Abstract
Though incidence is declining, the prognosis of lung cancer remains poor. This is likely due to lack of early detection and only recent developments in selective cancer therapies. Key immune cells involved in the pathogenesis of lung cancer include CD4+ T-lymphocytes, macrophages, dendritic cells, and natural killer cells. The growing understanding of these cells indicates a highly complex and intertwined network of their involvement in each stage of lung cancer. Immune cell types and numbers affect prognosis and could offer an opportunity for clinical therapeutic applications. However, an incomplete understanding of immune cell involvement and the underlying processes in lung cancer still remain. More investigation focusing on the role of the immune cells will further the understanding of lung carcinogenesis and develop novel therapeutic approaches for the treatment and management of patients with more specialized and selective lung cancer.
Keywords: Dendritic cells; Helper T cells; Immunotherapy; Lung cancer; Macrophages, Natural killer cells; Non-small cell lung cancer; Th17 cells; T-regulatory cells
Introduction
Lung cancer is the leading cause of cancer deaths in the United States, with an estimated 159,260 deaths (about 27% of all cancer deaths) expected in 2014 [1]. The lung cancer incidence rate has declined since the mid-1980s in men and late 1990s in women, associated with reduced smoking prevalence and increased tobacco control [2]. In spite of this reduced incidence, the prognosis remains poor. Lung cancers are classified as small cell (14%) or non-small cell (84%) lung carcinoma with 5-year survival at 6% and 18%, respectively [3]. Lack of effective early detection methods largely contribute to the poor prognosis. Over two-thirds of patients present with regional lymph node involvement or distant disease [4], whereas early detection at a localized stage (currently only 15% of diagnoses) increases 5-year survival to nearly 60% [3].
In acting as the natural defense of human body against disease, the immune system inevitably plays a critical and multifaceted role in lung cancer. The immune editing hypothesis discusses immune involvement in controlling quantity and quality of tumor development [5]. Immunosuppression [6] and immune cell tumor infiltration [7,8] are respectively associated with incidence and recurrence rates of lung and other cancers, suggesting that evaluation of the immune response in and around a tumor should be included in prognosis and treatment decisions [9]. However, the immune defense against cancer is clearly prone to malfunction and even counterproductive normal action. Chronic immune activation and inflammation [10], particularly humoral-mediated [11], are just some of the pathways implicated in tumor genesis and development. This diverse, and often paradoxical, immune involvement creates wide implications for immunotherapy [12–14] and vaccination [15] for treatment and prevention of lung cancer.
The goal of this article is to critically review the available literature concerning the cellular and molecular interplay between the immune system and lung cancer. In addition, current therapeutic modalities that harness the immune system against lung cancer are discussed. Particular focus is centered on immune cells and molecular signaling in lung cancer. However, where evidence is lacking, information is drawn from studies of parallel pathology.
Lung carcinogenesis
Many factors play a causative role in the pathogenesis of lung cancer, including genetic susceptibility and occupational or environmental carcinogens. Exposure to a number of factors, including asbestos, certain metals, radon, some organic chemicals, pre-existing lung disease, diet and familial history, are pre-disposing factors for the development of lung cancer [3,16,17]. Tobacco smoking is the overwhelming cause of lung cancer, estimated at 85% of cases [18]. Within the over 5,000 identified constituents, 73 compounds have been classified by the International Agency for Research on Cancer (IARC) as having sufficient evidence for carcinogenicity, of which over 20 compounds are known lung carcinogens [19]. These include polycyclic aromatic hydrocarbons (PAH), tobacco-specific N-nitrosamines, volatile hydrocarbons including 1,3-butadiene, and metals and metal compounds including cadmium. [20]. These carcinogens act in numerous ways to promote oncogenesis (Figure 1). The triggering of IKK-and JNK1-dependent inflammation has been suggested as one of the molecular mechanisms of tobacco smoke-dependent tumorigenesis [21]. Cancer-related inflammation is a well-described factor in oncogenesis and tumor promotion, so will not be discussed in this article [10,22,23].
Figure 1. Cigarette smoke and other carcinogens promote lung cancer by inducing aberrant receptor activation, cytogenetic effects, and excess inflammation.
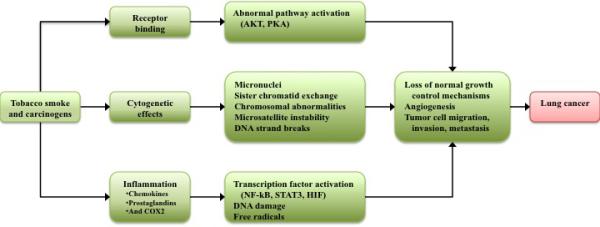
These pathways ultimately contribute a pro-tumorigenic environment through abnormalities in the control of cellular growth, angiogenesis, and pro-survival signaling. COX2, cyclooxygenase-2; HIF, hypoxia inducible factor; STAT3, signal transducer and activator of transcription-3
While still not completely understood, the mechanisms underlying genotoxicity of tobacco smoke have been previously reviewed [19,24,25]. DNA strand breaks are of particular importance, with generation of reactive oxygen and nitrogen species as the suggested primary cause [25]. Additionally, numerous somatic gene alterations known as “driver mutations” have been implicated in the development of lung cell metaplasia. These driver mutations are generally transformative in nature, inducing cell change to malignancy via upregulation of mitogenic growth signals. Additionally, these cancer cells exhibit “oncogene addiction” in which the tumor cells are dependent on the driver signal for survival [18]. Specific driver mutations in lung cancer have been previously reviewed [26–28], the most well-known of which include epidermal growth factor (EGF) mutation, anaplastic lymphoma kinase (ALK) translocation, and RAS gene family mutations (including KRAS, NRAS, HRAS).
CD4+ T helper cells
CD4+ T-lymphocytes play crucial roles in steering the immune response, particularly in tumor development and/or rejection [29] (Figure 2). Distinct T helper subsets modulate immune and inflammatory responses through secretion of cytokines and cell activation [30]. For example, Th1 and Th2 cells orchestrate the immune response to be cytotoxic-mediated or humoral, respectively [31]. In non-small cell lung carcinoma (NSCLC) patients, the Th1/Th2 cell ratio in peripheral blood is a well-characterized positive prognostic factor [32], with a low ratio increasing five- year survival by nearly 25% versus patients with a high ratio [32]. These two major cell types have dominated the field until recently [31].
Figure 2. Schema of CD4+ T cell development.
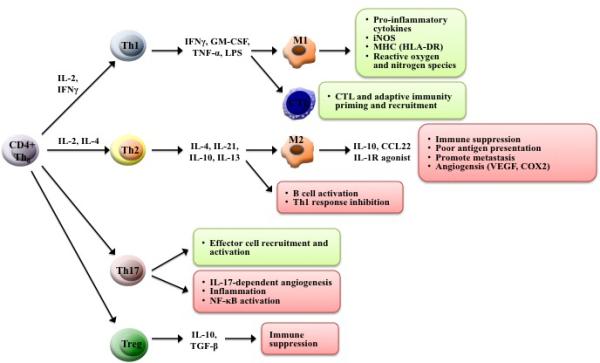
Cytokine presence influences mature T cell fate and subsequent immune responses. Th1 cells elicit a generally anti-tumorigenic response by activating M1 macrophages and cytotoxic adaptive immune cells. Th2 and Treg cells have a generally pro-tumor effect. Th17 cells have a dual role in lung cancer.
IL-17-producing Th17 cells are a CD4+ T cell subset that act as an important component in tissue inflammation and immune promotion [33]. There is currently extensive debate over the role of these cells in cancer [34], but the evidence seems to paint a multi-faceted picture of Th17 cells and associated cytokines in both anti- and pro-tumorigenic functions [35]. Th17 cells and IL17 enhance tumor cell proliferation and angiogenesis [36] but, also have been shown to induce tumor eradication [35]. While CD4+ T-lymphocytes were initially identified as solely immune promoting, recent advances have illuminated inhibitory functions. In particular, immunosuppressive CD4+ CD25+ regulatory T cells (Tregs) constitute a high proportion of tumor-infiltrating lymphocytes in NSCLC, impeding the immune response and correlating with poor prognosis [37]. Traditionally, Th1/Th2 cell balance has been the large focus of lung cancer immunity research [32]. However, the recent and growing understanding of Treg and Th17 cells has implicated a complex and intertwined role of these cells in lung cancer [38]. Overall, due to the extensive immunoregulatory nature of CD4+ cells, these cells are of high focus for cancer therapy; in particular, production of vaccines that harness these cells has potential and much current interest [15].
Th1 and Th2 cells in immune modulation
Differentiation of naïve CD4+ T cells into subtypes of specialized phenotypes is a keystone in the normal functioning immune system. The first major groups initially studied are the Th1 and Th2 cells, distinguished primarily by cytokine production [29,31]. Th1 cells are characterized by production of pro-inflammatory cytokines IFN-γ, TNF-α and TNF-β that stimulate both innate and cell-mediated cytolytic immune responses. Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13. The Th2 response promotes immunoglobulin class switching, eosinophil recruitment and, most notably, promote the humoral immune response.
The Th1-derived cytokines clearly facilitate tumor rejection and anti-tumor progression. A recent study [39] demonstrated these anti-tumor effects of Th1 cytokines in a pancreatic β-cell cancer mouse model. In this experiment, the combined action of Th1-produced IFN-γ and TNF drove Tag-expressing cancers into senescence by permanently arresting growth utilizing STAT1 and TNFR1 (also known as TNFRSF1A) signaling in addition to p16INK4a. In a study [40] involving three distinct tumor models (RM-1, DA3, and methylcholanthrene [MCA] induction of fibrosarcoma), mice deficient in IFN-γ were more susceptible to tumor metastasis to the lung. In the same study, IFN-γ was demonstrated to play a role in early protection from metastasis as well as controlling the growth rate of sarcomas. Additionally, inflammation driven by tumor-specific Th1 cells were shown to effectively protect against myeloma and B-cell lymphoma in mice [41]. In this study, Th1-secreted IFN-γ-induced macrophages were directly cytotoxic to cancer cells and secreted angiostatic chemokines.
TNF-α, another key Th1 cytokine, plays a role in the priming, proliferation, and recruitment of tumor-specific T cells, among a number of other innate immune cells. TNF-α knockout in a pancreatic cancer mouse model expressed signs of tumor development and progression at a significantly earlier age, supporting the critical role of TNF- α in immune response promotion and immune surveillance [42]. Regarding adaptive immunity, Th1 cells primarily activate and induce proliferation of CD8+ cytotoxic T lymphocyte proliferation directed specifically against cancer cells. Concomitant high CD8+ T cell and high CD4+ T cell infiltration has been demonstrated to significantly increase survival rate in NSCLC patients [43].
T helper type 2 cells, on the other hand, are not effective in tumor rejection [9]. Rather, the cytokines produced by Th2 cells often have pro-tumor and some immunosuppressive effects. Human NSCLC cells have been shown to produce type 2 cytokines both in situ and in vitro [44], contributing to a pro-tumor microenvironment and suggesting NSCLC to favor a Th2 environment. IL-4, a key Th2 cytokine, induces B cell activation and maturation and Th2 cell differentiation. In cancer progression, IL-4 promotes lung (as well as pancreatic islet and mammary) tumor growth and metastasis by inducing cathepsin protease activity in tumor-associated macrophages [45]. The IL-4 - 590T/C polymorphism down-regulates IL-4 expression and has been found to be associated with decreased susceptibility to NSCLC [46]. IL-6 is similarly pro-tumorigenic in lung carcinogenesis by promoting STAT3 and NF-kB pathways, which in turn cooperate to activate prosurvival, antiapoptotic, and proangiogenic signals [47].
Th2 lymphocytes-derived IL-10 has also been found to promote lung cancer. In IL-10 transgenic mice, Lewis lung carcinoma cells were shown to grow more aggressively than in controls [48]. This was found to be due to immunosuppression caused by reduced antigen presentation capacity, CTL generation, and type 1 cytokine production in the IL-10 transgenic mice [49]. Additionally, expression of IL-10 by NSCLC cells has been linked to significantly poorer prognosis than those without IL-10 production [50]. In parallel, IL-13 also promotes tumor growth and/or survival through direct action on neoplastic cells and suppresses cell-mediated immunity [51]. Moreover, B lymphocytes themselves, a core component of the Th2 response and humoral immunity, are associated with inhibition of Th1 anti-tumor immunity [11] and with premalignant progression through promoting chronic inflammation [52].
Th1/Th2 balance has been well studied in regards to cancer progression. Th1 cell infiltration versus Th2 cells is associated with a better prognosis in NSCLC [32]. Independently, concurrent infiltration of NSCLC tumors with CD8+ T cells is also a positive prognostic factor [43]. Similarly, in colorectal cancer, Th1 differentiation with cytotoxic and memory components in tumors negatively correlated with microscopic evidence of early signs of tumor dissemination around the tumor and metastasis in distant organs [53].
Immunity mediated by CD4+ T cells, however, is not limited to a clearly dichotomous Th1 versus Th2 paradigm. Some Th1 cytokines have been implicated in tumor promotion as well. TNF-α is a notable culprit for having a seemingly counterintuitive dual role in cancer progression that has developed a very interesting history since its discovery. While TNF- α is crucial in the acute phase reaction and anti-tumor functions, the requirement of repeated local administration and the endotoxic symptoms at high doses limit TNF- α as an anti-tumor agent [54]. Furthermore, some recent evidence has illuminated pro-tumorigenic effects of TNF-α. In skin carcinogenesis, TNF-α activates both AP-1 (Activator Protein-1, a tumor promoting transcription factor) and nuclear factor-κB (NF-κB), which at normal expression opposes epidermal proliferation [22,55]. The TNF-α signaling involving both AP-1 and NF-κB has been implicated in all steps of tumorigenesis in a variety of other cancers [56]. In lung cancer, NSCLC patients exhibit high levels of serum TNF-α [57] with possibly even a positive prognostic value [58]. TNF-α expression within NSCLC tumors may hold some prognostic value. In a study of 133 patients with surgically resected NSCLC [59], expression of TNF-α was increased in tumor islets of patients with above median survival, indicating tumor islet TNF-α density as a favorable independent prognostic factor. Interestingly, in this study, stromal TNF-α density was an independent predictor of reduced survival. Studies have investigated TNF-α polymorphisms and lung cancer susceptibility, but the evidence is unclear as to a simple dose-dependent relationship [60,61]. Ultimately, the several transcription factors that cytokines activate individually determine tumor promotion or inhibition.
Traditionally, CD4+ cells play an indirect, albeit crucial, role in immune response. CD4+ CTLs with cytotoxic potential impart a more direct role for CD4+ cell-mediated immunity in both infection and malignancy. Their lytic activity is thought to be primarily enacted through the Fas:FasL cytotoxic pathway, though other investigations demonstrate CD4+ CTL secretion of perforin, granzyme B, granulysin [62]. While much remains to be learned about these cells, they offer a potential avenue for utilization of the host immune system in cancer immunotherapy.
Genetically engineering lymphocytes offer their potential in highly selective cancer immunotherapy. Morgan et al. [63] conferred specific tumor recognition in autologous lymphocytes from peripheral blood of metastatic melanoma patients using a retrovirus engineered to encode a T cell receptor. Adoptive transfer of these cells into 15 patients resulted in levels exceeding 10% of blood lymphocytes for at least two months after administration. Two patients retained high levels of engineered cells at 1 year after infusion and demonstrated objective regression of metastatic melanoma lesions.
To combat cancer immune evasion, vaccines are a particular area of research attempting to stimulate and direct the immune system toward the cancer. Texopi (OSE-2101, formerly EP2101 or IDM-2101) was designed to induce CTL responses against five TAAs frequently over-expressed in NSCLC: carcinoembryonic antigen (CEA), p53, HER2/neu, and MAGE-2 and −356. The vaccine incorporates nine synthetic peptides from these TAAs, which represent CTL epitopes, and one pan-DR epitope designed to augment the CTL response in patients expressing HLA-A2. The phase II study (NCT00104780) of OSE-2101 efficacy in 63 patients with metastatic NSCLC reported one year survival rate for the treated group to be 60%, with a median survival at 17.3 months [64]. Survival also correlated with the number of epitope peptides incorporated in the patient's immune response. After a long pause in drug development, the FDA and European Medicine's Agency recently gave approval for OSE Pharma to begin phase III trials of OSE-2101 in early 2015. This phase III trial will recruit 500 stage IIIb/IV NSCLC patients from the US and Europe to compare OSE-2101 to standard of care, docetaxel or pemetrexed [65]. Such reports demonstrate the pivotal role of CD4+ T-helper cells in cancer immunology, and suggest potential for utilizing these cells in concert with other immune modulators as effective cancer immunotherapy.
The Th17 and Treg paradigm
Th17 cells are potent inflammatory mediators responsible for controlling certain extracellular infections through production of IL-17A, IL-17F, IL-21 and IL-22 [29]. They have also been associated with the pathogenesis of various autoimmune diseases [66]. Current evidence suggests a complicated and even contradictory Th17 cell involvement in cancer (Figure 3). Several lines of evidence support Th17 promotion of a protective antitumor immune response, most notably through an indirect mechanism of effector immune cell recruitment and activation [35]. The high plasticity and diverse generation of these cells complicate Th17 cell function in cancer, but two distinct Th17 differentiation fates can add clarity. Th17 cells differentiation induced by the combination of TGF-β and IL-21 secrete IL-17, but not IFN-γ; the combination of IL-6, IL-1β and IL-23 without TGF-β induced Th1-polarized Th17 cells with high expression of IL-2, IL-33 and IL- 18r1, coexpression of RORC (RAR-related orphan receptor C) and T-bet, and significantly enhanced ability to produce IFN-γ [67]. The IL-23/IL-6-induced cell type constitutes the anti-tumor activity of Th17 cells due to synergistic action of IL-17 and IFN-γ that stimulates CXCL9- and CXCL10-dependent recruitment of tumor-infiltrating effector T cells, as demonstrated in ovarian cancer[68]. The same study found high Th17 cell numbers to correlate with high NK cell levels in the same tumor microenvironment. In lung cancer patients, increased accumulation of Th17 cells in malignant pleural effusion predicted improved patient survival [69].
Figure 3. Th17 cell subsets offer distinct roles in tumor progression.

Th17 cells induced by TGF-β and IL-21 generally elicit a pro-tumor effect, whereas those induced by IL-6 and IL-23 are anti-tumorigenic due to the recruitment of cytotoxic lymphocytes (CTL) and the promotion of innate immune cells.. Levels of IL-17 and IFNγ are implicated in these opposing Th17 activities.
The neoplasm-promoting effects of Th17 cells are reportedly derived from their expression of the IL-17 cytokine, which can mediate angiogenesis[70], upregulation of survival genes, NF-κB signal activation [71], and excessive inflammation. In a NSCLC mouse model, IL-17 promoted CXCR-2-dependent angiogenesis and tumor growth [72]. Another study demonstrated the acceleration of lung cancer development at least in part by IL-17 promotion of inflammation [36]. Li et al. [73] investigated NSCLC cell and mouse models, revealing direct IL-17 promotion of NSCLC cell metastasis both in vitro and in vivo. In human NSCLC patients, increased expression of IL-17 is linked to significantly lower disease-free and overall survival [74]. While high IL-17-associated angiogenesis and pro-tumor activity have been established, production of IL-17 solely or principally by Th17 cells has yet to be conclusively demonstrated. IL-17 can also be produced by some epithelial cells, NK cells, iNKT cells, αβ and γδ-T cells, neutrophils, and macrophages[67]. Interestingly, macrophages from IL-10-deficient and IL-10R-deficient mice demonstrate phenotypic plasticity with the ability to express IL-17 after LPS stimulation [75]. The same study demonstrated similar ability of CD4+ T cells.
Even with the complex and perhaps unclear role of these cells in cancer, therapeutic prospects of Th17 cell manipulation have been considered. Martin-Orozco et al. [76] investigated the effects of adoptive T cell therapy in IL-17A-deficient mice. Without treatment, these mice were more susceptible to developing lung melanoma. Adoptive tumor-specific Th17 cell therapy prevented tumor development through activation of tumor-specific CD8+ T cells, CCR6-dependent promotion of intratumoral dendritic cell recruitment, and promotion of lymph nodal CD8α+ dendritic cell presentation of tumor material. Use of Th17 cells as a therapeutic target has also been discussed in acute myeloid leukemia [77]. In patients with relapse, or high risk thereof, and no clinical anti-leukemic graft versus host disease (GVHD), promotion of Th17 cell activity may be a therapeutic option. However, better characterization of the role of Th17 cells in GVHD development and cancer in general will further elucidate the potential and risk of this therapy.
The CD4+CD25highFoxp3+ regulatory T cells (Tregs), comprised of two main subsets, primarily function to maintain self-tolerance and immune homeostasis. Naturally occurring regulatory T cells (nTreg) produce inhibitory effector cytokines and actively modulate immune responses. Inducible regulatory T cells (iTreg) produce large amounts of IL-10 and TGF-β and exhibit nonspecific immune-suppressive activity [29]. In the context of cancer, these extensive inhibitory effects of Treg cells may serve as favorable to tumor development and growth. Patients with NSCLC present with increased Treg cell numbers in tumor tissue and peripheral blood that were found to promote tumor growth, correlated with lymph node metastasis [78]. In a lung cancer model, tumor-derived COX-2/PGE2 induced Treg cell activity and Foxp3 expression, a Treg-specific transcription factor [79]. In the same study, mice with inhibited COX-2 showed a reduction in Treg cell frequency and activity, in Foxp3+ tumor-infiltrating lymphocyte numbers, and in overall tumor burden. Administration of PGE2 or Treg cell transfer reversed these effects, supporting COX-2 as an important regulator of Treg cells and a potential avenue for lung cancer therapy. Prognostically, tumor infiltration by Treg cells was found to be positively correlated with intratumoral COX-2 expression and was also associated with a worse recurrence-free survival (RFS) rate of NSCLC patients [37]. Currently a number of questions still remain regarding Treg cells in cancer [80]. Specifically, the stage and role of Treg proliferation in cancer onset or development, the role of organ- or tumor-specific Treg cells, and the possibility of long-term depletion of Treg cells and connection to autoimmunity are some areas of interest.
The recently emerged Treg and Th17 paradigm has drastically improved current knowledge of CD4+ T helper cell functionality, particularly in inflammation and autoimmune disease. The involvement of these cells in tumor pathogenesis is still being unraveled, but has potential in both understanding and treating cancer. In both SCLC and NSCLC, overexpression of TGF-β, an essential cytokine in generation of both Treg and Th17 cells, correlates with disease stage [81] and, interestingly, may elicit early-stage suppressive but late-stage promoting activity on tumor cells [82]. This is thought to be due to early stage low levels of TGF-β synergizing with IL-6 and IL-21 to promote Th17 differentiation, whereas late stage high TGF-β levels will favor a Treg response [38]. Levels of Th17 and Treg cells are known to correlate with NSCLC stage [83]. While there is some evidence on the negative prognostic value of Treg cells[84], the impact of Th17 cells[34] and Th17/Treg cell ratios in lung cancer has yet to be clearly defined.
Therapies focusing on Th17 and Treg cells (see Figure 4 and Table 2) have grown tremendously in recent years. Cytokine therapy has gained momentum for cancer therapy. IL-23 promotes Th17 cell differentiation and contributes to inflammatory processes. In mouse models, IL-23 knockouts expressed IL-6, IL-17A, and IL-22 at lower levels than controls and developed fewer and smaller colonic adenomas when subjected to CAC induction[85]. This indirect effect of IL-23 offers a potential therapeutic target for upstream inhibition of Th17 cells and other pro-tumor cytokines. Another study using transplantable tumor models in mice suggests IFN-dependent Treg-derived IL-10 can limit Th17-mediated inflammation in the tumor microenvironment. Considering the ambivalent role of Th17 cells, such therapies will only prove efficacious in cancers exacerbated by Th17-based tumor inflammation, not the mere presence of the cells. On the contrary, a number of preclinical experiments have demonstrated significant anti-tumor responses due to infusion of Th17 cells into mice. Further work is required to more clearly uncover the Th17 roles in cancer and translate current preclinical knowledge into therapeutic eradication of tumor tissue in clinic.
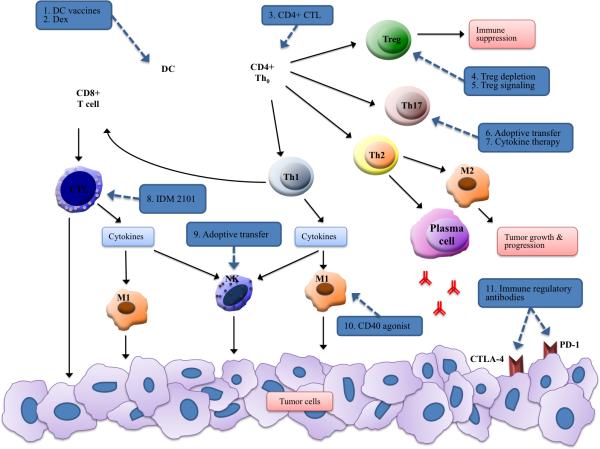
Figure 4. Immune cell targets of current clinical and pre-clinical immunotherapies.
The complex involvement of each immune cell, whether directly or indirectly, in cancer progression offers a vast array of potential immunotherapeutic targets. Current approaches include vaccines (often targeted to specific cells), adoptive transfer of cells activated ex vivo, and immune regulatory antibodies that target specific immune signaling pathways. Details of each therapy in regards to their mechanism of action are provided in Table 2.
Table 2.
Mechanism of action of various immunotherapies in lung cancer.
| Therapy | Description |
|---|---|
| 1. DC vaccines | Vaccines utilize various antigens including HER2, CEA, WT-1, MAGE-2, and surviving. [15] Tumor lysate has also been used to activate autologous DCs. [114] The TG4010 vaccine uses a modified vaccinia virus expressing MUC1 protein and IL-2. [141] |
| 2. Dex | Autologous DCs are used to purify secreted DC-derived exosomes (Dex) loaded with HLA-restricted NY-ESO-1, MAGE-1, MAGE-3, and MART-1 peptides. (NCT01159288) |
| 3. CD4+ CTL | Adoptive transfer of tumor-specific CD4+ cytotoxic cells activated ex vivo with various engineered viruses. [63] |
| 4. Treg depletion | DAB389IL-2, a recombinant IL-2 diphtheria toxin conjugate, depletes Treg cells in cancer patients [86-87] |
| 5. Treg signaling | Targeting VEGF-A reduces Treg-dependent immunosuppression. [87] Inhibitors of PI3 kinase p110δ isoform in Tregs are linked to CD8+ CTL proliferation and solid tumor regression. [88] |
| 6. Adoptive transfer | Adoptive transfer of tumor-specific Th17 cells activates tumor-specific CD8+ T cells, DC recruitment and increased DC tumor antigen presentation. [76] |
| 7. Cytokine therapy | Targeting excess IL-17 may reduce associated angiogenesis, survival gene upregulation, NF-κB activation, and inflammation. Targeting IL-23 may reduce Th17 differentiation and inflammation. [85] |
| 8. IDM 2101 (OSE-2101) | Vaccine designed to induce CTL responses. Contains five frequent NSCLC tumor antigens (CEA, p53, HER2/neu, MAGE-2, MAGE-356). (NCT00104780) [64-65] |
| 9. Adoptive transfer | Reinfusion of NK cells activated ex vivo using the 14 amino acid sequence (TKD) of Hsp70 plus low dose IL-2. (NCT02118415) |
| 10. CD40 agonist | Agonist CD40 antibody therapy stimulated M1 polarized response and IL-12 production leading to tumor regression. [104-105] |
| 11. Immune regulatory antibodies | Tremelimumab and Ipilimumab are two CTLA-4 specific antibodies currently in clinical trials. CTLA-4 is involved in Treg-induced immune inhibition. [156-158] PD-1 provides immune inhibitory signals through multiple immunosuppressive pathways. CT-011 and nivolumab (BMS-936558 or MDX-1106) are PD-1 inhibitory antibodies in clinical trials. (nivolumab: NCT00730639, NCT01454102) |
Therapies involving Treg cells are also in early stages of development. Murine models suggest that selective elimination of Treg cells alone or in combination with other treatment approaches could induce regression of already established tumors. In a melanoma mouse model, 90-95% depletion of Treg cells resulted in regression of established tumors by inducing activation of tumor-specific CD8+ T cells and enhanced tumor infiltration. [86]. In a similar study, effect of Treg depletion was investigated in colon cancer-bearing mice and colorectal cancer patients by targeting VEGF-A [87]. This suggests a tumor escape mechanism in which VEGF-A mediates Treg-dependent immunosuppression and has future implications in cancer immunotherapy. Regarding a separate pathway, inhibitors against the p110δ isoform of phosphoinositide-3-OH (PI3) kinase show marked therapeutic efficacy in some leukemias. In mice models of various solid tumors, the study linked inactivation of p110δ in Treg cells with CD8+ CTL proliferation and induction of solid tumor regression [88]. This suggests p110δ inhibitors as another target to break Treg cell-mediated immune tolerance to cancer.
Docetaxel has been shown to exhibit some activity to decrease the density of regulatory T cells. Docetaxel treatment reduced numbers of in vitro Treg cells originally obtained from NSLC patients [89]. In this study, two subsets of Treg cells were found where one Treg subset secreted more IFN-γ and less TGF-β. Additionally, the same study observed a reduced Treg cell population in peripheral blood from NSCLC patients after four cycles of docetaxel-based chemotherapy. In another small study, effect of a low dose of cyclophosphamide (CTX) was investigated for selective depletion of Treg cells in conjunction with pemetrexed-based NSCLC chemotherapy [90]. While Treg cell numbers were unaffected, activated T-effector cells were increased, suggesting CTX may affect Treg cell function. Lastly, there is some interest in chemotherapy combined with COX-2 inhibitors to deplete Treg cells. In a phase II study, 120 patients with stage IIIb/IV NSCLC patients were treated with erlotinib and apricoxib or placebo [91]. While marginal benefit was observed in a subpopulation, the primary endpoint of the trial was not met and some issues with toxicity may exist. While still in early stages, depletion of Treg cells has strong potential to enhance the different immunotherapeutic modalities and warrants further investigation.
Macrophages
Tissue-dwelling macrophages constitute a heterogeneous population of cells characteristic for their functional plasticity. They thus play a diverse role in innate immunity and cancer, with particular emphasis on their abilities to phagocytosis and release inflammatory cytokines. The lung is a prime organ exhibiting macrophage heterogeneity, with functions ranging from the alveolar or intravascular macrophage clearance of particles and microorganisms to the interstitial macrophage role in limiting inflammation, fibrosis, and antigen presentation [92]. The paradigm of M1 and M2 polarization encompasses the extremes of macrophage functionality and closely mirrors the Th1 and Th2 paradigm. Tumor-associated macrophages (TAMs) are dominated by M2 phenotypic macrophages exhibiting an notably immunosuppressive IL-10 and TGF-β cytokine expression profile, poor antigen-presenting capacity and suppress Th1 adaptive immunity [93]. The M2 phenotype also produces mediators that promote metastasis and angiogenesis, such as vascular endothelial growth factor and COX-2-derived PGE2 [94], implicating M2 macrophages as strong pro-tumor cells.
M1 macrophages (promoted by lipopolysaccharides and IFNγ) exhibit immunostimulatory Th1-orienting properties including high pro-inflammatory cytokine expression, inducible nitric oxide synthase (iNOS), major histocompatibility complex (MHC) molecules, and reactive oxygen and nitrogen intermediates [22]. This cell subset has been positively associated with survival of NSCLC patients [95].
Current research in therapeutic and clinical application of the molecular signaling directing the M1/M2 paradigm is underway. In a Lewis lung carcinoma model, mice not expressing interleukin receptor-associated kinase (IRAK)-M exhibited a five-fold reduction in tumor growth and an M1-dominated macrophage response compared to wild type [96]. Conversely, the same study demonstrated IRAK-M induction in macrophages by human lung cancer cells that was dependent on TGF-β. Together, this data suggests TGF-β-dependent IRAK-M expression as a mechanism for lung tumor avoidance of the anti-tumor macrophage response and a potential molecular target for therapy. In addition, NF-κB and HIF-1 have been identified as master regulators in transcriptional control of TAM phenotype. Inhibition of both the NF-κB inhibitory p50-subunit in human monocytes [97] and the IKKβ-dependent NF-κB activation pathway in ovarian cancer cells [98], favors the M1 phenotype response. As such, early IKKβ-dependent NF-κB activation may trigger cancer-related inflammation whereas the p50-dependent regulatory pathway may tune and promote M2 associated smoldering inflammation [94]. As a therapeutic approach, restoration of NF-kB activity in TAM is a potential strategy for repolarization to the M1 phenotype, thereby inducing M1 inflammation and cytotoxicity [93].
Hypoxia-inducible factors (HIF) overexpression, caused by intratumorial hypoxia and genetic alterations, has been linked to tumor-promoting activity (including angiogenesis, cell survival, glucose metabolism and invasion), regulation of TAM attraction, and increased patient mortality in several cancer types [93]. Additionally, TAMs respond to tumor hypoxia with up-regulation of both HIF-1 and HIF-2, which have been linked to the angiogenic [99], infiltration and inflammatory [100] activities of these cells. The HIF expression in hypoxic tumor microenvironment is particularly notable in most solid tumors including NSCLC [101] and is being developed as a potential therapeutic target in pre-clinical and early clinical development [102]. Recent investigations involving HIF, however, have yet to utilize its role in macrophage infiltration and mediation of inflammation as a target to repolarize the macrophage response in cancer therapy.
The extensive cytokine and signaling network involving M1/M2 cells also offers a wide variety of drug targets, including TAM recruitment, survival, mechanisms of activation and polarization, immunosuppression, angiogenic activity and matrix remodeling. In preclinical models, toll-like receptors (TLR) in conjunction with cytokines such as IFN-γ have been shown to induce M1 polarization through pro-inflammatory signaling [103]. However, more evidence is needed on their potential to reprogram already polarized macrophages. In a review on TAM in human cancer, Heusinkveld et al. [104] reported TLR agonists as M2 TAM activator that alters M2 marker expression, but not functionality, and suggests TLR may not play a major role when CD4+ T cells are already fully polarized. The ability for TLR agonists to repolarize macrophages, therefore, is questionable and yet to be demonstrated. A recent study [105] observed macrophage-mediated tumor regression in both pancreatic cancer patients and a mouse model, upon treatment with the combination of an agonist CD40 antibody with gemcitabine chemotherapy. The CD40 therapy stimulated macrophage recruitment and production of IL-12, as opposed to IL-10 in the untreated mice, suggesting an M1 polarized response. Since IFN-γ is necessary for M1 polarization, it is likely that the non-specific expression of CD40 activated several cell types and provided the necessary pro-inflammatory cytokines that enabled this macrophage-mediated effect [104].
Dendritic cells
Dendritic cells (DCs) are a heterogeneous group of innate immune cells that are essential in antigen presentation and activation of naïve T cells. However, DCs have been implicated in inducing immune tolerance [106], offering the possibility of dual function in cancer immunity. This also likely contributes to the unclear understanding currently held of DC tumor-infiltration. In a study investigating tumor-infiltrating immune cells and NSCLC patient survival, mature DC numbers was positively associated with positively associated with patient's survival time (in an univariate analysis but not in a multivariate analysis, which calls for caution in using DC number to predict patient outcome)[107]. Another study similarly found the density of mature DCs in tertiary lymphoid structures to be associated with a favorable outcome in both early-stage and treated late-stage NSCLC [108]. These studies, however, single out mature DCs, which may not be the primary DC population infiltrating tumors.
To address this issue of immunization versus tumor-specific tolerance generated by DCs, Perrot et al. [109] characterized DC populations isolated from surgical biopsy specimens in NSCLC patients with no pre-surgical tumor treatment. Compared with peripheral blood, tumor-infiltrating CD11chigh myeloid DCs exhibited a “semi-mature” phenotype, expressing a higher, but limited, level of five markers chosen to indicate DC maturity (CD80/B7-1, CD86/B7-2, the DC activation marker CD83, HLA-DR, and CD208/DC-LAMP). CD11c- plasmacytoid DCs isolated were immature. Interestingly, a third subset was found to express intermediate levels of CD11c with low levels of co-stimulatory molecules and high levels of the immune-inhibitory molecule B7-H1. In vitro TLR stimulation of these cells resulted in only partial maturation, limited cytokine secretion, and sustained poor antigen presentation and migratory ability. An in vitro study[110] of DCs co-cultured with lung carcinoma cells (squamous cell carcinoma and adenocarcinoma cell lines) attributed this inhibited DC state to the tumor microenvironment. Compared to DCs not exposed to tumor cells, co-culture with lung carcinoma cells increased DC expression of TGF-β1, decreased immature DC expression of CD86 and HLA-DR, and decreased mature DC expression of CD86 and production of TNF-α and IL-12 p70. Furthermore, lung carcinoma cells induced mature DC production of TGF-β1, causing them to poorly activate CD4+ T cells and abnormally frequently activate Treg immune-suppressive, tumor-promoting cells. Other mechanisms of DC suppression or bypass have been suggested. Overexpression of the TIM-3 receptor on DCs in Lewis lung cancer microenvironment suppresses pattern-recognition receptor-mediated innate immune responses to nucleic acids[111]. Similarly, in vitro blockade of up-regulated B7-H1 on tumor-associated DCs improved DC-mediated antitumor immunity in ovarian carcinoma [112]. More work is required to further elucidate the underlying mechanism and clinical significance of these cancer-associated DC subpopulations. Regardless of the dysfunction of these DCs, however, work is already underway to direct DCs toward therapeutic, anticancer functionality.
While still primarily preclinical in evidence, dendritic cell-derived exosomes have therapeutic potential in NSCLC treatment. These DC-derived exosomes are secreted nanovesicles originating from late endosomal compartments and have been shown to modulate immune responses through MHC class II-dependent stimulation or other costimulatory mechanisms [113]. A phase II clinical trial (NCT01159288) is currently underway, assessing the efficacy of DC-derived exosomes immunotherapy in 47 stage IIIB to IV NSCLC patients. Autologous DCs will be used to purify DC-derived exosomes loaded with HLA-restricted NY-ESO-1, MAGE-1, MAGE-3, and MART-1 peptides. The primary endpoint is progression-free survival at 4 months post-chemotherapy.
DC vaccines are another area of interest (see Figure 4 and Table 2). A recent phase I study of the ad.p53 DC vaccine (an adenovirus used to generate DC vaccines directed against p53 epitpoes) administered with indoximod (an indoleamine 2,3 dioxygenase pathway inhibitor) [116]. Indoximod+Ad.p53DC vaccine therapy was well tolerated and may have a chemosensitizing effect for subsequent chemotherapy. A follow-up phase II trial in metastatic breast cancer is evaluating this treatment followed by carboplatin/gemcitabine therapy (NCT01042535). Belagenpumatucel-L (Lucanix) is a genetically modified tumor cell vaccine that inhibits TGF-β2, a known immunosuppressor found to have antagonistic effects on natural killer cells and dendritic cells (discussed further below). While DC-based vaccines are still in early clinical trials, the central role of DCs in linking innate and adaptive immunity offers great potential for cancer immunotherapy.
Natural killer cells
Natural killer (NK) cells are recognized as a subset of cytotoxic innate lymphoid cells that offer vital function in the immune cytokine network. Activating NK cell receptors detect ligands and cells in “distress”, infectious nonself ligands, and TLR ligands [117]. Additionally, NK cells express inhibitory MHC class I-specific receptors to gauge the absence of this constitutively expressed self molecule on potential target cells. Cytokines, including type I interferons, IL-12, and IL-18, are also involved in NK cell activation, upon which cytolytic granules are released for targeted cell disruption and cytokines for immune response propagation[118]. While IFN-α is the primary NK-produced cytokine, Th2-associated cytokines, such as IL-5 and IL-13, and the regulatory IL-10 cytokine may be released. While NK cells are known to demonstrate effective anti-tumor activity, evidence has arisen demonstrating tumor-infiltration numbers as well as intratumoral NK functionality both hinder NK control of malignancy. An in vitro analysis of NSCLC tissue suggests profound phenotypic alteration of NK cells [119]. Intratumoral NK cells exhibited defects in deegranulation and IFN-γ production. Another study noted release of soluble factors by NSCLC cells that inhibited granzyme B, perforin and IFNγ expression in intratumoral NK cells [120]. These data suggest local impairment of NK cells by the NSCLC tumor microenvironment. Carrega and colleagues [121] characterized the tumor-infiltrating NK cell population in NSCLC tissue, observing that the CD56brightCD16-NK cell subset was particularly enriched. These cells indeed displayed activation markers, including NKp44, CD69, and HLA-DR, but exhibited markedly lower cytolytic potential when compared to peripheral blood NK cells. Additionally, these CD56brightCD16- NK cells demonstrate pro-angiogenic activity, with production of VEGF, placental growth factor, and IL-8/CXCL8 [122]. Differences in tumor-infiltration by immune cells have also been noted in malignant versus non-malignant NSCLC tissues. Malignant tumor areas were reported to have high Treg presence and minimal NK cell presence, whereas non-malignant areas were oppositely populated, with NK cells demonstrating strong cytolytic activity ex vivo [123]. The NK cell suppression by Treg cells does play a role in tumor progression [124] and could hold associations with severity of disease.
The influence of NK cells on both innate and adaptive immunity makes these cells attractive for therapeutic development. IL-2 is a well defined activator of NK cell cytotoxic activity against previously resistant cell targets, and has been incorporated into treatment of metastatic renal cell carcinoma and AIDS-associated lymphoma [125]. IL-2 activation of peripheral blood mononuclear cells demonstrate increased cytotoxic activity against primary lung cancer cells, which is further promoted by IL-12 [126].
Adoptive transfer of NK cells is another therapeutic approach currently being studied in various cancer types. Krause et al.[127] investigated treatment of a NSCLC patient and 11 colorectal cancer patients with autologous transfer of NK cells activated ex vivo by the 14 amino acid sequence (TKD) of heat shock protein 70 (Hsp70) plus low-dose IL-2. The NK cell reinfusion exhibited minimal adverse effects and demonstrated promising immunological results. A phase II RCT is currently underway to test treatment efficacy in 90 NSCLC patients (NCT02118415). Preliminary studies suggest NK cell reinfussion to be safe and have potential antitumor activity. Further investigations are required to develop optimal NK cell therapy schema, particularly on a large-scale clinical grade NK cell expansion. Additionally, much is yet to be learned about NK cell development, differentiation, and various subset role, particularly in light of NK cell expansion.
CD8+ Cytotoxic T Lymphocytes
CD8+ Cytotoxic T lymphocytes (CTLs) are effector cells of adaptive immunity and have the capability of specifically recognizing and destroying cancer cells. However, tumors have numerous mechanisms to escape immune surveillance, particularly the impairment of T lymphocytes. NSCLC tumor microenvironments induce immunosuppressive phenotypes on tumor-residing DCs by upregulating B7-H3 [128]. This is thought to play a crucial role in mediating T cell suppressive effects of DCs. As discussed above, Treg cells also contribute to cancer cell escape from antitumor immunity through immune suppression. None-theless, CTLs compose a portion of tumor-infiltrating lymphocytes, whose intra-tumor presence is a positive prognostic factor [129] and has predictive value in response to chemotherapy. Higher Treg/CTL ratios in tumor sites was found to be an indicator for poor response to platinum-based chemotherapy in advanced NSCLC [130]. Ultimately, CD8+ CTLs are thought to modify the tumor stroma and epithelium to reduce disease progression and metastasis [129], indicating an overall protective and anti-tumoral role of CTLs.
The CD8+ T lymphocytes are a primary target of interest in development of immunotherapy and NSCLC treatment since tumor antigen-specific CD8+ T lymphocytes infiltrating the tumor express high levels of programmed cell death-1 (PD-1) and become functionally impaired. Therefore, one promising approach involves the modulation of PD-1 protein on immune cells to overcome immune resistance. These drug therapies are discussed below, including nivolumab, an IgG4 monoclonal antibody that blocks PD-1 ligand 1 (PD-L1) binding to PD-1. In particular, the development of adoptive T-cell therapy (ATcT), involving the autologous transfusion of T lymphocytes to elicit an anti-tumor response. There are two primary approaches to ATcT: ex vivo clonal expansion of T cells (often isolated from tumor infiltrating lymphocytes) and genetic manipulation of T-cells (to express a T cell receptor or antibody fragment allowing tumor-derived antigen recognition) [131,132]. Several phase II trials of ATcT techniques are currently underway, including targeting CEA (NCT01723306), NY-ESO-1 (NCT00670748) for patients with NYESO-1 expressing metastatic cancers, and mesothelin (phase I/II, NCT01583686) for mesothelin expressing metastatic cancers or mesothelioma. The forefront of genetic engineered T cells for cancer immunotherapy involves chimeric antigen receptor (CAR) technology, consisting of a junction between antibody components at the membrane surface and intracellular tails to induce T cell proliferation and activity, thereby allowing MHC independence in T cell targeting [131]. Most CAR T cell therapies in clinical trials are directed toward hematological malignancies, with use in solid tumors in far less development [133]. Early data in a number of malignancy types have recently been presented, including neuroblastoma [134], colon cancer [135], metastatic epithelial tumors [136], and high-grade glioma [137]. Many challenges remain to be addressed, as with any rising therapeutic technology: solid tumor treatment poses physical barrier to CAR T cells, particularly in areas of low vasculature or hostile microenvironment, tumor heterogeneity, and tumor cell “evolution” and accumulation of mutations. Interestingly, a recent study on CAR T cells in solid tumors reported CAR T-cell intratumoral inhibition similar to tumor-induced inhibition or normal TILs [138]. While this therapeutic modality is very early in development with many challenges to address, it offers high potential and much excitement for highly specific treatment with less toxicity.
Vaccine therapy
Tumor escape mechanisms from the immune system pose as formidable challenge to cancer treatment. Current focus on modulation of the host immune response to cancer cells encompasses vaccine approaches that couple immunogenic adjuvant agents to tumor antigens. Additionally strategies used to bolster this immune response include genetically modifying autologous tumor cells or allogeneic cell lines to secrete immunostimulatory molecules and expressing the antigen in a viral vector, which can also be designed to encode co-stimulatory molecules or cytokines [15]. Ongoing immunotherapy clinical trials are summarized (Table 1) and various immunotherapy modalities are explored (Figure 4). In addition, the underlying mechanisms of action of immunotherapies in lung cancer are described in Table 2.
Table 1.
Selected ongoing clinical trials of immunotherapeutic vaccines and other approaches.
| Intervention | Study design | N | Stage | Study population | Endpoint | References | |
|---|---|---|---|---|---|---|---|
| IDM-2101 | Nine-epitope HLA-A2-specific vaccine | Phase II open label, single arm | 63 | Stage IIIb, IV NSCLC | HLA-A2 positive patients | Primary: OS. Secondary: safety, immune response | NCT00104780 (Barve et al., 2008) |
| Dendritic cell-derived exosome (Dex) | Tumor antigen-loaded Dex vaccine | Phase II open label, single arm | 47 | Stage IIIb, IV NSCLC | Response or stable after primary chemotherapy | Primary: PFS | NCT01159288 |
| TIME trial | TG4010 vaccine modified Vaccinia virus expressing MUC1 and IL-2 vs. first-line therapy alone | Phase IIb/III randomized, double blind, placebo controlled | 1000 | Stage IV NSCLC | Naïve to first line therapy | Phase II primary: PFS. Phase III primary: OS. Secondary: ORR, duration of response, safety | NCT01383148 |
| MAGRIT (discontinued) | MAGE-A3 vaccine | Phase III randomized, double blind, placebo controlled | 2278 | Completely resected, stage IB, II, IIIA NSCLC | MAGE-A3 tumor expression | Primary: DFS. Secondary: OS, lung-cancer-specific survival, Anti-MAGE-A3 and anti-protein D seropositivity rate, adverse events | NCT00480025 |
| START (discontinued) | Tecemotide (L-BLP25) full protein vaccine of MUC1 and IL-2 | Phase III randomized, double-blind, placebo controlled | 1513 | Unresectable stage III NSCLC | Response or stable after primary CRT. ≥2 cycles platinum-based chemotherapy, ≥50 Gy radiation | Primary: OS. Secondary: time to symptom progression, time to disease progression, 1, 2, and 3 year survival, safety | NCT00409188 |
| Racotumomab | N-Glycolil-GM3 ganglioside and racotumomab vs. BSC | Phase III randomized, open label | 1082 | Stage IIIa (unresectable), IIIb, IV NSCLC | Response or stable after first line therapy | Primary: OS. Secondary: PFS, adverse events, immune response | NCT01460472 |
| STOP | Lucanix (Belagenpumatucel-L) allogenic cell tumor vaccine | Phase III randomized, double blind, placebo controlled | 506 | Stage III, IV NSCLC | Response or stable after primary platinum-based CRT | Primary: OS. Secondary: PFS, quality of life, time to progression, overall tumor response, response duration, CNS metastasis rate, adverse events | NCT00676507 |
| REVEL | Ramucirumab and docetaxel vs. chemotherapy alone | Phase III randomized, double blind, placebo controlled | 1242 | Stage IV NSCLC | Disease progression after primary platinum-based chemotherapy | Primary: OS. Secondary: PFS, objective response rate, disease control rate, change from baseline, Cmax and Cmin of Ramucirumab, anti-Ramucirumab antibody response | NCT01168973 |
| Ipilimumab | CTLA-4 specific mAb in addition to Paclitaxel and Carboplatin vs chemotherapy alone | Phase III randomized, double blind, placebo controlled | 920 | Stage IV NSCLC | Squamous cell histology | Primary: OS Secondary: PFS | NCT01285609 |
| Nivolumab | PD-1 specific monoclonal antibody | Phase I non-randomized, open label | 311 | Unresectable NSCLC, melanoma, RCC, prostate cancer | 1-5 prior therapies for advanced or recurrent disease | Primary: Safety and tolerability. Secondary: Immune response, pharmacokinetic profile, efficacy | NCT00730639 |
| Nitedanib | Angiokinase inhibitor | Phase III randomized, double blind, placebo controlled | 1314 | Stage IIIb/IV NSCLC | Recurrent NSCLC progressing after first-line chemotherapy | Primary: PFS Secondary: OS | NCT00805194 |
OS = overall survival, ORR = overall response rate, PFS = progression-free survival, BSC = best supportive care, DFS = disease-free survival, RCC = renal cell carcinoma, CRT = chemoradiotherapy
The melanoma-associated antigen A3 (MAGE-A3) is a full protein vaccine comprised of a recombinant fusion protein (MAGE-A3 and protein D of Haemophilus influenzae) and ASO2B as an immune response-enhancing adjuvant. Results of the phase II randomized controlled trial (RCT) were promising, showing some improvement in disease-free survival and overall survival with no significant safety concerns. [139]. However, the subsequent phase III MAGRIT (NCT00480025) was recently terminated after data from the trial announced in March 2014 revealed that it did not meet its primary endpoints of disease free survival (overall, chemotherapy naïve, or gene signature positive sub-populations) [140]. Results from the final analysis are expected to be released in 2015.
The mucinous glycoprotein-1 (MUC1) is another vaccine target for NSCLC treatment. The TG4010 vaccine is a full protein vaccine based on a recombinant viral vector expressing the full MUC1 protein and IL-2 as an immunostimulant [141]. The phase IIb/III TIME trial (NCT01383148) will assess TG4010 first-line therapy in stage IV NSCLC patients [142]. Earlier phase IIB results of TG4010 plus cisplatin and gemcitabine versus chemotherapy alone demonstrated an enhancing effect of TG4010, improving 6 month progression-free survival (43.2% vs 35.1%)[143]. Tecemotide (L-BLP25 vaccine) liposomally delivers a 25 amino acid sequence from MUC1 with a monophosphoryl lipid A adjuvant. The results from the completed phase III START trial have recently been published [144]. In this double-blind RCT (NCT00409188), 1513 stage III NSCLC patients who responded to or had stable disease after first-line chemoradiotherapy were randomly assigned to tecemotide or placebo administered weekly for 8 weeks, and then every 6 weeks until disease progression or withdrawal. While no significant difference in overall survival was observed, median overall survival in patients who received previous concurrent chemoradiotherapy was improved with tecemotide treatment (adjusted HR 0·78, 0·64–0·95; p=0·016). Adverse effects observed with a greater than 2% frequency include dyspnea, metastasis to the central nervous system, and pneumonia. These findings prompted two further phase III studies, START2 (NCT02049151) and INSPIRE (NCT01015443, or EMR63325-012) [145]. However, due to the negative results from the INSPIRE trial, development plan of tecemotide was terminated in September 2014 [146].
Additional vaccines in various stages of pre-clinical and clinical trials incorporate a diverse array of tumor antigens or tumor-promoting pathways. Vaccines that target epidermal growth factor (EGF) or its cell membrane receptor (EGFR), often overexpressed in epithelial tumors including lung cancer, have been evaluated in early clinical trials [15]. Racotumomab (formerly IE10), which incorporates a Neu-glycosylated sialic acid-containing ganglioside (NeuGc-GM3) expressed on tumor cell surfaces, was evaluated in a phase II/III trial [147,148], with promising extension of both overall and progression-free survival and well tolerated effects. Use of racotumomab as switch maintenance therapy followed by second line therapy is currently being investigated in a phase III trial (NCT01460472). Belagenpumatucel-L is an allogeneic cell tumor vaccine that was recently evaluated in the phase III STOP trial (NCT00676507). While the STOP trial did not meet the primary endpoint, specific subgroups had marked improvement in survival. Median overall survival was considerably higher in patients pretreated with radiation (40.1 vs 10.3 months, HR 0.45, p = 0.014) and in patients with stage IIIB/IV non-adenocarcinoma randomized within 12 weeks of chemotherapy completion (19.9 vs 12.3 months, HR 0.55, p = 0.036) [149]. While vaccine therapy are as promising as they are diverse, a number of innate challenges [150] limit treatment solely by vaccination. Different approaches to cancer immunotherapy may complement vaccine therapy and allow for more specialized treatment of cancers.
Beyond vaccine therapy
A growing approach to cancer treatment utilizes antibodies that target particular tumor-promoting pathways. Angiogenesis can be modulated by blocking vascular endothelial growth factor receptors (VEGFRs). The recently released REVEL (NCT 01168973) study reports a 14% reduced risk of death with second-line treatment of NSCLC using ramucirumab, a recombinant human monoclonal antibody that binds with high affinity to the extracellular domain of VEGFR-2 [151], combined with docetaxel [152]. Nintedanib (BIBF 1120) is an oral angiokinase inhibitor that targets VEGFRs, fibroblast growth factor receptors (FGFR) and platelet derived growth factors (PDGFR). Results from the phase III LUME-Lung1 trial (NCT00805194) assessing docetaxel plus nintedanib as second-line therapy in NSCLC demonstrated significantly improved overall survival (median 10.9 vs 7.9 months, HR 0.75, p = 0.0073) and progression-free survival (median 3.4 vs 2.7 months, HR 0.79, p = 0.0019) [153]. While some Grade 3 or worse adverse events were associated with docetaxel plus nintedanib in this analysis, extended investigation demonstrated no increase in the frequency of antiangiogenic-specific adverse effects, except grade 1-2 bleeding events in SCC patients, when adding nintedanib to docetaxel for NSCLC therapy [154]. Results of the phase III LUME-Lung2 trial (NCT00806819) were analyzed to assess the effect of second-line nintedanib plus pemetrexed chemotherapy in NSCLC patients. Nintedanib plus pemetrexed significantly improved progression-free survival (median 4.4 vs 3.6 mo, HR 0.83, p = 0.04), but did not show a significant difference in overall survival with a manageable safety profile [155].
Self-regulatory checkpoints of the immune system are a continued challenge facing therapy by vaccine alone. Immune regulatory antibodies that abrogate immune inhibition (see Figure 4 and Table 2) have developing clinical application [150]. The most thoroughly investigated and clinically developed of these immunomodulatory strategies targets cytotoxic T lymphocyte antigen (CTLA)-4. This CD28:B7 immunoglobulin superfamily member is expressed at low levels on Tregs [80] and naïve effector T cells and is involved Treg-induced immune inhibition [38]. Tremelimumab is an anti-CTLA-4 human monoclonal antibody that primarily tested in advanced melanoma. The recent phase III RCT of tremelimumab failed to show a significant survival advantage over standard-of-care chemotherapy in first line treatment of patients with metastatic melanoma [156].
Ipilimumab, another CTLA-4-specific human monoclonal antibody, has demonstrated an overall survival benefit in a phase III trial for advanced melanoma patients [157] and clinical potential in NSCLC therapy. In the double blind phase II randomiuzed controlled trial in 204 chemotherapy-naïve stage IIIB/IV NSCLC patients, effect of ipilimumab (concurrent or phased) plus paclitaxel and carboplatin chemotherapy versus chemotherapy alone was compared [158]. The primary endpoint, immune-related progression-free survival, and progression-free survival both improved in the two ipilimumab groups compared with the controls (significant only for phased ipilimumab with a predefined significant p value of 0.1). The subsequent phase III trial will assess efficacy of this combination treatment in an estimated 920 squamous cell carcinoma patients (NCT01285609).
Programmed cell death protein 1 (PD-1) is a second inhibitory receptor that serves as a promising immunomodulation target. Expressed on T cells, B cells, and myeloid cells after activation, PD-1 interaction with its ligand provides immune inhibitory signals through multiple immunosuppressive pathways [159]. CT-011 and nivolumab (BMS-936558 or MDX-1106) are two PD-1 inhibitory antibodies that have been developed and clinically tested to show toleration to the treatment and disease response in solid tumor patients [150]. A phase I trial of nivolumab (NCT00730639) demonstrated objective responses in patients with NSCLC, melanoma, and renal-cell carcinoma with some adverse affects that do not appear to preclude its use. Clinical trials involving immunologic and molecular-marker correlates (NCT01354431 and NCT01358721) are under way, and phase 3 trials are expected. Additionally, first-line treatment of NSCLC with Nivolumab and ipilimumab is currently being evaluated (NCT01454102), with interim phase I data that were recently presented [160], suggesting nivolumab + ipilimumab immunotherapy to be feasible and demonstrating antitumor activity in both PD-L1+ and PD-L1− patients.
Expert Commentary and Five-Year View
The recent advances in cellular immunology have redefined the current understanding of lung cancer. However, learning from data on anti-CTLA-4 therapy, for example, the survival benefit from immunomodulation is only limited to a specific subset of patients (5.8% - 22%) suggested to be determined by tumor-specific CTL presence [78]. Similarly, a large number of other approaches, including vaccines, are not all-inclusive therapies, but rather specifically designed for certain tumor types. The early vaccine clinical trials have yielded mixed results, which could be due to several reasons [150,161]. Firstly, identifying antigens expressed exclusively and/or predominantly by the tumor poses significant difficulty. Additionally, overcoming immune tolerance to the tumor antigens (particularly in the lung) requires further investigation. This will likely require further use of immune stimulating agents in combination with immune checkpoint blockade to provoke a sufficient anti-tumor response. A number of combination therapies that have been mentioned previously include DC/CIK adoptive transfer in conjunction with erlotinib therapy [114], the TG4010 vaccine with cisplatin and gemcitabine [143], nintedanib plus docetaxel [153], and nivolumab plus ipilumab [160]. Overall, these combinations of therapies have improved efficacy and thus address the problems associated with mono-therapy. Lastly, finding the ideal patient population for each therapy warrants further studies, considering that the majority of clinical trials in new immunotherapy agents recruit advanced stage cancer. A move toward personalized medical treatment (whether molecular, genetically, or based on cancer progression) could be more effective. A recent study [162] featured in JAMA investigates the feasibility of incorporating genomic testing into clinical care for the stratification of lung cancer treatment. Ten oncogenes were tested in metastatic lung adenocarcinoma patients to guide targeted treatment in 28% of the 1007 patients. This genotype-directed therapy demonstrated a marked increase in the median survival (HR 0.69, 95%CI 0.53-0.9, P = .006). In the overall clinical setting, such subtyping of tumor cells has only recently gained momentum in the past few years.
Future diagnosis and treatment of lung cancers should be directed by extensive characterization of the tumor microenvironment. Biopsy should not be used solely for basic cancer type, but rather could include immune cell type infiltration, molecular markers and expression on tumor cells, and even tumor cell mutation genotyping. A major step towards individualized lung cancer therapy has been the targeting of specific driver mutations such as EGFR mutations (EGFR tyrosine kinase inhibitors (EGFR-TKI), including erlotinib, gefitinib and afatinib) and ALK translocations (ALK tyrosine kinase inhibitors, including crizotinib, certitinib). While these therapies of high specificity do provide a favorable prognosis to a specific subset of NSCLC patients, they are prone to confounding by other molecular pathways resulting in the development of acquired resistance to EGFR-TKI therapy. While not completely understood, some pathways such as secondary EGFR mutations or MET oncogene amplification have been implicated [163]. KRAS mutation, along with several other markers, has been identified as a predictor for EGFR-TKI resistance [164]. Common clinical practice of screening for such genotypes could aid in further individualizing therapeutic approach, thereby improving survival and quality of life.
Adoptive transfer of various immune cells is of growing interest in immunotherapy, with particular emphasis on T cell adoptive transfer. CAR T cells, in particular, have high potential with data now emerging from the earliest clinical trials. Early successful CAR T cells can expand in vivo more than 1000-fold compared to the initial level and persist in peripheral blood and bone marrow for at least six months with continued expression of CAR; They also demonstrated effective anti-tumor responses, but did cause some serious side effects that will require further investigation [133]. Nonetheless, this immunotherapy approach holds much promise.
With the explosion in understanding of cellular immunology of cancer, a diverse array of therapeutic approaches is developing. Immunotherapy may also be individualized in lung cancer treatment based on tumor-infiltrating immune cell profiles. Continued clinical trials are needed to evaluate novel combinations of the growing immunotherapy arsenal in lung cancer, including immunomodulation and systemic therapies.
Key issues.
The well-characterized Th1/Th2 cell paradigm implicates Th1 cells in a generally anti-tumorigenic response, whereas Th2 cells have a pro-tumor effect; these cells influence cancer response through cytokine release and immune cell modulation.
Th17 cells have been very recently discovered and have a still unclear role in lung cancer; IL-23/IL-6-induced subtypes have been linked to anti-tumor activity, whereas excessive IL-17 may promote angiogenesis, tumor growth, and excessive inflammation.
Targeting Treg-mediated immune inhibition offers therapeutic potential, by utilizing Treg cell depletion, cytokine-induced immunomodulation, or other mechanisms.
The roles of macrophage subtypes mirror those of the Th1/Th2 paradigm in lung cancer; induced M1 macrophage polarization or inhibition of the M2 macrophage response may offer therapeutic value.
Due to the direct effect on adaptive immunity, dendritic cells may mediate effective anti-tumor functionality or tumor immune escape through abnormally lowered activity; DC-based immunotherapies are of particular interest, including vaccines and DC-derived exosomes which may both be directed at particular cancer cells.
Adoptive transfer of NK cells offers a highly selective potential therapeutic approach with minimal adverse effects; ex vivo expansion of these cells on a clinical grade large-scale is still in development.
Cytotoxic T cells are of particular interest for immunotherapy, especially through adoptive T cell transfer, which is performed by either ex vivo expansion of endogenous T cells (generally isolated tumor infiltrating lymphocytes) or genetic modification (eg CAR T cells to allow MHC independent anti-tumor activity).
Vaccines targeting a wide array of tumor antigens are in various stages of clinical testing; some antigens targeted include MAGE, MUC-1, EGF or EGFR, HER2, CEA, and WT-1.
Combination therapy of vaccines in conjunction with immunomodulatory antibodies and/or chemo-radiotherapies may improve treatment; the most well-characterized targets for inhibitory antibodies are CTLA-4 and PD-1.
Acknowledgments
This work was supported by research grants from the National Institutes of Health, USA to DK Agrawal.
Footnotes
Disclaimer
The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as: *of interest
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics , 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. doi:10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Moolgavkar SH, Holford TR, Levy DT, Kong CY, Foy M, Clarke L, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975-2000. J Natl Cancer Inst. 2012;104:541–8. doi: 10.1093/jnci/djs136. doi:10.1093/jnci/djs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer facts and figures 2014. Atlanta: 2014. [Google Scholar]
- 4.Hirsch FR, Franklin WA, Gazdar AF, Bunn PA. Early Detection of Lung Cancer : Clinical Perspectives of Recent Advances in Biology and Radiology. Clin Cancer Res. 2001;7:5–22. [PubMed] [Google Scholar]
- 5.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. doi:10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 6.Engels E a, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. doi:10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. doi:10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 8.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–71. doi: 10.1158/0008-5472.CAN-10-2907. doi:10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. doi:10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 10.O'Callaghan D, O'Donnell D, O'Connell F, O'Byrne K. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024–36. doi: 10.1097/jto.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 11.Tan T-T, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–16. doi: 10.1016/j.coi.2007.01.001. doi:10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Langer CJ. Emerging Immunotherapies in the Treatment of Non-Small Cell Lung Cancer (NSCLC): The Role of Immune Checkpoint Inhibitors. Am J Clin Oncol. 2014;00:1–9. doi: 10.1097/COC.0000000000000059. doi:10.1097/COC.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg S a, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. doi:10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies M. New modalities of cancer treatment for NSCLC: focus on immunotherapy. Cancer Manag Res. 2014;6:63–75. doi: 10.2147/CMAR.S57550. doi:10.2147/CMAR.S57550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly R, Giaccone G. Lung cancer - Vaccines. Cancer J. 2011;17:302–8. doi: 10.1097/PPO.0b013e318233e6b4. doi:10.1097/PPO.0b013e318233e6b4.Lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano T, Haro A, Shikada Y, Maruyama R, Maehara Y. Non-small cell lung cancer in never smokers as a representative “non-smoking-associated lung cancer”: epidemiology and clinical features. Int J Clin Oncol. 2011;16:287–93. doi: 10.1007/s10147-010-0160-8. doi:10.1007/s10147-010-0160-8. [DOI] [PubMed] [Google Scholar]
- 17.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei A a. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. doi: 10.4065/83.5.584. doi:10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med. 2011;32:703–40. doi: 10.1016/j.ccm.2011.08.003. doi:10.1016/j.ccm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–32. doi: 10.1002/ijc.27816. doi:10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon, France: 2004. [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell. 2010;17:89–97. doi: 10.1016/j.ccr.2009.12.008. doi:10.1016/j.ccr.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. doi:10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. doi:10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Beane J, Vick J, Schembri F, Anderlind C, Gower A, Campbell J, et al. Characterizing the impact of smoking and lung cancer on the airway transcriptome using RNA-Seq. Cancer Prev Res. 2011;4:803–17. doi: 10.1158/1940-6207.CAPR-11-0212. doi:10.1158/1940-6207.CAPR-11-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–74. doi: 10.1016/j.mrrev.2004.02.001. doi:10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Ogino S, Galon J, Fuchs C, Dranoff G. Cancer Immunology - Analysis of Host and Tumor Factors for Personalized Medicine. Nat Rev Clin Oncol. 2011;8:617–32. doi: 10.1038/nrclinonc.2011.122. doi:10.1038/nrclinonc.2011.122.Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–51. doi: 10.1038/sj.onc.1205803. doi:10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 28.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80. doi: 10.1016/S1470-2045(10)70087-5. doi:10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 29.Wan YY, Flavell R a. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. doi:10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–62. doi: 10.1111/j.1600-065X.2010.00951.x. doi:10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations. Annu Rev Immunol. 2012;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. doi:10.1146/annurev-immunol-030409-101212.Differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito N, Suzuki Y, Taniguchi Y, Ishiguro K, Nakamura H, Ohgi S. Prognostic significance of T helper 1 and 2 and T cytotoxic 1 and 2 cells in patients with non-small cell lung cancer. Anticancer Res. 2005;25:2027–31. [PubMed] [Google Scholar]
- 33.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. doi:10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 34.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–9. doi: 10.1093/carcin/bgr019. doi:10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–56. doi: 10.1038/nri2742. doi:10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111:5664–9. doi: 10.1073/pnas.1319051111. doi:10.1073/pnas.1319051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu K, Nakata M, Hirami Y, Yukawa T, Maeda A, Tanemoto K. Tumor-infiltrating Foxp3+ regulatory T cells are correlated with cyclooxygenase-2 expression and are associated with recurrence in resected non-small cell lung cancer. J Thorac Oncol. 2010;5:585–90. doi: 10.1097/JTO.0b013e3181d60fd7. doi:10.1097/JTO.0b013e3181d60fd7. [DOI] [PubMed] [Google Scholar]
- 38.Duan M-C, Zhong X-N, Liu G-N, Wei J-R. The Treg/Th17 Paradigm in Lung Cancer. J Immunol Res. 2014;2014:1–9. doi: 10.1155/2014/730380. doi:10.1155/2014/730380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. doi:10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 40.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–7. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 41.Haabeth OAW, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. doi:10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, et al. TNF- α is critical for antitumor but not antiviral T cell immunity in mice. J Clin Investig. 2007;117:3833–45. doi: 10.1172/JCI32567. doi:10.1172/JCI32567.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–80. doi: 10.1038/sj.bjc.6602934. doi:10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neurath MF, Finotto S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine Growth Factor Rev. 2012;23:315–22. doi: 10.1016/j.cytogfr.2012.08.009. doi:10.1016/j.cytogfr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Gocheva V, Wang H-W, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. doi:10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Shi W, Yu G, Lin L, Yang B, Li J, et al. Interleukin-4 -590T/C polymorphism influences the susceptibility to nonsmall cell lung cancer. DNA Cell Biol. 2012;31:797–800. doi: 10.1089/dna.2011.1425. doi:10.1089/dna.2011.1425. [DOI] [PubMed] [Google Scholar]
- 47.Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M, Evans CM, et al. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res. 2011;4:51–64. doi: 10.1158/1940-6207.CAPR-10-0180. doi:10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagenbaugh a, Sharma S, Dubinett SM, Wei SH, Aranda R, Cheroutre H, et al. Altered immune responses in interleukin 10 transgenic mice. J Exp Med. 1997;185:2101–10. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, et al. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J Immunol. 1999;163:5020–8. [PubMed] [Google Scholar]
- 50.Hatanaka H, Abe Y, Kamiya T, Morino F, Nagata J, Tokunaga T, et al. Clinical implications of interleukin ( IL ) -IO induced by non-small-cell lung cancer. Ann Oncol. 2000:815–9. doi: 10.1023/a:1008375208574. [DOI] [PubMed] [Google Scholar]
- 51.Terabe M, Park JM, Berzofsky J a. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. doi:10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. doi:10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 53.Pagès F, Galon J, Dieu-Nosjean M-C, Tartour E, Sautès-Fridman C, Fridman W-H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. doi:10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 54.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. doi:10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 55.Zhang JY, Green CL, Tao S, Khavari P a. NF-kappaB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18:17–22. doi: 10.1101/gad.1160904. doi:10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sethi G, Sung B, Aggarwal BB. TNF: A master switch for inflammation to cancer. Front Biosci. 2008:5094–107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 57.Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 2009;64:219–25. doi: 10.1016/j.lungcan.2008.08.015. doi:10.1016/j.lungcan.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Boldrini L, Calcinai a, Samaritani E, Pistolesi F, Mussi a, Lucchi M, et al. Tumour necrosis factor-alpha and transforming growth factor-beta are significantly associated with better prognosis in non-small cell lung carcinoma: putative relation with BCL-2-mediated neovascularization. Br J Cancer. 2000;83:480–6. doi: 10.1054/bjoc.2000.1345. doi:10.1054/bjoc.2000.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohri CM, Shikotra A, Green RH, Waller D a, Bradding P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer. 2010;10:323. doi: 10.1186/1471-2407-10-323. doi:10.1186/1471-2407-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colakogullari M, Ulukaya E, Oral AY, Aymak F, Basturk B, Ursavas A, et al. The involvement of IL-10 , IL-6 , IFN- g , TNF- a and TGF- b gene polymorphisms among Turkish lung cancer patients. Cell Biochem Funct. 2008;26:283–90. doi: 10.1002/cbf.1419. doi:10.1002/cbf. [DOI] [PubMed] [Google Scholar]
- 61.Shih C-M, Lee Y-L, Chiou H-L, Chen W, Chang G-C, Chou M-C, et al. Association of TNF-alpha polymorphism with susceptibility to and severity of non-small cell lung cancer. Lung Cancer. 2006;52:15–20. doi: 10.1016/j.lungcan.2005.11.011. doi:10.1016/j.lungcan.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 62.Brown DM. Cytolytic CD4 cells: Direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. doi: 10.1016/j.cellimm.2010.02.008. doi:10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan R a, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. doi:10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barve M, Bender J, Senzer N, Cunningham C, Greco FA, McCune D, et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26:4418–25. doi: 10.1200/JCO.2008.16.6462. doi:10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 65.PR Newswire Agreement on Phase 3 Trial Protocol of OSE Pharma's OSE-2101 From the FDA and EMA. 2014 [Google Scholar]
- 66.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32:603–11. doi: 10.1016/j.it.2011.08.003. doi:10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Middleton GW, Annels NE, Pandha HS. Are we ready to start studies of Th17 cell manipulation as a therapy for cancer? Cancer Immunol Immunother. 2012;61:1–7. doi: 10.1007/s00262-011-1151-y. doi:10.1007/s00262-011-1151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kryczek I, Banerjee M, Cheng P. Phenotype , distribution , generation , and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. doi:10.1182/blood-2009-03-208249.An. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Z-J, Zhou Q, Gu Y-Y, Qin S-M, Ma W-L, Xin J-B, et al. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J Immunol. 2010;185:6348–54. doi: 10.4049/jimmunol.1001728. doi:10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–64. doi: 10.1084/jem.20090207. doi:10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin W, Karin M. A cytokine-mediated link between innate immunity , inflammation , and cancer. J Clin Investig. 2007;117:1175–83. doi: 10.1172/JCI31537. doi:10.1172/JCI31537.data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, et al. IL-17 Enhances the Net Angiogenic Activity and In Vivo Growth of Human Non-Small Cell Lung Cancer in SCID Mice through Promoting CXCR-2-Dependent Angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. doi:10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 73.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett. 2012;148:144–50. doi: 10.1016/j.imlet.2012.10.011. doi:10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–54. doi: 10.1016/j.lungcan.2009.11.013. doi:10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 75.Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–13. doi: 10.1002/eji.200838331. doi:10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. doi:10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ersvær E, Melve GK, Bruserud O. Future perspectives: should Th17 cells be considered as a possible therapeutic target in acute myeloid leukemia patients receiving allogeneic stem cell transplantation? Cancer Immunol Immunother. 2011;60:1669–81. doi: 10.1007/s00262-011-1118-z. doi:10.1007/s00262-011-1118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aerts JG, Hegmans JP. Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res. 2013;73:2381–8. doi: 10.1158/0008-5472.CAN-12-3932. doi:10.1158/0008-5472.CAN-12-3932. [DOI] [PubMed] [Google Scholar]
- 79.Sharma S, Yang S, Zhu L, Reckamp K, Gardner B. Tumor Cyclooxygenase-2 / Prostaglandin E 2 − Dependent Promotion of FOXP3 Expression and CD4 + CD25 + T Regulatory Cell Activities in Lung Cancer. Cancer Res. 2005;65:5211–20. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 80.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11. doi: 10.1182/blood-2006-02-002774. doi:10.1182/blood-2006-02-002774.Supported. [DOI] [PubMed] [Google Scholar]
- 81.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factorbeta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91:964–71. [PubMed] [Google Scholar]
- 82.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–3. doi: 10.1073/pnas.1633291100. doi:10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li S, Li Y, Qu X, Liu X, Liang J. Detection and significance of TregFoxP3+ and Th17 cells in peripheral blood of non-small cell lung cancer patients. Arch Med Sci. 2014;2:232–9. doi: 10.5114/aoms.2014.42573. doi:10.5114/aoms.2014.42573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. doi:10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Wang K, Grivennikov SI, Karin M. Implications of anti-cytokine therapy in colorectal cancer and autoimmune diseases. Ann Rheum Dis. 2013;72(Suppl 2):ii100–3. doi: 10.1136/annrheumdis-2012-202201. doi:10.1136/annrheumdis-2012-202201. [DOI] [PubMed] [Google Scholar]
- 86.Li X, Kostareli E, Suffner J, Garbi N, Hämmerling GJ. Efficient Treg depletion induces T-cell infiltration and rejection of large tumors. Eur J Immunol. 2010;40:3325–35. doi: 10.1002/eji.201041093. doi:10.1002/eji.201041093. [DOI] [PubMed] [Google Scholar]
- 87.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–49. doi: 10.1158/0008-5472.CAN-12-2325. doi:10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 88.Ali K, Soond DR, Piñeiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014 doi: 10.1038/nature13444. doi:10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J-Y, Duan X-F, Wang L-P, Xu Y-J, Huang L, Zhang T-F, et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res. 2014;2014:286170. doi: 10.1155/2014/286170. doi:10.1155/2014/286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alistair C, Alison M, Bruce R, Richard L, Michael M, Anna N. Optimising regulatory T cell (Treg) depletion in combination with chemotherapy for enhanced anti-tumour immunity. Front Immunol. 2013;4 Conference abstract presented August 2013. doi:10.3389/conf.fimmu.2013.02.00925. [Google Scholar]
- 91.Gitlitz BJ, Bernstein E, Santos ES, Otterson GA, Milne G, Syto M, et al. A Randomized, Placebo-Controlled, Multicenter, Biomarker-Selected, Phase 2 Study of Apricoxib in Combination with Erlotinib in Patients with Advanced Non–Small-Cell Lung Cancer. J Thorac Oncol. 2014;9:577–82. doi: 10.1097/JTO.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneberger D, Aharonson-Raz K, Singh B. Monocyte and macrophage heterogeneity and Toll-like receptors in the lung. Cell Tissue Res. 2011;343:97–106. doi: 10.1007/s00441-010-1032-2. doi:10.1007/s00441-010-1032-2. [DOI] [PubMed] [Google Scholar]
- 93.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. doi:10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 94.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. doi:10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 95.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. doi:10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Standiford TJ, Kuick R, Bhan U, Chen J, Newstead M, Keshamouni VG. TGF-β-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene. 2011;30:2475–84. doi: 10.1038/onc.2010.619. doi:10.1038/onc.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–83. doi: 10.1073/pnas.0809784106. doi:10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagemann T, Lawrence T, McNeish I, Charles K a, Kulbe H, Thompson RG, et al. “Re-educating” tumorassociated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–8. doi: 10.1084/jem.20080108. doi:10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. doi:10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 100.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, et al. HIF-1α Is Essential for Myeloid Cell-Mediated Inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. doi:10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. doi: 10.1093/jnci/djm135. doi:10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 102.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–6. doi: 10.1111/j.1582-4934.2009.00876.x. doi:10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. doi:10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 104.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. doi:10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. doi:10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. doi:10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 107.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, et al. The number and microlocalization of tumor-associated immune cells are associated with patient's survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. doi:10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–44. doi: 10.1164/rccm.201309-1611OC. doi:10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 109.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, et al. Dendritic Cells Infiltrating Human Non-Small Cell Lung Cancer Are Blocked at Immature Stage. J Immunol. 2007;178:2763–9. doi: 10.4049/jimmunol.178.5.2763. doi:10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 110.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–807. doi: 10.4049/jimmunol.0712671. doi:10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 111.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42. doi: 10.1038/ni.2376. doi:10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Curiel T, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell – mediated antitumor immunity. Nat Med. 2003;9:5–10. doi: 10.1038/nm863. doi:10.1038/nm. [DOI] [PubMed] [Google Scholar]
- 113.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. 2010;70:1281–5. doi: 10.1158/0008-5472.CAN-09-3276. doi:10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 114.Shi S-B, Tang X-Y, Tian J, Chang C-X, Li P, Qi J-L. Efficacy of erlotinib plus dendritic cells and cytokineinduced killer cells in maintenance therapy of advanced non-small cell lung cancer. J Immunother. 2014;37:250–5. doi: 10.1097/CJI.0000000000000015. doi:10.1097/CJI.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 115.Chen R, Deng X, Wu H, Peng P, Wen B, Li F, et al. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: A systematic review and meta-analysis. Int Immunopharmacol. 2014;22:451–64. doi: 10.1016/j.intimp.2014.07.019. doi:10.1016/j.intimp.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 116.Soliman HH, Minton SE, Han HS, Ismail-khan R, Janssen W, Streicher H, et al. A phase I study of ad.p53 DC vaccine in combination with indoximod in metastatic solid tumors. J Clin Oncol. 2014;31(Suppl) abstr 30609. [Google Scholar]
- 117.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. doi:10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–63. doi: 10.1111/j.1365-2567.2009.03167.x. doi:10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. doi:10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 120.Hodge G, Barnawi J, Jurisevic C, Moffat D, Holmes M, Reynolds PN, et al. Lung cancer is associated with decreased expression of perforin, granzyme B and IFNγ by infiltrating lung tissue T cells, NKT-like and NK cells. Clin Exp Immunol. 2014:1–24. doi: 10.1111/cei.12392. doi:10.1111/cei.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–75. doi: 10.1002/cncr.23239. doi:10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 122.Bruno A, Focaccetti C, Pagani A, Imperatori AS, Spagnoletti M, Rotolo N, et al. The Proangiogenic Phenotype of Natural Killer Cells in Patients with Non – Small Cell Lung Cancer. Neoplasia. 2013;15:133–142. IN7. doi: 10.1593/neo.121758. doi:10.1593/neo.121758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esendagli G, Bruderek K, Goldmann T, Busche a, Branscheid D, Vollmer E, et al. Malignant and nonmalignant lung tissue areas are differentially populated by natural killer cells and regulatory T cells in non-small cell lung cancer. Lung Cancer. 2008;59:32–40. doi: 10.1016/j.lungcan.2007.07.022. doi:10.1016/j.lungcan.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 124.Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–38. doi: 10.1111/j.1600-065X.2006.00445.x. doi:10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 125.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266:154–81. doi: 10.1111/j.1365-2796.2009.02121.x. doi:10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 126.Hiraki A, Kiura K, Yamane H, Nogami N, Tabata M, Takigawa N, et al. Interleukin-12 augments cytolytic activity of peripheral blood mononuclear cells against autologous lung cancer cells in combination with IL-2. Lung Cancer. 2002;35:329–33. doi: 10.1016/s0169-5002(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 127.Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, et al. Treatment of Colon and Lung Cancer Patients with ex Vivo Heat Shock Protein 70-Peptide-Activated , Autologous Natural Killer Cells : A Clinical Phase I Trial. Clin Cancer Res. 2004;10:3699–707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 128.Schneider T, Hoffmann H, Dienemann H, SChnabel P, Enk A, Ring S, et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac. 2011;6:1162–8. doi: 10.1097/JTO.0b013e31821c421d. doi:10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 129.Bremnes RM, Sirera R, Al-saad S, Camps C, Busund L. The Role of Tumor-Infiltrating Immune Cells and Chronic Inflammation at the Tumor Site on Cancer Development , Progression , and Prognosis. J Thorac Oncol. 2011;6:824–33. doi: 10.1097/JTO.0b013e3182037b76. [DOI] [PubMed] [Google Scholar]
- 130.Liu H, Zhang T, Ye J, Li H, Huang J, Li X, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–56. doi: 10.1007/s00262-012-1231-7. doi:10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu R, Forget M, Chacon J, Bernatchez C, Haymaker C, Chen JQ, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–75. doi: 10.1097/PPO.0b013e31824d4465. doi:10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Wang X. Beyond chemotherapy and targeted therapy: adoptive cellular therapy in non-small cell lung cancer. Mol Biol Rep. 2014;41:6317–23. doi: 10.1007/s11033-014-3514-x. doi:10.1007/s11033-014-3514-x. [DOI] [PubMed] [Google Scholar]
- 133.Chmielewski M, Hombach A a, Abken H. Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells. Front Immunol. 2013;4:371. doi: 10.3389/fimmu.2013.00371. doi:10.3389/fimmu.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ozkaynak MF, Gilman AL, Yu AL, London WB, Sondel PM, Smith MA, et al. A comprehensive safety trial of chimeric antibody 14.18 (ch14.18) with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: A Children's Oncology Group study. J Clin Oncol. 2014;32(suppl) abstr 10044. [Google Scholar]
- 135.De Portilla F, Salazar R, Santos C, Sanchez A, Duran H, Sanjuan X, et al. A phase 1 mechanism of action study of intratumoral or intravenous administration of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus in colon cancer patients undergoing resection of primary tumor. J Clin Oncol. 2014;32(suppl) abstr TPS3112. [Google Scholar]
- 136.Calvo E, Gil-martin M, Machiels JH, Rottey S, Beadle JW, Blanc C, et al. A first-in-class, first-in-human phase I study of enadenotucirev, an oncolytic Ad11/Ad3 chimeric group B adenovirus, administered intravenously in patients with metastatic epithelial tumors. J Clin Oncol. 2014;32(suppl) abstr 3103. [Google Scholar]
- 137.Hegde M, Wakefield A, Brawley VS, Grada Z, Tiara T. Genetic modification of T cells with a novel bispecific chimeric antigen receptor to enhance the control of high-grade glioma (HGG). J Clin Oncol. 2014;32(suppl) abstr10027. [Google Scholar]
- 138.Moon EK, Wang L-C, Dolfi D V, Wilson CB, Ranganathan R, Sun J, et al. Multifactorial T-cell Hypofunction That Is Reversible Can Limit the Efficacy of Chimeric Antigen Receptor-Transduced Human T cells in Solid Tumors. Clin Cancer Res. 2014;20:4262–73. doi: 10.1158/1078-0432.CCR-13-2627. doi:10.1158/1078-0432.CCR-13-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, et al. Adjuvant MAGEA3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol. 2013;31:2396–403. doi: 10.1200/JCO.2012.43.7103. doi:10.1200/JCO.2012.43.7103. [DOI] [PubMed] [Google Scholar]
- 140.Chustecka Z. MAGE A3 Vaccine Now Dropped for Lung Cancer. Medscape. 2014 [Google Scholar]
- 141.Cuppens K, Vansteenkiste J. Vaccination therapy for non-small-cell lung cancer. Curr Opin Oncol. 2014;26:165–70. doi: 10.1097/CCO.0000000000000052. doi:10.1097/CCO.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 142.Quoix EA, Nemunaitis JJ, Burzykowski T, Bastien B, Lacoste G. TIME: A phase IIb/III randomized, double-blind, placebo-controlled study comparing first-line therapy with or without TG4010 immunotherapy product in patients with stage IV non-small cell lung cancer (NSCLC). J Clin Oncol. 2012;30(suppl) abstr TPS7610. [Google Scholar]
- 143.Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12:1125–33. doi: 10.1016/S1470-2045(11)70259-5. doi:10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 144.Butts C, Socinski M a, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. doi:10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 145.Wu Y-L, Park K, Soo R a, Sun Y, Tyroller K, Wages D, et al. INSPIRE: A phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer. 2011;11:430. doi: 10.1186/1471-2407-11-430. doi:10.1186/1471-2407-11-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chustecka Z. Vaccine for Lung Cancer Dropped From Development. Medscape. 2014 [Google Scholar]
- 147.Santiesteban E, Perez L, Alfonso S, Neninger E, Acosta S, Flores Y, et al. Safety and Efficacy of Racotumomab-Alum Vaccine as Second-Line Therapy for Advanced Non-Small Cell Lung Cancer. 2014:844–50. [Google Scholar]
- 148.Alfonso S, Valdés-Zayas A, Santiesteban ER, Flores YI, Areces F, Hernández M, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20:3660–71. doi: 10.1158/1078-0432.CCR-13-1674. doi:10.1158/1078-0432.CCR-13-1674. [DOI] [PubMed] [Google Scholar]
- 149.Giaccone G, Bazhenova L, Nemunaitis J, Juhasz E, Ramlau R, van den Heuvel M, et al. A phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small-cell lung cancer (NSCLC). Eur. Cancer Congr. 2013;49 doi: 10.1016/j.ejca.2015.07.035. Abstract LBA2. Presented September 28, 2013. [DOI] [PubMed] [Google Scholar]
- 150.Postow M, Callahan M, Wolchok J. Beyond Cancer Vaccines: A Reason for Future Optimism with Immunomodulatory Therapy. Cancer J. 2011;17:372–8. doi: 10.1097/PPO.0b013e31823261db. doi:10.1097/PPO.0b013e31823261db.Beyond. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garon EB, Cao D, Alexandris E, John WJ, Yurasov S, Perol M. A randomized, double-blind, phase III study of Docetaxel and Ramucirumab versus Docetaxel and placebo in the treatment of stage IV nonsmall-cell lung cancer after disease progression after 1 previous platinum-based therapy (REVEL): treatment rationale an. Clin Lung Cancer. 2012;13:505–9. doi: 10.1016/j.cllc.2012.06.007. doi:10.1016/j.cllc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 152.Perol M, Ciuleanu T-E, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. REVEL: A randomized, double-blind, phase III study of docetaxel (DOC) and ramucirumab (RAM; IMC-1121B) versus DOC and placebo (PL) in the second-line treatment of stage IV non- small cell lung cancer (NSCLC) following disease progression after one prior p. J Clin Oncol. 2014;32(suppl) abstr LBA8006^. [Google Scholar]
- 153.Reck M, Kaiser R, Mellemgaard A, Douillard J-Y, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–55. doi: 10.1016/S1470-2045(13)70586-2. doi:10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 154.Reck M, Mellemgaard A, Orlov S V, Krzakowski MJ, Von Pawel J, Gottfried M, et al. Antiangiogenicspecific adverse events (AEs) in patients with non-small cell lung cancer (NSCLC) treated with nintedanib (N) and docetaxel (D). J Clin Oncol. 2014;32(suppl) abstr 8100. [Google Scholar]
- 155.Hanna NH, Kaiser R, Sullivan RN, Aren OR, Tiangco B, Zvirbule Z, et al. Lume-lung 2: A multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy. J Clin Oncol. 2013;31(suppl) abstr 8034. [Google Scholar]
- 156.Ribas A, Kefford R, Marshall M a, Punt CJ a, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–22. doi: 10.1200/JCO.2012.44.6112. doi:10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hodi F, O'Day S, McDermott DF, Weber R, Sosman J, Haanen J, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. doi:10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 159.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13:e301–10. doi: 10.1016/S1470-2045(12)70126-2. doi:10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Antonia SJ, Gettinger SN, Quan L, Chow M, Rosalyn A, Borghaei H, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol. 2014;1(suppl) abstr 8023. [Google Scholar]
- 161.Ramlogan-Steel CA, Steel JC, Morris JC. Lung Cancer Vaccines : Current Status and Future Prospects. Transl Lung Cancer Res. 2014;3:46–52. doi: 10.3978/j.issn.2218-6751.2013.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate a J, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. doi:10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Engelman J a, Jänne P a. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–9. doi: 10.1158/1078-0432.CCR-07-2248. doi:10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 164.Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. doi:10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]