Abstract
BACKGROUND
Cell block (CB) preparation during the endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA) procedure plays an important role in the diagnosis of lung cancer and recovery of cellular material for molecular characterization of the tumor. However, the efficiency of the conventional method of CB preparation is suboptimal.
METHODS
In the current study, the “tissue coagulum clot” cell block (TCC-CB) method was used to prepare the CBs and its efficiency was compared with that of the conventional saline rinse cell block (NR-CB) method. A total of 84 consecutive TCC-CBs (106 lymph nodes [LNs] and 14 lung lesions) and 28 consecutive cases of NR-CB (39 LNs and 3 lung lesions) obtained within the same time period were included in the current study.
RESULTS
In the TCC-CB specimens, 94 of 106 LN cases (88.7%) yielded sufficient diagnostic material, as did 11 of 14 lung lesions (78.6%). In the NR-CB group, which was used as the control, 22 of 39 LN specimens (56.4%) and none of 3 lung specimens (0%) were found to provide sufficient diagnostic material. Although the average size of the LNs in the study group were not significantly different from those in the control group (1.76 cm vs 1.82 cm; P>.05), the overall nondiagnostic rates in the TCC-CB and NR-CB groups were 11.2% and 43.6%, respectively (P<.001). The nondiagnostic rates of the lung specimens were 15.4% in the TCC-CB group and 100% in the NR-CB group (P<.05). In addition, immunohistochemistry studies and epidermal growth factor receptor (EGFR)/KRAS mutational analyses were performed in 26 and 14 TCC-CB cases, respectively. With the exception of 1 case, all of them had satisfactory results.
CONCLUSIONS
The data from the current study demonstrate that the TCC-CB method significantly increases the cellular yield of CB preparations without compromising cytomorphological characterization of tumor cells.
Keywords: fine-needle aspiration cytology, cell block preparation, improvement of cellularity, endobronchial ultrasound-guided transbronchial fine-needle aspiration (EBUS-TBNA), lung cancer
INTRODUCTION
Lung cancer is among the most common malignancies in the world and is one of the leading causes of cancer-related death in the United States.1 Greater than 80% of lung cancers are classified as non-small cell lung carcinoma (NSCLC), which includes nonsquamous cell carcinoma (adenocarcinoma and large cell carcinoma) and squamous cell carcinoma.2 Approximately 40% to 60% of NSCLC patients have either locally advanced or metastatic disease at the time of diagnosis.1,2 The current conventional treatments, such as surgical resection and/or conventional combination of cytotoxic chemotherapy and radiotherapy, have reached a plateau of effectiveness in treating patients with advanced NSCLC.3 In recent years, considerable progress has been made in identifying novel molecular targets and developing biological agents that may be administered to patients with NSCLC.4–8 These studies have shown that the identification of prognostic and predictive biomarkers has changed the diagnostic and therapeutic paradigm from primarily morphological assessment to morphological assessment plus molecular analysis of tumor to further personalize therapy, with the goal of improving long-term lung cancer survival.8–12 For example, the first-line therapeutic option for patients with nonsquamous NSCLC is pemetrexed but not for those with the squamous cell subtype,10,11 whereas a tyrosine kinase inhibitor is used for patients with epidermal growth factor receptor (EGFR) gene mutations.12 Thus, the demand for adequate tissue sampling for both morphological assessment and molecular studies has increased dramatically.13,14. Although larger tissue samples such as surgical biopsy or resected tumor tissues are preferred for morphological and molecular studies,13,14 often cytological samples obtained by minimally invasive procedures such as transbronchial fine-needle aspiration (TBNA) are the only type of specimen available.13,14 This is particularly common in patients with locally advanced and/or metastatic NSCLC.
Our previous study and others have shown that endoscopic transbronchial procedures, particularly endobronchial ultrasound-guided TBNA (EBUS-TBNA), are effective procedures for obtaining tissue samples to diagnose and stage lung cancers.15–20 Several types of cytological material can be obtained during bronchoscopy, including brushing, washing, lavage, fine-needle aspiration (FNA), and core needle biopsy samples.21–23 From these materials, smears, cytospins, monolayer ThinPrep specimens, cell blocks (CB), and other cytological preparations can be made.21–23 Among them, core needle biopsy and CB preparations from aspirated material are particularly important because they may provide additional morphological information and be used for further immunohistochemistry and molecular tests.13,14 However, in some patients with lung cancer, core needle biopsy, including percutaneous computed tomography-guided FNA, cannot be performed because of the size and location of the lesion and/or coexisting medical conditions. Therefore, CB preparations from bronchoscopy-directed TBNA are a frequently used method to sample the lesion and harvest tumor cells for diagnostic purposes.
The commonly used method of CB preparation is to rinse the aspiration needle with normal saline, spin down and collect the material, and then process the pellet in the laboratory.24–27 Our experience, and that of others, has shown that a variable amount of material and/or tissue is recovered using this conventional method, and the efficiency of this method for obtaining adequate tissue samples is limited.23–27 The need for a more efficient method to recover cellular material and prepare cellular tissue is growing, as clinical trials and treatment options not only depend on morphological assessment but also on molecular characteristics of lung cancers.13,14,28
To improve cellular yield on CB sections for histologic, immunohistochemical (IHC), and molecular marker analysis, we have adopted and are routinely applying the so-called “tissue coagulum clot” CB (TCC-CB) method to harvest cellular material from the aspiration needle during EBUS-TBNA procedures.15 In this study, we compared CB preparation using the TCC-CB method with the conventional normal saline needle rinse CB (NR-CB) method. The diagnostic rate, cellular yield, and adequacy for ancillary studies were compared between these 2 methods. The purpose of the current study was to identify an advantageous method for CB preparation during EBUS-TBNA and to improve the cellularity on CB sections to provide optimal diagnostic information for the management of patients with lung cancer.
MATERIALS AND METHODS
Data Collection
In the current study, a total of 112 patients who underwent EBUS-TBNA for the evaluation of suspected thoracic malignancies were selected over a period of 12 months at the Johns Hopkins Hospitals. Among them, 84 consecutive cases had cytological smears and CB preparations using the TCC-CB method and 28 consecutive cases used the NR-CB method and were included as independent controls. Clinical information such as the size and location of the lesions was also correlated.
EBUS-TBNA Procedure
Under moderate sedation by intravenous fentanyl and midazolam and topical anesthesia with 1% lidocaine, or via general anesthesia with intravenous propofol, a standard bronchoscope (Olympus BF 160, BF-1T160, BF 180 series video bronchoscopes; Olympus America, Center Valley, Pa) was inserted into the patient’s airway. After the initial inspection and visualization, the bronchoscope was removed and an EBUS bronchoscope (Olympus BF UC 160F/BF UC 180F 7.5-megahertz [MHz] convex probe linear array puncture scope) was introduced into the airway. Ultrasound scanning of the mediastinum and hilum was performed to identify visible lymph nodes (LNs). In a subset of the patients (14 of 84 patients), the EBUS bronchoscope was also used for imaging lung parenchymal masses that were located at the central peribronchial area and for evaluating an appropriate needle puncture access and route. Once identified, the LNs or lung masses were sampled with Olympus ViziShot 22-gauge and/or 21-gauge needles under real-time ultrasound guidance. A wire stylet within the lumen of the aspiration needle was used to remove possible intraluminal bronchial epithelium and cartilage that might obstruct the needle lumen. The stylet was removed and a VacLok syringe (Qosina, Edgewood, NY) was attached to aspirate biopsy material under negative suction pressure (10–20 mL of air in the syringe left under negative pressure). During the procedure, an immediate onsite evaluation of adequacy was performed by an experienced cytotechnologist using air-dried slides stained by the Diff-Quik method. An immediate assessment was given to clinicians (R.C.W.Y., D.F-K., and L.Y.) after each pass. If onsite evaluation was adequate, revealing cancer cells or lymphocytes, material from additional aspirations was processed for CB preparations. Conversely, if the onsite evaluation was inadequate, more passes might be directed and performed, depending on clinical suspicion and the patient’s medical condition.
Preparation of Cytological Smears
Smears were prepared using both air-dried and wet-fixed methods. The air-dried smears were stained with the Diff-Quik method and used for the immediate onsite evaluation. Additional smears were wet-fixed with 95% alcohol and stained using the Papanicolaou test in the cytopathology laboratory.
CB Preparation Using TCC-CB
To prepare CBs using the TCC-CB method, the material within the 21-gauge/22-gauge cytology needle was gently pushed out using the wire stylet that came with the needle kit, instead of using a syringe to expel the material into saline for CB preparation as is usually done. As the material streamed out from the needle tip, it was collected onto a pre-cut piece of filter paper with the needle tip directed in a tight circular motion to build up a cone-shaped coagulum of tissue and blood mixture (Fig. 1). The clot was slightly air dried on the filter paper, mostly to ensure that the coagulum was congealed and the cellular elements were not quickly dispersed in a liquid medium. The tissue coagulum was wrapped with filter paper, gently slid into a formalin container, and processed in the histology laboratory. After histological processing, 4–5 micron sections were mounted on uncharged glass slides. Sections stained with hematoxylin and eosin (H&E) were used to assess cellularity and morphology.
FIGURE 1.
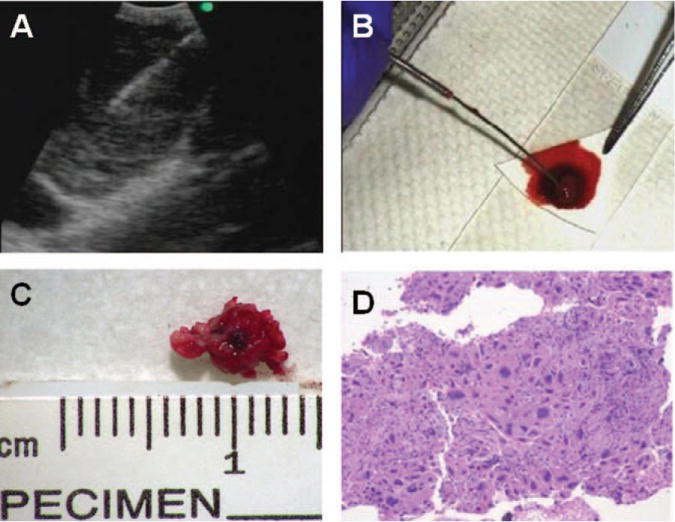
Cell block preparation using the tissue coagulum clot (TCC-CB) method is shown. (A) Endobronchial ultrasound imaging of the station 7 lymph node with a metastatic, poorly differentiated adenocarcinoma is shown. (B) A TCC-CB is shown on the filter paper. (C) The actual size of the TCC-CB is shown. (D) Hematoxylin and eosin staining of the TCC-CB section from the same lymph node with a metastatic, poorly differentiated adenocarcinoma as shown in Panels A through C is shown (× 200).
Preparation of CBs Using NR-CB
The cellular material in the aspiration needle was rinsed out with 20 mL of saline into a centrifuge tube. After centrifugation in the cytology laboratory, the cell pellets were harvested and wrapped with a filter paper. It was then fixed in formalin and processed in the histology laboratory using the same method as for TCC-CB preparation. The CB section was cut and stained in the same way as for the TCC-CB method.
Evaluation of Data
If lymphocytes were found in LN sampling, or if bronchial epithelium or pigmented alveolar macrophages were found in lung sampling, the specimen was designated as “adequate.” If lymphocytes were found in LN sampling or bronchial epithelium or pigmented macrophages were found in lung sampling without evidence of tumor, the specimen was designated as “reactive” or “benign/negative for malignancy.” If there was only blood and no lymphoid cells present and/or no bronchial epithelial cells identified, the case was interpreted as a “nondiagnostic specimen.”
Biostatistical analyses were performed using SAS Version 9.2 (SAS Institute Inc, Cary, NC). Chi-square and Fisher exact tests were used to calculate the P value. If the α value was <.05, it was considered to be statistically significant (P <.05).
RESULTS
The patients ranged in age from 31 to 83 years with a median age of 62 years. The male:female ratio was 1:1.11. Seventy-eight of 112 (69.6 %) patients were current or former smokers. Of the 112 patients, 95 patients had only LNs sampled, 8 patients had only a lung lesion sampled, and 9 patients had both LNs and lung lesions sampled. Eighty-four patients were in the TCC-CB group, including 106 LNs and 14 lung lesions, whereas 28 patients were in the NR-CB group as independent controls, including 39 LNs and 3 lung lesions. Taken together, a total of 145 LNs and 17 lung lesions were sampled.
We evaluated the cellular material on CB sections, and cytological diagnoses are summarized in Table 1. In the TCC-CB group, 62 of the total of 106 LNs (58.5%) were diagnosed as benign/reactive conditions, including 13 cases of granulomatous inflammation. Twenty-nine LNs (27.4%) were diagnosed as malignant. Of the 29 malignant cases, 27 were primary lung malignancies and 2 cases were distant metastases to the lung from known extrathoracic malignancies, including 1 case each of breast and salivary duct carcinoma. In the NR-CB group, 17 of 39 LNs (43.6%) were diagnosed as benign/reactive conditions, including 2 cases of granulomatous inflammation. Four LNs (10.3%) were diagnosed as malignant, and all were primary lung malignancies. In the TCC-CB group, 8 lung lesions were diagnosed as malignant, 4 were diagnosed as atypical/suspicious for carcinoma, and 2 were nondiagnostic. In NR-CB group, all 3 lung lesions were considered to be nondiagnostic because of a lack of bronchial epithelium. Taken together, in the TCC-CB specimens, 94 of 106 LNs (88.7%) and 11 of 14 lung lesions (78.6%) yielded sufficient diagnostic material, whereas in the NR-CB control group, 22 of 39 LNs (56.4%) and none of 3 lung lesions (0%) provided sufficient diagnostic material. The diagnostic rate was significantly higher when using the TCC-CB method in LN sampling (P<.001, P=.00004186) as well as lung sampling (P <.05, P = .0368).
Table 1.
Comparison of CB Diagnoses Between TCC-CB and NR-CB Methods
| Diagnosis | NR-CB | TCC-CB | ||
|---|---|---|---|---|
| LNs | Lung | LNs | Lung | |
| Benign | 17 (43.6%) | 0 | 62 (58.5%) | 0 |
| Reactive | 15 | 49 | ||
| Granuloma | 2 | 13 | ||
| Malignancy (lung primary) | 4 (10.3%) | 0 | 27 (25.5%) | 7 (50.0%) |
| Adenoca | 4 | 17 | 1 | |
| SCC | 0 | 6 | 2 | |
| SCLC | 0 | 2 | 4 | |
| Lymphoma | 0 | 2 | ||
| Metastasis | 0 | 0 | 2 (1.9%) | 1 (7.1%) |
| Atypical/suspicious | 1 (2.6%) | 0 | 3 (2.8%) | 4 (28.6%) |
| Nondiagnostic | 17 (43.6%)a | 3 (100%)b | 12 (11.3%)a | 2 (14.3%)b |
| Total | 39 (100%) | 3 (100) | 106 (100%) | 14 (100%) |
Adenoca, adenocarcinoma; CB, cell block; LNs, lymph nodes; NR-CB, normal saline rinse cell block method; SCC, squamous cell carcinoma; SCLC, small cell lung carcinoma; TCC-CB, tissue coagulum clot cell block method.
P<.001 (P=.00004186).
P<.05 (P =.0368).
In the lung lesions sampled, epithelial cells, inflammatory cells, and blood were found on CB sections from both TCC-CB and NR-CB preparations (Fig. 2). Of the 8 malignant cases in the TCC-CB group, 7 cases were primary lung malignancies and 1 case was a metastasis to the lung from a known pancreatic carcinoma. Among satisfactory cases, the diagnostic material (ie, tumor cells) were well preserved on the CB sections prepared using the TCC-CB method (Figs. 1 and 2).
FIGURE 2.
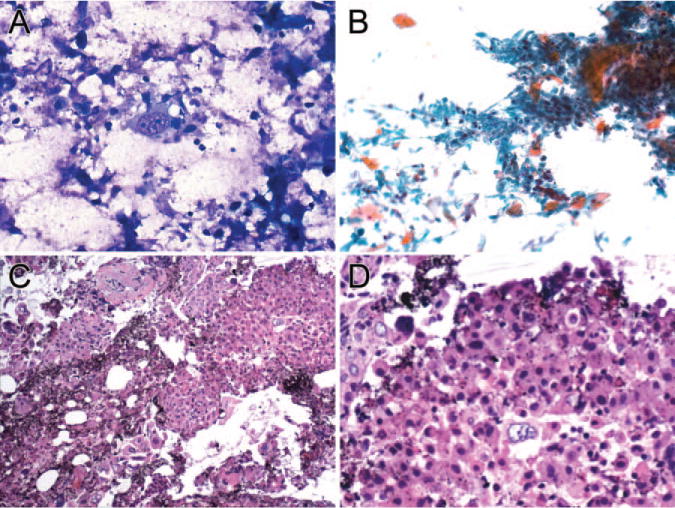
Endobronchial ultrasound-guided transbronchial needle aspiration of a squamous cell carcinoma of the lung is shown. (A) Diff-Quik staining of tumor cells is shown (×160). (B) Papanicolaou staining of tumor cells is shown (×64). (C) Hematoxylin and eosin (H&E) staining of the tissue coagulum clot cell block (TCC-CB) section is shown (×200). (D) H&E staining of the TCC-CB section is shown (× 600). Cytological features of malignancy, such as hyperchromasia, an irregular nuclear membrane, prominent nucleoli, and a high nuclear:cytoplamic ratio, are well preserved.
The size of the LNs ranged from 0.5 cm to 3.4 cm. The average size of LNs in the TCC-CB and NR-CB groups were 1.76 cm and 1.82 cm, respectively; they were not statistically different (P>.05, P=.219). The most sampled LN stations were 4 right (4R) and 7, including 33 cases (33 of 106; 31.1%) in the TCC-CB group and 13 cases (13 of 39; 33.3%) in the NR-CB group. The overall locations of the LN station and the diagnostic rate between the TCC-CB and NR-CB groups are summarized in Table 2. Again, the current study data indicate that the TCC-CB group yielded a significantly higher diagnostic rate than the NR-CB group (P<.001, P=.00004186).
Table 2.
Comparison of Satisfactory and Nondiagnostic Cases in Lymph Node Samplings Between the TCC-CB and NR-CB Methods
| Locations | NR-CB | TCC-CB | ||
|---|---|---|---|---|
| Satisfactory | Non-Dx | Satisfactory | Non-Dx | |
| 2R | 0 | 0 | 4 | 0 |
| 3R | 0 | 0 | 1 | 0 |
| 4L | 1 | 2 | 5 | 1 |
| 4R | 10 | 3 | 28 | 5 |
| 7 | 3 | 5 | 25 | 2 |
| 10L | 1 | 0 | 0 | 1 |
| 10R | 0 | 1 | 1 | 0 |
| 11L | 2 | 2 | 13 | 1 |
| 11R | 4 | 4 | 12 | 2 |
| 12L | 0 | 0 | 1 | 0 |
| 12R | 1 | 0 | 4 | 0 |
| Total | 22 (56.4%) | 17 (43.6%) | 94 (88.7%) | 12 (11.3%) P<.001 |
L, left; Non-Dx, nondiagnostic specimen due to lack of lymphocytes on cell block section; NR-CB: normal saline rinse cell block method; R, right; TCC-CB, tissue coagulum clot cell block method.
We semiquantitatively evaluated the tumor cellularity relative to nontumor cellularity (eg, lymphocytes and benign respiratory epithelial cells) on CB sections using a ×20 magnification objective (Fig. 3). The overall tumor cellularity on CB sections in both the TCC-CB and NR-CB groups is summarized in Table 3. In the TCC-CB group, 42.5% and 23.6% of cases, respectively, revealed 20% to 50% and > 50% tumor cellularity. In contrast, in the NR-CB group, only 17.9% and 10.3% of cases, respectively, demonstrated 20% to 50% and >50% tumor cellularity. Taken together, the overall percentage of cases with > 20% tumor cellularity was 66.1% in the TCC-CB group and 28.2% in the NR-CB group. The TCC-CB group demonstrated a significantly higher tumor cellularity compared with the NR-CB group (P <.001, P=.0004).
FIGURE 3.
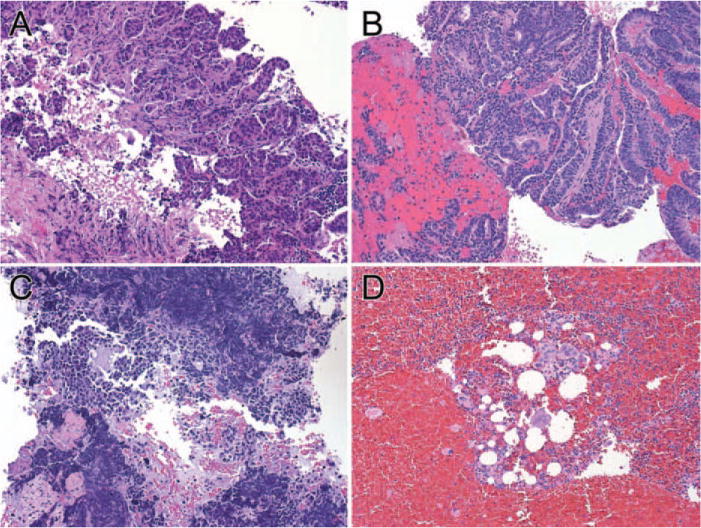
Comparison of tumor cellularity on cell block (CB) sections prepared using the tissue coagulum clot method is shown. (A and B) Sections show >50% tumor cellularity in cases of lung adenocarcinoma. (C) Section shows approximately 20% tumor cellularity. (D) Section shows <10% tumor cellularity (H&E stain, × 64).
Table 3.
Comparison of Tumor Cellularity and Non-Diagnostic Rate on CB Sections Prepared With 2 Different Methods
| Tumor Cellularity | NR-CB | TCC-CB | ||
|---|---|---|---|---|
| LNsa | Lungb | LNsa | Lungb | |
| Nondiagnosticc | 17 (43.6%) | 3 (100%) | 12 (11.3%) | 2 (14.3%) |
| >50% | 4 (10.3%) | 0 | 25 (23.6%) | 4 (28.6%) |
| 20%–50% | 7 (17.9%) | 0 | 45 (42.5%) | 3 (21.4%) |
| <20% | 11 (28.2%) | 0 | 24 (22.6%) | 5 (35.7%) |
| Total | 39 (100%) | 3 (100%) | 106 (100%) | 14 (100%) |
CB, cell block; LNs, lymph nodes; NR-CB, normal saline rinse cell block method; TCC-CB, tissue coagulum clot cell block method.
P value for LNs: P<.001, P =.0004.
P value for lungs: P <.05, P =.0368.
Nondiagnostic indicates that there were no lymphocytes or alveolar cells on the CB section.
In addition, ancillary studies were performed in 40 cases in the TCC-CB group, including 26 cases stained with multiple IHC markers and 14 cases analyzed for tumor genetic mutations (EGFR and KRAS). Among IHC study cases, 21 cases were LNs and 5 cases were lung lesions. Twenty-five of 26 cases (96.2%) had satisfactory results (Fig. 4). In these cases, IHC results provided additional diagnostic and/or differential diagnostic information for the tumor. Of 14 cases analyzed for genetic mutations, including 13 LNs and 1 lung lesion, EGFR and KRAS mutational analyses had satisfactory results (the sequencing data are not shown here). Among 14 cases, 8 cases were stage IV, 5 cases were stage III, and 1 case was a stage II NSCLC (AJCC Cancer Staging Manual, Seventh Edition).
FIGURE 4.
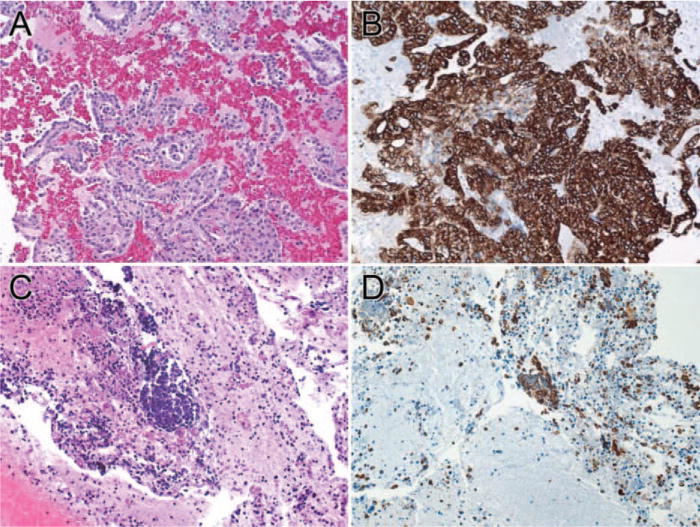
Immunohistochemical staining of cytokeratin 7 (CK7) in adenocarcinomas of the lung prepared with the tissue coagulum clot cell block method (A and B, × 64) and conventional normal saline needle rinse cell block method (C and D, × 64) (A and C: H&E stain).
DISCUSSION
The results of the current study demonstrate that the routine application of the TCC-CB method to EBUS-TBNA samples significantly increases the efficiency and adequacy of tissue recovery from the procedure. The technique is safe and cost-effective and carries no increased risk to patients. Most importantly, it is able to provide sufficient material for a variety of ancillary studies, including IHC and molecular analysis of the tumor.
CB preparation was first introduced in late 1960s and early 1970s.29,30 Since that time, it has been widely used in the preparation of a variety of cytological specimens, particularly in aspirated needle and effusion specimens.23–27,31,32. The commonly used method for CB preparation is to rinse out cellular material from the aspiration needle with saline into a centrifuge tube. The material is then harvested by centrifugation, fixed in formalin, and later processed in the cytology or histopathology laboratories. The main purpose for the preparation of CBs is to recover and concentrate the cellular material to be used for routine diagnosis and other necessary IHC or ancillary studies. Data have shown that CB preparations can provide additional information, including architectural/morphological features of the tumor24,25,27 and IHC data in the diagnosis of a variety of cytological specimens, particularly when searching for the primary site of a metastatic tumor.32
Although precious aspiration material can be recovered by rinsing the aspiration needle and subsequently concentrating it in the laboratory, the cellular yield of CBs is inconsistent and varies widely.25–27 The reasons for this varying yield are several-fold. CB yield depends not only on the skill of the FNA operator to sample the lesion and the method of processing specimens, but also is related to the nature of the lesion, such as its location and size.15–19 To improve the cellular yield of CB sections, a variety of preparative methods have been proposed, including adding thrombin, plasma, and agar gel, as well as the use of commercial assay kits.31,33–36 These methods are primarily designed to concentrate cellular material after it is collected from aspiration needles rather than to protect such material from loss before the preparative procedure.
The first critical step in the preparation of a satisfactory CB is the protection of cellular material during EBUS-TBNA and the efficient recovery of the material from the aspirate needle. However, during the first step of CB preparation using the conventional method, 20 to 30 mL of saline was generally used to rinse the aspiration needle; thus, the cellular material was diluted. It is now possible to overcome this limitation by using the TCC-CB method because this technique is different from rinsing an aspirate needle with saline. In the TCC-CB method, the lesion was first sampled as usual by mechanically moving the needle tip back and forth. The mixture of cellular material and blood was then aspirated into the lumen of the needle, in which a tissue coagulum was formed. By pushing the melange of tissue coagulum out with a stylet rather than by forced spraying and dispersion of the material with a syringe filled with saline, the minimally disturbed tissue clot functions as a carrier to bring down all the cellular material in the needle lumen without further disrupting or diluting it. In the current study, all samples were collected from the same lesion in each patient by the same bronchoscopist to minimize the sampling error and bias. The data from the current study demonstrate that of 145 cases of LN aspirations, the diagnostic rate was 88.7% (94 of 106 cases) using the TCC-CB method versus 56.4% (22 of 39 cases) using the NR-CB method. Similar results were found in peribronchial parenchymal lung lesions. Of 17 cases of lung mass aspirations, the diagnostic rate was 85.7% (12 of 14 cases) using the TCC-CB method versus 0% (none of 3 cases) using the NR-CB method. Although the diagnostic rate in lung lesions appears to follow the same trend as noted in LN sampling, we had fewer specimens in the lung group than in the LN group, and therefore our observation needs to be tested further in a larger patient cohort. Nevertheless, the current study data indicate that using a naturally formed tissue coagulum clot from needle aspiration is clearly superior to conventional aspiration needle rinse in the recovery of cellular material. This method allows us to recover and prevent the loss of precious diagnostic material from an aspiration needle and subsequently to improve cellular yield on CB sections.
We compared the cytomorphology of aspiration material on CB sections that were prepared using both methods. There was no identifiable difference with regard to the nuclear and cytoplasmic characterization of the cells. Cytological features of malignancy, such as hyperchromasia, an irregular nuclear membrane, prominent nucleoli, and a high nuclear: cytoplasmic ratio, were all well preserved.
We further investigated the efficiency and usefulness of TCC-CB for IHC and mutational testing in tumor cases. IHC staining with multiple markers was performed in 26 cases. With the exception of 1 case with insufficient material, the cases (25 of 26; 96.2%) had satisfactory results, thereby providing additional diagnostic and/or differential diagnostic information for the tumor. We did not find any differences with regard to IHC staining patterns between TCC-CB and NR-CB sections, because TCC-CB and NR-CB sections were fixed and stained in an identical manner. In addition, the TCC-CB method makes an excellent preparation for lung cancer molecular studies. EGFR and KRAS mutational analyses were ordered in 14 tumor cases. In our institution, the threshold of adequate tumor cells for isolating DNA was set at ≥ 40% of tumor cells on CB section. By using this criterion, we had satisfactory results with 14 of 14 cases in EGFR and KRAS mutational analyses. Remarkably, among 14 cases, the number of stage III and stage IV lung cancers were 5 and 8 cases, respectively. The data from this study and others have demonstrated that many lung cancer patients are diagnosed at a late stage of the disease1,2,37, and they may not be surgical candidates because of unresectable tumor or other medical conditions. Therefore, the EBUS-TBNA procedure is the optimal option to sample the tumor, provide diagnostic information, and acquire sufficient tumor material for studying prognostic and predictive tumor markers.15–19 The use of the EBUS bronchoscope permits real-time image guidance to provide increased accuracy in puncturing the target lesion of interest. For patients with locally advanced or metastatic disease, the ability to consistently obtain a sufficient amount of tumor tissue by using minimally invasive EBUS-TBNA is crucial for both morphological and molecular analysis of the tumor, because a combination of cytomorphologic and molecular characterization of the tumor is required for the optimization of the targeted or individualized therapy.
Recently, several predictive and prognostic molecular biomarkers have been identified, particularly using mediastinal lymph node aspirates collected by EBUS-TBNA.38 In the study, the expression of cell cycle-related proteins in response to chemotherapy was investigated in the pursuit of refining predictive markers for chemotherapy response.38 Furthermore, additional predictive genetic markers such as excision repair cross-complementation group 1 (ERCC1) and ribonucleotide reductase M1 (RRM1) have been identified28,39; these biomarkers are used to predict specific chemotherapy resistance to platinum and gemcitabine, whereas prognostic markers have the ability to estimate patient outcome independent of treatment modality.39 Obviously, obtaining adequate tumor tissue is vital for these advanced molecular characterizations of the tumor, and the current study demonstrated that the TCC-CB method has an improved ability and potential to provide such needed tumor material for the study.
Finally, there are some limitations to the current study. For example, this study was not a blinded study. To reduce interpretation bias, CB preparations were evaluated independently among cytopathologists. In addition, 2 CB preparation methods were not performed in the same patients; therefore, to reduce sampling error bias, we collected cases consecutively over a period of 12 months.
In summary, the data from the current study demonstrate that the TCC-CB method has significantly increased the efficiency and adequacy of cellular yield from minimally invasive bronchoscopic EBUS-TBNA. It does not change the cytomorphological characteristics of tumor cells. By using this technique, we are able to provide sufficient material for a variety of ancillary studies, including IHC and molecular analysis of the tumor. The technique is simple and without any increased risk to patients. Although it has been challenging for both clinicians and cytopathologists to constantly obtain adequate cellular material from FNA procedures, the emergence and development of targeted therapies will only increase the demand. We believe that this optimal goal can be achieved with multidisciplinary effort and collaboration between bronchoscopists and cytopathologists.
Acknowledgments
We acknowledge the development of the tissue coagulum clot technique by Dr. Yasafuku, Dr. Kurimoto, Dr. Nakajima, Dr. Fujisawa, Dr. Miyazawa, and colleagues and thank them for their generous expenditure of time to teach us (R.C.Y.) and to allow us to refine this technique in our practice.
FUNDING SUPPORT: This work is partially supported by the Drs. Ji and Li family foundation (to Q.K.L.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 4.Chen HY, Yu SL, Chen CH, et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11–20. doi: 10.1056/NEJMoa060096. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Lee W, Jiang Z, Liu J, et al. The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature. 2010;465:473–477. doi: 10.1038/nature09004. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Moran T, Queralt C, et al. Spanish Lung Cancer Group Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 8.Ganti AK, Huang CH, Klein MA, Keefe S, Kelley MJ. Lung cancer management in 2010. Oncology (Williston Park) 2011;25:64–73. [PubMed] [Google Scholar]
- 9.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 10.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advancedstage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 11.Scagliotti GV, Germonpre P, Bosquee L, et al. A randomized phase II study of bortezomib and pemetrexed, in combination or alone, in patients with previously treated advanced non-small-cell lung cancer. Lung Cancer. 2010;68:420–426. doi: 10.1016/j.lungcan.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch FR, Wynes MW, Gandara DR, Bunn PA., Jr The tissue is the issue: personalized medicine for non-small cell lung cancer. Clin Cancer Res. 2010;16:4909–4911. doi: 10.1158/1078-0432.CCR-10-2005. [DOI] [PubMed] [Google Scholar]
- 14.Cagle PT, Allen TC, Dacic S, et al. Revolution in lung cancer: new challenges for the surgical pathologist. Arch Pathol Lab Med. 2011;135:110–116. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]
- 15.Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126:122–128. doi: 10.1378/chest.126.1.122. [DOI] [PubMed] [Google Scholar]
- 16.Jacob-Ampuero MP, Haas AR, Ciocca V, Bibbo M. Cytologic accuracy of samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration at Thomas Jefferson University Hospital. Acta Cytol. 2008;52:687–690. doi: 10.1159/000325622. [DOI] [PubMed] [Google Scholar]
- 17.Alsharif M, Andrade RS, Groth SS, Stelow EB, Pambuccian SE. Endobronchial ultrasound-guided transbronchial fine-needle aspiration: the University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol. 2008;130:434–443. doi: 10.1309/BLLQF8KDHWW6MJNQ. [DOI] [PubMed] [Google Scholar]
- 18.Feller-Kopman D, Yung RCW, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: a retrospective study with histology correlation. Cancer Cytopathol. 2009;117:482–490. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 19.Stoll LM, Yung RC, Clark DP, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. Cancer Cytopathol. 2010;118:278–286. doi: 10.1002/cncy.20103. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Song K, Zervos M, et al. The diagnostic value of endobronchial ultrasound-guided needle biopsy in lung cancer and mediastinal adenopathy. Diagn Cytopathol. 2010;38:337–342. doi: 10.1002/dc.21195. [DOI] [PubMed] [Google Scholar]
- 21.Lam WK, So SY, Hsu C, Yu DY. Fibreoptic bronchoscopy in the diagnosis of bronchial cancer: comparison of washings, brushings and biopsies in central and peripheral tumours. Clin Oncol. 1983;9:35–42. [PubMed] [Google Scholar]
- 22.Mak VH, Johnston ID, Hetzel MR, Grubb C. Value of washings and brushings at fibreoptic bronchoscopy in the diagnosis of lung cancer. Thorax. 1990;45:373–376. doi: 10.1136/thx.45.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yung RC. Tissue diagnosis of suspected lung cancer: selecting between bronchoscopy, transthoracic needle aspiration, and resectional biopsy. Respir Care Clin N Am. 2003;9:51–76. doi: 10.1016/s1078-5337(02)00083-7. [DOI] [PubMed] [Google Scholar]
- 24.Karnauchow PN, Bonin RE. “Cell-block” technique for fine needle aspiration biopsy. J Clin Pathol. 1982;35:688. doi: 10.1136/jcp.35.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burt AD, Smillie D, Cowan MD, Adams FG. Fine needle aspiration cytology: experience with a cell block technique. J Clin Pathol. 1986;39:114–115. doi: 10.1136/jcp.39.1.114-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson NJ, Gogel HK, Williams WL, Mettler FA., Jr Processing of aspiration cytology samples. An alternative method. Acta Cytol. 1986;30:409–412. [PubMed] [Google Scholar]
- 27.Rosell A, Monso E, Lores L, et al. Cytology of bronchial biopsy rinse fluid to improve the diagnostic yield for lung cancer. Eur Respir J. 1998;12:1415–1418. doi: 10.1183/09031936.98.12061415. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado F, Jett JR. Advances in the diagnosis of lung cancer: contribution of molecular biology to bronchoscopic diagnosis. Curr Opin Pulm Med. 2010;16:315–320. doi: 10.1097/MCP.0b013e328337f938. [DOI] [PubMed] [Google Scholar]
- 29.Constantian HM, De Girolami E. Urothelial tumors detected by cytology: new cell block technique. J Urol. 1973;109:304–307. doi: 10.1016/s0022-5347(17)60412-9. [DOI] [PubMed] [Google Scholar]
- 30.De Girolami E. Applications of plasma-thrombin cell block in diagnostic cytology, Part II: II: digestive and respiratory tracts, breast and effusions. Pathol Annu. 1977;12(pt 2):91–110. [PubMed] [Google Scholar]
- 31.Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86–90. doi: 10.1002/dc.20963. [DOI] [PubMed] [Google Scholar]
- 32.Fetsch PA, Simsir A, Brosky K, Abati A. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26:61–66. doi: 10.1002/dc.10039. [DOI] [PubMed] [Google Scholar]
- 33.Zito FA, Gadaleta CD, Salvatore C, et al. A modified cell block technique for fine needle aspiration cytology. Acta Cytol. 1995;39:93–99. [PubMed] [Google Scholar]
- 34.Krogerus LA, Andersson LC. A simple method for the preparation of paraffin-embedded cell blocks from fine needle aspirates, effusions and brushings. Acta Cytol. 1988;32:585–587. [PubMed] [Google Scholar]
- 35.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko C, Kobayashi TK, Hasegawa K, Udagawa Y, Iwai M. A cell-block preparation using glucomannan extracted from Amorphophallus konjac. Diagn Cytopathol. 2010;38:652–656. doi: 10.1002/dc.21280. [DOI] [PubMed] [Google Scholar]
- 37.Munfus-McCray D, Harada S, Adams C, et al. EGFR and KRAS mutations in metastatic lung adenocarcinomas. Hum Pathol. 2011;42:1447–1453. doi: 10.1016/j.humpath.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Mohamed S, Yasufuku K, Nakajima T, et al. Analysis of cell cycle-related proteins in mediastinal lymph nodes of patients with N2-NSCLC obtained by EBUS-TBNA: relevance to chemotherapy response. Thorax. 2008;63:642–647. doi: 10.1136/thx.2007.090324. [DOI] [PubMed] [Google Scholar]
- 39.Bepler G, Bequm M, Simon GR. Molecular analysis-based treatment strategies for non-small cell lung cancer. Cancer Control. 2008;15:130–139. doi: 10.1177/107327480801500205. [DOI] [PubMed] [Google Scholar]


