Abstract
Background
This study sought to estimate the severity, etiology, and clinical importance of treatment-related lymphopenia in patients with stage III non-small-cell lung cancer.
Methods
Serial lymphocyte counts and survival were analyzed retrospectively in 47 patients accounting for known prognostic factors.
Results
Total lymphocyte counts (TLCs) were normal before therapy and did not change following neoadjuvant chemotherapy. Following radiation, TLC fell by 67% (median 500 cells/mm3, p <.00001). Multivariate analysis revealed an association between severe TLC and survival (HR 1.70, 95% CI: 0.8–3.6).
Conclusions
Rapid and severe lymphopenia occurred in 50% of patients following radiation which was associated with reduced survival.
Keywords: Lymphopenia, Non-small-cell lung cancer, Radiation, Chemotherapy, Treatment-related toxicities
INTRODUCTION
The immune system in general, and cellular immunity in particular, is thought to play a central role in cancer suppression. Pretreatment lymphopenia has been documented to be a poor prognostic factor in patients with cancers of the lung, breast, and colorectum as well as sarcomas and lymphomas (1, 2). In addition, pathologic studies have found that patients with intense lymphocytic infiltration of their solid tumors have longer survivals rates than those who do not (3–6). These and other related observations led to extensive efforts to develop vaccines and other immunologic approaches in an attempt to enhance the immune system’s effect against invasive cancers (7–9).
Although the association between pretreatment lymphopenia and survival is well known, lymphopenia that occurs following the administration of antineoplastic therapies has been previously described but has only recently been associated with patient outcomes. In 2011, a prospective multicenter study monitoring lymphocyte counts in patients with newly diagnosed high-grade gliomas was reported (10). Lymphocyte and CD4 counts were assessed before and for 1 year after the administration of concurrent radiation and temozolomide. The median CD4 counts prior to radiation and chemotherapy were 664 cells/mm3. However, CD4 counts fell significantly after 2 months of treatment resulting in 40% of patients having CD4 counts fewer than 200 cells/mm3. CD4 counts in these patients remained persistently low during the entire 12 months of observation. In addition, patients with CD4 counts <200 cells/mm3 at 2 months had shorter survival than those with higher counts (median 13.1 vs. 19.7 months, p = .002). The deaths were from progressive disease rather than opportunistic infection. Multivariate analysis determined that a patients CD4 count at 2 months was an independent predictor of survival.
Patients with resectable pancreatic adenocarcinoma also receive postoperative radiation and chemotherapy. A recently reported study found that this patient population had normal lymphocyte counts following surgery but 2 months after radiation and chemotherapy 45% of patients had developed severe lymphopenia (11). Multivariate analysis revealed that this severe lymphopenia was also independently associated with early death from progressive cancer. This suggests that treatment-related lymphopenia seen in patients with glioblastoma was not primarily related to high-dose glucocorticoids or temozolomide, as these are not routinely administered to patients with pancreatic cancer.
This retrospective study was performed to determine if patients with stage III NSCLC develop severe lymphopenia after radiation and chemotherapy and if this is most likely secondary to the administration of radiation or chemotherapy. In addition, this study sought a preliminary view of the relationship between treatment-related lymphopenia and survival in this patient population.
PATIENTS AND METHODS
Study population
This study was reviewed and approved by the Institutional Review Board of The Johns Hopkins University. Four hundred eighty patients with stage III lung cancer were retrospectively identified using a database of lung cancer patients at The Johns Hopkins Hospital as having been seen between 1996 and 2008. The following eligibility criteria were used to select the study population: (1) ≥18 years of age, (2) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, (3) baseline and follow-up complete blood counts performed at The Johns Hopkins Hospital and thus accessible through the Electronic Patient Record (EPR), and (4) treatment with concurrent chemoradiation therapy at our institution. Patients were excluded if they had received chemotherapy or radiation prior to their lung cancer treatment. Since the majority of these patients were excluded because they were eventually treated elsewhere or they did not have baseline and/or follow-up total lymphocyte counts (TLCs) in the EPR, 47 stage III patients constituted the study population.
Treatment and total lymphocyte counts examination
Information relating to known prognostic factors in NSCLC was obtained from the medical records of each patient (12, 13). Surgical procedures were categorized as biopsy, wedge resection, lobectomy, or pneumonectomy. Additional data collected included ECOG performance status, smoking history, clinical staging, histology, tumor grade and differentiation, resection margins, dose and duration of radiation, and chemotherapy treatments.
Patients were divided into two groups: those who received two cycles of neoadjuvant chemotherapy followed by concurrent chemoradiation and those who received only concurrent chemoradiation. In patients who received neoadjuvant chemotherapy, TLCs were collected before starting neoadjuvant chemotherapy, monthly during treatment, before beginning chemoradiation, and monthly thereafter for a total of 24 months. In patients who received only chemoradiation, lymphocyte counts were collected before beginning chemoradiation and monthly thereafter. Baseline TLCs were classified as normal (≥1000 cells/mm3) or abnormal (<1000 cell/mm3). Following the initiation of antineoplastic treatment, the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0) was used to classify the severity of treatment-related lymphopenia.
The TLCs at 2 months after the initiation of radiation and chemotherapy were dichotomized to CTCAE grade 0–II versus grade III–V for the relevant analyses. For patients with missing lymphocyte counts at 2 months, the TLCs at 1 month were used instead. Overall survival time was measured from the date of diagnosis to the date of death due to any cause. Survival was censored if the subject was alive at the time of last follow-up.
Statistical analysis
Patient baseline characteristics were summarized using descriptive statistics. Chi-square test statistics were used for proportional comparison. Student’s t-test and paired t-test were used for continuous data between and within group comparison, respectively. Survival probability was estimated using the Kaplan–Meier method (14). The confidence interval of median overall survival time was constructed by the method of Brookmeyer–Crowley (15). The log-rank test was used to compare survival distributions for patients with lymphocyte counts <500 versus ≥500 cells/mm3 2 months after starting radiation therapy (16). Univariate analysis was used to assess an association between known prognostic factors and overall survival. Important patient characteristics associated with survival were identified in the univariate analysis using a p value of ≤.4. These characteristics and baseline lymphocyte counts were selected as covariates to construct the multivariate proportional hazards regression model. The proportional hazards regression model was used to estimate the hazard ratio (HR) for death attributable to prognostic factors. All p values are reported as two sided and all analyses were conducted using SAS software (version 9.1, SAS Institute).
RESULTS
Baseline characteristics of patients
Forty-seven adults met the required eligibility criteria. Baseline demographic information on these patients is provided in Table 1. The median age of the patients was 59 years (range 43–79) and 77% of the patients were over the age of 55. Sixty-four percent were female, 83% were Caucasian, and 64% had an ECOG performance status of zero. Seventy-four percent were stage IIIA and 26% were stage IIIB, 70% had adenocarcinoma, 30% had squamous cell carcinoma, and 68% were poorly differentiated. Forty-three percent of patients had only a biopsy, while surgery was performed in 57% of patients. Surgery included lobectomy (21 patients), pneumonectomy (3 patients), and wedge resection (3 patients).
Table 1.
Baseline Characteristics of All Patients and Those With Lymphocyte Counts Above and Below 500 cells/mm3 at 2 Months
| All Patients (N = 47) | Patients With Lymphocyte Counts ≥500 at 2 Months (N = 24) | Patients With Lymphocyte Counts <500 at 2 Months (N= 23) | p-Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age: median (range) | 59 (37–79) | 59.5 (47–78) | 59 (37–79) | .56 |
| Age >55 years (%) | 36 (77) | 19 (80) | 17 (74) | .67 |
| Male: no. (%) | 17 (36) | 6 (25) | 11 (48) | .84 |
| Caucasian: no. (%) | 39 (83) | 20 (83) | 19 (83) | .95 |
| ECOG performance = 0: no. (%) | 30 (64) | 16 (67) | 14 (61) | .68 |
| Smoking >30 pack-year: no. (%) | 31 (66) | 17 (71) | 14 (61) | .47 |
| Baseline laboratory data | ||||
| Total lymphocyte count: median (range) | 1510 (570–3336) | 1557 (760–3336) | 1500 (570–2680) | .63 |
| Lymphocyte ≥ 1000: no. (%) | 42 (89) | 21 (88) | 21 (91) | .67 |
| WBC: median (range) | 7190 (1660–18800) | 6645 (1660–15270) | 7770 (2730–18800) | .27 |
| Hematocrit: median (range) | 37.6 (27.2–46.4) | 38.6 (31.7–46.4) | 36.9 (27.2–46.3) | .29 |
| Platelet: median (range) | 283 (82–700) | 285 (82–625) | 274 (98–700) | .67 |
| Tumor staging data | ||||
| Clinical stage IIIA: no. (%) | 35 (74) | 19 (79) | 16 (70) | .45 |
| Clinical stage IIIB: no. (%) | 12 (26) | 5 (21) | 7(30) | |
| Histology of adenocarcinoma: no. (%) | 33 (70) | 16 (67) | 17 (74) | .59 |
| Histology of squamous carcinoma: no. (%) | 14 (30) | 8 (33) | 6 (26) | .59 |
| Poorly differentiated pathology: no. (%) | 32 (68) | 16 (67) | 16 (70) | .83 |
| Moderately differentiated pathology: no. (%) | 15 (32) | 8 (53) | 7 (47) | |
| Surgical resected: no. (%) | 27 (57) | 16 (67) | 11 (48) | .25 |
| Positive margins: no. (%) | 10 (21) | 4 (17) | 6 (26) | .43 |
| Adjuvant treatment data | ||||
| Radiation dose (Gy): median (range) | 60 (43.2–70.2) | 60 (43.2–64.0) | 59.4 (43.2–70.2) | .98 |
| Concurrent chemotherapy (taxol/carboplatin): no. (%) | 37 (79) | 18 (75) | 19 (83) | .52 |
| Neoadj. chemotherapy: no. (%) | 20 (43) | 10 (42) | 10 (43) | .90 |
For the purposes of analysis, patients were divided into two groups depending on whether neoadjuvant chemotherapy was administered prior to concurrent chemoradiation. Twenty (43%) of the 47 patients received neoadjuvant chemotherapy which consisted of two cycles of taxol/carboplatin (85%) or gemcitabine/carboplatin (15%). These patients then went on to receive concurrent chemoradiation (median dose 60.0 Gy) with taxol/carboplatin (95%) or gemcitabine/carboplatin (5%). Twenty-seven patients (57%) received only concurrent chemoradiation (median dose 54.0 Gy). This was administered with taxol/carboplatin (66%), etoposide/cisplatin (30%), or vinblastine/etoposide (4%). The decisions as to which therapy the patient received were largely determined by tumor stage with stage IIIB patients receiving neoadjuvant chemotherapy in an attempt to reduce tumor and radiation field size prior to proceeding with concurrent chemoradiation.
Total lymphocyte counts over time
In the 20 patients who received neoadjuvant chemotherapy, the median TLC prior to chemotherapy was 1190 cells/mm3 (range 399–3760 cells/mm3). Following two cycles of chemotherapy, TLCs were largely unchanged (median 1500 cells/mm3, range 570–2680 cells/mm3) resulting in TLCs within the normal range in 85% of patients prior to beginning their concurrent chemoradiation. However, 2 months after receiving the concurrent chemoradiation, the TLCs fell by 68% to a median of 480 cells/mm3 (range 131–1300 cells/mm3; p <.00001) and 50% of patients had TLC <500 cells/mm3 (see Figure 1). In the 27 patients who received only concurrent chemoradiation, the initial TLCs were normal (median 1570 cells/mm3, range 640–3336 cells/mm3) in 93% of patients. Two months after receiving chemoradiation, their TLC had fallen by 68% (median 500 cells/mm3, range 196–2040 cells/mm3; p <.00001) and 48% had TLC <500 cells/mm3 (see Figure 1).
Figure 1.
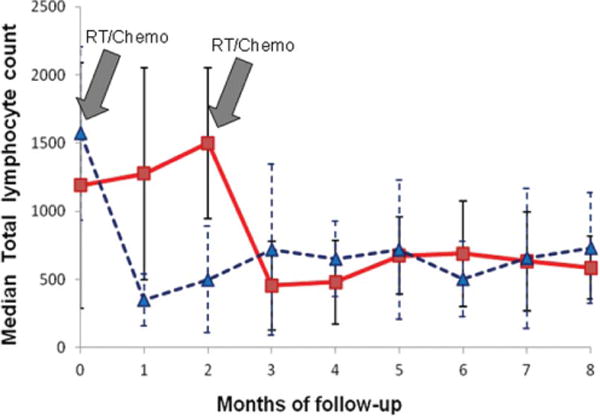
Median total lymphocyte counts dropped significantly with the addition of radiation. Twenty patients (solid red line) received two cycles of neoadjuvant chemotherapy followed by concurrent chemoradiation. Twenty-seven patients (broken blue line) received only concurrent chemoradiation. While neoadjuvant chemotherapy did not reduce TLCs, 2 months after beginning combined radiation/chemotherapy (2nd gray arrow), TLCs fell by 68%. Gray arrows depict the start of radiation exposure in both treatments.
The summary data from all 47 patients demonstrate that TLCs were normal (above 1,000 cells/mm3) in 89% of patients prior to receiving concurrent chemoradiation. Ten patients had missing lymphocyte counts at 2 months, and in these patients, the TLCs at 1 month were used. Two months after initiating radiation and chemotherapy, the median TLC in all patients fell rapidly from a median of 1510 cells/mm3 (range 570–3336 cells/mm3) to 500 cells/mm3 (range 131–2040 cells/mm3; p <.00001) with a 67% overall reduction. Twenty-three patients (49%) had a TLC below 500 cells/mm3. The reduction in the median TLC occurred irrespective of the concurrently administered chemotherapy regimen. As noted in Table 1, there was no significant difference in the baseline demographic, staging, pathology, laboratory, or adjuvant treatment data in patients whose TLCs remained above 500 cells/mm3 or fell significantly at 2 months after chemoradiation treatment. In addition, as shown in Figure 1, once the TLCs were reduced, they remained low for many months thereafter.
Median overall survival
The median overall survival for all patients was 24.8 months (95% CI: 19.6–35.8). The median overall survival of patients whose lymphocyte counts were fewer than 500 cells/mm3 at 2 months after beginning radiation was 21.8 months compared with 27.3 months for patients who had higher lymphocyte counts (p = .38, log-rank test). Kaplan–Meier survival curves for patients with TLC <500 cells/mm3 versus ≥ 500 cells/mm3 at 2 months are presented in Figure 2. The cause of death in this patient population was progressive cancer in the vast majority of patients. Serious opportunistic infections were not observed.
Figure 2.
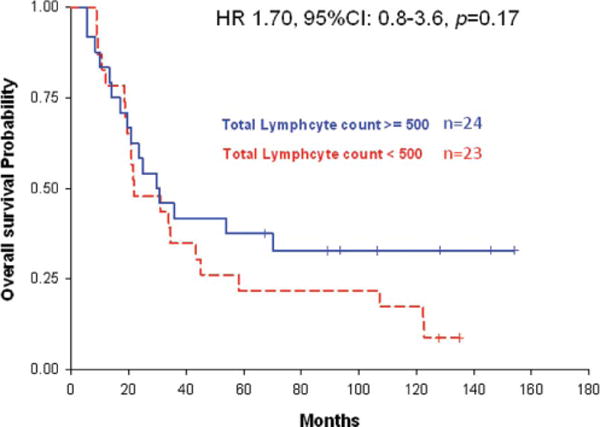
Kaplan–Meier survival curves for patients with total lymphocyte counts above (solid blue line) and below (broken red line) 500 cells/mm3.
Associations between patient characteristics and survival
Table 2 presents univariate and multivariate associations between patient characteristics and survival in these 47 patients. No significant univariate associations were identified between potential prognostic factors and overall survival. Multivariate analysis revealed a 70% higher hazard rate of death attributable to lower TLCs at 2 months after chemoradiation compared with TLCs ≥500 cells/mm3 (HR = 1.70; 95% CI: 0.8–3.6) adjusting for radiation dose, patient age, and whether or not patients received neoadjuvant chemotherapy. Treatment with different chemotherapy regimen was also not significantly associated with different lymphocyte count or survival.
Table 2.
Univariate and Multivariate Associations Between Patient Characteristics and Survival
| Characteristic | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Univariate associations | ||
| Age: ≤55 vs. >55 | 0.46 (0.20–1.06) | .07 |
| ECOG: 0 vs. 1 | 1.04 (0.52–2.08) | .91 |
| Staging: IIIA vs. IIIB | 0.97 (0.47–2.02) | .94 |
| Lymph count at 2 months: <500 vs. ≥500* | 1.34 (0.70–2.57) | .38 |
| Pathology: moderate differentiated vs. poorly differentiated | 0.86 (0.42–1.74) | .67 |
| Histology: adenocarcinoma vs. squamous | 0.89 (0.44–1.79) | .75 |
| Neoadjuvant chemotherapy: no vs. yes | 0.70 (0.36–1.35) | .29 |
| Radiation dose | 1.02 (0.97–1.08) | .38 |
| Multivariate associations | ||
| Age: ≤55 vs. >55 | 0.42 (0.14–1.25) | .12 |
| Radiation dose | 1.03 (0.97–1.09) | .39 |
| Lymph count at 2 months: <500 vs. ≥500 | 1.70 (0.80–3.61) | .17 |
| Neoadj chemotherapy (stratified by stage): no vs. yes | 0.79 (0.35–1.78) | .56 |
Lymphocyte count at 2 months is dichotomized at 500 cells/mm3 (per the CTCAE NIH grade III–IV treatment-induced lymphopenia).
DISCUSSION
This retrospective analysis of patients with stage III NSCLC demonstrates that prior to the initiation of radiation and chemotherapy, median TLCs were normal. However, 2 months after initiating radiation, lymphocyte counts were reduced by a median of 67% and 49% of patients had TLCs fewer than 500 cells/mm3. The timing, frequency, and severity of this treatment-induced lymphopenia are comparable to that seen in patients with newly diagnosed high-grade gliomas and resected pancreatic cancers (10, 11).
Of particular importance is the observation that patients who received two cycles of neoadjuvant chemotherapy experienced no fall in their TLCs. However, 2 months after the addition of radiation, nearly 50% of patients had grade III–IV lymphopenia (TLC <500 cells/mm3). This was also observed in patients who received chemoradiation alone. This strongly suggests that radiation is a significant factor responsible for the reduction in TLCs. Different chemotherapy regimens were administered as neoadjuvant therapy and concurrent therapy with radiation. The changes in TLCs were similar following the different regimens. These findings are consistent with those seen in patients with glioblastoma and pancreatic cancer and suggest that the observed treatment-related immunosuppression is not related to a specific chemotherapeutic agent. Also similar to the findings in patients with glioblastoma and pancreatic cancer, this severe treatment-related lymphopenia persists for months to years after chemoradiation is administered.
Furthermore, patients who developed grade III–IV lymphopenia 2 months after starting concurrent radiation and chemotherapy had shorter survival times than those whose TLCs remained higher (21.8 vs. 27.3 months, p = .17). Although this difference was not statistically significant possibly due to small sample size, multivariate analysis revealed a strikingly higher hazard rate of death associated with lower TLCs at 2 months after chemoradiation compared with TLC ≥500 cells/mm3 (HR = 1.70; 95% CI: 0.8–3.6). This finding suggests that future studies with larger sample sizes are likely to provide significant survival results, as were noted in patients with glioblastoma and pancreatic cancer (10, 11).
Prior evidence suggests that the role of lymphocytes may be important in the control of human cancers. Lymphopenia prior to the initiation of antineoplastic therapy has been demonstrated to predict a poor prognosis in metastatic breast cancer, advanced soft tissue sarcoma, and non-Hodgkin’s lymphoma (2). This has also been associated with a lower efficacy of chemotherapy in lung cancer, colorectal cancer, and breast cancer (1).
Although it is well documented that pretreatment lymphopenia is associated with poor outcomes, only recently has posttreatment lymphopenia been associated with inferior survival outcomes (10, 11). Severe posttreatment lymphopenia has also been reported in stage III NSCLC treated with concurrent paclitaxel and radiation (17). Fifteen patients with stage IIIA/B NSCLC were treated with weekly paclitaxel (dose range from 50 to 86 mg/m2) and simultaneous daily radiation (a total dose of 56 Gy). Fourteen patients were analyzed for response and toxicity. Their pretreatment lymphocyte counts were normal (1800 cells/mm3 ±780). However, grade III–IV lymphopenia (TLC <500 cells/mm3) was found in 12 of 14 patients at the end of the chemoradiation treatment. Unlike the toxic effect on neutrophils, this lymphopenia occurred independently of the dose level of paclitaxel. However, this study did not determine whether the etiology of this treatment-related lymphopenia in stage III NSCLC was due to radiation or chemotherapy and did not examine the relationship between treatment-induced lymphopenia and overall survival.
Our data suggest that radiation is likely to play a major role in the development of treatment-related lymphopenia. This is plausible given the extreme sensitivity of lymphocytes to radiation and the previously demonstrated severe lymphopenia that occurs when radiation alone is administered to partial brain fields or to blood passing through extracorporeal radiation source (18, 19). Radiation for high-grade gliomas is administered 5 days each week for 6 weeks to a limited brain field which is devoid of bone marrow and lymphatic tissue. Preliminary data with computer modeling of the dose of radiation administered to circulating lymphocytes as they pass through the radiation beam used to treat glioblastomas (60 Gy in 30 fractions) suggest that nearly 100% of circulating lymphocytes receive sufficient dose to explain the observed lymphopenia (20).
The findings of this retrospective study are consistent with previous reports on newly diagnosed high-grade gliomas and resected pancreatic cancers (10, 11). We have shown that 49% of patients with stage III NSCLC who received concurrent chemoradiation develop grade III–IV lymphopenia 2 months after initiating therapy. Reductions in TLCs occurred irrespective of the concurrently administered chemotherapy with radiation, which is consistent with the finding in pancreatic cancers where similar lymphopenia was shown in patients who received either 5-FU or gemcitabine along with radiation. More importantly, TLC levels remained stable following neoadjuvant chemotherapy but fell to grade III–IV lymphopenia levels in nearly half of the patients 2 months after the initiation of radiation. These results, coupled with those from patients with glioblastomas and pancreatic cancer, suggest that the treatment-related immunosuppression is more likely to be related to radiation than to chemotherapy. Given this treatment-related lymphopenia appears to be common, severe, and associated with inferior survival in patients with NSCLC, prospective studies are needed to confirm and expand upon these novel findings. If severe treatment-related lymphopenia is ultimately shown to be contributed to shortened survival, additional research to discover and test treatment approaches that preserve or restore immunologic function will be important to improve the outcome of patients with NSCLC.
Acknowledgments
We thank Mr. Craig Hooker for the database assistance.
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest.
References
- 1.Lissoni P, Brivio F, Fumagalli L, Messina G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A, Gardani GS. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–140. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 2.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, Guastalla JP, Bachelot T, Perol D, Chabaud S, Hogendoorn PC, Cassier P, Dufresne A, Blay JY. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell in-filtration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 5.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlin AM, Henriksson ML, Van Guelpen B, Stenling R, Oberg A, Rutegard J, Palmqvist R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 7.Winter H, van den Engel NK, Rusan M, Schupp N, Poehlein CH, Hu HM, Hatz RA, Urba WJ, Jauch KW, Fox BA, Ruttinger D. Active-specific immunotherapy for non-small cell lung cancer. J Thorac Dis. 2011;3:105–114. doi: 10.3978/j.issn.2072-1439.2010.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodolfo M, Zilocchi C, Melani C, Cappetti B, Arioli I, Parmiani G, Colombo MP. Immunotherapy of experimental metastases by vaccination with interleukin gene-transduced adenocarcinoma cells sharing tumor-associated antigens. Comparison between IL-12 and IL-2 gene-transduced tumor cell vaccines. J Immunol. 1996;157:5536–5542. [PubMed] [Google Scholar]
- 9.Wang J, Zou ZH, Xia HL, He JX, Zhong NS, Tao AL. Strengths and weaknesses of immunotherapy for advanced non-small-cell lung cancer: a meta-analysis of 12 randomized controlled trials. PLoS One. 2012;7:e32695. doi: 10.1371/journal.pone.0032695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balmanoukian AS, Ye X, Herman JM, Laheru D, Grossman SA. Effect of treatment-related lymphopenia on survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socinski MA, Zhang C, Herndon JE, 2nd, Dillman RO, Clamon G, Vokes E, Akerley W, Crawford J, Perry MC, Seagren SL, Green MR. Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: analysis of factors influencing survival and toxicity. Ann Oncol. 2004;15:1033–1041. doi: 10.1093/annonc/mdh282. [DOI] [PubMed] [Google Scholar]
- 13.Lee DS, Kim YS, Kang JH, Lee SN, Kim YK, Ahn MI, Han DH, Yoo I, Wang YP, Park JG, Yoon SC, Jang HS, Choi BO. Clinical responses and prognostic indicators of concurrent chemoradiation for non-small cell lung cancer. Cancer Res Treat. 2011;43:32–41. doi: 10.4143/crt.2011.43.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 16.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–83. [Google Scholar]
- 17.Reckzeh B, Merte H, Pflüger K, Pfab R, Wolf M, Havemann K. Severe lymphocytopenia and interstitial pneumonia in patients treated with paclitaxel and simultaneous radiotherapy for non-small-cell lung cancer. J Clin Oncol. 1996;14:1071–1076. doi: 10.1200/JCO.1996.14.4.1071. [DOI] [PubMed] [Google Scholar]
- 18.Weeke E. The development of lymphopenia in uremic patients undergoing extracorporeal irradiation of the blood with portable beta units. Radiat Res. 1973;56(3):554–559. [PubMed] [Google Scholar]
- 19.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 20.Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]


