Abstract
Swallowing and swallowing disorders have garnered continuing interest over the past several decades. Electroencephalography (EEG) is an inexpensive and non-invasive procedure with very high temporal resolution which enables analysis of short and fast swallowing events, as well as an analysis of the organizational and behavioral aspects of cortical motor preparation, swallowing execution and swallowing regulation. EEG is a powerful technique which can be used alone or in combination with other techniques for monitoring swallowing, detection of swallowing motor imagery for diagnostic or biofeedback purposes, or to modulate and measure the effects of swallowing rehabilitation. This paper provides a review of the existing literature which has deployed EEG in the investigation of oropharyngeal swallowing, smell, taste and texture related to swallowing, cortical pre-motor activation in swallowing, and swallowing motor imagery detection. Furthermore, this paper provides a brief review of the different modalities of brain imaging techniques used to study swallowing brain activities, as well as the EEG components of interest for studies on swallowing and on swallowing motor imagery. Lastly, this paper provides directions for future swallowing investigations using EEG.
Keywords: Swallowing, dysphagia, neurology of swallowing, electroencephalography, sensation, cortical potential
1 Introduction
In humans, swallowing is an essential motor activity by which food, liquids, and saliva pass from the oral cavity to the stomach. It is considered one of the most complex aerodigestive sensorimotor activities due to the high level of coordinated efforts required to accomplish the swallowing task and the multiple central and peripheral subsystems involved. The system that plans, coordinates and executes the oropharyngeal swallowing sequence actively targets muscle groups in the head, neck, and upper thorax via activation of a broad range of regions in the brain and brainstem (Ertekin et al., 2003; Stevenson and Allaire, 1991).
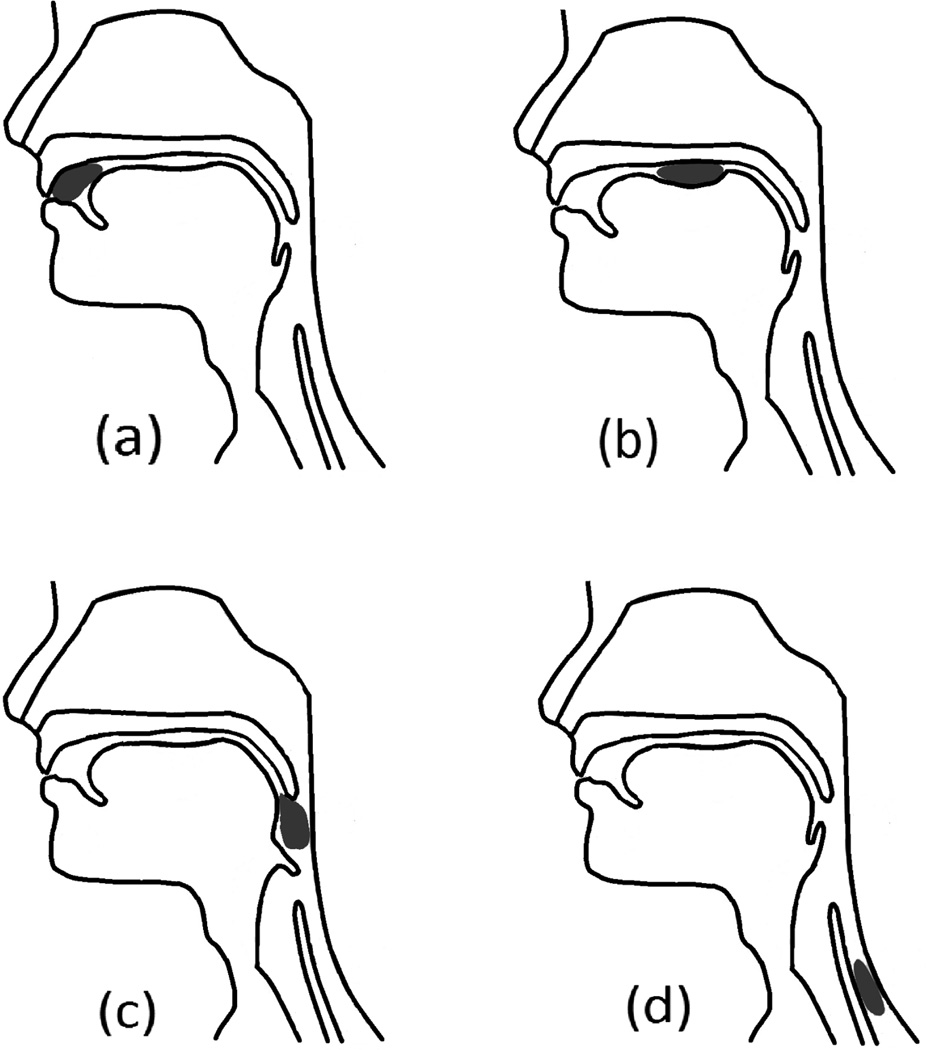
In an effort to discretely describe the numerous sensorimotor events that occur during the relatively short duration of an oropharyngeal swallow, Logemann (1993) described four distinct swallowing phases which occur in a somewhat overlapping temporal sequence (Figure 1). During the first swallowing phase, the oral preparatory phase, solid food is reduced into a swallowable form (bolus) while tactile, kinesthetic, proprioceptive, and taste sensory input are delivered to the brainstem and cortical centers (Hughes et al., 1987). Depending on the information collected by oral sensory receptors about the bolus, the brainstem generates signals which result in the activation of masticatory and other oral and pharyngeal motor activities. During the oral transit phase, the tongue propels the bolus posteriorly while the pharynx and tongue act as a piston-like propulsion hydrostat to enable the transfer of the bolus to the pharynx (Nicosia and Robbins, 2001; Miller and Watkin, 1996). During the next phase, the pharyngeal phase, the bolus is propelled toward the digestive system while numerous and complex short-duration sensorimotor events produce closure of the airway and nasopharynx along with opening of the esophagus (Hughes et al., 1987) while receptors continue to transmit information about the size, shape, temperature, taste, and speed of motion of the food or liquids being processed (Miller, 1999). During the fourth phase, the esophageal phase, the bolus is propelled toward the lower esophageal sphincter, which momentarily relaxes and enables clearance into the stomach (Goyal et al., 2001).
Figure 1.
Four swallowing phases: (a) oral preparatory phase; (b) oral transit phase; (c) pharyngeal phase; (d) esophageal phase.
Historically, it was believed that only the brainstem centers were responsible for controlling swallowing. However, later studies emphasized the importance of the cerebral cortex during this action (Martin and Sessle, 1993). Other parts of the brain which have demonstrated activity during swallowing include: the faciale areas of the sensorimotor cortices, the premotor cortex, the anterior cingulate cortex, the insular cortex, the frontal operculum, the cerebellum, etc. (Hamdy et al., 1999a,b; Martin et al., 2001; Kern et al., 2001a; Mosier and Bereznaya, 2001; Zald and Pardo, 1999).
Swallowing difficulties (i.e., dysphagia) may occur for a variety of reasons, the most common of which include neurological conditions such as stroke (Gottlieb et al., 1996), traumatic brain injuries (Lazarus and Logemann, 1987), cerebral palsy (Rogers et al., 1994), and Parkinson’s or other neurodegenerative diseases (Murray, 1999). Dysphagia is responsible for increasing the risk of other adverse medical conditions (i.e., dehydration (Smithard et al., 1996), malnutrition (Foley et al., 2009), failure of the immune system Curran and Groher (1990), and respiratory infection (Marik and Kaplan, 2003)), which can lead to additional medical complications and even death in some severe cases. Patients with acute stroke, for example, will have a three-fold increase in their adjusted mortality risk if they develop dysphagia-related pneumomia after stroke, when compared to patients for whom pneumonia can be mitigated by aggressive intervention (Katzan et al., 2003). Dysphagia can significantly influence the quality of life, particularly when a strict change of diet is required during the rehabilitation process to mitigate adverse events caused by dysphagia. Likewise, diseases that cause dysphagia, medical and surgical treatments that patients undergo, can produce disrupted sensory functions (i.e. taste and smell). These changes can occur due to systemic diseases (Ship, 1999), surgical outcomes, radiation therapy (Rose-Ped et al., 2002), chemotherapy (Schwartz et al., 1993), changes in taste bud cell responses (Fukunaga et al., 2005), changes in olfactory sensitivity (Murphy et al., 1991), xerostomia (reduction in salivary flow), a common side effect of numerous medications, or brain damage (Daniels and Foundas, 1997). Conversely, studies have demonstrated that oral and pharyngeal sensory input via a sour flavored material produces a facilitative effect upon the onset of swallow-related pharyngeal motor activity (Pelletier and Lawless, 2003). Therefore, since the diminished or enhanced taste and smell can either contribute or reverse some effects of dysphagia, the importance of these senses needs to be considered in order to understand their contribution to recovery from dysphagia after neural injury and their role in swallowing-related cerebral plasticity.
Neurologically, it is known that dysphagia is caused by lesions in the brain (Cichero and Murdoch, 2006). However, it has been shown that the brain has the ability to reorganize the sensory and motor cortex (Rosenkranz et al., 2008; Davenport et al., 2011; Robbins et al., 2008), a principle referred to as cerebral plasticity, which correlates with patient rehabilitation (Hamdy et al., 2000; Doeltgen and Huckabee, 2012). This leads us to the notion that in order to improve swallowing rehabilitation with a patient affected by dysphagia, swallowing should be analyzed from the prospective of brain activity.
Electroencephalography (EEG) is one of the techniques currently used for investigating brain activity during different tasks. EEG records neural electrical activity via a cap placed over the cranium which provides direct information about neural behavior in the brain (Nunez and Srinivasan, 2006). Normal swallowing has a distinctive EEG wave representation (Stern and Engel, 2005) which can be disturbed in cases of neurological conditions which causes dysphagia (Wilson and Oliver, 1988; Saint-Martin et al., 1999). Thus, EEG is considered a suitable technique for analyzing cortical brain activity during swallowing (Huckabee et al., 2003; Hiraoka, 2004). Current EEG swallowing investigations concentrate on the voluntary part of swallowing (i.e., oral preparatory and oral transit phase), components of swallowing related to sensation and peripheral innervation of swallowing (i.e., the motor component and sensory feedback), and motor imagery of swallowing. Despite the short history of the use of EEG to monitor swallowing activity, the advantages this technique provides suggests its usefulness for this task. The purpose of this article is to present current literature about the neurological origins of swallowing, as well as to describe the contributions of the studies which used EEG for swallowing investigations. The intent of this review is to give direction to future EEG swallowing investigations.
2 A brief comparative overview of brain imaging modalities used for swallowing studies
Studies which used advanced imaging techniques, such as functional magnetic resonance imaging (fMRI), positron emission tomography (PET), or magnetoencephalography (MEG), provided significant contributions to the field through their investigations of brain activity during swallowing. fMRI, together with PET, could be considered the gold standard for investigating central neural activation during swallowing, as these imaging techniques provide good spatial representations of changes in brain activity. Through the use of fMRI and PET, it is possible to identify specific lesions and examine the lesions’ influence on central neural activity related to swallowing (Hamdy et al., 1999a,b; Kern et al., 2001a; Hartnick et al., 2001; Kern et al., 2001b; Mosier et al., 1999). On the other hand, because of poor temporal resolution and the numerous short-duration events occurring during swallowing and its neural activation patterns, most of these studies could not clearly recognize the differences between motor and sensory signals. Instead, these studies simply demonstrated that many different brain regions activated during swallowing (Kern et al., 2001a). Kern et al. (2001a) claimed that the activation of the cortical component related to swallowing overlaps with the activation of the cortical component related to other non-swallowing or pre-swallowing related orofacial movements. A further limitation of these techniques is that they only provide indirect measures of neural activity via changes in cranial blood flow (fMRI (Jueptner and Weiller, 1995)) or metabolic activity (PET (Cook et al., 1998)) instead of directly recording the electrical output of these cells. On the other hand, MEG records the magnetic field generated by neural activity in the brain. This technique is known for having comparatively high temporal resolution. In addition, MEG has been shown to be useful for determining the role of the premotor cortex during initiation and regulation of swallowing (Hamdy et al., 1999a; Abe et al., 2003). However, MEG is limited to monitoring surface activity only, as it cannot propagate a magnetic field from areas that generate neural impulses in deeper layers of brain tissue (Hillebrand and Barnes, 2002). This all suggests that accurate information about brain activation in different swallowing stages is not easily accessible or acquired (i.e., motor planning for swallowing, the oral volitional components of swallowing, the pharyngeal pattern-response components, and the sensory feedback activated through swallowing).
Besides its significantly lower cost, EEG exhibits some advantages over fMRI and PET by capturing information that these two techniques fail to transduce. EEG describes temporal and spatial representations of the cortical excitatory potential and the inhibitory potential, as well as their interaction with and influence on cerebral metabolism. This means that EEG can directly describe the immediate outcomes associated with cerebral metabolic activity (Jordan, 1993). EEG has a very high temporal resolution (i.e., 0.5–130 ms), which ensures that it is capable of taking many samples of neural activity throughout the duration of the swallow, while the temporal resolutions of fMRI (i.e. 1–3 s) and PET (i.e. tens of seconds to minutes) are too slow to be useful in analysis of swallowing events whose durations are measured in milliseconds. Another important advantage of EEG over both fMRI and PET has to do with the position of the patient while performing the test. During fMRI and PET testing, the patient is required to lie down in the supine position and remain completely still. In the case of voluntary swallowing activity the supine position is not natural for healthy persons and dangerous for people with dysphagia and can cause abnormal muscle and brain activity when compared to a typical sitting position used by most people when eating and drinking (Drake et al., 1997; Malandraki et al., 2011). EEG allows for testing in any natural head or body position used by the individuals when eating and drinking, since the equipment is much smaller and less restrictive than these alternative recording techniques. This results in the greater face validity of our research methods and a much simpler and more comfortable testing procedure for the patient.
3 EEG components of interest for current swallowing studies
EEG is a time domain acquisition modality that records neural electrical activation along the interface of the scalp (Niedermeyer and da Silva, 2005) with electrodes positioned according to the 10–20 international electrode system (Jasper, 1958). As a noninvasive and affordable technique, EEG is broadly used in clinics for diagnosing and investigating a wide range of diseases as well as in research (Mormann et al., 2000; Kupfer et al., 1978; Jordan, 1993; Powner, 1976; Young, 2000).
3.1 Pre-processing of the EEG
Before analyzing the EEG during swallowing to extract EEG components of interest, it is desirable to first pre-process the EEG. Standard processing of the EEG signals includes resampling, filtering, and artifact removal. EEG recordings produce waveforms which, besides components of interest, also contain noise. Studies showed that very high frequencies represent only noise without any useful information and should be removed through filtering (Sullivan et al., 2007).
During EEG recordings, a device provides conversion of the analog signal into a digital time series representation. The sampling rate of the data is specified before recording. According to the Nyquist criterion (Araki and Yamamoto, 1986), the minimum sampling frequency should be greater than twice the maximum frequency which is expected in EEG signals. This implies that in order to capture very high frequency information we should also choose a very high sampling frequency. The filtered EEG can be downsampled to reduce storage space. Previous EEG swallowing studies utilized a sampling frequency between 200 Hz to 512 Hz (Mizoguchi et al., 2002; Ohla et al., 2009, 2010; Franken et al., 2011; Hummel et al., 2010; Singh et al., 2011; Yoshida et al., 2003; Satow et al., 2004; Hiraoka, 2004; Yang et al., 2014). In the next step, raw signals are usually band-pass filtered. For the lower bound of the band-pass filter, previous studies used a frequency range of 0 to 0.35 Hz, while for the upper bound a frequency range of 30 to 100 Hz was used (Mizoguchi et al., 2002; Ohla et al., 2009, 2010; Franken et al., 2011; Hummel et al., 2010; Singh et al., 2011; Yoshida et al., 2003; Satow et al., 2004; Hiraoka, 2004).
EEG signals are also very sensitive to artifacts. The most common artifacts are due to eyeblinks, eye-movements, muscle activity, or some other external factors. Artifacts are undesired signals that may introduce changes in the EEG measurements and affect the signal of interest (Urigüen and Garcia-Zapirain, 2015). Even though artifacts can affect EEG signals, most of the past EEG swallowing studies did not consider artifacts removal. However, some of them removed artifacts caused by eye movement (Mizoguchi et al., 2002; Singh et al., 2011; Huckabee et al., 2003).
3.2 Traditional EEG analysis
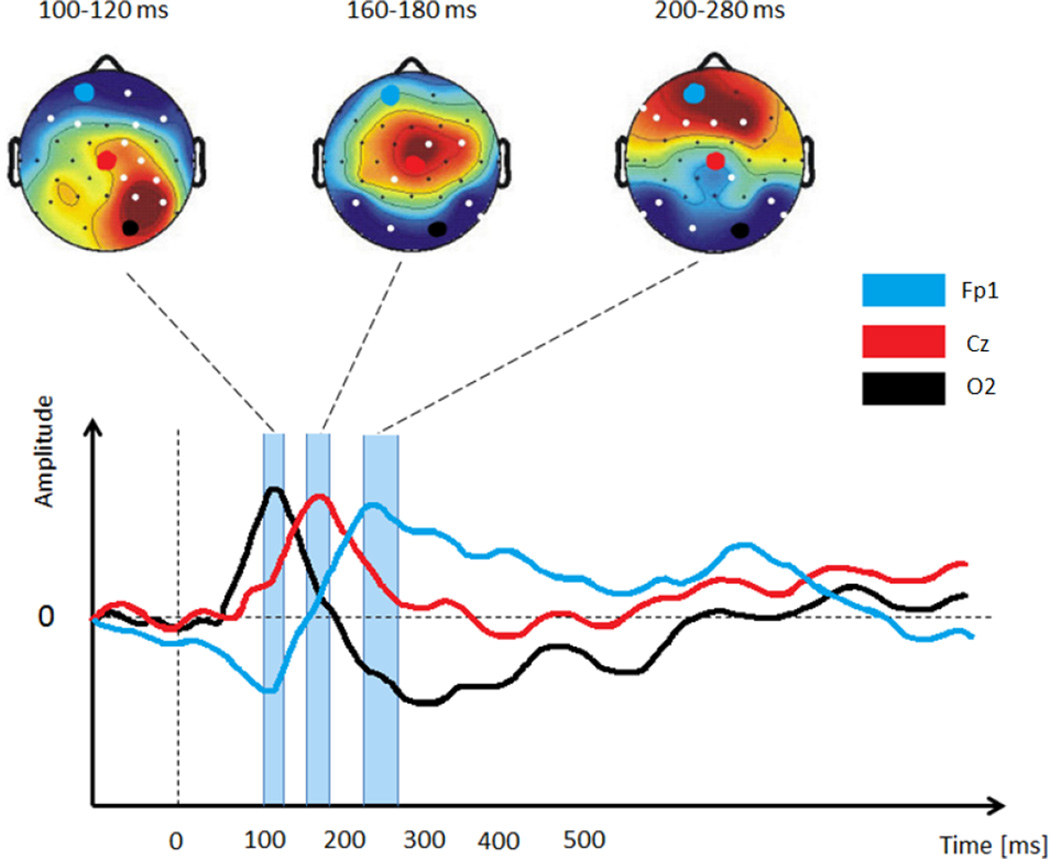
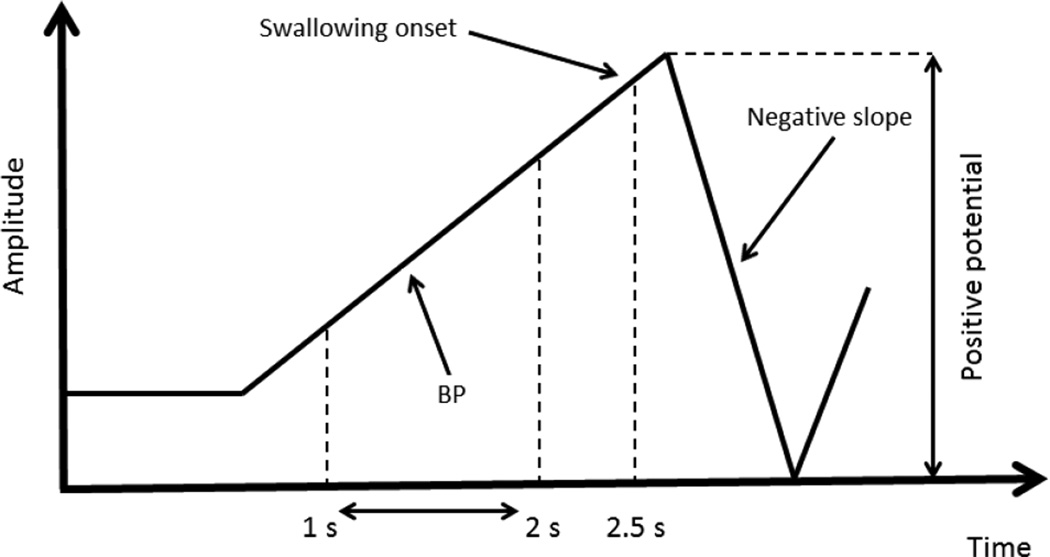
EEG studies which investigated swallowing activity and swallowing components related to sensation were interested in characterizing the changes in the frequency bands of interest between different conditions, as well as defining the brain regions involved in swallowing. The EEG power spectrum is controlled by the brain’s homeostatic system, which produces rhythmic electrical activity of various frequencies through the actions of neurons and the neurotransmitters (Llinás, 1988; Da Silva, 1996; McCormick, 1992). In literature, frequencies of the waveforms that EEG signals produce can be divided into different frequency bands: Delta (up to 4 Hz), Theta (4 – 8 Hz), Alpha (8 – 16 Hz), Beta (16 – 32 Hz), and Gamma (higher than 32 Hz) (Michel et al., 1992). For defining the brain regions involved in swallowing, previous studies investigated event related potentials (ERP), Bereitschafts potentials (BP) and movement-related cortical potentials (MRCP). ERP are positive and negative voltage deflections, which are observed as positive and negative changes or excursions in the waveform. ERP waveforms are very often investigated in EEG studies. ERP is made of averaged ongoing EEG signals which are time-locked to the response event, and provide information about the discharge pattern of large populations of neurons related to some cognitive or sensory-motor process (Kutas and Federmeier, 2000). For better understanding we illustrated an example of the observed ERP in Figure 2. BP and MRCP are characteristic features of EEG signals which describe the cortical activity associated with motor responses. BP is observed as the negative potential which occurs 1 – 2 seconds before a voluntary motor action, and its amplitude depends on the motor demands of the task. The MRCP contains two components: the BP and the negative slope (Figure 3). The contingent negative variation (CNV) is a negative cortical potential with a slow drop, which occurs between two successively instructed tasks (Walter et al., 1964; Rektor et al., 2005). CNV and MRCP have functional differences in the cognitive process that occurs at the beginning of movement.
Figure 2.
An illustrative sample of ERP activation across different time ranges/windows (i.e., the three vertical rectangular boxes shown exist between roughly 100–300 ms). Each ERP activation respectively captures the peak of one of the three illustrated EEG signals. As ERP are quick voltages which are generated in the cerebral cortex due to some event or swallowing-related stimulus, they are very useful for identifying brain regions involved in the summation of fast and short duration events such as sensory stimulation (e.g. taste and smell).
Figure 3.
An illustrative sample of MRCP contains two components: Bereishafts potential (BP) which occurs 1.5s before swallowing onset, and negative slope which occurs after swallowing onset. Besides identifying brain regions involved in swallowing, MRCP allows distinguishing between cortical motor preparation (BP), cortical control of swallowing execution (swallowing onset), and cortical swallowing regulation (negative slope).
3.3 Advanced EEG analysis
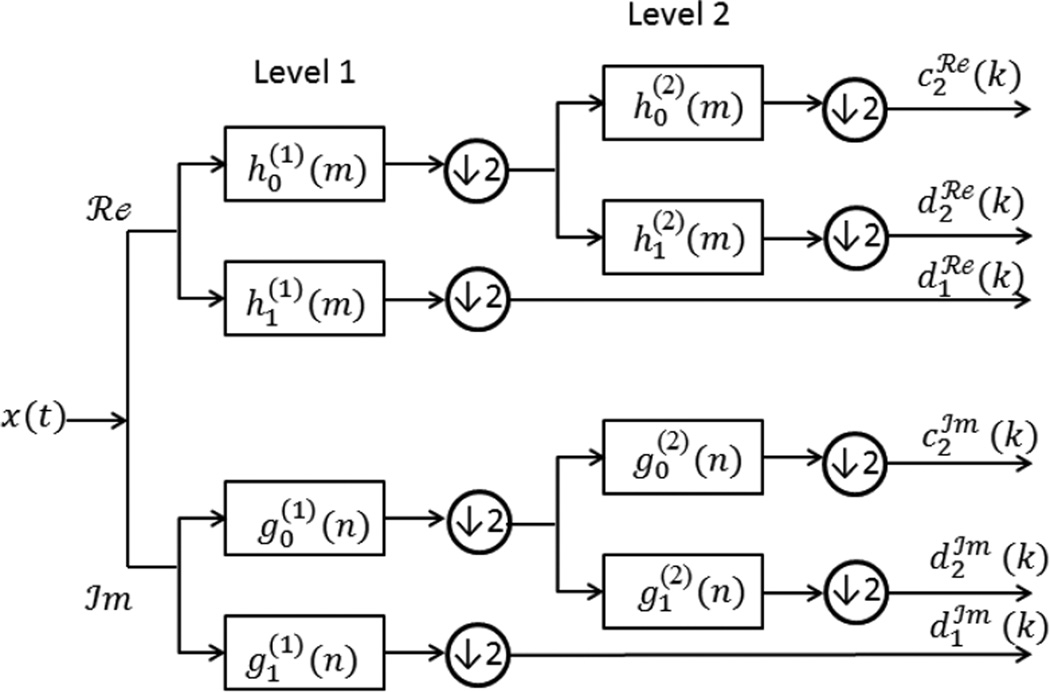
EEG features such as the frequency band of interest, ERP, BP, and MRCP describe the shape of the EEG waveform in the time domain. However, detection of certain events within the EEG signals can be conducted by extracting mathematical features that describe the signal’s behavior in the frequency domain. Several studies investigated swallowing motor imagery detection and classification of the EEG signals. They extracted features based on the dual-tree complex wavelet transform (Selesnick, 2004; Yang et al., 2013, 2012). The dual-tree complex wavelet transform is a shift invariant version of the standard wavelet transform and is directionally selective in the higher dimensions (Selesnick et al., 2005). The dual-tree of the decomposition is implemented such that two parallel discrete wavelet transforms use low-pass and high-pass filters on each scale (Figure 4). In the first level, wavelets pair and are one sample offset from the wavelets pair and . In the second level, each wavelet pair forms an approximate Hilbert transform pair. Studies showed that signals decomposed with the dual-tree complex wavelet transform provided distinctive signal characteristics (e.g., mean, variance, skewness, kurtosis, power energy of the coefficients of the dual-tree complex wavelet transform, the phase information at each level and direction of the dual-tree complex wavelet transform‘, the coarse representation of the coefficients, etc.) that enable motor imagery of swallowing detection. Swallowing motor imagery detection from the decomposed signals can be obtained by applying some of the classification methods.
Figure 4.
An illustrative scheme of the dual-tree complex wavelet transform. x(t) is an input signal. The first pair of wavelets (Level 1) are offset from each others by one half. Another pair of wavelets (Level 2) are forming an approximate Hilbert transform pair.
Common spatial pattern algorithm method has already been shown as useful in human-to-computer interface design studies (Lotte and Guan, 2011; Grosse-Wentrup and Buss, 2008; Townsend et al., 2006). This algorithm provides an optimal decomposition which enables transform of different data classes into a common space. Applied on the data sets, the common spatial pattern algorithm finds the direction where features of different classes should be projected on the plane such that differences between classes are maximized (Ge et al., 2014). In EEG studies related to swallowing motor imagery, common spatial pattern algorithm has been used for detection of tongue movement motor imagery (Naeem et al., 2006; Ang et al., 2012; Ge et al., 2014).
4 EEG studies related to swallowing
4.1 Smell
Previous studies reported that EEG signals are sensitive to changes in odor, suggesting that EEG is suitable for investigating and analyzing brain activity during olfaction (Lorig, 1989; Lorig and Schwartz, 1988a; Klemm et al., 1992). Most of the olfactory-based EEG studies reported changes in signals with respect to different stimuli. The observed changes in EEG signals were not ubiquitous across all of the EEG olfactory studies, however, due to differences in investigation methodologies. Moncrieff published one of the earliest studies on this topic (Moncrieff, 1962). Moncrieff used five subjects with eight bilaterally placed electrodes on each subject. The results of his study showed changes in the alpha band of the signals for almost all stimuli. Moncrieff used anachronistic EEG recording procedures and a large number of stimuli. Also, the experimental conditions were not very well controlled, which accounts for the differing results observed in recent studies.
Even though recent studies have improved the standards for experimental control, differing results exist among reported studies. Lorig and Schwartz (1988b) and Klemm et al. (1992) found no changes in the alpha band activity, while Lorig et al. (1991) later reported a reduction in the alpha band power for different stimuli. Other studies also reported conflicting results, such as both a reduction (Stacher et al., 1979; Lorig and Schwartz, 1988a) and an increase (Klemm et al., 1992) of the theta activity for differing odor stimuli. In one portion of their study, Lorig and Schwartz (1988b) found that for different stimuli, alpha and theta activity exhibited changes along different brain hemispheres. Reasons for these observed differences could include environmental conditions during recording sessions, the specific odor stimuli which were used, the number of electrodes, and that the recording duration all differed among studies. One study, by Martin (1998), investigated the influence of food’s odor on the EEG signals. Martin found that theta activity was significantly reduced for chocolate stimuli in comparison with either almond and cumin stimuli. He also found similar results (i.e., reduced theta activity) for spearmint when compared with an the control (i.e., no odor). These results could be attributed to the psychological experience of the pleasant odor property. The results of these studies are summarized in Table 1.
Table 1.
Summary of published studies which investigated the human response to odor using EEG.
| Study | Number of subjects | Stimuli | Electrodes | Findings |
|---|---|---|---|---|
| Moncrieff (1962) | 5 | Floral perfume, alcohol, lavender, methyl antrhrantilate, lemon-grass oil, cinnamon, citral, pyridine, ammonium sulphide | 4 on the left side and 4 on the right side | Changes in alpha band activity |
| Stacher et al. (1979) | 16 | Chives placed on bread and butter, frying butter, eggs, bacon | F7, F8, T5, T6 | Reduction in theta band activity |
| Loring et al. (1988) - first part | 13 | Spiced apple, eucalyptus, lavender | Cz | Reduction in theta band activity |
| Loring et al. (1988) - second part | 10 | Five fragrances diluted with distilled water | F7, F8, T5, T6 | Increase or decrease of alpha and theta activity along different hemisphere |
| Loring et al (1991) | 16 | Different concentrations of spiced apple, lavender oil | F7, F8, T5, T6 | Reduction in alpha band activity |
| Klemm et al. (1992) | 16 | Birch tar, galbanum, jasmine, heliotropine, lavender, lemon, peppermint, room-air | FP1, FP2, F3, F4, F7, F8, C3, C4, T3, T4, T5, T6, P3, P4, O1, O2, Fz, Cz, Pz | Increase in theta band activity |
| Stacher et al. (1998) - first part | 21 | Chocolate, spearmint, almond, strawberry, vegetable, garlic, onion, cumin | FP1, FP2, F3, F4, F7, F8, C3, C4, T3, T4, T5, T6, P3, P4, O1, O2, Fz, Cz, Pz | Reduction in theta band activity |
4.2 Taste and texture
Recent EEG studies which investigated the gustatory brain waveform concentrated on analyzing the ERP. The first positive (P1) gustatory ERP peak for a salt stimulus was reported by Mizoguchi et al. (2002) and Wada (2005), and the P1 gustatory ERP peak for electric taste (applying an electrical current to the participants tongue) was reported by Ohla et al. (2009, 2010). Each of these studies reported the P1 gustatory ERP peak as latencies spanning between 130 and 150 ms. In these studies, the P1 deflection was higher for frontal electrodes, where this deflection is assumed to have origins in the insula, the middle temporal gyrus, and the anterior cingulate cortex (Ohla et al., 2010). The fist negative (N1) deflection with a latency time of 200 ms was reported by Ohla et al. (2009, 2010) for an electric taste stimuli in the regions of the cranial vertex and bilateral insula. Mizoguchi et al. (2002) reported the N1 deflection for salt at a latency of 256 ms in the region of the cranial vertex. Early ERP studies which focused on ERP deflection (i.e., Min and Sakamoto (1998), and Franken et al. (2011)) were hindered by the gustatory and tactile stimulation, which influenced their interpretation of the reported results. These studies were not concentrated on brain regions where deflection occurred, but rather on the difference in potential between different tastes. It was found that sweetness in stimuli influenced the evoked potential. P1 deflection at latencies higher than 500 ms were reported by Funakoshi and Kawamura (1971), Kobal (1985), Plattig et al. (1988), Hummel et al. (2010), and Singh et al. (2011). However, it is not known if these late peaks have origins from the same process that produces early peaks, nor is it known if their origins are due to variations in the administration of different stimuli. The results of these studies are summarized in Table 2.
Table 2.
Summary of published studies which investigated the human response to taste using EEG.
| Study | Number of subjects | Stimuli | Electrodes | Findings |
|---|---|---|---|---|
| Kobal (1985) | 5 | Acetic acid | 12 electrodes | P300, N410, P660, N860 |
| Plattig et al. (1988) | 21 | NaCl, Tartaric acid, Sucrose, Quinine HCl, Water | Cz | N1000, P2300 for NaCl; P2300 for Tartaric acid; N2300 for Quinine HCl |
| Min and Sakamoto (1998) | 10 | NaCl, sucrose, tartaric acid, quinine HCl, artificial saliva | Cz | P50, P180 |
| Mizoguchi et al. (2002) | 5 | NaCl | Fz, Cz, Pz, T3, T4 | P127, N263, P432 |
| Wada (2005) | 11 | Glucose, NaCl, artificial saliva | Cz | P72197 for glucise, P84188 for Nacl |
| Olha et al. (2009) | 17 | 11.5 µA electrogustatory stimuli | 64 electrodes | P130, N220, P390 |
| Olha et al. (2010) | 17 | 11.5 µA and 360.5 µA electrogustatory stimuli | 64 electrodes | P134, N219, P390 for 11.5 µA; P124, N186, P347 for 360.5 µA |
| Hummel et al. (2010) | 17 | 70% acetic acid, 100% acetic acid | Cz, Fz, Pz | N390, P601 for 100% acetic acid |
| Singh et al. (2011) | 17 | NaCl, MSG | Fz, Cz, Pz, C3, C4 | N506, P718 for NaCl |
4.3 Cortical pre-motor activation in swallowing
The supplementary motor area is not frequently the focus of swallowing analysis, but it is known that the supplementary motor area is involved in the planning and initiation of the voluntary movement phases of swallowing (Parent, 1996). Knowing this leads us to the investigation of MRCP in the case of volitional swallowing. This investigation could provide information about sequential cerebral processing, and it could enable us to distinguish between cortical motor preparation, cortical control of swallowing execution, and cortical swallowing regulation. However, Yoshida et al. (2003) showed the importance of MRCP in the diagnosis of oral motor dysfunction, and their results could be instrumental in the treatment of dysphagia. CNV gives information about cognitive functions in the case of swallowing activities initiated by a command, and together with MRCP, CNV offers important information about brain activity.
In the analysis of MRCP, it was assumed that the MRCP can provide information about cortical motor planning. Similar assumptions were used in the case of analyzing volitional activity such as finger movement. Huckabee et al. (2003) was the first to use the EEG technique in a swallowing study. They investigated the role of the cerebral cortex in the motor planning of swallowing as well as the initiation of swallowing. They found the BP before the onset of volitional swallowing at the supplementary motor cortex. The same study by Huckabee et al. also claimed that, contrary to other volitional activities, the primary motor cortex is not involved in the volitional swallowing task. Satow et al. (2004) later reported BP activity in both hemispheres (i.e., the central area of the cranial vertex with additional involvement of the cerebral cortex) during a swallowing activity. They found that the role of the cerebral cortex during a swallowing activity is very similar to its role involving tongue movement. These differing results between Huckabee et al. and Satow et al. could be attributed to the choice of the stimuli which were used in the two studies. Huckabee investigated dry swallowing (i.e., saliva swallowing), while Satow used water as a stimulus. Hiraoka (2004) later documented differences in the cortical activation during saliva and water swallows. He showed that the amplitude of the positive potential is significantly higher for water swallows compared with those for saliva swallows. This can be attributed to the bolus size used in this study. Here it was assumed that the saliva bolus was smaller than the water bolus. These results signify that information registered with different types of receptors in the oral cavity and oropharynx activate different cortical processes, where the particular activated cortical process depends on the type of information registered by the receptors. With these results, he claimed that the cortical preparatory process greatly depends on the type of the swallowing task.
Satow also found the BP in the case of swallowing and tongue movement. There was not a difference in the BP’s amplitude between these two tasks. However, in the case of swallowing activities, the BP occurred earlier than in the case of tongue movement. Several years after Satow, Nonaka et al. (2009) investigated brain activity in the case of volitional and command swallowing. He compared CNV waveforms in the case of the command swallowing task with the MRCP in the case of the volitional swallowing task. He found that the CNV, in the case of the command swallowing task, had both larger amplitude and longer duration compared with the volitional swallowing task. This finding was observed from the Cz electrode signal which reflects the activity of the supplementary motor area. This means that supplementary motor area activation starts earlier and has greater activation in the case of complex motor tasks. The results of these studies are summarized in Table 3.
Table 3.
Summary of published studies which investigated the MRCP during swallowing using EEG.
| Study | Number of subjects | Stimuli | Electrodes | Findings |
|---|---|---|---|---|
| Huckabee et al. (2003) | 20 | Saliva swallowing | Cz, FCz, FC1z, FC2z | BP at the supplementary motor cortex |
| Satow et al. (2004) | 8 | Water swallowing | Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2 | BP in both hemi-spheres at the central area of the cranial vertex; Activation of cerebral cortex |
| Hiraoka (2004) | 7 | Saliva swallowing, water swallowing | C3, Cz, C4 | higher positive potential amplitude for water swallowing compared with saliva swallowing |
| Nonaka et al. (2009) | 10 | Command saliva swallowing, volitional saliva swallowing | Fz, Cz, Pz, C3, C4 | higher amplitude and longer duration of the CNV for the command swallowing task compared to the MRCP for volitional swallowing task |
4.4 Motor imagery
Motor imagery involves envisioning certain motor activity with the absence of the actual activation. Morash et al. (2008) showed that EEG signals can detect the imagery action of hand, foot, and tongue movements. Previous studies showed that the Fugl-Meyer assessment of motor recovery after a stroke is significantly higher with patients who participated in the motor imagery rehabilitation approach (Ang et al., 2014a). Since motor imagery cortical activity can be detected with the EEG, integration of the EEG with motor imagery has been shown as a promising human-to-computer interface approach, which can benefit stroke rehabilitation (Ang et al., 2014b; Morone et al., 2015). This benefit led to the conclusion that motor imagery can be used during the dysphagia rehabilitation after stroke or other brain injury (Faralli et al., 2013; Kober and Wood, 2014). Current studies related to EEG motor imagery of swallowing concentrate on the detection of the motor imagery of swallows and tongue movements. Naeem et al. (2006) investigated the advantages of common spatial patterns of pre-processing algorithms over the ICA pre-processing algorithm for motor imagery detection of the tongue movement. Their study showed that signals pre-processed with common spatial patterns provide better classification accuracy (i.e., between 33% and 84%) than the signals pre-processed with the ICA algorithm. Ang et al. (2012) used the filter bank common spatial pattern algorithm in order to detect the EEG motor imagery of tongue movement. Filter bank common spatial pattern algorithm showed a cross-validation classification accuracy of 90.3%. Ge et al. (2014) also used the common spatial pattern algorithm for detection of the EEG motor imagery of tongue movement. They investigated if motor imagery of the tongue movement can be detected with only one EEG electrode. Their study showed that motor imagery can be detected using a single EEG channel from the forehead area.
In their first study related to detection of the EEG swallowing motor imagery, Yang et al. (2012), introduced a novel approach for feature extraction based on the dual-tree complex wavelet transform. They documented that an approach based on dual-tree complex wavelet transform has a 9.95% higher detection accuracy of swallowing motor imagery than other existing methods. Later, Yang et al. (2013) introduced a method based on model adaptation for swallowing motor imagery detection. This method achieved an average accuracy of 74.29% and 72.64%. Furthermore, Yang et al. (2014) used feature extraction based on the DTCWT of the EEG signals in order to perform detection of motor imagery of swallows. Their results showed a cross-validation classification accuracy of 70.89%. Though the efficacy of this method in rehabilitating impaired swallowing was not evaluated in this study, this method may warrant further research to determine its potential as a dysphagia rehabilitation modality. The results of these studies are summarized in Table 4.
Table 4.
Summary of published studies which were concentrate on detection of motor imagery of swallows and motor imagery of tongue movements using EEG.
| Study | Number of subjects | Stimuli | Used Method | Findings |
|---|---|---|---|---|
| Naeem et al. (2006) | 8 | Common spatial patterns | 22 | electrodes Classification accuracy between 33% and 84% |
| Ang et al. (2012) | 9 | Filter bank common spatial pattern algorithm | 22 electrodes | Classification accuracy of 90.3% |
| Yang et al. (2012) | 9 | Dual-tree complex wavelet transform | 34 electrodes | Dual-tree complex wavelet transform based approach showed 9.95% higher accuracy than existing methods |
| Yang et al. (2013) | 10 | Model adaptation | 34 electrodes | classification accuracy of 74.29% and 72.64% |
| Ge et al. (2014) | 3 | Common spatial patterns | C3, Cz, C4, Fp1, Fpz, Fp2, | Motor imagery can be detected using single channel from the forehead area |
| Yang et al. (2014) | 10 | Dual-tree complex wavelet transform | 34 electrodes | classification accuracy of 70.89% |
5 Remarks and future perspectives
This paper provides an overview of findings from the neurology and neurology-related fields where the EEG technique was used. This paper discussed past and present findings which have been collected, compared, and expounded upon to give new directions for future swallowing studies. Moving swallowing investigations toward uncharted and novel directions will hopefully lead to an improvement in diagnosing dysphagia and its rehabilitation.
According to the Tables 1, 2, 3, and 4, previous studies mostly involved low number of EEG electrodes for investigating the brain activity during swallowing and swallowing motor imagery. Because previous imaging studies showed that swallowing activated many cortical regions (Hamdy et al., 1999a,b; Martin et al., 2001; Kern et al., 2001a; Mosier and Bereznaya, 2001; Zald and Pardo, 1999), recording neural activation from all of the activated cortical regions associated with swallowing would not be possible with such a design. In the future, the impact of electrode population size should be considered in the designs of swallowing studies with EEG.
In the case of dysphagia, patients usually have to adjust their diet according to a speech language pathologist’s recommendation. Very often patients with a swallowing disorder have difficulties swallowing thin liquids such as water or juice (O’Gara, 1990). In order to manage this problem and to help the patient perform safe swallowing, consuming thicker fluids is sometimes recommended. In fact, these thicker fluids were initially developed specifically for people suffering from dysphagia. Thicker fluids flow more slowly and, because they adhere to aerodigestive mucosa, the bolus transit time is increased. This compensates for short delays in airway closure during the pharyngeal phase and increases overall airway protection. The influence of viscosity on swallowing characteristics was investigated by numerous groups using a variety of techniques (Jestrović et al., 2013; Clavé et al., 2006; Steele and Van Lieshout, 2004). However, it was shown that the cortical activation observed using EEG is different when comparing saliva and water swallowing (Hiraoka, 2004). Future investigations should concentrate on the analysis of the EEG signals during consumption of thicker liquids, as well as solid boluses. These investigations could provide a better understanding as to how texture modification provides short-term benefit to dysphagic patients.
The difference between command and non-command swallowing of healthy people (Nonaka et al., 2009) explains how the processing of language and the awareness of the swallowing act may be important for performing the swallowing activity. This means that swallowing safety, in the case of dysphagia, may be highly influenced by manipulation of the linguistic system (Daniels, 2000; Nonaka et al., 2009; Nagy et al., 2013). Because CNV and MRCP describe differences between non-command and command swallowing, these parameters may provide a metric to assess a person’s swallowing ability. Therefore, it would be informative to investigate the CNV and MRCP with dysphagic subjects during both non-command and command swallowing.
One of the most challenging problems inherent in the analysis of acquired central neural swallowing data is difficulty determining when each of the swallowing phases begins and ends, and if the swallowing is healthy or unhealthy. Unlike gold standards for investigation the brain activity (i.e., fMRI and PET), EEG is not sensitive to any nearby metal objects (e.g., implants), so it can be easily combined with other testing techniques that deploy instrumentation containing metal components such as imaging technology (fiberoptic endoscopic evaluation of swallowing, videofluoroscopy (VFSS)). Concurrent imaging together with EEG sampling during swallowing could provide more information about swallowing that EEG alone cannot obtain, (e.g. swallowing start and stop points) and that can be compared to the EEG signals (i.e., if swallowing is healthy or unhealthy, etc.), and could be important for future developments in the field. Incorporating EEG together with a videofluoroscopic swallowing study (Logemann, 1993; Tabaee et al., 2006) could also provide the chance to investigate the relationships between neural activity and the biomechanics of oropharyngeal swallowing phases. In the case of dysphagia, aspiration often occurs during the pharyngeal phase. By performing a simultaneous recording on patients with both EEG and VFSS, a speech language pathologist can demarcate the swallowing phase segment duration using the VFSS and correlate EEG signals to physiological events. Analysis of segmented signals would enable a better understanding of brain activation during each swallowing phase. Finally, single treatment modalities do not provide a complete set of information regarding the effects of treatment because treatments are often combined. However, individual treatment modalities are designed to address specific impairments in swallow physiology and are then combined with other treatments that address other impairments. Understanding the effects of each component of a combined treatment program on the EEG signal will elucidate whether and how each modality may contribute to treatment-induced cerebral plasticity.
The ability to combine EEG with other techniques could be used for a number of different applications and may provide better insights into the swallowing function. For example, it could be beneficial to combine EEG with pharyngeal neuromuscular stimulation. Neuromuscular electrical stimulation activates muscles and peripheral motor nerves and may be able to recover normal swallowing control by strengthening the muscles that were weakened by a stroke or other neurological condition, though the evidence supporting its efficacy is mixed. A number of studies showed advantages of the neuromuscular electrical stimulation (NMES) as a safe and effective treatment which provides better swallowing function (Freed et al., 2001; Ludlow et al., 2007; Leelamanit et al., 2002; Blumenfeld et al., 2006; Kiger et al., 2006). However, the majority of these studies were poorly controlled and tainted by design flaws that rendered their results equivocal at best. In a meta-analysis published by Carnaby-Mann and Crary (2007), 81 studies of NMES for dysphagia treatment were evaluated. 74 of these studies were rejected from the analysis due to poor evidence quality, and the remaining 7 studies were significantly heterogeneous. Although a small but significant effect size was measured, the authors cautioned: “Because of the small number of studies and low methodological grading for these studies, caution should be taken in interpreting this finding.” Hence, the use of EEG to monitor brain activity during neuromuscular electrical stimulation and other dysphagia treatments warrants significant further investigation to determine whether it may be a worthwhile addition to the treatment armamentatium Further, EEG can also be combined with some other commonly used techniques for screening or diagnosis of dysphagia such as fiberoptic endoscopic evaluation, or with some techniques which are still under development such as cervical auscultation. Combining EEG with other techniques could provide additional information about swallowing which may provide deeper insight into brain activity during swallowing.
Since the motor imagery of swallowing may evolve into an adjunct to dysphagia rehabilitation, it would be highly desirable to evaluate the efficacy of this approach in combination with EEG in future swallowing investigations. This could eventually lead to the development of advanced human-to-computer interface based applications. A previous study produced a 70.89% classification accuracy of swallowing-imagery detection using the dual-tree complex wavelet transform feature. Better accuracy could be achieved by employing more features, or perhaps using an alternative analysis technique such as graph theory or signal processing on graph. However, translation of this result to improved swallowing function in humans with dysphagia needs to be established to advance the clinical utility of this method.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute Of Child Health & Human Development of the National Institutes of Health under Award Number R01HD074819. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Iva Jestrović, Email: ivj2@pitt.edu.
James L. Coyle, Email: jcoyle@pitt.edu.
Ervin Sejdić, Email: esejdic@ieee.org.
References
- Abe S, Wantanabe Y, Shintani M, Tazaki M, Takahashi M, Yamane G, et al. Magnetoencephalographic study of the starting point of voluntary swallowing. Cranio. 2003;21(1):46–49. doi: 10.1080/08869634.2003.11746231. [DOI] [PubMed] [Google Scholar]
- Ang KK, Chin ZY, Wang C, Guan C, Zhang H. Filter bank common spatial pattern algorithm on BCI competition IV datasets 2a and 2b. Frontiers in Neuroscience. 2012;6(39) doi: 10.3389/fnins.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK, Chua KSG, Phua KS, Wang C, Chin ZY, Kuah CWK, Low W, Guan C. A randomized controlled trial of EEG-based motor imagery brain-computer interface robotic rehabilitation for stroke. Clinical EEG and Neuroscience. 2014a:1–11. doi: 10.1177/1550059414522229. [DOI] [PubMed] [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhou L, Tang KY, Joseph GJE, Kuah CWK, Chua KSG. Brain-computer interface-based robotic end effector system for wrist and hand rehabilitation: results of a three-armed randomized controlled trial for chronic stroke. Frontiers in Neuroengineering. 2014b;7(30) doi: 10.3389/fneng.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki M, Yamamoto K. Multivariable multirate sampled-data systems: state-space description, transfer characteristics, and nyquist criterion. IEEE Transactions on Automatic Control. 1986;31(2):145–154. [Google Scholar]
- Blumenfeld L, Hahn Y, LePage A, Leonard R, Belafsky PC. Transcutaneous electrical stimulation versus traditional dysphagia therapy: a nonconcurrent cohort study. Otolaryngology–Head and Neck Surgery. 2006;135(5):754–757. doi: 10.1016/j.otohns.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Carnaby-Mann GD, Crary MA. Examining the evidence on neuromuscular electrical stimulation for swallowing: a meta-analysis. Archives of Otolaryngology–Head & Neck Surgery. 2007;133(6):564–571. doi: 10.1001/archotol.133.6.564. [DOI] [PubMed] [Google Scholar]
- Cichero JA, Murdoch BE. Dysphagia: foundation, theory and practice. New York City, NY: John Wiley & Sons; 2006. [Google Scholar]
- Clavé P, De Kraa M, Arreola V, Girvent M, Farré R, Palomera E, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Alimentary Pharmacology & Therapeutics. 2006;24(9):1385–1394. doi: 10.1111/j.1365-2036.2006.03118.x. [DOI] [PubMed] [Google Scholar]
- Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. Journal of Clinical Oncology. 1998;16(10):3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- Curran J, Groher ME. Development and dissemination of an aspiration risk reduction diet. Dysphagia. 1990;5(1):6–12. doi: 10.1007/BF02407388. [DOI] [PubMed] [Google Scholar]
- Da Silva FL. Comprehensive human physiology. Springer; 1996. The generation of electric and magnetic signals of the brain by local networks; pp. 509–531. [Google Scholar]
- Daniels SK. Swallowing apraxia: A disorder of the praxis system? Dysphagia. 2000;15(3):159–166. doi: 10.1007/s004550010019. [DOI] [PubMed] [Google Scholar]
- Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia. 1997;12(3):146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Bolser DC, Morris KF. Swallow remodeling of respiratory neural networks. Head & Neck. 2011;33(1):8–13. doi: 10.1002/hed.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Huckabee M-L. Swallowing neurorehabilitation: from the research laboratory to routine clinical application. Archives of Physical Medicine and Rehabilitation. 2012;93(2):207–213. doi: 10.1016/j.apmr.2011.08.030. [DOI] [PubMed] [Google Scholar]
- Drake W, O’Donoghue S, Bartram C, Lindsay J, Greenwood R. Case study eating in side lying facilitates rehabilitation in neurogenic dysphagia. Brain Injury. 1997;11(2):137–142. doi: 10.1080/026990597123737. [DOI] [PubMed] [Google Scholar]
- Ertekin C, Aydogdu I, et al. Neurophysiology of swallowing. Clinical Neurophysiology. 2003;114(12):2226–2244. doi: 10.1016/s1388-2457(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Faralli A, Bigoni M, Mauro A, Rossi F, Carulli D. Noninvasive strategies to promote functional recovery after stroke. Neural Plasticity. 2013;2013 doi: 10.1155/2013/854597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. Journal of Rehabilitation Medicine. 2009;41(9):707–713. doi: 10.2340/16501977-0415. [DOI] [PubMed] [Google Scholar]
- Franken IH, Huijding J, Nijs IM, Van Strien JW. Electrophysiology of appetitive taste and appetitive taste conditioning in humans. Biological Psychology. 2011;86(3):273–278. doi: 10.1016/j.biopsycho.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Freed ML, Freed L, Chatburn RL, Christian M. Electrical stimulation for swallowing disorders caused by stroke. Respiratory care. 2001;5(46):466–474. [PubMed] [Google Scholar]
- Fukunaga A, Uematsu H, Sugimoto K. Influences of aging on taste perception and oral somatic sensation. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(1):109–113. doi: 10.1093/gerona/60.1.109. [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Kawamura Y. Summated cerebral evoked responses to taste stimuli in man. Electroencephalography and Clinical Neurophysiology. 1971;30(3):205–209. doi: 10.1016/0013-4694(71)90055-1. [DOI] [PubMed] [Google Scholar]
- Ge S, Wang R, Yu D. Classification of four-class motor imagery employing single-channel electroencephalography. PloS One. 2014;9(6):e98019. doi: 10.1371/journal.pone.0098019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D, Kipnis M, Sister E, Vardi Y, Brill S. Validation of the 50 ml3 drinking test for evaluation of post-stroke dysphagia. Disability and Rehabilitation. 1996;18(10):529–532. doi: 10.3109/09638289609166040. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Padmanabhan R, Sang Q. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. The American Journal of Medicine. 2001;111(8):95–105. doi: 10.1016/s0002-9343(01)00863-4. [DOI] [PubMed] [Google Scholar]
- Grosse-Wentrup M, Buss M. Multiclass common spatial patterns and information theoretic feature extraction. IEEE Transactions on Biomedical Engineering. 2008;55(8):1991–2000. doi: 10.1109/TBME.2008.921154. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1999a;277(1):219–225. doi: 10.1152/ajpgi.1999.277.1.G219. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Thompson DG. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clinical Science. 2000;99(2):151–157. [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H2 150 PET activation. Journal of Neurophysiology. 1999b;81(4):1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- Hartnick CJ, Rudolph C, Willging JP, Holland SK. Functional magnetic resonance imaging of the pediatric swallow: imaging the cortex and the brainstem. The Laryngoscope. 2001;111(7):1183–1191. doi: 10.1097/00005537-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Barnes G. A quantitative assessment of the sensitivity of whole-head meg to activity in the adult human cortex. Neuroimage. 2002;16(3):638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- Hiraoka K. Movement-related cortical potentials associated with saliva and water bolus swallowing. Dysphagia. 2004;19(3):155–159. doi: 10.1007/s00455-004-0002-9. [DOI] [PubMed] [Google Scholar]
- Huckabee ML, Deecke L, Cannito MP, Gould HJ, Mayr W. Cortical control mechanisms in volitional swallowing: the Bereitschaftspotential. Brain Topography. 2003;16(1):3–17. doi: 10.1023/a:1025671914949. [DOI] [PubMed] [Google Scholar]
- Hughes CV, Fox PC, Marmary Y, Chih-Ko Yeh B, Sonies BC. Oral-pharyngeal dysphagia: a common sequela of salivary gland dysfunction. Dysphagia. 1987;1(4):173–177. [Google Scholar]
- Hummel T, Genow A, Landis BN. Clinical assessment of human gustatory function using event related potentials. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81(4):459–464. doi: 10.1136/jnnp.2009.183699. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten twenty electrode system of the international federation. Electroencephalography and Clinical Neurophysiology. 1958;10(2):371–375. [PubMed] [Google Scholar]
- Jestrović I, Dudik JM, Luan B, Coyle JL, Sejdić E. The effects of increased fluid viscosity on swallowing sounds in healthy adults. Biomedical Engineering Online. 2013;12(1):90–90. doi: 10.1186/1475-925X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience intensive care unit. Journal of Clinical Neurophysiology. 1993;10(4):445–475. doi: 10.1097/00004691-199310000-00006. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2(2):148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- Katzan I, Cebul R, Husak S, Dawson N, Baker D. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, et al. Swallow-related cerebral cortical activity maps are not specific to deglutition. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001a;280(4):531–538. doi: 10.1152/ajpgi.2001.280.4.G531. [DOI] [PubMed] [Google Scholar]
- Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001b;280(3):354–360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- Kiger M, Brown CS, Watkins L. Dysphagia management: An analysis of patient outcomes using vitalstim therapy compared to traditional swallow therapy. Dysphagia. 2006;21(4):243–253. doi: 10.1007/s00455-006-9056-1. [DOI] [PubMed] [Google Scholar]
- Klemm W, Lutes S, Hendrix D, Warrenburg S. Topographical EEG maps of human responses to odors. Chemical Senses. 1992;17(3):347–361. [Google Scholar]
- Kobal G. Gustatory evoked potentials in man. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1985;62(6):449–454. doi: 10.1016/0168-5597(85)90055-3. [DOI] [PubMed] [Google Scholar]
- Kober S, Wood G. Changes in hemodynamic signals accompanying motor imagery and motor execution of swallowing: A near-infrared spectroscopy study. NeuroImage. 2014;93:1–10. doi: 10.1016/j.neuroimage.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Foster F, Coble P, McPartland R, Ulrich R. The application of EEG sleep for the differential diagnosis of affective disorders. The American Journal of Psychiatry. 1978;135(1):69–74. doi: 10.1176/ajp.135.1.69. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Lazarus C, Logemann J. Swallowing disorders in closed head trauma patients. Archives of Physical Medicine and Rehabilitatione. 1987;68(2):79–84. [PubMed] [Google Scholar]
- Leelamanit V, Limsakul C, Geater A. Synchronized electrical stimulation in treating pharyngeal dysphagia. The Laryngoscope. 2002;112(12):2204–2210. doi: 10.1097/00005537-200212000-00015. [DOI] [PubMed] [Google Scholar]
- Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Logemann JA. Evaluation and treatment of swallowing disorders. PRO-ED Inc, San Diego: College-Hill Press; 1993. [Google Scholar]
- Lorig TS. Human EEG and odor response. Progress in Neurobiology. 1989;33(5):387–398. doi: 10.1016/0301-0082(89)90007-5. [DOI] [PubMed] [Google Scholar]
- Lorig TS, Huffman E, DeMartino A, DeMarco J. The effects of low concentration odors on EEG activity and behavior. Journal of Psychophysiology. 1991;5(1):69–77. [Google Scholar]
- Lorig TS, Schwartz GE. Brain and odor: I. alteration of human EEG by odor administration. Psychobiology. 1988a;16(3):281–284. [Google Scholar]
- Lorig TS, Schwartz GE. EEG activity during relaxation and food imagery. Imagination, Cognition and Personality. 1988b;8(3):201–208. [Google Scholar]
- Lotte F, Guan C. Regularizing common spatial patterns to improve BCI designs: unified theory and new algorithms. IEEE Transactions on biomedical Engineering. 2011;58(2):355–362. doi: 10.1109/TBME.2010.2082539. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia. 2007;22(1):1–10. doi: 10.1007/s00455-006-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki GA, Johnson S, Robbins J. Functional MRI of swallowing: from neurophysiology to neuroplasticity. Head & Neck. 2011;33(1):14–20. doi: 10.1002/hed.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. CHEST Journal. 2003;124(1):328–336. doi: 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- Martin GN. Human electroencephalographic (EEG) response to olfactory stimulation: two experiments using the aroma of food. International Journal of Psychophysiology. 1998;30(3):287–302. doi: 10.1016/s0167-8760(98)00025-7. [DOI] [PubMed] [Google Scholar]
- Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiology. 2001;85(2):938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8(3):195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Michel C, Lehmann D, Henggeler B, Brandeis D. Localization of the sources of EEG delta, theta, alpha and beta frequency bands using the FFT dipole approximation. Electroencephalography and Clinical Neurophysiology. 1992;82(1):38–44. doi: 10.1016/0013-4694(92)90180-p. [DOI] [PubMed] [Google Scholar]
- Miller AJ. The neuroscientific principles of swallowing and dysphagia. San Diego, CA: Singular Publishing Group San Diego; 1999. [Google Scholar]
- Miller JL, Watkin KL. The influence of bolus volume and viscosity on anterior lingual force during the oral stage of swallowing. Dysphagia. 1996;11(2):117–124. doi: 10.1007/BF00417901. [DOI] [PubMed] [Google Scholar]
- Min BC, Sakamoto K. Influence of sweet suppressing agent on gustatory brain evoked potentials generated by taste stimuli. Journal of Physiological Anthropology. 1998;17(1):9–17. doi: 10.2114/jpa.17.9. [DOI] [PubMed] [Google Scholar]
- Mizoguchi C, Kobayakawa T, Saito S, Ogawa H. Gustatory evoked cortical activity in humans studied by simultaneous EEG and MEG recording. Chemical Senses. 2002;27(7):629–634. doi: 10.1093/chemse/27.7.629. [DOI] [PubMed] [Google Scholar]
- Moncrieff R. Effect of odours on EEG records. Perfumery Essential Oil Rec. 1962;53(1):757–760. [Google Scholar]
- Morash V, Bai O, Furlani S, Lin P, Hallett M. Classifying eeg signals preceding right hand, left hand, tongue, and right foot movements and motor imageries. Clinical Neurophysiology. 2008;119(11):2570–2578. doi: 10.1016/j.clinph.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, E Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena. 2000;144(3):358–369. [Google Scholar]
- Morone G, Pisotta I, Pichiorri F, Kleih S, Paolucci S, Molinari M, Cincotti F, Kübler A, Mattia D. Proof of principle of a brain-computer interface approach to support post-stroke arm rehabilitation in hospitalized patients: design, acceptability, and usability. Archives of Physical Medicine and Rehabilitation. 2015;96(3):71–78. doi: 10.1016/j.apmr.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Mosier K, Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Experimental Brain Research. 2001;140(3):280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier K, Patel R, Liu W-C, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. The Laryngoscope. 1999;109(9):1417–1423. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: elderly versus young persons. The American Journal of Psychology. 1991;104:161–192. [PubMed] [Google Scholar]
- Murray J. Manual of dysphagia assessment in adults. San Diego, CA: Singular; 1999. [Google Scholar]
- Naeem M, Brunner C, Leeb R, Graimann B, Pfurtscheller G. Seperability of four-class motor imagery data using independent components analysis. Journal of Neural Engineering. 2006;3(3):208. doi: 10.1088/1741-2560/3/3/003. [DOI] [PubMed] [Google Scholar]
- Nagy A, Leigh C, Hori SF, Molfenter SM, Shariff T, Steele CM. Timing differences between cued and noncued swallows in healthy young adults. Dysphagia. 2013;28(3):428–434. doi: 10.1007/s00455-013-9456-y. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Robbins J. The fluid mechanics of bolus ejection from the oral cavity. Journal of Biomechanics. 2001;34(12):1537–1544. doi: 10.1016/s0021-9290(01)00147-6. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, da Silva FHL. Electroencephalography: basic principles, clinical applications, and related fields. Philadelphia, PA: Wolters Kluwer Health; 2005. [Google Scholar]
- Nonaka T, Yoshida M, Yamaguchi T, Uchida A, Ohba H, Oka S, et al. Contingent negative variations associated with command swallowing in humans. Clinical Neurophysiology. 2009;120(10):1845–1851. doi: 10.1016/j.clinph.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford, UK: Oxford university press; 2006. [Google Scholar]
- O’Gara JA. Dietary adjustments and nutritional therapy during treatment for oralpharyngeal dysphagia. Dysphagia. 1990;4(4):209–212. doi: 10.1007/BF02407267. [DOI] [PubMed] [Google Scholar]
- Ohla K, Hudry J, le Coutre J. The cortical chronometry of electrogustatory event-related potentials. Brain Topography. 2009;22(2):73–82. doi: 10.1007/s10548-009-0076-7. [DOI] [PubMed] [Google Scholar]
- Ohla K, Toepel U, le Coutre J, Hudry J. Electrical neuroimaging reveals intensity-dependent activation of human cortical gustatory and somatosensory areas by electric taste. Biological Psychology. 2010;85(3):446–455. doi: 10.1016/j.biopsycho.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Parent A. Carpenters Human Neuroanatomy. Baltimore, MD: Williams and Wilkins; 1996. [Google Scholar]
- Pelletier CA, Lawless HT. Effect of citric acid and citric acid–sucrose mixtures on swallowing in neurogenic oropharyngeal dysphagia. Dysphagia. 2003;18(4):231–241. doi: 10.1007/s00455-003-0013-y. [DOI] [PubMed] [Google Scholar]
- Plattig KH, Dazert S, Maeyama T. A new gustometer for computer evaluation of taste responses in men and animals. Acta Oto-Laryngologica. 1988;105(458):123–128. doi: 10.3109/00016488809125115. [DOI] [PubMed] [Google Scholar]
- Powner DJ. Drug-associated isoelectric EEGs: a hazard in brain-death certification. The Journal of American Medical Association. 1976;236(10):1123–1123. [PubMed] [Google Scholar]
- Rektor I, Bareš M, Brázdil M, Kaňovskỳ P, Rektorová I, Sochurková D, et al. Cognitive-and movement-related potentials recorded in the human basal ganglia. Movement Disorders. 2005;20(5):562–568. doi: 10.1002/mds.20368. [DOI] [PubMed] [Google Scholar]
- Robbins J, Butler SG, Daniels SK, Gross RD, Langmore S, Lazarus CL, et al. Swallowing and dysphagia rehabilitation: translating principles of neural plasticity into clinically oriented evidence. Journal of Speech, Language, and Hearing Research. 2008;51(1):276–300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- Rogers B, Arvedson J, Buck G, Smart P, Msall M. Characteristics of dysphagia in children with cerebral palsy. Dysphagia. 1994;9(1):69–73. doi: 10.1007/BF00262762. [DOI] [PubMed] [Google Scholar]
- Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers: the patients perspective. Cancer Nursing. 2002;25(6):461–467. doi: 10.1097/00002820-200212000-00010. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Butler K, Williamon A, Cordivari C, Lees A, Rothwell J. Sensorimotor reorganization by proprioceptive training in musician’s dystonia and writer’s cramp. Neurology. 2008;70(4):304–315. doi: 10.1212/01.wnl.0000296829.66406.14. [DOI] [PubMed] [Google Scholar]
- Saint-Martin A, Petiau C, Massa R, Maquet P, Marescaux C, Hirsch E, et al. Idiopathic rolandic epilepsy with interictal facial myoclonia and oromotor deficit: a longitudinal EEG and PET study. Epilepsia. 1999;40(5):614–620. doi: 10.1111/j.1528-1157.1999.tb05564.x. [DOI] [PubMed] [Google Scholar]
- Satow T, Ikeda A, Yamamoto JI, Begum T, Thuy DHD, Matsuhashi Maa. Role of primary sensorimotor cortex and supplementary motor area in volitional swallowing: a movement-related cortical potential study. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;287(2):459–470. doi: 10.1152/ajpgi.00323.2003. [DOI] [PubMed] [Google Scholar]
- Schwartz LK, Weiffenbach JM, Valdez IH, Fox PC. Taste intensity performance in patients irradiated to the head and neck. Physiology & Behavior. 1993;53(4):671–677. doi: 10.1016/0031-9384(93)90172-c. [DOI] [PubMed] [Google Scholar]
- Selesnick IW. The double-density dual-tree DWT. IEEE Transactions on Signal Processing. 2004;52(5):1304–1314. [Google Scholar]
- Selesnick IW, Baraniuk RG, Kingsbury NC. The dual-tree complex wavelet transform. IEEE Signal Processing Magazine. 2005;22(6):123–151. [Google Scholar]
- Ship JA. The influence of aging on oral health and consequences for taste and smell. Physiology & Behavior. 1999;66(2):209–215. doi: 10.1016/s0031-9384(98)00267-4. [DOI] [PubMed] [Google Scholar]
- Singh PB, Iannilli E, Hummel T. Segregation of gustatory cortex in response to salt and umami taste studied through event-related potentials. NeuroReport. 2011;22(6):299–303. doi: 10.1097/WNR.0b013e32834601e8. [DOI] [PubMed] [Google Scholar]
- Smithard D, O’neill P, Park C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996;27(7):1200–1204. doi: 10.1161/01.str.27.7.1200. [DOI] [PubMed] [Google Scholar]
- Stacher G, Bauer H, Steinringer H. Cholecystokinin decreases appetite and activation evoked by stimuli arising from the preparation of a meal in man. Physiology & Behavior. 1979;23(2):325–331. doi: 10.1016/0031-9384(79)90374-3. [DOI] [PubMed] [Google Scholar]
- Steele CM, Van Lieshout PH. Influence of bolus consistency on lingual behaviors in sequential swallowing. Dysphagia. 2004;19(3):192–206. doi: 10.1007/s00455-004-0006-5. [DOI] [PubMed] [Google Scholar]
- Stern JM, Engel J. Atlas of EEG patterns. Netherlands: Wolters Kluwer Health, Alphen aan den Rijn; 2005. [Google Scholar]
- Stevenson RD, Allaire JH. The development of normal feeding and swallowing. Pediatric Clinics of North America. 1991;38(6):1439–1453. doi: 10.1016/s0031-3955(16)38229-3. [DOI] [PubMed] [Google Scholar]
- Sullivan TJ, Deiss SR, Cauwenberghs G. Proc. IEEE Biomedical Circuits and Systems Conference. Vol. 2007. IEEE; 2007. A low-noise, non-contact EEG/ECG sensor; pp. 154–157. [Google Scholar]
- Tabaee A, Johnson P, Gartner C, Kalwerisky K, Desloge R, Stewart M. Patient-controlled comparison of flexible endoscopic evaluation of swallowing with sensory testing FEESST and videofluoroscopy. The Laryngoscope. 2006;116(5):821–825. doi: 10.1097/01.mlg.0000214670.40604.45. [DOI] [PubMed] [Google Scholar]
- Townsend G, Graimann B, Pfurtscheller G. A comparison of common spatial patterns with complex band power features in a four-class bci experiment. IEEE Transactions on Biomedical Engineering. 2006;53(4):642–651. doi: 10.1109/TBME.2006.870237. [DOI] [PubMed] [Google Scholar]
- Urigüen JA, Garcia-Zapirain B. EEG artifact removalxstate-of-the-art and guidelines. Journal of Neural Engineering. 2015;12(3):1–23. doi: 10.1088/1741-2560/12/3/031001. [DOI] [PubMed] [Google Scholar]
- Wada M. Evoked responses to taste stimulations. The International Tinnitus Journal. 2005;11(1):43–47. [PubMed] [Google Scholar]
- Walter W, Cooper R, Aldridge V, McCallum W, Winter A. Contingent negative variation: an electric sign of sensori-motor association and expectancy in the human brain. Nature. 1964;203(1):380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wilson GN, Oliver W. Further delineation of the G syndrome: a manageable genetic cause of infantile dysphagia. Journal of Medical Genetics. 1988;25(3):157–163. doi: 10.1136/jmg.25.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Guan C, Ang KK, Wang C, Phua KS, Yin CTK, et al. 35th Annual International Conference of the IEEE, Engineering in Medicine and Biology Society (EMBC) Vol. 2013. IEEE; 2013. Feature consistency-based model adaptation in session-to-session classification: A study using motor imagery of swallow EEG signals; pp. 429–432. [DOI] [PubMed] [Google Scholar]
- Yang H, Guan C, Ang KK, Wang CC, Phua KS, Yu J. Dynamic initiation and dual-tree complex wavelet feature-based classification of motor imagery of swallow eeg signals; Neural Networks (IJCNN), The 2012 International Joint Conference on. IEEE; 2012. pp. 1–6. [Google Scholar]
- Yang H, Guan C, Chua KSG, Wang CC, Soon PK, Tang CKY, Ang KK. Detection of motor imagery of swallow eeg signals based on the dual-tree complex wavelet transform and adaptive model selection. Journal of Neural Engineering. 2014;11(3):035016. doi: 10.1088/1741-2560/11/3/035016. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kaji R, Kohara N, Murase N, Ikeda A, Shibasaki H, et al. Movement-related cortical potentials before jaw excursions in oromandibular dystonia. Movement Disorders. 2003;18(1):94–100. doi: 10.1002/mds.10296. [DOI] [PubMed] [Google Scholar]
- Young GB. The EEG in coma. Journal of Clinical Neurophysiology. 2000;17(5):473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Annals of Neurology. 1999;46(3):281–286. [PubMed] [Google Scholar]