Abstract
BACKGROUND
Surgery remains the only potentially curative option for patients with hepatocellular carcinoma (HCC) and fibrolamellar carcinoma (FLC). We sought to investigate the differences over time in surgically managed FLC compared with conventional HCC using population-based data.
STUDY DESIGN
Using SEER data, we identified 7,225 patients with surgically managed FLC or HCC from 1986 to 2008. We examined differences in clinicopathologic and surgical factors associated with long-term survival.
RESULTS
Of the 7,225 patients, the majority had HCC (n = 7,135; 99%) vs FLC (n = 90; 1%). Patients with FLC were younger (25 years vs 59 years) and more often were women (44% vs 27%) than patients with HCC (both p < 0.001). Regional disease was more common among patients with FLC (42.2%) vs patients with HCC (22.1%) (p < 0.001). More than one-third of patients with FLC (36.9%) were operatively managed with a hemi-hepatectomy compared with patients with HCC, who were more often managed with a liver transplant (p < 0.001). On univariable analysis, there was a marked difference in overall survival, with patients with FLC surviving a median of 75 months vs 43 months for HCC (hazard ratio [HR]: 0.59; p = 0.001). There was a marked difference in survival when patients were stratified by localized (FLC, 78 months vs HCC, 49 months; p = 0.012) vs regional disease (FLC, 46 months vs HCC, 23 months; p = 0.002.
CONCLUSIONS
Patients with FLC have many clinicopathologic features that are different from those of patients with HCC, including younger age and female sex. Despite a higher likelihood of advanced disease at the time of diagnosis, surgically treated FLC patients had better long-term outcomes than patients with conventional HCC.
In 2013, the American Cancer Society estimates there will be 28,720 new cases of primary liver cancer, with 20,550 deaths in the United States.1 Primary liver cancers include hepatocellular carcinoma (HCC), intrahepatic cholangio-carcinoma, and the more uncommon fibrolamellar carcinoma (FLC) variant of HCC. It is estimated that FLC comprises approximately 1% of all HCC based on population data derived from the SEER data maintained by the National Cancer Institute (NCI).2 Fibrolamellar carcinoma is distinct from HCC in both its clinical and pathologic manifestations, often affecting younger patients and having a higher incidence in women.2,3 Importantly, FLC has been shown to have a general better prognosis than HCC, leading to significant interest in maximizing the intent for cure in this often young and otherwise healthy patient population.2–6 In recent years, advances in surgical care and improvements in preoperative imaging and surgical techniques have decreased the morbidity associated with liver resection.7,8 However, the distinct clinicopathologic features of FLC compared with HCC, including no association with cirrhosis, necessitate a better understanding of factors influencing outcomes in patients with operable FLC vs HCC.
To date, there have been several small institutional series summarizing the surgical management of patients with FLC. Unfortunately, these studies have been limited by small sample sizes due to the rarity of FLC, and therefore they have lacked the statistical power to draw meaningful conclusions.3,9,10 Population-based data may help better characterize patterns of care among patients with low-incidence cancers such as FLC. Using population-based data, more patients can be included in the study cohort and data on therapeutic management beyond that delivered just at tertiary referral centers can be examined. Although general epidemiologic trends have been examined using national data, FLC has yet to be examined from a surgical perspective using the SEER database.2 As such, the objective of this study was to characterize the surgical management of patients with FLC, as well as define national trends in the specific use of operative procedures and treatments among patients with FLC. In addition, we sought to examine whether there have been improvements in survival among patients with surgically managed FLC vs HCC on a population basis over time.
METHODS
Data source
This study was a retrospective analysis of prospectively collected data from the SEER database maintained by the NCI. The SEER data derive from 20 cancer registries, representing approximately 28% of the United States population.11 These data include information on patient demographics, tumor and disease characteristics, course of treatment, use of cancer-directed surgery and medical therapy, survival, and cause of death for individuals diagnosed with cancer.
Study population
Patients with a diagnosis of primary liver cancer from 1986 to 2008 within the SEER database were identified by the International Classification of Diseases for Oncology (ICD-O-3) topography, behavior, and histology codes using SEER*Stat software.12 Patients with intrahepatic cholangiocarcinoma and other biliary tract cancers were excluded from the cohort. We chose 1986 as the first year of the study because FLC (8171) was not recognized and coded as a distinct entity separate from HCC until that year. To fully capture all of the incident cases of HCC from 1986 to 2008, we included all histology codes relevant to HCC as determined by a gastrointestinal pathologist at the Johns Hopkins Hospital (RAA). Histologies relevant to HCC included hepatocarcinoma (8170), hepatocellular carcinoma— scirrhous (8172), hepatocellular carcinoma—spindle cell (8173), hepatocellular carcinoma—clear (8174), and hepatocellular carcinoma—pleomorphic (8175); collectively, these histologies comprised the HCC population of our study. We excluded other histology codes specific to cholangiocarcinoma from the analysis and included only patients undergoing a cancer-directed operation that was histologically or microscopically confirmed. Finally, we selected only patients with local or regional disease as defined by the SEER historical stage in the study population; patients with metastatic disease were excluded. The SEER historic stage is often used when dealing with datasets that span several revisions of the American Joint Committee on Cancer (AJCC) staging manual in order to ensure some uniformity of staging data over time. The historic stage has been standardized and simplified to ensure consistent definitions over time and is preferred to the AJCC staging system that is more commonly used in the clinical settings. Localized cancer is defined by SEER as cancer that is limited to the organ in which it began, without evidence of spread outside of the primary organ. For hepatic malignancies this is defined as no evidence of spread to regional or distant lymph nodes and no evidence of extrahepatic disease. Regional cancer is cancer that has spread beyond the original (primary) site to nearby lymph nodes or organs and tissues including nodal stations in or along the hepatic pedicle, inferior vena cava, hepatic artery, porta hepatis, and periportal basins. Distant cancer is cancer that has spread from the primary site to distant organs or distant lymph nodes (eg, para-aortic, peri-pancreatic, and retroperitoneal). For further information regarding staging of hepatic malignancies by SEER, please see Appendix C, available at http://seer.cancer.gov/manuals/2010/appendixc.html.
Outcomes and predictor variables
Using SEER*Stat data, clinicopathologic information on patient age, sex, race, year of treatment, grade, cancer stage, and the type of liver-directed procedure performed were collected from the SEER database. Operations were classified as ablative procedures only (eg, radiofrequency ablation), hepatic resection or liver transplantation, and surgery not otherwise specified. The extent-of-surgery variable was available and more detailed within SEER starting in 1998. When available, the types of operations were further divided, transforming the extent-of-surgery variables within SEER over the latter 11-year time period of the study. Types of operative procedures included partial hepatectomy, hemihepatectomy, extended hepatectomy (≥5 segments), and liver transplantation.13 Furthermore, we examined liver-directed procedures that used ablative procedures combined with hepatic resection. Information regarding receipt and type of systemic therapy are not reliably available within the SEER database and are therefore not included in the analysis. Variables were transformed into categorical and indicator variables where appropriate.
Statistical analyses
Median values with standard deviations were used to describe continuous data, with discrete variables displayed as totals and frequencies. Cells with fewer than 11 cases per variable cell were relabeled as “<11 (<%)” in compliance with the NCI data-usage agreement for reporting of SEER-Medicare data. This data usage agreement serves to protect patient confidentiality when the data are stratified, thereby decreasing the proportion of patients with those particular characteristics represented in a table that could allow identification of specific patients. Although the masking of cells <11 is usually used with SEER-Medicare data, we chose to incorporate this safeguard given the rarity of FLC to maximally protect patient confidentiality. Univariable comparisons were assessed using the 2-sample Student’s t-test for continuous variables and the chi-square test for dichotomous and categorical variables. The cases were separated into quartiles (1986 to 2001, 2002 to 2004, 2005 to 2006, and 2007 to 2008) based on the year of FLC or HCC diagnosis. Trends in ordinal data were evaluated using the linear-by-linear association test.14
Cumulative event rates were calculated using the method of Kaplan and Meier15 and survival curves were compared using the log-rank test. Overall survival time was calculated from the date of diagnosis for FLC or HCC to the date of last follow-up as recorded in the SEER database. To account for perioperative complications such as hepatic insufficiency, which would be presumed to be higher in patients with HCC given the association of cirrhosis, we assessed mortality at 30 and 90 days after diagnosis. Kaplan-Meier analyses were then performed with patients who died at 30 or 90 days excluded from the analysis to determine if there was a difference from the overall cohort. Univariable and multivariable modeling of survival were performed using Cox proportional hazards models.16 Covariates were included in the multivariable Cox model based on statistical significance in the univariable models (p ≤ 0.30). The model was validated by checking against a forward stepwise Wald selection model as described by Hosmer and Lemeshow.17,18 The overall fit of the multivariable models was assessed using the likelihood ratio test. Relative risks were expressed as hazard ratios (HR) with a 95% CI. Next, the final model was evaluated for goodness-of-fit using the method proposed by May and Hosmer.17,19 Schoenfeld residuals and log-log plots were used to examine adherence to the proportional hazards assumption. All reported p values are 2-tailed, and for all tests, p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Version 21.0.
RESULTS
Patient and primary tumor characteristics
Using the SEER database, we identified 45,255 patients diagnosed with FLC or HCC between 1986 and 2008. After excluding all patients who did not have cancer-directed operations performed, 8,815 patients remained. Finally, only patients with histologically or microscopically confirmed FLR or HCC who had regional or localized disease were included, leaving 7,225 patients available for analysis.
The demographic and clinical characteristics of the 7,225 patients with surgically managed FLC or HCC are outlined in Table 1. The median age for patients with FLC was 25.0 years (SD 18.5 years) compared with 59.0 years (SD 12.0) for patients with HCC (p < 0.001). A slight majority of patients with FLC were men (n = 50; 55.6%); almost three-quarters of the patients with HCC were men (n = 5,234; 73.4%; p < 0.001). Overall, the majority of patients were white (FLC, 83.3% and HCC, 63.4%). Patients with FLC were less likely to be Asian or Pacific Islander than patients with HCC (4.4% vs 26.3%; p < 0.001). No patient or tumor characteristic changed over the time periods examined for either FLC or HCC (all p > 0.05). However, more than half of the patients with FLC were diagnosed from 2001 to 2008 (n = 47; 58.9%), while cases of HCC were distributed more homogenously over the 23-year study period.
Table 1.
Demographic and Clinical Characteristics of Patients with Fibrolamellar Carcinoma and Hepatocellular Carcinoma (n = 7,225) Undergoing Liver-Directed Therapy from 1986 to 2008
| Variable | FLC (n = 90) | HCC (n = 7,135) | p Value |
|---|---|---|---|
| Demographics | |||
| Median age, y, ± SD | 25.0 ± 18.5 | 59.0 ± 12.0 | <0.001 |
| Age range, y | 7–84 | 1–96 | – |
| Sex, n (%) | <0.001 | ||
| Female | 40 (44.4) | 1,901 (26.6) | <0.001 |
| Male | 50 (55.6) | 5,234 (73.4) | <0.001 |
| Year of diagnosis quartile, n (%) | |||
| 1986–2001 | 43 (47.8) | 1,993 (27.9) | <0.001 |
| 2002–2004 | 20 (22.2) | 1,864 (26.1) | 0.401 |
| 2005–2006 | 15 (16.7) | 1,636 (22.9) | 0.159 |
| 2007–2008 | 12 (13.3) | 1,642 (23.0) | 0.030 |
| Race, n (%) | |||
| White | 75 (83.3) | 4,522 (63.4) | <0.001 |
| Asian or Pacific Islander | <11 (<12.2) | 1,873 (26.3) | <0.001 |
| Black | <11 (<12.2) | 642 (9.0) | 0.741 |
| Other/Unknown | <11 (<12.2) | 98 (1.4) | 0.497 |
| Pathologic characteristics, n (%) | |||
| SEER historic stage | |||
| Localized | 52 (57.8) | 5,557 (77.9) | <0.001 |
| Regional | 38 (42.2) | 1,578 (22.1) | <0.001 |
| Grade, n (%) | |||
| Well-differentiated | <11 (<12.2) | 1,741 (24.4) | <0.001 |
| Moderately differentiated | 14 (15.6) | 2,328 (32.6) | <0.001 |
| Poorly differentiated | <11 (<12.2) | 831 (11.6) | 0.072 |
| Undifferentiated | <11 (<12.2)) | 100 (1.4) | <0.001 |
| Unknown, n (%) | 64 (71.1) | 2,135 (29.9) | <0.001 |
| Liver-directed procedure, n (%) | |||
| Ablative procedure only | <11 (<12.2) | 1,954 (27.4) | <0.001 |
| Hepatic resection or liver transplant | 81 (90.0) | 5,061 (70.9) | <0.001 |
| Surgery not otherwise specified | <11 (<12.2) | 120 (1.7) | 0.230 |
Cells with fewer than 11 cases per variable cell were relabeled as <11 (<%) in compliance with the National Cancer Institute data-usage agreement for reporting of SEER data.
FLC, fibrolamellar carcinoma; HCC, hepatocellular carcinoma.
Patients with operatively managed FLC were less likely to have localized disease compared with HCC patients (57.8% vs 77.9%; p < 0.001). Overall, approximately one-quarter of patients had regional disease (n = 1,616; 22.3%), with regional disease being more common among patients with FLC (42.2%) vs patients with HCC (22.1%) (p < 0.001). The most prevalent histology code for patients with HCC (n = 1,658; 78.2%) was 8140 (“adenocarcinoma not otherwise specified”) (Table 1).12 The majority of patients with FLC had an unknown cancer grade (n = 64; 71%); 57.0% (n = 4,069) of patients with HCC had a well- or moderately differentiated cancer.
Trends in liver-directed management
Of the 7,225 patients who underwent liver-directed management of FLC or HCC, 71.2% (n = 5,142) were managed with hepatic resection or liver transplantation, while 27.7% (n = 2,000) had an ablative procedure only (Table 1). Overall, patients with FLC were more likely to undergo resection or transplantation (90.0% vs 70.9%; p < 0.001) and were less likely to be managed with ablation only (<12% vs 27.4%; p < 0.001) than patients with HCC. The use of major hepatic resection or transplantation (1986 to 2001, 31.7% vs 2002 to 2004, 51.1% vs 2005 to 2006, 48.6% vs 2007 to 2008, 48.0%) as well as the use of ablation alone (1986 to 2001, 16.4% vs 2002 to 2004, 31.2% vs 2005 to 2006, 33.4% vs 2007 to 2008, 29.3%) increased over the time periods examined (both p < 0.001). Beginning in 1998, more information was available regarding the extent of operation for 6,376 patients, including 65 patients with FLC (Table 2). A similar proportion of FLC and HCC patients were managed with partial hepatectomy (24.6% vs 19.0%; p = 0.250). Most patients with FLC were operatively managed with a hemihepatectomy (n = 24; 36.9%) vs patients with HCC, who were more often managed with liver transplantation (n = 1970; 31.2%) (both p < 0.001). Extended hepatic resection was rarely used in patients with HCC (n = 171; 2.7%); it was used in patients with FLC more often (n <11; <16.9%; p < 0.001). Men and women with FLC were equally as likely to be operatively managed with a major hepatic resection (p = 0.531). However, patients who underwent a liver resection of greater than 3 segments (ie, a major hepatectomy) were more likely to be younger compared with patients who were managed with other liver-directed modalities (median age 19.0 years vs 35 years, respectively; p < 0.001). When patients who underwent hepatic transplantation were added to the definition of major hepatectomy, the difference between the median ages of patients undergoing major hepatectomy/transplantation compared with minor hepatectomies or ablations was still present but less pronounced (median age 20.0 years vs 28.0 years, respectively; p = 0.006). The use of major hepatic resection among patients with FLC did not increase over time (1986 to 2001, 50.0% vs 2002 to 2004, 55.0% vs 2005 to 2006, 66.7% vs 2007 to 2008, 55.0%; p = 0.114). Among patients with HCC who underwent combined resection and ablation (n = 186), hepatic resection consisted of partial hepatectomy or wedge resections in the vast majority of patients (n = 159; 85.5%).
Table 2.
Details of Liver-Directed Procedure among Patients with Fibrolamellar Carcinoma or Hepatocellular Carcinoma from 1998 to 2008
| Variable | FLC (n = 65) | HCC (n = 6,311) | p Value |
|---|---|---|---|
| Type of liver-directed procedure | |||
| Surgery not otherwise specified | <11 (<16.9) | 50 (0.8) | 0.230 |
| Ablative procedure only | <11 (<16.9) | 1,902 (30.1) | <0.001 |
| Partial hepatectomy | 16 (24.6) | 1,197 (19.0) | 0.250 |
| Minor hepatectomy | 16 (24.6) | 1,197 (19.0) | 0.250 |
| Hemihepatectomy | 24 (36.9) | 1,021 (16.2) | <0.001 |
| Extended hepatectomy (>5 segments) | <11 (<16.9) | 171 (2.7) | <0.001 |
| Liver transplant | <11 (<16.9) | 1,970 (31.2) | <0.001 |
Cells with fewer than 11 cases per variable cell were relabeled as <11 (<%) in compliance with the National Cancer Institute data-usage agreement for reporting of SEER data.
FLC, fibrolamellar carcinoma; HCC, hepatocellular carcinoma.
Long-term outcomes
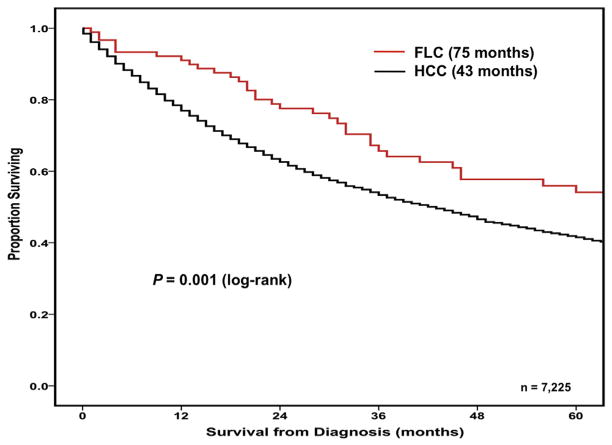
The overall median survival of patients with surgically managed FLC was 75.0 months (95% CI 52.3 to 97.7 months), which was longer than the median survival of 43.0 months (95% CI 40.6 to 45.4 months) for patients with HCC (p = 0.001) (Fig. 1). Among individuals managed with a liver-directed procedure, FLC and HCC patients had 1-, 3-, and 5-year survivals of 91.0%, 65.7%, and 54.1% vs 77.1%, 53.6%, and 41.7%, respectively (all p < 0.001). In the statistical model that included patients with either FLC or HCC, several clinicopathologic factors were associated with worse survival on univariable analyses including age greater than 58 years, race, high tumor grade, the use of ablative procedures, and the presence of regional disease (all p < 0.05; Table 3). In addition, the year of diagnosis was associated with outcome, as patients treated in later time periods had anywhere from a 20% to 60% increased hazard of death compared with patients diagnosed between 1986 and 2001 (Table 3). After using multivariable analysis to control for competing clinicopathologic risk factors, age greater than 58 years (HR 1.41; [95% CI 1.32 to 1.51]; p < 0.001), treatment with ablation alone (HR 1.79; [95% CI 1.66 to 1.93]; p < 0.001), the presence of regional disease (HR 1.55; [95% CI 1.44 to 1.66]; p < 0.001), and a later time period of diagnosis (referent 1986 to 2001 vs 2005 to 2006, HR 1.35; p < 0.001) remained independently associated with a worse outcome (Table 3).
Figure 1.
Overall survival of patients with fibrolamellar carcinoma (FLC) (n = 90; median survival: 75 months) or hepatocellular carcinoma (HCC) (n = 7,135; median survival 43 months) managed with a liver-directed procedure from the time of diagnosis in SEER from 1986 to 2008 (p = 0.001).
Table 3.
Cox Regression Analyses of Variables Associated with Survival after Liver-Directed Procedures for Patients with Fibrolamellar Carcinoma or Hepatocellular Carcinoma from 1998 to 2008
| Prognostic factor | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p Value | Hazard ratio | 95% CI | p Value | |
| Age > 58y | 1.51 | 1.42–1.62 | <0.001 | 1.41 | 1.32–1.51 | <0.001* |
|
| ||||||
| Female sex | 1.024 | 0.95–1.10 | 0.516 | – | – | – |
|
| ||||||
| Year of diagnosis quartile | ||||||
|
| ||||||
| 1986–2001 | – | Reference | – | – | – | |
|
| ||||||
| 2002–2004 | 1.62 | 1.42–1.86 | <0.001 | 1.74 | 1.52–1.99 | <0.001* |
|
| ||||||
| 2005–2006 | 1.35 | 1.18–1.55 | <0.001 | 1.35 | 1.18–1.55 | <0.001* |
|
| ||||||
| 2007–2008 | 1.20 | 1.04–1.39 | <0.001 | 1.15 | 1.01–1.33 | 0.049* |
|
| ||||||
| Race | ||||||
|
| ||||||
| White | – | Reference | – | – | – | |
|
| ||||||
| Asian/Pacific Islander | 1.62 | 1.16–2.26 | 0.005 | 1.44 | 1.03–2.02 | 0.032* |
|
| ||||||
| Black | 1.52 | 1.09–2.14 | 0.014 | 1.23 | 0.88–1.74 | 0.217 |
|
| ||||||
| Other | 2.05 | 1.45–2.90 | <0.001 | 1.86 | 1.31–2.63 | <0.001* |
|
| ||||||
| Regional disease | 1.62 | 1.51–1.75 | <0.001 | 1.55 | 1.44–1.66 | <0.001* |
|
| ||||||
| Grade | ||||||
|
| ||||||
| Well-differentiated | – | Reference | – | – | – | |
|
| ||||||
| Moderately differentiated | 1.20 | 1.09–1.32 | <0.001 | 0.74 | 0.70–0.82 | <0.001* |
|
| ||||||
| Poorly differentiated | 1.77 | 1.77–1.58 | <0.001 | 0.96 | 0.89–1.05 | 0.399 |
|
| ||||||
| Undifferentiated | 1.67 | 1.28–2.18 | <0.001 | 1.40 | 1.25–1.56 | <0.001* |
|
| ||||||
| Unknown | 1.44 | 1.32–1.58 | <0.001 | 1.26 | 0.96–1.63 | 0.089 |
|
| ||||||
| Liver-directed procedure | ||||||
|
| ||||||
| Resection or hepatic transplant | – | Reference | – | – | – | |
|
| ||||||
| Ablation only | 1.52 | 1.67–1.92 | <0.001 | 1.79 | 1.66–1.93 | <0.001* |
|
| ||||||
| Surgery, not otherwise specified | 1.79 | 1.23–1.86 | <0.001 | 1.39 | 1.13–1.71 | 0.002* |
|
| ||||||
| Hepatocellular carcinoma | 1.69 | 1.23–2.32 | 0.001 | 1.65 | 1.20–2.28 | 0.002* |
Significant.
Overall, there were 184 patients who died within 30 days of diagnosis of their primary liver cancer; all of these patients had HCC. When these 184 patients were excluded from the analysis, there was little impact on the median survival, with almost identical results obtained (median survival for FLC with all patients, 75.0 months and with <30-day survivors excluded, 75.0 months; median survival for HCC with all patients, 43 months and with <30-day survivors excluded, 44 months). When the data were examined at 90 days after diagnosis, there were 622 patients (8.6% overall) who died within this time period. Of the 622 patients, 3 had FLC (3.3% of the 90 patients with FLC) and 619 had HCC (8.7% of the 7,135 patients with HCC). Again, there was no decrease in median survival for patients with FLC when patients who died within 90 days after diagnosis were excluded from analysis (75.0 months), and there was the expected slight increase in the median survival from 43.0 to 48.0 months for patients with HCC. Given the similarities in the survival, we decided to use the entire cohort and continue to define survival starting from the date of diagnosis for patients with FLC and HCC.
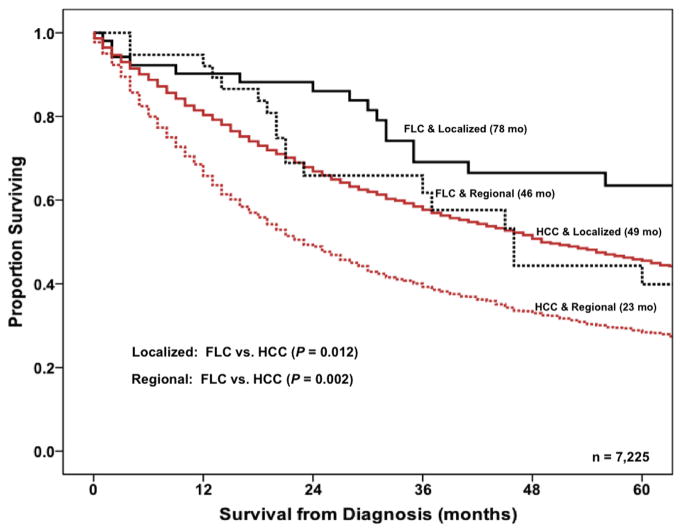
When HCC vs FLC was examined on univariable analysis, HCC was associated with worse survival (HR 1.69, 95% CI 1.23 to 2.32; p < 0.001), and the increased risk of death with HCC relative to FLC remained on multivariable analysis (HR 1.65; 95% CI 1.20 to 2.28; p = 0.002). Overall survival of patients was then stratified by both tumor subtype (ie, HCC vs FLC) and SEER summary stage designation (ie, local vs regional). Of note, patients with FLC had a better prognosis compared with HCC patients regardless of stage designation. Specifically, patients with FLC and localized disease had a median survival of 78 months vs 49 months for patients with HCC and localized disease (p = 0.012). Similarly, the prognosis of patients with FLC and regional disease was better (46 months) compared with patients who had HCC and regional disease (23 months) (p = 0.002) (Fig. 2).
Figure 2.
Overall survival of patients with fibrolamellar carcinoma (FLC) or hepatocellular carcinoma (HCC) stratified by the SEER summary stage designation (localized vs regional) from 1986 to 2008. Medians months of survival: FLC and localized (78 months) vs HCC and localized (49 months) (p = 0.012); FLC and regional (46 months) vs HCC and regional (23 months) (p = 0.002).
In examining only those patients with FLC, there were no factors associated with outcomes on univariable analysis. For example, among the 65 FLC patients undergoing surgery between 1998 and 2008 for whom detailed operative data were available, patients who underwent a major hepatectomy or transplantation (n = 42) had a comparable median survival to patients who were managed with either a minor hepatectomy or RFA alone (n = 23) (75 months vs 60 months; p = 0.643). On multivariable analysis, the use of ablation only did, however, tend to be associated with a worse outcome (HR 3.01; [95% CI 1.02 to 8.87]; p = 0.046) (Table 4).
Table 4.
Cox regression Analyses of Variables Associated with Survival after Liver-Directed Procedure for Patients with Fibrolamellar Carcinoma (n = 90) from 1986–2008
| Prognostic factor | Univariate
|
Multivariate
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p Value | Hazard ratio | 95% CI | p Value | |
| Age >25 y | 1.47 | 0.78–2.77 | 0.237 | 1.61 | 0.84–3.11 | 0.155 |
|
| ||||||
| Female sex | 1.03 | 0.55–1.94 | 0.925 | – | – | – |
|
| ||||||
| Year of diagnosis quartile | ||||||
|
| ||||||
| 1986–2001 | – | Reference | – | – | – | |
|
| ||||||
| 2002–2004 | 1.08 | 0.51–2.31 | 0.835 | – | – | – |
|
| ||||||
| 2006–2006 | 0.71 | 0.21–2.28 | 0.595 | – | – | – |
|
| ||||||
| 2007–2008 | 1.38 | 0.30–6.41 | 0.682 | – | – | – |
|
| ||||||
| Regional disease | 1.41 | 0.75–2.66 | 0.283 | 1.87 | 0.95–3.67 | 0.070 |
|
| ||||||
| Liver-directed procedure | ||||||
|
| ||||||
| Resection or hepatic transplant | – | Reference | – | – | – | |
|
| ||||||
| Ablation only | 2.53 | 0.89–7.20 | 0.118 | 3.01 | 1.02–8.87 | 0.046* |
|
| ||||||
| Surgery, not otherwise specified | 2.16 | 0.66–7.05 | 0.081 | 2.40 | 0.72–8.03 | 0.157 |
Significant.
DISCUSSION
Fibrolamellar hepatocellular carcinoma is a rare malignancy, with an estimated age-adjusted incidence of 0.02 per 100,000 in the US.2 Despite its relative rarity, FLC is one of the more common primary liver cancers among adolescents and young adults with no underlying cirrhosis or hepatitis.20 Fibrolamellar hepatocellular carcinoma was first described by Edmondson in 1956, and since that time there has been little progress in understanding the biologic underpinnings or clinical behavior of this disease.6,21 Although several studies have reported on the general epidemiology of FLC,2,22 no report has focused on a population-based analysis of FLC patients specifically managed with surgery. In fact, the published literature on the outcomes of surgically treated patients with FLC is limited both in quantity and in quality, with most studies being either case reports or small retrospective single-center series.23 Due to the limited data on the topic, our group previously reported a systemic review and meta-analysis detailing the treatment and prognosis of patients with FLC based on a pooled analysis of individual data available in the literature.23 This study expands and complements this previous work by focusing on population-based, rather than individual institution-based, data. In this study, we analyzed a well-validated, prospectively collected nationwide database representing approximately one-quarter of the cases in the US over a 23-year span. In doing this, we were able to amass – to the best of our knowledge – the largest cohort of surgically treated patients with FLC to date. We report that patients with FLC were younger and more likely to be women compared with patients diagnosed with HCC. Perhaps more importantly, we noted that patients with FLC had a substantially higher incidence of regional disease, but in spite of this, FLC patients had better outcomes than patients with HCC.
Whether patients with FLC have better outcomes than patients with conventional HCC has been somewhat controversial. Data from the Childhood Liver Tumor Strategy Group (SIOPEL) in Europe has suggested that the fibrolamellar variant of HCC does not have a better survival than conventional HCC.24 In this study, the authors reported data on 24 FLC and 38 HCC patients and noted that the overall 3-year survival was no different among FLC (42%) vs HCC (33%) patients (p = 0.24). Data from this study, however, were difficult to interpret. First, while the difference in survival was not statistically significant, there was a suggestion that FLC patients did do better; in turn, the lack of significance may have been due to the small sample size and a type II statistical error. Second, the patients included in the study were heterogeneous with regard to treatment: 45 patients received chemotherapy alone and only 17 patients underwent surgical resection. The SEER data presented here do not contain information on receipt of systemic therapy, making comparisons to the SIOPEL data difficult. In contrast, several small series that have included only surgical patients have indeed suggested a better prognosis for FLC compared with conventional HCC.3–6 In one study by Ang and colleagues,6 the authors reported a median survival for FLC close to 7 years, which is significantly longer than the median survival traditionally associated with conventional HCC. In this study, we noted that patients with surgically treated FLC had excellent clinical outcomes, with a median survival of 6.1 years. Interestingly, survival estimates derived from data based on the population-based SEER dataset were quite comparable to the data we previously reported using a meta-analysis of individual patient data from 19 different studies.23 In fact, the median and 5-year overall survival were nearly identical when comparing survival data from this study (median, 73 months; 5-year, 54.1%) and the previous meta-analysis (median, 74 months; 5-year, 59%).
The better prognosis of FLC relative to conventional HCC is sometimes attributed to younger patient age or other confounding factors, such as differences in surgical therapy. In this study, we did note that patients with FLC were younger than patients with conventional HCC. Specifically, the median age of patients with conventional HCC was more than twice that of patients with FLC (59.0 years vs 25.0 years). The predilection for FLC to develop in a younger patient population has previously been well established, and it is clear that there are major differences in age among patients with FLC vs HCC.2 In looking at modes of liver-directed procedures, we similarly noted differences. The FLC patients were more often treated with resection or transplantation and were less likely to be managed with ablation only as compared with HCC patients. In order to account for these differences in age and treatment approaches among FLC vs HCC patients, we included these variables in the multivariable model and found that HCC remained independently associated with a 65% increased risk of death relative to FLC (Table 3).
Another intriguing finding of this study regarded regional disease. Unlike conventional HCC, FLC is believed to more commonly metastasize to regional lymph nodes. In this study, we did note that the incidence of regional disease among patients with FLC was quite high, at more than 40%—nearly double the incidence noted in conventional HCC. The presence of regional disease, as opposed to localized disease, portended a worse prognosis for both FLC and HCC patients. In fact, in multivariable analysis, the presence of regional disease was independently associated with shorter overall survival (HR 1.55 [1.44 to 1.66], p < 0.001). In contrast, patients with FLC who had only localized disease experienced the best survival. As depicted in Figure 2, it was interesting to note that the long-term outcomes of FLC patients with regional disease were similar to those of patients with conventional localized HCC disease. We take these data to further illustrate the unique, and still not understood, biologic underpinnings of FLC compared with HCC.
This study has certain limitations that should be considered in interpreting the data. First, the SEER database, although prospectively collected and well validated, provides limited data on therapy other than surgery. Although the linked SEER-Medicare database may have provided more data, its use was not feasible given that most patients with FLC are well below 65 years of age. Because patients with HCC may have a lower performance status than younger FLC patients, it would have been ideal to control for comorbidities and other perioperative risk factors in the survival analyses. However, these data and other data such as receipt of systemic therapy were not available in the SEER dataset. In addition, the SEER dataset reflects surgical practice throughout the last 23 years in the entire country, which may limit the extrapolation of our findings to specific patient populations. Taking a national perspective did, however, provide for a wider overview of the overall trends in surgical management and prognosis of patients with FLC.
CONCLUSIONS
In conclusion, patients with operatively managed FLC had different clinicopathologic characteristics than those with HCC, including younger age and a higher incidence among women. Despite a higher likelihood of advanced disease at the time of diagnosis, surgically treated FLC patients had better long-term outcomes than patients with conventional HCC. Although long-term outcomes after surgical management were better than those after conventional HCC, 5-year survival among FLC patients was still only 54%. Given the rarity of the tumor, future efforts should focus on multi-institutional collaborations to improve our ability to understand the clinical behavior, as well as better identify potential therapeutic targets for FLC.
Abbreviations and Acronyms
- HCC
hepatocellular carcinoma
- FLC
fibrolamellar carcinoma
- HR
hazard ratio
- NCI
National Cancer Institute
- OS
overall survival
Footnotes
Author Contributions
Study conception and design: Mayo, Mavros, Pawlik
Acquisition of data: Mayo, Mavros, Nathan, Pawlik
Analysis and interpretation of data: Mayo, Mavros, Nathan, Cosgrove, Herman, Kamel, Anders, Pawlik
Drafting of manuscript: Mayo, Mavros, Pawlik
Critical revision: Mayo, Mavros, Nathan, Cosgrove, Herman, Kamel, Anders, Pawlik
Final approval: Mayo, Mavros, Nathan, Cosgrove, Herman, Kamel, Anders, Pawlik
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004;39:798–803. doi: 10.1002/hep.20096. [DOI] [PubMed] [Google Scholar]
- 3.Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331–1338. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 4.Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinicopathologic features. Cancer. 1980;46:372–379. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Nagorney DM, Adson MA, Weiland LH, et al. Fibrolamellar hepatoma. Am J Surg. 1985;149:113–119. doi: 10.1016/s0002-9610(85)80019-2. [DOI] [PubMed] [Google Scholar]
- 6.Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 8.Mayo SC, Shore AD, Nathan H, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford) 2011;13:473–482. doi: 10.1111/j.1477-2574.2011.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniaci V, Davidson BR, Rolles K, et al. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35:617–621. doi: 10.1016/j.ejso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Ringe B, Wittekind C, Weimann A, et al. Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet. 1992;175:299–305. [PubMed] [Google Scholar]
- 11.National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch. [Accessed November 7, 2013];Surveillance, Epidemiology, and End Results (SEER) program. ( http://www.seer.cancer.gov) SEER* Stat database: Incidence, SEER 17 regs public-use, Nov 2005 sub (1973–2003 varying), linked to county attributes, total U.S., 1969–2003 counties. Released April 2006, based on the November 2005 submission.
- 12.Fritz AG. International Classification of Diseases for Oncology: ICD-O. 3. Geneva: World Health Organization; 2000. [Google Scholar]
- 13.Strasberg SM The International Hepato-Pancreato-Biliary Association. The Brisbane: 2000 Terminology of Liver Anatomy and Resections. HBP. 2000;2:333–339. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agresti A. Introduction to categorical data analysis. New York: Wiley; 1996. pp. 182–185.pp. 187–189.pp. 201pp. 217pp. 279 [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2. New York: John Wiley and Sons, Inc; 1999. [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York: John Wiley and Sons, Inc; 2000. [Google Scholar]
- 19.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa T, Federle MP, Grazioli L, et al. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213:352–361. doi: 10.1148/radiology.213.2.r99nv31352. [DOI] [PubMed] [Google Scholar]
- 21.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–186. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 22.Eggert T, McGlynn KA, Duffy A, et al. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000–10. Gut. 2013;62:1667–1668. doi: 10.1136/gutjnl-2013-305164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–830. doi: 10.1016/j.jamcollsurg.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Weeda VB, Murawski M, McCabe AJ, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma - Results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer. 2013;49:2698–2704. doi: 10.1016/j.ejca.2013.04.012. [DOI] [PubMed] [Google Scholar]