Abstract
This article provides an overview of the biological function of a recently discovered cytokine, interleukin-37 (IL-37), formerly referred to as IL-1F7, and its role in chronic inflammation and autoimmune disease. Much has been discovered about IL-37 in the last decade, including its ability to down-regulate systemic and local inflammation by lowering levels of pro-inflammatory molecules. Here, we critically reviewed the published reports. Future research is necessary in order to understand the receptor-dependent effects of IL-37, its intracellular and extracellular functions in both normal and diseased states, and its potential role as a biomarker and pharmacological target in human disease.
Keywords: Adaptive immunity, Autoimmune disease, Inflammation, Innate immunity, IL-37
Introduction
There are 11 cytokines in the interleukin 1 (IL-1) family. Seven of these are pro-inflammatory agonists in the innate immune system produced after stimulation by Toll-like receptors (TLR): IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ [1]. Two are naturally occurring receptor antagonists: IL-1Ra and IL-36Ra. No function has yet been demonstrated for IL-1F10. With the exception of IL-18 and IL-33, these IL-1 family members all map to chromosome 2 [2].
IL-37 is the most recently discovered IL-1 family member. It was previously called IL-1F7 before Nold and colleagues (2010) renamed it. Synonyms of IL-37 are included in Table 1, along with the original publications in which they were described. IL-37 is located in the cytoplasm and nucleus. Smad-3 plays a role in the translocation of IL-37 to the nucleus [2]. IL-37 is also secreted. These details will be discussed in the subsequent section of this review.
Table 1.
Synonyms of IL-37, listed in the chronological order of published reports.
Alternative splicing of the human gene for IL-37 gives rise to five different isoforms: IL-37a, IL-37b, IL-37c, IL-37d, and IL-37e. The isoforms IL-37c and IL-37e are not predicted to be functional cytokines. These five isoforms are expressed in different areas of the body (Table 2). Since IL-37b is most likely to be biologically functional [3], and it contains the most complete set of exons, this review will primarily focus on IL-37b.
Table 2.
Isoforms of IL-37, their expression and biological role [2].
| Isoform | Expression | Biological Role |
|---|---|---|
| IL-37a | Brain, lymph nodes, thymus, bone marrow, lung, testis, placenta, uterus, skin, colon, NK, monocytes, stimulated B cells, keratinocytes | May be functional |
| IL-37b | Heart, lymph nodes, thymus, bone marrow, lung, testis, placenta, uterus, skin, colon, NK, monocytes, stimulated B cells, keratinocytes | Biologically functional isoform with the greatest amount of exons. |
| IL-37c | Kidney, lymph nodes, thymus, bone marrow, lung, testis, placenta, uterus, skin, colon, NK, monocytes, stimulated B cells, keratinocytes | Non-functional, due to abnormal folding |
| IL-37d | Testis and bone marrow | May be functional |
| IL-37e | Testis and bone marrow | Non-functional, due to abnormal folding |
In the last decade, there have been intense investigations on the anti-inflammatory actions of IL-37. IL-37 suppresses pro-inflammatory cytokine production, including IL-1A, IL-6, CCL-12, CSF-1, CSF-2, CXCL-13, IL-1β, IL23-A and IL1RA (IL-1 receptor antagonist). However, it spares anti-inflammatory cytokines, including IL-10. IL-37 also inhibits dendritic cell activation, and plays a role in adaptive immunity [4]. IL-37 binds to IL-18BP, and subsequently binds IL-18Rβ, inhibiting the pro-inflammatory activity of IL-18. IL-37 also binds to the IL-18R α-chain, but with much lower affinity than IL-18.
Although more information about IL-37 is detailed in the following sections, the major role of IL-37 is to provide a negative feedback mechanism to suppress excessive inflammation in both the innate and adaptive immune system.
Mechanism of action of IL-37
IL-37 Receptors and Interactions
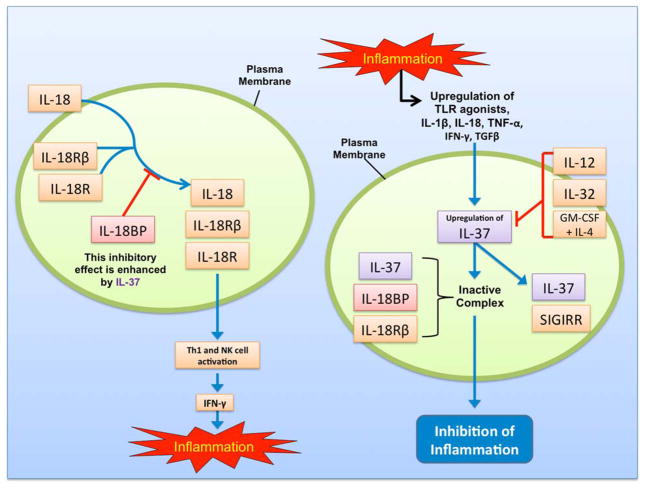
In order to understand the anti-inflammatory role of IL-37, it is critical to discuss IL-1 family receptors. These receptors have an intracellular TIR domain that begins the pro-inflammatory signaling cascade in the innate immune system [3]. A signaling ligand-binding chain relevant to IL-37 is IL-18Rα. IL-18, an important inducer of interferon-γ (IFN-γ), promotes Th1 lymphocytes and natural killer (NK) cell activation by binding to IL-18Rα and recruiting an accessory protein, IL-18Rβ [3]. IL-18-binding protein (IL-18BP) prevents binding of IL-18 to IL-18Rα and IL-18Rβ.
IL-37 has the ability to non-competitively bind both IL-18BP and IL-18Rα [2], making IL-37 an inhibitor of IL-18. This is supported by the fact that IL-37 and IL-18 share critical amino acid residues [5]. The affinity of IL-37b (generated in E. coli) for IL-18Rα is 50 times lower than the affinity of IL-18 for IL-18Rα [6]. The affinity of IL-37b for IL-18BP is also very weak, although IL-37b does increase the inhibitory effect of exogenous IL-18BP in a limited manner [7]. It is suggested that IL-37 binds IL-18BP, and subsequently binds IL-18Rβ, resulting in an inactive complex that prevents an increase in IFN-γ levels. However, IL-37 has not been proven to be a receptor antagonist or agonist for IL-18 [6].
IL-18Rα bound to IL-37 may be able to recruit a molecule other than IL-18Rβ (such as TIR8/SIGIRR, TIGIRR-1, or TIGIRR-2) in order to deliver an inhibitory signal [3]. For instance, IL-37 binds to SIGIRR, an inhibitory IL-1R family member, and to IL-18Rα [8]. This was observed in two different cells transfected with IL-37: LPS-stimulated RAW macrophages and IL-1β-treated A549 cells. When SIGIRR was silenced, there was only a 34% reduction in IL-1β, as opposed to an 83% reduction in IL-37 transfected THP-1 macrophages with an intact SIGIRR gene (Figure 1).
Figure 1.
Schematic diagram depicting mechanisms of action of IL-37
Production and Processing of IL-37
A variety of normal tissues and diseased tissues express IL-37 with differential expression of its five different isoforms (Table 2). IL-37 protein is associated with plasma cells, and it is constitutively expressed in the cytoplasm of monocytes and peripheral blood mononuclear cells (PBMCs) [3]. Cytoplasmic IL-37 is located near the Golgi apparatus, endoplasmic reticulum, and plasma membrane, suggesting that IL-37 could be released by secretory vesicles [6].
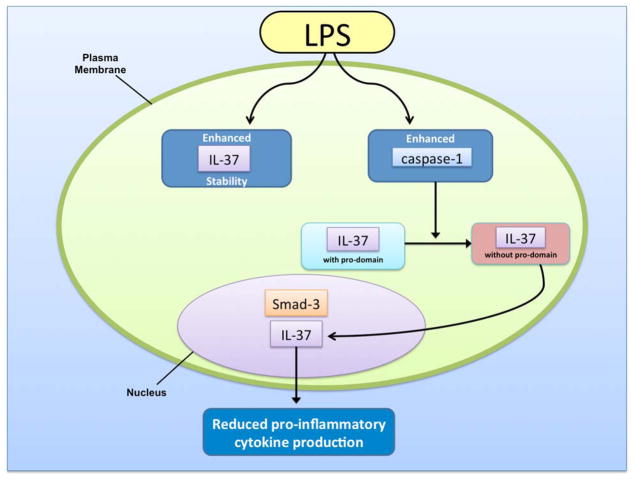
Transcript stability depends upon expression of an instability element in exon 5 [3]. This instability sequence limits the half-life of IL-37 mRNA. Stimulation with lipopolysaccharide (LPS) enhances the stability of the IL-37b transcript in monocytes, thereby increasing intracellular protein expression during inflammatory conditions [9]. As previously mentioned, IL-18 is structurally similar to IL-37. Therefore, it makes sense that IL-18 stability is also enhanced with LPS stimulation [9].
Protein processing of IL-37b resembles that of other IL-1 family members. The N-terminal sequence of exon 1, which is present in all isoforms except IL-37a, contains a pro-domain that is cleaved by capsase-1 before maturation [6]. Once IL-37b is in the mature form, it is known to translocate to the nucleus upon LPS stimulation [10]. However, this is significantly reduced when a capsase-1 inhibitor is present.
Approximately 25% of LPS-induced endogenous IL-37 translocates to the nucleus [9] (Figure 2). It functions by reducing the production of pro-inflammatory cytokines induced by Toll-like receptor (TLR) agonists, IL-1, and tumor necrosis factor (TNF) [2]. IL-37 is released from the cell due to a loss of membrane integrity during cell death. The exact function of IL-37 released with cell necrosis remains unknown. Also, we still do not fully know whether IL-37 released from viable cells in vivo is the precursor or a mature cytokine cleaved by caspase-1. However, we do know now that mature IL-37 translocates into the nucleus in caspase-1-dependent manner, suggesting the release of mature form of IL-37 upon cell necrosis. Thus, IL-37 has both intra- and extra-cellular roles.
Figure 2.
Translocation of IL-37 to the nucleus in response to stimulation with LPS.
Biological Role of IL-37
Whereas IL-37 in PBMCs is up-regulated by TLR agonists such as IL-1β, IL-18, TNF-α, IFN-γ, and TGFβ, it is down-regulated by IL-12, IL-32, and GM-CSF plus IL-4 [2]. Because GM-CSF plus IL-4 stimulates the differentiation of monocytes to dendritic cells, there is an inverse relationship between expression of IL-37 and the activation of dendritic cells [3]. Therefore, the anti-inflammatory effects of IL-37 are most evident when dendritic cell activity is reduced.
In murine macrophage-like RAW264.7 cells overexpressing IL-37b, decreased levels of IL-1α, TNFα, IL-6, GM-CSF, M-CSF, and IL-1Ra, and increased levels of IL-13 were observed after inflammatory stimulation [2]. Also, in IL-37 transgenic mice challenged with LPS, there was significant reduction in the plasma level of many pro-inflammatory cytokines, including IL-6, IL-1β, IL-17, and IFN-γ with increase in plasma IL-4, IL-10 and IL-13 [2]. Based on these findings, it is possible that IL-37 increases Th2 immune deviation by increasing IL-4 and IL-13, together with the inhibition of Th1 and Th17 immune response. However, since cells other than Th2 lymphocytes could also release IL-4 and IL-13, it is critical to examine the effect of IL-37 on IL-5 and determine the direct or indirect effect of IL-37 to confirm Th2 immune deviation. Furthermore, IL-37 has the ability to decrease IL-1α and IL-6 cytokines as well as IL-18 and IFN-γ that are associated with a Th1 response, further supporting the regulatory role of IL-37 in controlling the pro-inflammatory immune response. In essence, these findings support the role of IL-37 in the immune deviation by regulating Th1, Th2 and Th17 cells under in vivo conditions.
The functional role of IL-37 was further studied by neutralizing secreted IL-37 in transfected RAW macrophages and in transgenic mice expressing IL-37 [11]. RAW IL-37 cells pre-incubated with goat anti-human IL-37-IgG were stimulated with LPS. These cells produced significantly less IL-6 than mock transfected cells. Because intracellular IL-37 is not targeted by anti-IL-37-IgG, it was not neutralized. Next, IL-37 transgenic mice pre-treated with the same antibody were challenged with LPS. Whereas anti-IL-37-IgG did not affect wild type mice, there was a 2.5-fold increase in serum IL-6 levels in transgenic mice pre-treated with anti-IL-37-IgG. Therefore, this antibody neutralized secreted IL-37, abrogating the anti-inflammatory effect of IL-37 after LPS administration in vivo.
Smad-3, a transcriptional modulator in the transforming growth factor-β (TGF-β) pathway, plays an important role in the biological effects of IL-37b by contributing to translocation of IL-37b to the nucleus [2]. In RAW264.7 cells and THP-1 overexpressing IL-37b, inhibition of Smad-3 (via anti-Smad-3 RNA) resulted in increased production of inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-8, and TNFα) compared to cells with normal Smad-3 levels. These results were confirmed in vivo where IL-37 transgenic mice with inhibited Smad-3 had increased levels of pro-inflammatory cytokines after LPS challenge [2]. Thus, inhibition of Smad3 abrogates IL-37 function.
Once IL-37b forms a functional complex with Smad-3, gene transcription is affected. Transcription of pro-inflammatory cytokines is suppressed via phosphorylation of STAT-1 to STAT-4, suppression of c-Jun, phosphorylation of p38 MAPK, and phosphorylation of GSK-3a/b [12]. By rendering certain aspects of pro-inflammatory signaling cascades inactive, IL-37 diminishes our innate immune response upon TLR stimulation (Figure 2).
Genetics of IL-37
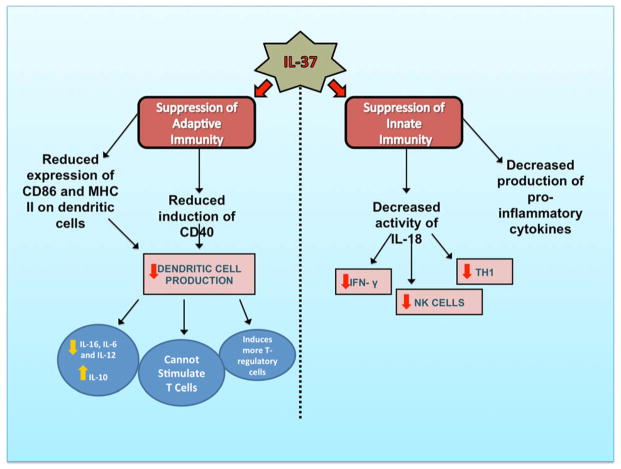
Whereas the gene for IL-37 is expressed on chromosome 2 in humans, mice have not been found to naturally express a gene corresponding to IL-37 [3]. Thus, transgenic mice expressing IL-37 have been compared to wild type mice in numerous studies. IL-37 protects transgenic mice from the consequences of LPS-induced shock such as hypothermia, metabolic acidosis, dehydration, increased potassium, and liver damage (compared to wild-type mice) [2]. Furthermore, transgenic IL-37 mice have decreased levels of pro-inflammatory cytokines, but it is important to note that there are no differences in anti-inflammatory cytokines (such as I-309, IL-13, and IL-10). IL-37 transgenic mice also have decreased activation of dendritic cells, which have a marked reduction in their expression of CD86 and MHC II after LPS challenge [2]. Continued utilization of transgenic IL-37 mice will be of use in future studies exploring the role of IL-37 in disease (Figure 3).
Figure 3.
An overview of the role of IL-37 in innate and adaptive immunity.
Role of IL-37 in inflammatory and autoimmune diseases
General Overview
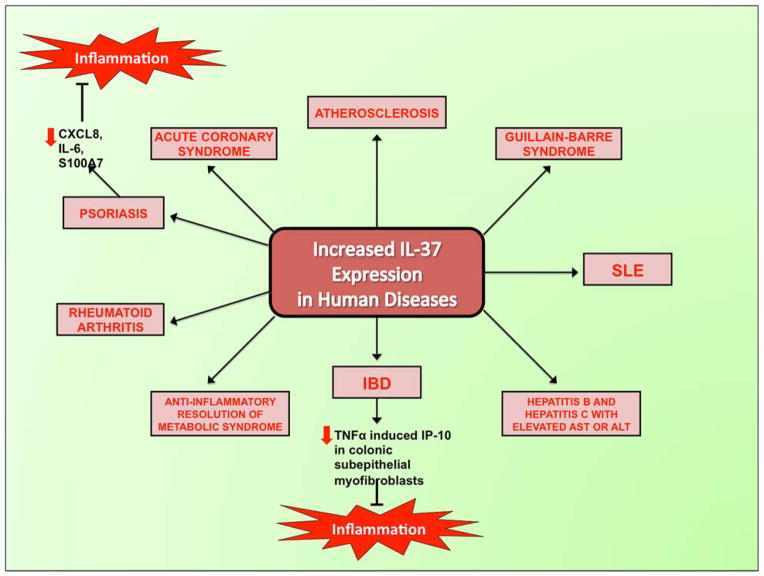
Increased expression of IL-37 has been found in several chronic inflammatory and autoimmune diseases. For instance, unpublished data demonstrates IL-37 up-regulation in Mycobacterium avium infections, atherosclerotic plaques, psoriatic plaques, Crohn’s disease, and lupus [3] (Figure 4). Furthermore, high levels of IL-37 are found in synovial tissue of active rheumatoid arthritis (RA), suggesting a protective effect of IL-37 in RA, a joint disease characterized by chronic inflammation [2].
Figure 4.
Increased expression of IL-37 in human diseases and induction of inflammation.
There are higher levels of the IL-37 protein in ductal mammary carcinomas than in melanoma, stroma of colon carcinoma, and lung carcinoma [6]. Furthermore, enhanced IL-37 staining in plasma cells and lymphoma cells may correlate with immunoglobulin production and B cell activation in multiple myeloma, B cell lymphoma, colon carcinoma, and inflammatory bowel disease [6]. Role of IL-37 in contact hypersensitivity and other skin diseases
By investigating the role of IL-37 in skin contact hypersensitivity, researchers have found that IL-37 not only suppresses innate immunity, but also inhibits adaptive immunity [4]. In transgenic IL-37 mice, contact hypersensitivity to a hapten antigen is impaired, as demonstrated by reduction in ear swelling compared to wild-type. Transgenic mice demonstrate reduced LPS induction of CD40, a co-stimulatory molecule for dendritic cells. Transgenic mice also have dendritic cells with decreased LPS-induced secretion of IL-1b, IL-6 and IL-12, whereas IL-10 secretion is increased. Dendritic cells expressing IL-37 do not stimulate naïve T cells as effectively as wild-type counterparts, and they induce more T-regulatory cells. Contact hypersensitivity is impaired in wild-type mice when sensitized dendritic cells are adoptively transferred to wild-type mice. These mice have decreased CD8+ T cells and increased T-regulatory cells. Thus, both branches of the immune system are affected by IL-37 (Figure 3).
Recently discovered IL-37-related conditions involve the skin. First, the role of IL-37 was studied in two models of psoriasis, an immune-mediated skin disease characterized by red, scaly patches [13]. When IL-37 was introduced to a human keratinocyte cell line (HaCaT), there was marked suppression in the production of pro-inflammatory cytokines such as CXCL8, IL-6, and S100A7. There were also potent immunosuppressive effects in keratin 14 VEGF-A–transgenic mice treated with a plasmid encoding human IL-37. Thus, these authors suggest a therapeutic role of IL-37 in psoriasis. Furthermore, IL-37 has been shown to function as a novel immunomodulatory therapy for atopic dermatitis [14].
IL-37 in autoimmune diseases: IBD, SLE, and GBS
In order to investigate the role of IL-37 in inflammatory bowel disease (IBD), transgenic mice expressing IL-37 were compared to wild type counterparts [15]. Both groups of mice were subjected to dextran sulfate sodium (DSS)-induced colitis. Since IL-37 inhibits innate inflammation and immunity, transgenic mice had clinical disease scores that were reduced by 50%, and histological indices of colitis that were one-third less than wild type mice. Transgenic mice had decreased leukocyte recruitment to the colonic lamina propria and decreased release of IL-1β and TNFα. Bone marrow from transgenic mice administered to wild type mice conferred protection from colitis. Thus, IL-37 originating from hematopoietic cells is sufficient to exert protective anti-inflammatory effects (Figure 4).
The role of IL-37 has been investigated in human IBD, comprised of Crohn’s disease and ulcerative colitis [16]. Whereas normal colonic mucosa does not express IL-37, elevated IL-37 finds expression in the inflamed mucosa of IBD patients and in human colonic epithelial cells. IL-37 was also analyzed in the human colonic epithelial cell line T84 in order to explore its molecular mechanisms. IL-37b mRNA and protein expression increased in these cells due to TNF-α. This occurred via activation of nuclear factor (NF)-κB and activator protein (AP)-1. This regulation of IL-37b expression in intestinal epithelial cells is different from the regulation observed in PBMCs. Lastly, these authors showed that IL-37b inhibits TNF-α-induced interferon-γ-inducible protein (IP)-10 in colonic subepithelial myofibroblasts. Thus, IL-37 is predicted to play an important anti-inflammatory role in IBD via negative feedback.
Another recent study looks into the role of IL-37 in patients with systemic lupus erythematosus (SLE) and the ability of glucocorticoids to regulate plasma levels of IL-37 and mRNA expression in PBMCs [17]. These authors conclude that anti-inflammatory cytokines like IL-37 are increased in most autoimmune diseases in order to compensate for increased pro-inflammatory cytokines. However, the anti-inflammatory cytokines cannot always fully compensate.
Similar to autoimmune diseases like IBD and SLE, IL-37 is elevated in Guillain-Barré Syndrome (GBS). GBS involves an immune attack against the peripheral nervous system, causing ascending paralysis and changes in sensation or pain. GBS patients had significantly higher IL-37 concentrations in plasma and cerebrospinal fluid (CSF) than healthy controls [18]. Moreover, pro-inflammatory cytokines such as plasma IL-17A, IFN-γ and TNF-α were positively correlated with plasma levels of IL-37 in GBS patients. CSF levels of IL-37 and IL-17A, and plasma levels of TNF-α were positively correlated with GBS disability scale scores. When intravenous immunoglobulin was administered, these particular cytokines were reduced.
IL-37 in obesity
The role of IL-37 has also been investigated in morbid obesity, which is associated with chronic inflammation [19]. This study compared expression if IL-1 family members before and after laparoscopic adjustable gastric banding surgery. Levels of IL-37 mRNA transcripts were higher in subcutaneous and visceral fat than in the liver. After surgery, IL-37 expression in fat increased, whereas liver expression remained stable before and after surgery. Thus, the anti-inflammatory state with increased IL-37 favors weight reduction after gastric banding surgery, suggesting a rapid anti-inflammatory resolution of metabolic syndrome.
IL-37 in hepatic disorders
Hepatitis has also been shown to improve in mice with transient IL-37 expression [20]. Mice were injected with concanavalin A (ConA) so that they would develop severe acute hepatitis. Two hours after ConA injection, mice previously injected with human IL-37 plasmid-DNA (via a hydrodynamic tail vein procedure) had significantly reduced levels of IL-1α, IL-6, IL-5, and IL-9. These pro-inflammatory cytokines, but not anti-inflammatory IL-10, were also reduced after LPS stimulation in serum from mice transiently expressing IL-37. In both cases, the effect was only sustained for 24 hours. This study helps elucidate the extracellular role of IL-37 in ConA-induced hepatitis and LPS-induced sepsis.
In a related study, IL-37 was also found to protect against inflammation induced by hepatic ischemia/reperfusion in mice [21]. Both hepatocyte injury and neutrophil activation were reduced in vivo after IL-37 treatment via decreased levels of hepatic reactive oxygen species (ROS), as well as decreased levels of serum TNF-α and macrophage inflammatory protein-2 (MIP-2). ALT levels were reduced by approximately 34% in mice treated with IL-37, indicating decreased liver injury. In vitro, IL-37 reduced production of MIP-2 and KC after hepatocytes and Kupffer cells were stimulated with LPS. There was reduced oxidative injury via increased expression of Bcl-2 in hepatocytes. This subsequently reduced cell death. Therefore, IL-37 may prove to be a therapeutic target for inflammatory liver disease.
IL-37 and hepatitis was further studied by comparing patients with chronic hepatitis B virus infection (HBeAg positive), patients with chronic hepatitis C, and healthy controls [22]. Although not statistically significant, serum IL-37 was elevated in in chronic HBV patients with high virus loads. Treating these patients with Telbivudine for 48 weeks resulted in decreased serum levels of IL-37. Serum IL-37 concentrations in hepatitis B and C patients with elevated serum ALT or AST were significantly higher than patients with normal levels of ALT or AST. Thus, in a chronic inflammatory state like hepatitis, elevated IL-37 finds expression.
IL-37 in cardiovascular diseases
Another IL-37 related disease that deserves our attention is atherosclerosis. IL-37 is expressed in the foam-like cells of atherosclerotic plaques [3]. Therefore, the involvement of IL-37 in Acute Coronary Syndrome (ACS) has been elucidated recently [23]. ACS includes unstable angina pectoris and acute myocardial infarction. Compared to patients with stable angina pectoris and healthy controls, plasma levels of IL-37, IL-18, and IL-18BP were significantly increased in ACS. The levels of IL-37 positively correlated with increased IL-18 and CRP levels, which are the biomarkers of inflammation. Therefore, increased IL-37 possibly resulted from the excessive inflammatory response in ACS.
A common theme on the role of IL-37 in human disease is that increased levels of IL-37 serve to buffer the excessive inflammation that occurs in chronic and autoimmune diseases. Thus, IL-37 plays an integral role in monitoring and regulating the natural checks-and-balances inherent to the human immune system.
Expert commentary and five-year review
Much has been discovered about IL-37 in the last decade. Yet, the exact role and the underlying immunomodulatory mechanisms of IL-37 in various inflammatory diseases warrant further attention. Additional careful studies are required to establish IL-37 as a biomarker or diagnostic tool. Whereas IL-37b is generally focused upon, it would be critical to investigate the role of other functional isomers, especially IL-37a and IL-37d. Furthermore, the exact role of capsase-1 in producing mature IL-37 is still unknown. Similarly, the role of IL-37 as a potential IL-18 antagonist is yet to be confirmed. Intracellular and extracellular functions of IL-37 should continue to be differentiated. Since most of the current data in in experimental animal models, the next step is to examine the effect and the underlying mechanisms in human diseases. Such information would be critical in designing pharmacological agents that either enhance or suppress IL-37 activity and could help ameliorate chronic inflammatory and autoimmune disorders. Potential use of such agents has been shown in atopic dermatitis. However, with the advancement in our knowledge on the specific role of IL-37 isoforms, specific therapeutic approaches in other conditions could be developed.
Key Issues/Points.
Interleukin-37 (IL-37) is an anti-inflammatory cytokine that inhibits both innate and adaptive immunity by down-regulating pro-inflammatory molecules and pathways.
Alternative splicing of IL-37 gives rise to five different isoforms: a, b, c, d, and e.
IL-37b, the most functional isoform, finds expression in cells throughout the body.
IL-37 is up-regulated as a natural defense mechanism in inflammatory states and in various autoimmune diseases.
IL-37 expression in transgenic mice decreases the severity of inflammatory bowel disease, severe acute hepatitis, and hepatic ischemia and reperfusion.
Increased levels of IL-37 in various cells and tissues of humans have been found in several pathological conditions or procedures, including weight reduction after gastric banding surgery, rheumatoid arthritis (RA), inflammatory bowel disease (IBD), hepatitis B and C, systemic lupus erythematosus (SLE), Guillain-Barré syndrome (GBS), psoriasis, atherosclerosis, and acute coronary syndrome (ACS).
Future research should focus on the precise mechanism of action of IL-37, as well as potential therapeutic roles of IL-37 enhancement or suppression.
Footnotes
Disclaimer
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial & competing interest disclosure
This work was supported by research grants from the National Institutes of Health, USA to DK Agrawal. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special interest have been highlighted as:
• Of interest
- 1.Dinarello CA, Arend W, Sims J, et al. IL-1 family nomenclature. Nat Immunol. 2010;11(11):973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–24. doi: 10.1038/ni.1944. IL-37 function in transgenic mice and the role of Smad3 are elucidated in this paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22(3):127–47. doi: 10.1684/ecn.2011.0288. The binding of IL-37 to IL-18Rα and other mechanistic information about IL-37 is elucidated here. [DOI] [PubMed] [Google Scholar]
- 4•.Luo Y, Cai X, Wang S, et al. IL-37 suppresses contact hypersensitivity by inducing tolerogenic dendritic cells. Cytokine. 2013;63:283. By inhibiting dendritic cell activation, IL-37 plays a role in adaptive immunity. [Google Scholar]
- 5.Dinarello CA, Bufler P. Interleukin-37. Semin Immunol. 2013 doi: 10.1016/j.smim.2013.10.004. http://dox.doi.org/10.1016/j.smim.2013.10.004. [DOI] [PubMed]
- 6.Kumar S, Hannin CR, Brigham-Burke MR, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18(2):61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 7.Bufler P, Azam T, Gamboni-Robertson F, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Nat Acad Sci USA. 2002;99(21):13723–28. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nold MF, Nold-Petry CA, Lo C, et al. Interleukin 37 employs the IL-1 family inhibitory receptor SIGIRR and the alpha chain of the IL-18 receptor to suppress innate immunity. Cytokine. 2013;63:287. [Google Scholar]
- 9.Bufler P, Gamboni-Robertson F, Azam T, Kim S-H, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381(1):503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Kulk N, Nold MF, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180(8):5477–82. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 11.Bulau A-M, Gruschka A, Schwaiger R, Dinarello CA, Bufler P. Anti-IL-37 antibody abrogates the protection in IL-37 transgenic mice to LPS-induced septic shock. Cytokine. 2013;63(3):250. [Google Scholar]
- 12.Akdis M, Burgler S, Crameri R, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701, 721.e1–70. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 13•.Teng X, Hu Z, Wei X, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing pro-inflammatory cytokine production. J Immunol. 2014;192(1):1815–1823. doi: 10.4049/jimmunol.1300047. This study suggests that IL-37 could play a therapeutic role in psoriasis. [DOI] [PubMed] [Google Scholar]
- 14•.Harskamp CT, Armstrong AW. Immunology of atopic dermatitis: novel insights into mechanisms and immunomodulatory therapies. Semin Cutan Med Surg. 2013;32(3):132–9. doi: 10.12788/j.sder.0018. This study suggests that IL-37 could play a therapeutic role in atopic dermatitis. [DOI] [PubMed] [Google Scholar]
- 15•.McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Nat Acad Sci USA. 2011;108(40):16711–16. doi: 10.1073/pnas.1111982108. The bone marrow from transgenic mice expressing IL-37 protected mice from developing colitis, suggesting a possible therapeutic role. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Imaeda H, Takahashi K, Fujimoto T, et al. Epithelial expression of interleukin-37b in inflammatory bowel disease. Clin Exp Immunol. 2013;172(3):410–416. doi: 10.1111/cei.12061. This study helps apply knowledge from McNamee et al. (2011) to human subjects and demonstrates that IL-37 serves as a defense mechanism in IBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L, Qiu F, Fan Y, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33(1):111–117. doi: 10.1007/s10875-012-9791-z. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Zhao P, Xiguang S, Che Y, Jiang Y. Elevated levels of cerebrospinal fluid and plasma interleukin-37 in patients with Guillain-Barré Syndrome. Mediat Inflamm. 2013;8(1):1–9. doi: 10.1155/2013/639712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moschen AR, Molnar C, Enrich B, Geiger S, Ebenbichler CF, Tilg H. Adipose and liver expression of IL-1 family members in morbid obesity and effects of weight loss. Molecular Medicine. 2011;17(7–8):840–845. doi: 10.2119/molmed.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulau A-M, Fink M, Maucksch C, et al. In vivo expression of Interleukin-37 reduces local and systemic inflammation in concanavalin A-induced hepatitis. The Scientific World JOURNAL. 2011;11(1):2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai N, Van Sweringen HL, Belizaire RM, et al. Interluekin-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Ji HF, Cai YJ, et al. Serum Interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. J Interferon Cytokine Res. 2013;33(10):612–618. doi: 10.1089/jir.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediat Inflamm. 2014;3(1):1–9. doi: 10.1155/2014/165742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DE, Renshaw BR, Ketchem RR, et al. Four new members expand the interleukin-1 superfamily. J Biol Chem. 2000;275(2):1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, McDonnell PC, Lehrt R, et al. Identification and initial characterization of four novel members of the Interleukin-1 family. J Biol Chem. 2000;275(4):10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 26.Busfield SJ, Comrack CA, Yu G, et al. Identification and gene organization of three novel members of the IL-1 family on human chromosome 2. Genomics. 2000;66(1):213–216. doi: 10.1006/geno.2000.6184. [DOI] [PubMed] [Google Scholar]
- 27.Pan G, Risser P, Mao W, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-Rrp. Cytokine. 2001;13(1):1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 28.Ji Q, Zeng Q, Huang Y, et al. Elevated plasma IL-37, IL-18, and IL-18BP concentrations in patients with acute coronary syndrome. Mediat Inflamm. 2014;3(1):1–9. doi: 10.1155/2014/165742. [DOI] [PMC free article] [PubMed] [Google Scholar]