186–92. doi: 10.1097/MOH.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 54.Hernigou P, et al. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88(12):2565–72. doi: 10.2106/JBJS.E.01455. [DOI] [PubMed] [Google Scholar]
- 55.Glueck CJ, et al. Thrombophilia, hypofibrinolysis, the eNOS T-786C polymorphism, and multifocal osteonecrosis. J Bone Joint Surg Am. 2008;90(10):2220–9. doi: 10.2106/JBJS.G.00616. [DOI] [PubMed] [Google Scholar]
- 56.Zalavras CG, et al. Genetic background of osteonecrosis: associated with thrombophilic mutations? Clin Orthop Relat Res. 2004;422:251–5. doi: 10.1097/01.blo.0000127921.13253.e3. [DOI] [PubMed] [Google Scholar]
- 57.Hadjigeorgiou G, et al. Genetic association studies in osteonecrosis of the femoral head: mini review of the literature. Skelet Radiol. 2008;37(1):1–7. doi: 10.1007/s00256-007-0395-2. [DOI] [PubMed] [Google Scholar]
- 58.Jones LC, et al. Procoagulants and osteonecrosis. J Rheumatol. 2003;30(4):783–91. [PubMed] [Google Scholar]
- 59.Zalavras C, et al. Potential aetiological factors concerning the development of osteonecrosis of the femoral head. Eur J Clin Investig. 2000;30(3):215–21. doi: 10.1046/j.1365-2362.2000.00621.x. [DOI] [PubMed] [Google Scholar]
- 60.Berger CE, et al. Elevated levels of lipoprotein(a) in familial bone marrow edema syndrome of the hip. Clin Orthop Relat Res. 2000;377:126–31. doi: 10.1097/00003086-200008000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Cheras PA, et al. Hypercoagulability and hypofibrinolysis in primary osteoarthritis. Clin Orthop Relat Res. 1997;334:57–67. [PubMed] [Google Scholar]
- 62.Gallistl S, et al. The role of inherited thrombotic disorders in the etiology of Legg-Calve-Perthes disease. J Pediatr Orthop. 1999;19(1):82–3. [PubMed] [Google Scholar]
- 63.Kealey WD, et al. The role of coagulation abnormalities in the development of Perthes' disease. J Bone Joint Surg (Br) 2000;82(5):744–6. doi: 10.1302/0301-620X.82B5.10183. [DOI] [PubMed] [Google Scholar]
- 64.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head. A histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67(5):755–63. [PubMed] [Google Scholar]
- 65.Wang GJ, et al. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59(6):729–35. [PubMed] [Google Scholar]
- 66.Wang Y, et al. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–24. doi: 10.1097/01.blo.0000063602.67412.83. [DOI] [PubMed] [Google Scholar]
- 67.Ikemura S, et al. Lipid metabolism abnormalities in alcohol-treated rabbits: a morphometric and haematologic study comparing high and low alcohol doses. Int J Exp Pathol. 2011;92(4):290–5. doi: 10.1111/j.1365-2613.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet. 1987;1(8538):902–6. doi: 10.1016/S0140-6736(87)92870-4. [DOI] [PubMed] [Google Scholar]
- 69.•.Aaron RK, et al. Corticosteroid-associated avascular necrosis: dose relationships and early diagnosis. Ann N Y Acad Sci. 2011;1240:38–46. doi: 10.1111/j.1749-6632.2011.06218.x. [DOI] [PubMed] [Google Scholar]
- 70.Cruess RL. Steroid-induced osteonecrosis. J R Coll Surg Edinb. 1981;26(2):69–77. [PubMed] [Google Scholar]
- 71.Fisher DE, Bickel WH. Corticosteroid-induced avascular necrosis. A clinical study of seventy-seven patients. J Bone Joint Surg Am. 1971;53(5):859–73. [PubMed] [Google Scholar]
- 72.Vakil N, Sparberg M. Steroid related osteonecrosis in inflammatory bowel disease. Gastroenterology. 1989;96:62–7. doi: 10.1016/0016-5085(89)90764-6. [DOI] [PubMed] [Google Scholar]
- 73.Zizic TM, et al. Corticosteroid therapy associated with ischemic necrosis of bone in systemic lupus erythematosus. Am J Med. 1985;79(5):596–604. doi: 10.1016/0002-9343(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 74.Schroer WC. Current concepts on the pathogenesis of osteonecrosis of the femoral head. Orthop Rev. 1994;23(6):487–97. [PubMed] [Google Scholar]
- 75.Joo YB, et al. Prevalence, incidence, and associated factors of avascular necrosis in Korean patients with systemic lupus erythematosus: a nationwide epidemiologic study. Rheumatol Int. 2014. [DOI] [PubMed]
- 76.Arlet J. Nontraumatic avascular necrosis of the femoral head: past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed] [Google Scholar]
- 77.Patterson RJ, Bickel WH, Dahlin DC. Idiopathic avascular necrosis of the head of the femur. A study of fifty-two cases. J Bone Joint Surg Am. 1964;46:267–82. [PubMed] [Google Scholar]
- 78.Matsuo K, et al. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988;234:115–23. [PubMed] [Google Scholar]
- 79.Deegan PB, et al. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine. 2011;90(1):52–60. doi: 10.1097/MD.0b013e3182057be4. [DOI] [PubMed] [Google Scholar]
- 80.Poll LW, et al. MRI bone marrow findings in 63 patients with type I Gaucher disease. Röfo. 2010;182(11):979–85. doi: 10.1055/s-0029-1245410. [DOI] [PubMed] [Google Scholar]
- 81.Karimova EJ, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(12):1525–31. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 82.Niinimaki R, et al. Incidence of severe osteonecrosis requiring total joint arthroplasty in children and young adults treated for leukemia or lymphoma: a nationwide, register-based study in Finland and Denmark. J Adolesc Young Adult Oncol. 2013;2(4):138–44. doi: 10.1089/jayao.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Griffiths-Jones W, et al. the equivalence of remote electronic and paper patient reported outcome (PRO) collection. J Arthroplasty. 2014;29(11):2136–9. doi: 10.1016/j.arth.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Jones JP., Jr Intravascular coagulation and osteonecrosis. Clin Orthop Relat Res. 1992;277:41–53. [PubMed] [Google Scholar]
- 85.Jacobs B. Epidemiology of traumatic and nontraumatic osteonecrosis. Clin Orthop Relat Res. 1978;130:51–67. [PubMed] [Google Scholar]
- 86.Steib-Furno S, et al. Pregnancy-related hip diseases: incidence and diagnoses. Joint Bone Spine. 2007;74(4):373–8. doi: 10.1016/j.jbspin.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Sharareh B, Schwarzkopf R. Dysbaric osteonecrosis: a literature review of pathophysiology, clinical presentation, and management. Clin J Sport Med. 2014. [DOI] [PubMed]
- 88.Schulte CM, Beelen DW. Avascular osteonecrosis after allogeneic hematopoietic stem-cell transplantation: diagnosis and gender matter. Transplantation. 2004;78(7):1055–63. doi: 10.1097/01.TP.0000138026.40907.38. [DOI] [PubMed] [Google Scholar]
- 89.Jones L, Aaron RK. Circulatory pathology in osteonecrosis. In: Aaron RK, editor. Skeletal circulation in clinical practice. Singapore: World Scientific; 2015. [Google Scholar]


