Abstract
Objective
To establish the maximum tolerated dose of Clostridium novyi–NT spores in tumor-bearing dogs and evaluate spore germination within tumors and tumor response.
Animals
6 client-owned dogs.
Procedures
A standard dose-escalation study was planned, with maximum tolerated dose defined as the highest dose at which 0 or 1 of 6 dogs had dose-limiting toxicoses (DLT). Dogs received 1 dose of C novyi–NT spores IV. Toxicoses were graded and interventions performed according to specific guidelines. Grade 3 or higher toxicosis or any toxicosis combination that substantially affected patient status was considered DLT. Clinical response was measured by use of response evaluation criteria in solid tumors at 28 days.
Results
The first 2 dogs had DLT. The dose was decreased. Two of the next 4 dogs had DLT; therefore, dose administration was stopped because the study endpoint had been reached. The most common toxicosis was fever (n = 6 dogs). Two dogs developed abscesses (1 within a nasal carcinoma and 1 splenic abscess) attributable to C novyi–NT infection; both required surgical intervention. Clostridium novyi–NT was cultured from 1 of 6 tumors. Five dogs were available for response assessment (4 had stable disease; 1 had progressive disease).
Conclusions and Clinical Relevance
Results indicated that C novyi–NT can germinate within tumors of dogs. Toxicosis, although common and sometimes severe, was manageable with treatment. Further studies in dogs with superficial tumors may allow for continued dose escalation and provide information for use in clinical trials in veterinary and human oncology.
Malignant tumors have disorganized and immature vasculature, which leads to abnormal and inconsistent blood flow.1 In addition, the rapid cell growth of many tumors causes the size of the tumor to outgrow its existing blood supply. This combination of abnormal blood flow and rapid cell growth often leads to areas of hypoxia within tumors. These hypoxic areas represent locations within a tumor where the cells are protected from effective exposure to systemically administered chemotherapeutics. In addition, cells located in hypoxic areas tend to divide slowly (if at all) and are thus resistant to treatment modalities, such as traditional cytotoxic chemotherapy and radiation therapy, that specifically target rapidly dividing cells. Treatment strategies that target these hypoxic areas may provide a way to overcome this resistance and may result in improved anticancer activity while concurrently sparing healthy cells.
One method to target hypoxic areas of a tumor is to use attenuated anaerobic bacteria to lyse tumor cells and initiate an inflammatory immune response to the tumor. For close to a century, Clostridium spp have been evaluated when administered alone and in combination with standard cytotoxic treatments.2 Germination within tumors has been detected for almost all of the Clostridium spp studied, but severe (mostly lethal) toxicoses have been reported in laboratory animals.3–7 In mice that survive the treatment, there is tumor regrowth from well-oxygenated areas of the tumors, and even when this treatment is combined with chemotherapy or radiation therapy and hyperthermia, only partial tumor responses are detected.8–10 The administration of Clostridium spores to human cancer patients has been described in 2 reports,11,12 both of which indicated there was tumor lysis and abscessation.
Because of its motility and high sensitivity to oxygen, the potential antitumor efficacy of Clostridium novyi has been evaluated. Because of severe toxic effects (death) in initial studies, the lethal α-toxin from the wild-type strain was eliminated by selecting for clones without the toxin-associated phage episome, and the resulting strain, C novyi–NT, has been used in a preclinical study.13 Although no clinical toxicoses are observed when C novyi–NT spores are administered to immunocompetent mice and rabbits without tumors, histologic examination reveals minimal to moderate multifocal hepatitis.14 Preclinical studies13–17 in which investigators used a single IV injection of C novyi–NT spores in immunocompetent mice and rabbits bearing syngeneic tumors revealed a cure rate of up to 30%, which was higher when C novyi–NT spores were administered in combination with radiation, liposomal agents, and vascular-collapsing drugs. Responses to C novyi–NT are associated with an accumulation of inflammatory cells surrounding the hypoxic areas of infected tumors.15 Toxic effects detected after administration of C novyi–NT in mice and rabbits with tumors include lethargy, poor grooming, and death.14 Systemic hydration mitigates these toxic effects and prevents death without affecting the response of tumors to C novyi–NT.14 In addition, antimicrobial treatment prevents death but reduces the tumor response to C novyi–NT spores.14 Clostridium novyi–NT does not colonize non–tumor-associated hypoxic regions in rodents.14
Although C novyi–NT seems to be a promising novel treatment for cancer, reports of its use in companion animals or humans with naturally occurring tumors have not yet been published to the authors’ knowledge. A recent report18 illustrates the advantages of incorporating companion animal studies into anticancer drug development. For example, dogs share our environment and are genetically outbred and immunocompetent and develop tumors, many of which are extremely similar to tumors in humans. The opposite is true for most small laboratory animals used in experimental settings with implanted tumors. These critical distinctions suggest that studying naturally occurring cancer in privately owned dogs may provide results that are more clinically applicable to human oncology than are results obtained from studies of induced tumors in laboratory animal species.
A phase I study of C novyi–NT in veterinary cancer patients would improve knowledge about the efficacy and toxic effects of this treatment in patients with naturally occurring tumors, and this information could then be applied to future veterinary and human clinical trials. The primary purpose of the study reported here was to evaluate toxic effects and establish the MTD of C novyi–NT spores in dogs with naturally occurring tumors. Secondary goals were to evaluate evidence of spore germination within tumor tissue and assess tumor response to the treatment.
Materials and Methods
Animals
Client-owned dogs with naturally occurring tumors of any histopathologic type were recruited to participate in the study. Dogs for which standard treatment failed or whose owners declined such treatment were eligible for enrollment. In addition, dogs were required to weigh > 15 kg, have no evidence of organ dysfunction on the basis of physical examination or hematologic analysis, and have at least 1 primary or metastatic tumor > 2 cm in diameter. Dogs with evidence of active bacterial infection as determined on the basis of history or results of physical examination or a CBC were excluded from the study. Similarly, dogs currently receiving antimicrobial treatment because of confirmed or suspected infection were excluded from the study. The washout period from cytotoxic chemotherapy, radiation therapy, or corticosteroid administration was 3 weeks. In patients receiving NSAIDs, administration of the drug was discontinued 72 hours prior to C novyi–NT administration. The treatment protocol was approved by the University of Pennsylvania Institutional Animal Care and Use Committee, and informed owner consent was obtained for each dog in the study prior to enrollment.
Tumor staging was performed on the basis of the specific biological behavior of each dog’s tumor. A CBC, serum biochemical analysis, urinalysis, and thoracic radiography were performed on all dogs. Depending on the tumor type, some dogs underwent abdominal ultrasonography, neck ultrasonography, or computed tomography of the primary tumor.
The study was planned as a standard dose escalation experiment with 3 dogs/cohort. Analysis of preclinical data revealed that a dose of 2.5 × 108 spores/kg was associated with no clinical or histopathologic toxicosis in healthy rabbits14; therefore, a starting dose of 3 × 108 spores/kg was chosen for the first cohort. All dogs in a given cohort were administered spores before proceeding to the next cohort. If DLT was not detected, each of the dogs in the next cohort was scheduled to receive a 3-fold increase in dose. The DLT was defined as any single toxic effect more severe than grade 2 (according to the Veterinary Cooperative Oncology Group‘s common terminology criteria for adverse events19) or a combination of less severe toxic effects that substantially compromised the dog’s clinical condition. If DLT was detected in 0 of 3 dogs in a cohort, the dose was escalated for the next cohort. If DLT was detected in 1 of 3 dogs, an additional 3 dogs were enrolled in the same cohort at the same dose. If no DLT was detected in the additional 3 dogs, the dose was escalated for the next cohort. If ≥ 1 of the additional 3 dogs had DLT, the dose was decreased to the dose for the previous cohort. If DLT was detected in > 1 of 3 dogs within a cohort, the dose was decreased to the dose for the previous cohort. The MTD was defined as the highest dose at which 0 or 1 of 6 dogs had DLT. All toxic effects were recorded during the infusion and in-hospital monitoring period and at follow-up examinations. Toxic effects for which there was no grading scheme in the Veterinary Cooperative Oncology Group’s common terminology criteria for adverse events were graded according to presumed clinical severity as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4), and related to death (grade 5). Toxic effects were temporally described as acute (within 72 hours after spore administration), subacute (3 to 14 days after spore administration), and delayed (> 14 days after spore administration).
Drug administration and monitoring
The dose of C novyi–NT was administered IV following pretreatment assessment that consisted of physical examination, ECG evaluation, and measurement of rectal temperature, blood pressure, heart rate, and respiratory rate. The first 2 dogs were administered a test dose (10% of the total dose) and monitored for 30 minutes. If toxic effects were not detected, the remainder of the dose was administered. A test dose was not administered to the subsequent 4 dogs. These latter 4 dogs were administered diphenhydramine at home (2 mg/kg, PO) the night before and again at the hospital (2 mg/kg, IM) 30 minutes before spore administration because acute toxic effects detected in the first 2 dogs were attributed to a hypersensitivity reaction to the spores.
All dogs were hospitalized for continuous monitoring and IV administration of fluids for 72 hours after spore administration. Specific monitoring variables included rectal temperature, blood pressure, heart and respiratory rates, mental and overall status, and evidence of hives or erythema. A CBC and serum biochemical analyses were performed 72 hours after spore administration. Therapeutic interventions for expected toxic effects were implemented in accordance with a standard protocol, and other toxicoses were treated as necessary. Specific criteria for therapeutic interventions included rectal temperature > 39.7°C, systolic arterial blood pressure < 90 mm Hg, mean arterial blood pressure < 70 mm Hg, vomiting, and grade 2 nausea (lip smacking and hypersalivation). Examples of standard treatments included NSAID (carprofen) or antimicrobial (metronidazole, ticarcillin-clavulanate, or amoxicillin-clavulanate) administration for fever, increased IV administration of crystalloid fluids or colloid fluids for hypotension, and dolasetron or maropitant for nausea or vomiting.
Blood samples for anaerobic bacterial culture were collected at 0, 1, 3, 6, 24, and 72 hours (time of spore administration was designated as time 0). Fine-needle aspirates of tumors were obtained at 72 hours after spore administration when possible for cytologic examination and anaerobic culture. Dogs were returned for a physical examination, CBC, serum biochemical analysis, and urinalysis 1, 2, 4, 8, and 12 weeks after spore administration. Additional blood samples were collected for anaerobic bacterial culture, and when feasible, fine-needle aspirate samples of tumors were obtained for cytologic analysis and anaerobic culture 1, 2, and 4 weeks after spore administration. Owners were instructed to record any signs of illness in their dog in a daily log for 14 days after spore administration. If at any point after spore administration dogs developed a tumor-associated abscess and clinical signs or laboratory abnormalities consistent with sepsis, they received treatment, such as surgical debridement and antimicrobial treatment, at the discretion of the attending clinician.
Tumor response
Tumor response was assessed via tumor measurements and tumor staging at 4 weeks after spore administration. Tumor measurements were performed with hand calipers, ultrasonographic measurements, or computed tomographic measurements, depending on tumor location. The longest diameter of each target lesion was recorded. Response to spore administration was defined in accordance with response evaluation criteria in solid tumors (complete response, resolution of all target lesions; partial response, ≥ 30% decrease in the sum of the longest diameter of the target lesions, compared with the baseline sum of the longest diameter of the target lesions; stable disease, ≤ 30% decrease or < 20% increase in the sum of the longest diameter of the target lesions, compared with the baseline sum of the longest diameter of the target lesions; and progressive disease, ≥ 20% increase in tumor size or development of new tumors).20 Continued monitoring (physical examination, CBC, and serum biochemical analysis) was performed monthly in dogs with stable disease, a partial response, or a complete response until the study endpoint at 12 weeks after spore administration. Owners whose dogs were alive 12 weeks after spore administration were contacted periodically for updates on the dog’s status, and follow-up evaluations were scheduled as necessary. Dogs with progressive disease were removed from the study and were provided with appropriate palliative treatments according to owner and clinician discretion.
Endpoints for this study were the development of DLT, establishment of MTD, and the development of progressive disease. The response rate (complete response and partial response) was determined at 4 weeks after spore administration.
Results
Animals
Six dogs were enrolled in the study. Mean ± SD age was 8.7 ± 4 years, and there were 4 castrated males and 2 spayed females. Each dog was of a different breed; breeds included English Setter, Standard Poodle, Newfoundland, Labrador Retriever, Basset Hound, and mixed. Three dogs received C novyi–NT spores as the first treatment for their tumor. Prior treatment in the other 3 dogs included surgery (n = 1 dog), surgery and chemotherapy (1), and surgery, chemotherapy, and radiation therapy (1).
Toxicosis
The first 2 dogs (cohort 1) had DLT 1 hour after spore administration. The DLT in these dogs included a combination of grade 1 and grade 2 toxic events in one dog and a combination of grade 1, grade 2 and grade 4 toxic events in the other dog. Because of the severity of DLT in the first 2 dogs, the dose was decreased by 90% for the second cohort of dogs. In addition, a pretreatment protocol consisting of administration of diphenhydramine was initiated. One of the 3 dogs in the second cohort (3 × 107 spores/kg) had DLT as determined on the basis of a tumor abscess that required extensive surgical debridement; therefore, additional dogs were recruited for the second cohort. A second dog in the second cohort also had DLT as determined on the basis of a spleen abscess and resultant septic peritonitis. The study was stopped and further recruitment was discontinued because the starting dose remained above or equal to the MTD.
The most common acute toxic effect detected was fever, which was detected in all 6 dogs (Figure 1; Table 1). Five dogs were administered an NSAID (carprofen), which resulted in a decrease in rectal temperature. One dog in cohort 1 did not receive an NSAID. However, both dogs in cohort 1 received antimicrobial treatment (metronidazole, ticarcillin-clavulanate, and amoxicillin-clavulanate). Antimicrobial treatment in the dog of cohort 1 that did not receive an NSAID deviated from the study protocol when bacterial culture and susceptibility testing of an endotracheal wash sample revealed Escherichia coli that was resistant to amoxicillin-clavulanate. Treatment via IV administration of fluid boluses and antiemetic and antidiarrheal medications was effective at ameliorating other toxic effects. Treatment was not required for cardiac (ventricular arrhythmia) and neurologic (proprioceptive deficits) toxic effects.
Figure 1.
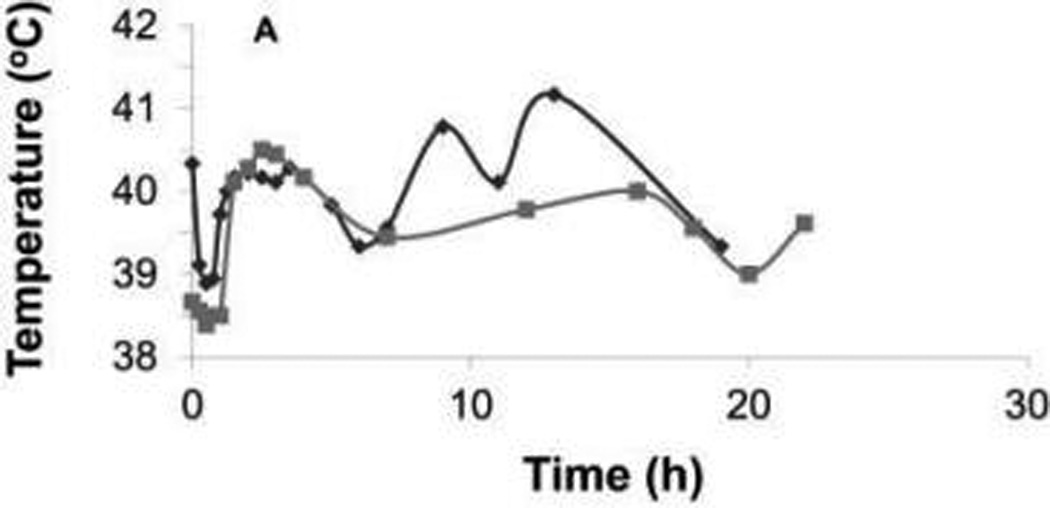
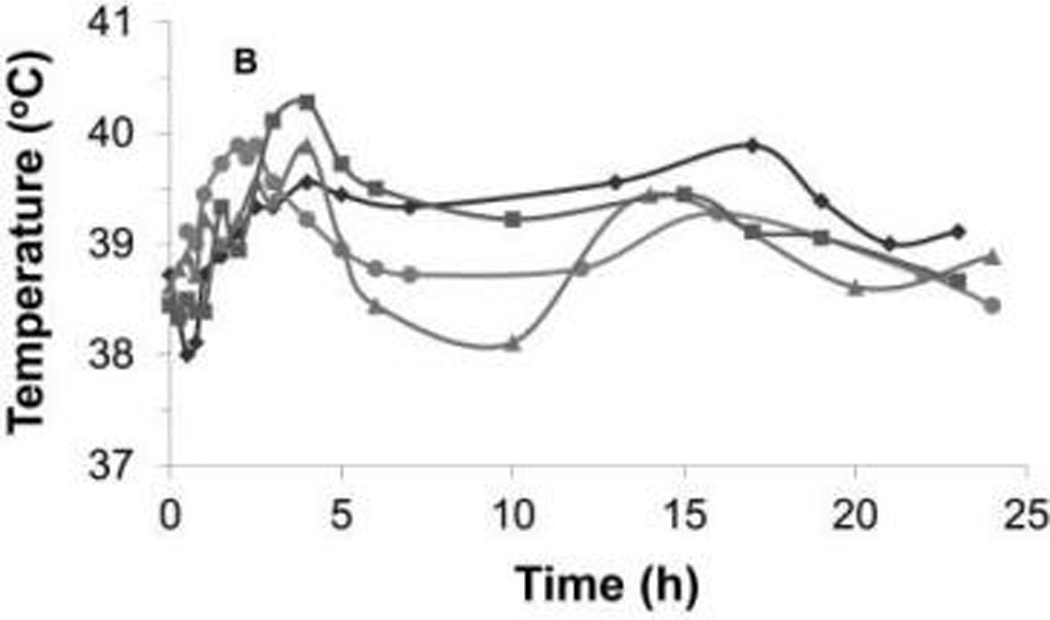
Rectal temperature during the first 24 hours after injection of Clostridium novyi–NT spores in each dog of cohort 1 (A) and cohort 2 (B). Notice that the maximum rectal temperature during the first 24 hours was higher in cohort 1 than in cohort 2, but the temperature decreased with medical interventions in each dog of both cohorts. Each symbol represents results for 1 dog.
Table 1.
Tumor diagnosis, dose of Clostridium novyi–NT spores, and type and severity of clinical toxic effects for each cohort of dogs.
| Acute toxic effects | Subacute toxic effects | |||||
|---|---|---|---|---|---|---|
| Cohort No. | Tumor diagnosis | Dose (No. of spores/kg) | Type | Grade | Type | Grade |
| 1 (2) | Subcutaneous hemangiosarcoma and lingual squamous cell carcinoma | 3 × 108 | Fever | 2 (2) | Inappetence | 2 (2) |
| Lethargy | 2 (1) | Diarrhea | 2 (1) | |||
| 1 (1) | ||||||
| Cardiac | 1 (2) | Fever | 1 (1) | |||
| Nausea | 2 (1) | |||||
| Diarrhea | 2 (1) | |||||
| Hypotension | 1 (1) | |||||
| Vomiting | 1 (1) | |||||
| Neurologic | 1 (1) | |||||
| 2 (4) | Maxillary osteosarcoma, nasal adenocarcinoma, shoulder fibrosarcoma, and anal sac adenocarcinoma | 3 × 107 | Fever | 1 (4) | Diarrhea | 2 (1) |
| Hypotension | 1 (2) | Inappetence | 2 (1) | |||
| 1 (1) | ||||||
| Nausea | 2 (2) | Fever | 2 (2) | |||
| 1 (1) | ||||||
Values in parentheses are the number of dogs.
Most acute hematologic and serum biochemical abnormalities were mild to moderate; however, one dog in cohort 1 required a blood transfusion because of grade 4 anemia and the other dog in cohort 1 was treated with supplemental electrolytes and fluids because of grade 1 hypokalemia, grade 2 hypophosphatemia, and grade 1 hypoproteinemia.
The most common subacute toxic effect was a decrease in appetite in 4 dogs (Table 1). Inappetence was treated with dolasetron in 2 dogs (1 dog in cohort 1 and 1 dog in cohort 2) but resolved without specific treatment in 2 dogs (1 dog in cohort 1 and 1 dog in cohort 2). One dog in cohort 1 developed grade 1 fever that resolved without specific intervention. Fever in 2 dogs in cohort 2 was associated with abscess development (Figure 2). Subacute hematologic and serum biochemical abnormalities were mild to moderate, and all resolved or there was improvement by day 14 after spore administration.
Figure 2.
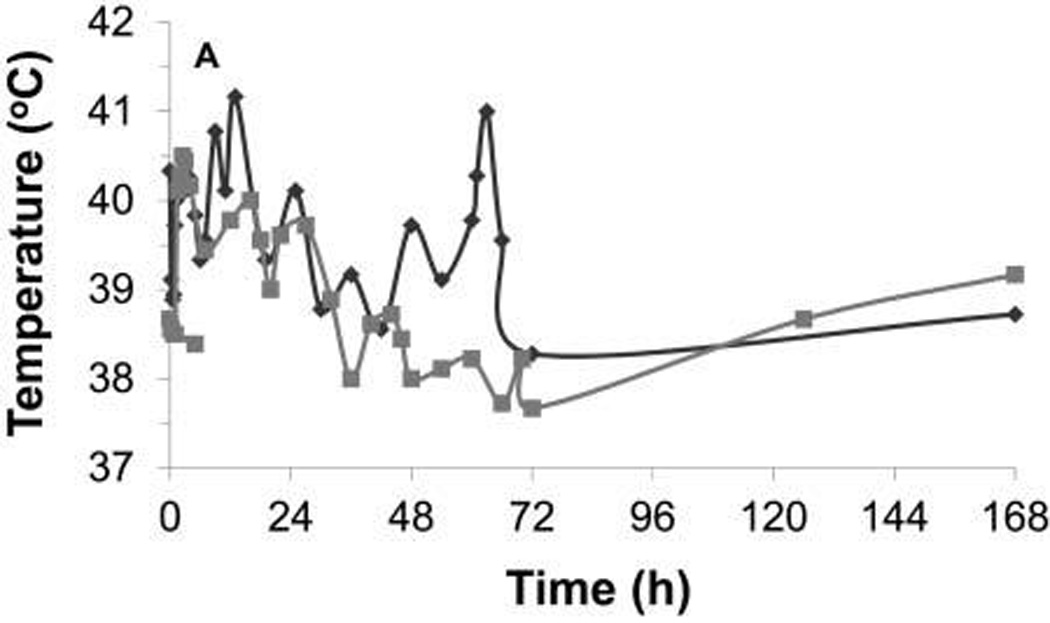
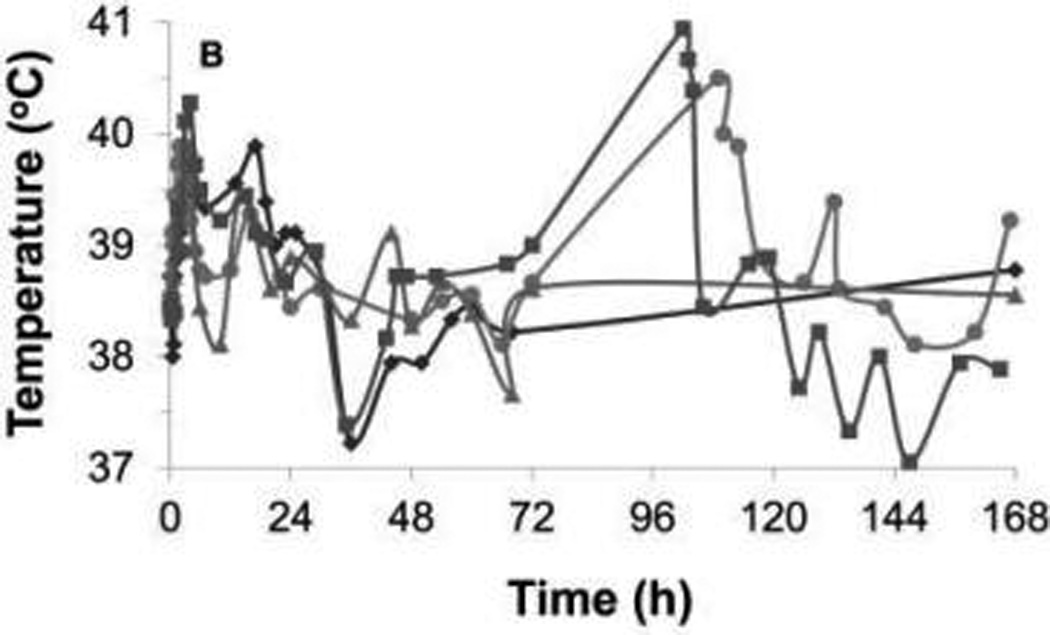
Rectal temperature during the first 7 days after injection of C novyi–NT spores in each dog of cohort 1 (A) and cohort 2 (B). In cohort 1, a fever developed in 1 dog at 60 hours, after carprofen and antimicrobial treatment had been discontinued for 35 hours; the fever resolved on reinstitution of these treatments. In cohort 2, 2 dogs that developed abscesses developed fevers on the fifth day after injection of spores. Each symbol represents results for 1 dog.
No delayed clinical toxic effects were detected. Delayed hematologic and serum biochemical abnormalities were mild, except for 1 episode of grade 3 azotemia in a dog of cohort 1. The azotemia resolved within 24 hours after initiation of IV administration of fluids and treatment with dolasetron and famotidine. Carprofen administration was discontinued in 1 dog in cohort 2 because of grade 1 elevations in alanine aminotransferase and alkaline phosphatase activities, which improved after discontinuation of carprofen administration.
Spore germination and tumor response
Three dogs (1 dog in cohort 1 and 2 dogs in cohort 2) developed abscesses 5 days after spore administration. Abscesses were located in a cervical lymph node with metastatic carcinoma of 1 dog in cohort 1, a nasal carcinoma of 1 dog in cohort 2, and the spleen of another dog in cohort 2 that had metastasis of an anal sac adenocarcinoma to the regional lymph nodes. Treatment for these dogs was determined on the basis of clinical status and abscess location; treatment included manual extrusion of purulent material followed by application of warm compresses, surgical debridement, and splenectomy and peritoneal lavage in 1 dog each. Additional treatment, consisting of IV administration of fluids, antimicrobials, NSAIDs, and/or pain medication, was provided at the discretion of the attending clinician. Cytologic evaluation of the abscessed lymph node from the dog in cohort 1 revealed suppurative inflammation and carcinoma but no bacteria, and results of anaerobic bacterial culture were negative. Inflammation and bacteria were seen in biopsy samples from both dogs in cohort 2, but carcinoma was seen in samples from only 1 dog. Anaerobic culture confirmed that the bacteria were Clostridium spp. Bacteria were identified as C novyi on the basis of DNA sequencing by use of a bacterial identification system,a and it was presumed that they were C novyi–NT.
Fine-needle aspirates were obtained from nonabscessed tumors of 2 additional dogs in cohort 2. Clostridium organisms were grown in anaerobic culture from a sample obtained on day 7 after spore administration from a maxillary osteosarcoma that extended into the oral cavity, but the organism was not available for analysis via the bacterial identification system.a Thus, confirmation that the organisms were C novyi–NT was not possible. Other bacterial cultures of tumor samples obtained from that dog yielded negative results. One fine-needle aspirate from a tumor of the other dog was obtained at day 59 after spore administration; anaerobic culture of that sample yielded negative results. Results of anaerobic cultures of blood samples yielded C novyi–NT for samples obtained at 1 (n = 6 dogs), 3 (6), 6 (5), 24 (2), and 72 (1) hours and 7 (2) and 14 (2) days after spore administration.
Progressive disease was detected in 1 dog in cohort 1 at day 14 after spore administration. This dog had a hemangiosarcoma and received a 14-day course of metronidazole and amoxicillin-clavulanate, which was followed by doxorubicin. Tumor staging for the remaining 5 dogs was performed on day 28 after spore administration. One dog in cohort 2 had progressive disease, and 4 dogs (1 dog in cohort 1 and 3 dogs in cohort 2) had stable disease. There was considerable regression in the size of the abscessed lymph node in the dog of cohort 1, but the size of metastatic retropharyngeal lymph nodes in that same dog did not change.
Follow-up evaluations
Five dogs died as a result of tumor progression at 28, 44, 44, 383, and 535 days after spore administration. One dog in cohort 1 died at 58 days after spore administration as a result of complications of previous glossectomy, presumed xerostomia attributable to previous radiation therapy, and recurrent aspiration pneumonia. Necropsy was performed on all dogs, and samples were obtained for bacteriologic culture. Necropsy results confirmed the original tumor diagnosis in all 6 dogs. Bacilli were observed in the spleen of 2 dogs (1 dog in cohort 1 and 1 dog in cohort 2) during histologic examination. Samples for bacteriologic culture were obtained from the spleen of one of those dogs, and growth of C novyi–NT was detected by use of bacteriologic culture and identification.a This dog did not require antimicrobials after spore administration because it had an acute fever that responded to NSAIDs. Necropsy samples for anaerobic culture were obtained in cohort 1 dogs from pleural effusion (n = 1 dog), the liver (1), and cervical and retropharyngeal lymph nodes (1) with cancerous metastases. Necropsy samples for anaerobic culture were obtained in cohort 2 dogs from the tumor (n = 4 dogs), liver (4), kidneys (4), muscle (4), spleen (3), lungs (3), sublumbar lymph nodes (1), mediastinal lymph node (1), and brain (1). Clostridium novyi–NT was cultured from the tumor sample obtained from 1 dog; all other samples yielded negative results when cultured for C novyi–NT.
Discussion
Results of the study reported here provide information regarding treatment feasibility, toxicoses, and potential antitumor effect of C novyi–NT spores in dogs with naturally occurring tumors. To the authors’ knowledge, this is the first report of the use of this agent in clinical patients with naturally occurring tumors, and the results can be used to develop additional clinical trials for canine and human patients.
Most toxic effects as a result of C novyi–NT spore administration in the present study occurred at 2 time points (within the first 24 hours and again at 5 days after spore administration). In mice and rabbits, spore germination occurs within 5 days after spore administration.14 The acute fevers in the present study responded quickly to treatment with NSAIDs and were not associated with other signs of sepsis in most dogs. Furthermore, the severity of the fever was mitigated by reduction in the C novyi–NT dose and pretreatment of dogs with diphenhydramine. Therefore, toxic effects within the first 24 hours after spore administration may have been attributable to a hypersensitivity or immune response to the spores, rather than a result of spore germination. It is worthy of mention that this toxic effect has not been detected in preclinical studies14,15 in animals. Abscesses consistently developed at 5 days after spore administration. The abscesses were associated with fever in 2 of 3 dogs. The dog that did not have a fever had a smaller abscess than did the other 2 dogs and was receiving antimicrobial treatment at the time of abscess formation, whereas the other 2 dogs were not. Interestingly, all 3 dogs that developed abscesses had carcinomas, and none of the dogs with sarcomas developed an abscess. This result suggests that C novyi–NT may be more likely to germinate in carcinomas.
Most toxicoses in the present study were mild to moderate and manageable with medication; however, there were severe (and potentially life-threatening) toxicoses necessitating aggressive surgical and medical treatment, and further enrollment of dogs was discontinued because of the toxic effects. Grade 4 anemia developed in a dog with hemangiosarcoma and was likely the result of tumor-related hemorrhage. Surgical debridement or removal of abscessed tissue was necessary in 2 dogs. In 1 dog, the location of the tumor (nasal carcinoma extending through the cribriform plate and around the right orbit) necessitated extensive surgical debridement. Evaluation of a biopsy specimen revealed carcinoma with necrosis and neutrophilic inflammation, and bacteriologic culture revealed growth of C novyi–NT. The abscess that developed in this dog confirmed the ability of C novyi–NT to germinate within and infect tumor tissue in dogs.
Positive results of bacterial culture of blood samples could have been attributable to spores or live bacteria, given that spores could germinate in culture and yield a positive result. It has been reported14 that C novyi–NT spores are sequestered in the spleen after IV injection, so it is not surprising that the bacteria were cultured from the spleen in 2 dogs in the present study. However, the non–tumor-related splenic abscess was an unexpected and serious toxic effect because a previous study14 in rodents did not reveal germination of C novyi–NT spores in non–tumor-related hypoxic tissue. Examination of the splenic biopsy specimen from this dog revealed bacterial colonies and areas of necrosis but no tumor, and to our knowledge, the present study is the first in which spore germination in non–tumor-associated tissue has been reported. However, it remains possible that preexisting tumor lesions had been colonized and eradicated by C novyi–NT. It is unknown whether tumor cells had been present previously in that dog’s spleen. However, the fact that there were areas of necrosis in the spleen suggests that the spleen may have contained tumor and that germinating spores eliminated the tumor cells, which left necrotic residual tissue. The spleen is considered a potential metastatic site for anal sac adenocarcinoma in dogs.21
Dose escalation and determination of MTD were not achieved as planned; DLT was detected in 2 patients in each dose cohort, so the study was discontinued before there was dose escalation. In fact, the dose was decreased because of the severity and acute nature of the DLT for the first 2 dogs. As such, the exact MTD of C novyi–NT spores in dogs with naturally occurring tumors is unknown. In addition, it is difficult to evaluate DLT in this study on the basis that abscess development is an expected and desired outcome because it indicates spore germination within tumors. The extensive nature of the tumor-related abscess and the non–tumor-related abscess was considered DLT because they were associated with high fevers and necessitated major surgical procedures.
It is clear from the results of the present study that C novyi–NT is associated with considerable risk of toxic effects in dogs. The DLTs were thought to be primarily related to an immediate hypersensitivity reaction to the spores as well as to abscess development from spore germination. To achieve the potential maximum antitumor effect, the goal is to delay the initiation of antimicrobial treatment as long as possible. The challenge is to deliver a dose that is high enough to establish abscessation and subsequent eradication of the tumor but low enough to prevent serious morbidity and death. Avoiding or delaying the use of antimicrobials may be necessary to determine the true potential of C novyi–NT as an anticancer agent, but this would require a carefully designed study with specific case selection. For example, exclusion of patients with internal tumors or tumors that would require extensive surgical debridement if an abscess developed could decrease the necessity for aggressive and expensive abscess treatment. In addition, screening patients via abdominal ultrasonography and excluding patients with nodules or tumors on the spleen may help to decrease the risk of spore germination and abscess development in this organ. Results from the present study can be used to design additional clinical trials in which investigators evaluate the antitumor efficacy of this agent and implement strategies to decrease its toxic effects. However, if severe toxicosis (eg, abscesses in nontumor tissues that require extensive treatment) continues to be detected, the risk-to-benefit ratio of administration of C novyi–NT in veterinary patients must be seriously considered.
Despite the limitations and small number of dogs in this study, the results are promising regarding future evaluation of C novyi–NT in canine and human patients with naturally occurring tumors. Toxic effects of the agent were medically and, when necessary, surgically manageable, and evidence of spore germination in the tumor of one dog and suspicion of germination in another dog suggests that C novyi–NT is able to colonize naturally occurring tumors in dogs. In addition, future studies with more specific patient selection may allow for more accurate determination of the feasibility, MTD, and antitumor efficacy of C novyi–NT in dogs with naturally occurring tumors, and the results of those studies may aid in the design of clinical trials in human cancer patients.
Acknowledgments
Supported by the Commonwealth Foundation, the Miracle Foundation, and National Institutes of Health grants CA062924 and CA129825.
Abbreviations
- DLT
Dose-limiting toxicity
- MTD
Maximum tolerated dose
Footnotes
Presented in part as an oral presentation at the American College of Veterinary Internal Medicine Forum, Anaheim, Calif, June 2010.
MicroSeq 500 16S rDNA Bacterial Identification Kit, Applied Biosystems, Foster City, Calif.
References
- 1.Sturk C, Dumont D. Angiogenesis. In: Tannock IF, Hill RP, Bristow RG, et al., editors. The basic science of oncology. 4th ed. New York: McGraw-Hill; 2005. pp. 231–248. [Google Scholar]
- 2.Barbe S, Van Mellaert L, Anne J. The use of clostridial spores for cancer treatment. J Appl Microbiol. 2006;101:571–578. doi: 10.1111/j.1365-2672.2006.02886.x. [DOI] [PubMed] [Google Scholar]
- 3.Torrey JC, Khan MC. The treatment of Flexner-Jobling rat carcinomas with bacterial proteolytic ferments. J Cancer Res. 1927;11:334–376. [Google Scholar]
- 4.Connell H. The study and treatment of cancer by proteolytic enzymes. Can Med Assoc J. 1935;33:364–370. [PMC free article] [PubMed] [Google Scholar]
- 5.Parker RC, Plummer HC, Siebenmann CO, et al. Effects of histolyticus infections and toxin on transplantable mouse tumors. Proc Soc Exp Biol Med. 1947;66:461–465. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 6.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:743–748. [PubMed] [Google Scholar]
- 7.Mose JR, Mose G. Oncolysis by clostridia. I. Activity of Clostridium butyricum (M-55) and other non-pathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 8.Thiele EH, Arison RN, Boxer GE. Oncolysis by clostridia III. Effects of clostridia and chemotherapeutic agents on rodent tumors. Cancer Res. 1964;24:222–233. [PubMed] [Google Scholar]
- 9.Schlechte H, Schwabe K, Mehnert WH, et al. Chemotherapy for tumours using clostridial oncolysis, antibiotics and cyclophosphamide: model trial on the UVT 15264 tumour. Arch Geschwulstforsch. 1982;52:41–48. [PubMed] [Google Scholar]
- 10.Gericke D, Dietzel F, Ruster I. Further progress with oncolysis due to local high frequency hyperthermia, local x-irradiation and apathogenic clostridia. J Microw Power. 1979;14:163–166. doi: 10.1080/16070658.1979.11689147. [DOI] [PubMed] [Google Scholar]
- 11.Carey RW, Holland JF, Whang HY, et al. Clostridial oncolysis in man. Eur J Cancer. 1967;3:37–46. [Google Scholar]
- 12.Heppner F, Mose JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir. 1978;42:123–125. doi: 10.1007/BF01406639. [DOI] [PubMed] [Google Scholar]
- 13.Dang LH, Bettegowda C, Huso DL, et al. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz LA, Cheong I, Foss CA, et al. Pharmacologic and toxicologic evaluation of C. novyi–NT spores. Toxicol Sci. 2005;88:562–575. doi: 10.1093/toxsci/kfi316. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal N, Bettegowda C, Cheong I, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheong I, Huang X, Bettegowda C, et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science. 2006;314:1308–1311. doi: 10.1126/science.1130651. [DOI] [PubMed] [Google Scholar]
- 17.Bettegowda C, Dang LH, Abrams R, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci U S A. 2003;100:15083–15088. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 19.Veterinary Co-operative Oncology Group. Common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. A consensus statement from the Veterinary Co-operative Oncology Group. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Turek MM, Withrow SJ. Perianal tumors. In: Withrow SJ, Vail DM, editors. Withrow and MacEwen’s small animal clinical oncology. 4th ed. Philadelphia: Saunders; 2007. pp. 503–510. [Google Scholar]


