Abstract
NOTCH regulates stem cells during normal development and stem-like cells in cancer but the roles of NOTCH in the lethal pediatric brain tumor diffuse intrinsic pontine glioma (DIPG) remain unknown. Because DIPGs express stem cell factors such as SOX2 and MYCN, we hypothesized that NOTCH activity would be critical for DIPG growth. We determined that primary DIPGs expressed high levels of NOTCH receptors, ligands, and downstream effectors. Treatment of the DIPG cell lines JHH-DIPG1 and SF7761 with the γ-secretase inhibitor MRK003 suppressed the level of the NOTCH effectors HES1, HES4, HES5, inhibited DIPG growth by 75%, and caused a 3-fold induction of apoptosis. Short hairpin RNAs targeting the canonical NOTCH pathway caused similar effects. Pre-treatment of DIPG cells with MRK003 suppressed clonogenic growth by more than 90% and enhanced the efficacy of radiation therapy. The high level of MYCN in DIPG led us to test sequential therapy with the bromodomain inhibitor JQ1 and MRK003, and we found that JQ1 and MRK003 inhibited DIPG growth and induced apoptosis. Together, these results suggest that dual targeting of NOTCH and MYCN in DIPG may be an effective therapeutic strategy in DIPG and that adding a γ-secretase inhibitor during radiation therapy may be efficacious initially or during re-irradiation.
Keywords: γ-Secretase inhibitor, Diffuse intrinsic pontine glioma, H3F3A, HES, MRK003, Pediatric brain tumor, Radiation
INTRODUCTION
Brain tumors are the most common solid tumor in children; diffuse intrinsic pontine glioma (DIPG) accounts for approximately 10% of pediatric brain malignancies (1). DIPG usually affects patients between 5 and 10 years of age (1). DIPG is an infiltrative, often high-grade (World Health Organization grade III or IV) glioma of the brainstem that is not amenable to surgical resection (2). Radiation therapy is the standard of care, and the median survival is less than 2 years (1). For the past 20 years there has been little progress in clinical trials testing new agents (3-5). Diagnosis is commonly made at the time of symptom onset based on characteristic radiographic appearance on magnetic resonance imaging; tissue biopsy for diagnosis is rarely performed (6). Although a limited archival series detected TP53 mutation as a common event in DIPG more than 20 years ago (7), only recently have a greater number of DIPG tissue specimens been collected at rapid autopsy, which has allowed more complete analysis of the genetic and epigenetic drivers of DIPGs (8-10). Some neurosurgeons have also demonstrated that stereotactic biopsy of pontine tumors in children can be safely accomplished, and this procedure, although not yet universally accepted, has also yielded tumor material for diagnostic and investigational purposes (11, 12). One of the few DIPG cell lines extant, SF7761, was derived from a pre-treatment stereotactic biopsy (13).The genetic studies demonstrate that DIPGs have distinct molecular characteristics compared to adult and pediatric glioblastomas (GBM), and that DIPG itself represents a biologically heterogeneous group of brainstem tumors (2, 14-19).
We have identified high-level expression of stem cell factors such as BMI1, SOX2, and nestin in primary DIPG tumors (2). DIPG tumors can be clustered by expression profiling into high MYCN and Hedgehog-expressing subgroups (20). These expression profile cohorts do not overlap with groups defined by H3F3A mutations (15, 16, 19), suggesting an added complexity to DIPG biology.
In light of the high level of stem cell marker expression in DIPG, we hypothesized that DIPG would also have significant NOTCH pathway activity. NOTCH is a stem cell pathway critical for the development of the nervous system that also is implicated in multiple cancer types (21). We have previously demonstrated the efficacy of NOTCH targeting in GBM, showing that NOTCH treatment depletes stem-like cells from GBM neurosphere cultures (22-24). Here, we extend these investigations to DIPG and determine that NOTCH blockade can augment radiation and enhance MYC-targeting bromodomain inhibition in this poor-prognosis tumor.
MATERIALS AND METHODS
Human Tissue Specimens
Human DIPG specimens were obtained postmortem in accordance with Institutional Review Board approvals with consent of the next of kin. Patient identifiers were removed prior to evaluation. Descriptive statistics of the patients are summarized in Supplemental Table 1.
Establishment of Orthotopic DIPG Xenografts
Nu/Nu mice were purchased from Charles River (Frederick, MD) and used in accordance with approved Johns Hopkins IACUC protocols. Orthotopic injections were performed after anesthesia with ketamine/xylazine, as previously described (25). Coordinates for brainstem injections were −5 from bregma, 1 to right, and 3.5 deep. A total of 100,000 cells in 5 μl of media were injected in each mouse.
Cell Culture
The JHH-DIPG1 cell line was derived from a rapid autopsy specimen. Tissue was removed from an area of brainstem tumor and placed into DMEM media on ice. Cells were dissociated by passing through gradually smaller size pipettes until a single cell suspension was obtained. More than 100,000 viable cells were injected into the striatum of immunodeficient (Nu/Nu) mice. After nearly 1 year, the mice showed signs of an intracranial tumor (hunching, weight loss) and were killed. On necropsy, a clear area of tumor was visible at the injection site and invading into the surrounding cerebral cortex. This area was excised and minced and placed into cell culture in “EF media”: 30% Ham's F12 media, 70% DMEM, 5% B27 regent 1% L-glutamine, 1% antibiotic-antimycotic (Life Technologies, Frederick, MD), 5 μg/ml heparin (Sigma-Aldrich, St. Louis, MO), 20 ng/ml FGF, and 20 ng/ml EGF (Peprotech, Rocky Hill, NJ). Within 7 days, neurospheres were visible in the culture. These neurospheres were propagated by splitting 1:2 after gently passing through a P1000 pipette tip. DIPG cell lines were grown in a humidified 37°C incubator with 5% CO2. The SF7761 cell line was derived as described, and is a kind gift of Rintaro Hashizume and Nalin Gupta (University of California, San Francisco, CA) (13). The SU-DIPG XIII cell line is a kind gift of Michelle Monje (Stanford University School of Medicine, Stanford, CA) (26). All cells were verified to be mycoplasma-free by PCR testing. Cell line identity testing was performed by the Johns Hopkins Genetic Resources Core Facility for the DIPG cell lines using a Promega StemElite kit (Promega, Madison, WI) to amplify 8 short tandem repeat loci plus a gender determining marker, Amelogenin. The PCR Product was electrophoresed on an ABI Prism 3730xl Genetic Analyzer. Data were analyzed using GeneMapper® v4.0 software (Applied Biosystems). DIPG cell lines were 100% human with no mouse DNA identified, and no cell line matched to any existing cell line in the ATCC database. The short tandem repeat profiles are reported in Supplemental Table 2.
DNA Sequencing
mRNA was extracted, reverse transcribed, and sequenced as previously described (20). PCR products were sent for direct Sanger sequencing at the Johns Hopkins Genetic Research Core Facility, and sequence chromatograms were analyzed visually to detect H3F3A K27M.
Drug Treatment
MRK003 was provided by Merck (Kenilworth, NJ). MRK003 was dissolved in dimethyl sulfoxide ([DMSO]; Life Technologies), and 10 mM stocks were stored at −80°C. MRK003 was used at 2 and 5 μM based on previous studies in GBM that showed that these were efficacious dose levels (24). JQ1 was provided by James Bradner (Dana-Farber Cancer Institute, Boston, MA) and diluted in DMSO according to his instructions (27).
Lentiviral Short Hairpin RNA Generation and Use
Validated core-binding factor 1 (CBF1) lentiviral short hairpin RNAs (shRNAs) were obtained through ThermoFisher (St. Louis, MO). They were TRCN0000016203 (target sequence GCTGGAATACAAGTTGAACAA) and TRCN0000016204 (target sequence CCCTAACGAATCAAACACAAA) corresponding the CBF shRNA#1 and #2 respectively (28). Control shRNAs were pLKO.1 scramble shRNA (Addgene plasmid 1864) (29) and pLKO.1 empty vector (Addgene plasmid 10878) (30). Short hairpin lentiviral particles were generated by co-transfecting VSVG envelope plasmid, delta8.9 and the shRNA of interest into 293T cells (31).
Assays of Cell Growth and Proliferation
Cell growth was determined by MTS assay using the Cell Titer 96 Aqueous One solution cell proliferation assay kit (Promega). Bromodeoxyuridine (BrdU) incorporation was measured as previously described (32).
Immunofluorescence Detection of Cleaved Caspase 3
Cells were triturated into single cells, washed with phosphate buffered saline (PBS), fixed in cytospin fluid, and cytospun onto Plus slides (Fisher). Cells were then processed for cleaved caspase 3 (CC3) detection, using an anti-CC3 antibody (Cell Signaling Technology, Beverly, MA) as described (33).
Western Blotting
Pellets were processed and protein was extracted as previously described (34). Forty μg of lysate for each sample per lane was run in precast NuPAGE Novex polyacrylamide gel 4% to 12% Bis-Tris Gels (1.0-mm thick, 10-well) in 1X Tris-glycine (Life Technologies, Grand Island, NY). Lysates were transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA), blocked in PBS containing 5% bovine serum albumin or nonfat dry milk powder, and incubated overnight with antibodies per manufacturer's directions. Blots were then washed several times with PBS containing 0.1% Tween 20 and incubated in peroxidase-conjugated immunoglobulin G diluted 1:5000 in blocking solution. After washing several times in PBS with 0.1% Tween 20, blots were developed with enhanced Western Lightning chemiluminescence reagent (Perkin Elmer, Waltham, MA) and exposed to film. Primary antibodies included HES1 (1:800 Aviva, San Diego, CA), NOTCH 3 (1:800, Cell Signaling Technologies, Inc.), JAG1 (1:800, Cell Signaling Technologies Inc.), DLL-1 (1:800 Cell Signaling Technologies, Inc.), glyceraldehyde 3-phosphate dehydrogenase ([GAPDH]; 1:5000, RDI, Flanders, NJ), and β-actin (1:500, mouse, Santa Cruz Biotechnology, Inc., Dallas, TX). Omission of primary antibody was used as a negative control.
Methylcellulose
Exponentially growing cells in EF media were treated with MRK003 or DMSO for 5 days, washed and then placed in EF media without MRK003 for 2 days to allow cells to recover. 1.5% methyl cellulose (Sigma-Aldrich) was made using EF media without MRK003 or DMSO. As described previously, 30,000 viable cells were plated in 2 mL of methylcellulose in each well of polyhema-coated 6-well plates (31). Fresh media/methylcellulose was added every 7 days, and the colonies were allowed to grow for 4 weeks. Colonies were then visualized by staining with nitro blue tetrazolium (Sigma-Aldrich) overnight at 37°C and quantified using MCID Elite software (Cambridge, UK) with a gate diameter of 50 μm.
Statistical Analyses
Student 2-sided t-tests were used to compare paired samples. ANOVA was used for experiments with multiple conditions, employing Tukey's multiple comparison correction. Statistics were performed using Microsoft Excel or GraphPad Prism. Error bars represent the standard deviation, unless otherwise indicated.
RESULTS
The JHH-DIPG1 Cell Line Forms Orthotopic Xenograft Tumors that Phenocopy DIPG
We established JHH-DIPG1 from a rapid autopsy specimen from a patient who was heavily pre-treated with radiation therapy and multiple lines of chemotherapy (Fig. 1A, B). After first passaging the tumor cells as a brain xenograft in Nu/Nu mice, we transitioned JHH-DIPG1 to grow as neurospheres in EGF/FGF containing media (Fig. 1C). Subsequent xenografting of 100,000 cells into either the striatum (Fig. 1D) or pons (Fig. 1E) of immunodeficient mice led to diffuse tumor infiltration of the brain parenchyma (Fig. 1F); neurologic signs appeared approximately 6 months after injection. Xenograft tumors derived from JHH-DIPG1 expressed stem cell markers such as SOX2 and nestin and were glial fibrillary acidic protein-positive and synaptophysin-negative (Fig. 1G-K), phenocopying primary DIPG tumors (2). JHH-DIPG1 and the primary tumor from which it was derived share a K27M mutation in the H3F3A gene, encoding the H3.3 histone (Fig. 1K, left) (19). For comparison, the chromatogram from a wild-type control sample is included (Fig. 1K, right).
Figure 1.
The JHH-DIPG1 cell line was derived from a rapid autopsy specimen and maintains the phenotype of diffuse intrinsic pontine glioma (DIPG) primary tumors. (A) Photo taken at autopsy shows a transverse cut through the midbrain. Arrows indicate areas of diffuse tumor infiltration. (B) Hematoxylin and eosin (H&E) stain of the primary DIPG tumor showing diffuse infiltration of the brain parenchyma. Magnification: 400X. (C) JHH-DIPG1 grows as neurospheres in cell culture. (D) JHH-DIPG1 forms invasive tumors when injected into the striatum of immunodeficient mice. Magnification: 4X. Tumor was injected on the right side and has subsequently invaded the cerebrum. (E) JHH-DIPG1 forms invasive tumors when injected into the brainstem. Magnification: 4X. (F) H&E stain of xenografted JHH-DIPG1, showing invasion into the brain parenchyma and high fidelity to the primary tumor. (G) SOX2 immunostain of JHH-DIPG1 with strong nuclear positivity in tumor cells. Magnification: 400X. (H) Nestin immunostain of JHHDIPG showing diffuse positivity in tumor cells. (I) Glial fibrillary acidic protein (GFAP) immunostain of JHH-DIPG1 xenograft showing positivity of tumor cells. Magnification: 400X. (J) Synaptophysin (SYP) immunostain of JHH-DIPG1 xenograft showing no immunoreactivity in tumor cells (bottom) and positive immunostaining of adjacent normal brain (top). Magnification: 400X. (K) Left, sequencing chromatogram of H3F3A gene in the primary tumor from which JHH-DIPG1 is derived, showing heterozygous K27M mutation (arrow). The half-height of the T peak under the arrow partially hides the half-height A peak of the chromatogram. Right, sequence and chromatogram from normal brain showing the wild-type sequence is included for comparison.
Primary DIPG Samples Express High Levels of NOTCH
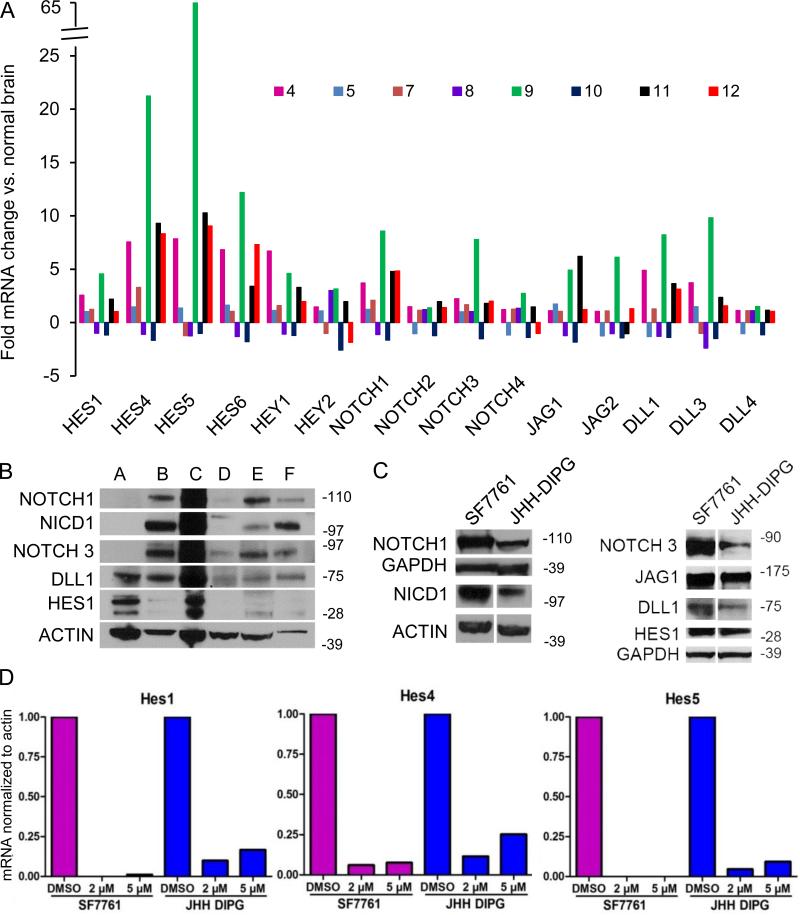
Because of the robust expression of stem cell markers in DIPG (2), we hypothesized that primary DIPG tumors and cell lines would express the stem cell factor NOTCH. We interrogated the NOTCH pathway at the RNA level in eight primary DIPG tumors and found that these tumors express high levels but heterogeneous amounts of NOTCH receptors, ligands, and effectors compared to normal brain samples (Fig. 2A). Descriptive characteristics of these 8 patients are listed in Supplemental Table 1.
Figure 2.
The NOTCH pathway is highly expressed in primary diffuse intrinsic pontine glioma (DIPG) and cell lines, and the γ-secretase inhibitor (GSI) MRK003 suppresses NOTCH effectors in DIPG cell lines. (A) Quantitative PCR showing fold change in NOTCH effectors, receptors, and ligands in DIPG compared to normal brain samples. The fold-increase or decrease compared to normal brain is shown for 8 primary DIPG tumors obtained at rapid autopsy. The ID numbers were assigned to de-identify tumors. (B) Western blot showing upregulation at the protein level of NOTCH receptors, ligands and the effector HES1 in six primary DIPG tumors. With 2 exceptions, these tumors do not overlap with those shown in (A). Tumor A corresponds to tumor 4 in (A); tumor C corresponds to tumor 11 in (A). (C) Western blot showing upregulation of NOTCH pathway in DIPG cell lines in culture. Left panel shows expression of NOTCH1 and the activated form of NOTCH, NICD1, in DIPG cell lines. Right panel shows expression of NOTCH3 and ligands DLL1 and JAG1 and effector HES1. (D) Bar graphs showing quantification of the reduction of HES1 (left), HES4 (middle), and HES5 mRNA expression (right) after 48-hour treatment with the GSI MRK003 or DMSO in DIPG cell lines SF7761 and JHH-DIPG1.
Six of the 8 samples showed coordinately increased expression of the NOTCH pathway, and 2 of the 8 samples showed coordinately decreased NOTCH pathway expression compared to normal brain. Only 1 of the tumors, DIPG tumor 9, was wild-type for H3F3A and HIST1H3B. DIPG tumor 8 had an H3K27M mutation in HIST1H3B, and the remainder of tumors had an H3K27M mutation in H3F3A. We next measured NOTCH pathway activation via Western blot in 6 primary tumor DIPG samples obtained from autopsy specimens; all tumors showed varying degrees of expression of NOTCH receptors, NOTCH intracellular domain (NICD) 1, and the one downstream target, HES1, for which we have a reliable antibody for Western blot (Fig. 2B). These DIPG tumors used for Western blotting were distinct from the tumors used for RNA extraction, with the exception of tumor ‘A,’ which corresponds to tumor 4 in Figure 2A, and tumor ‘C,’ which corresponds to tumor 11 in Figure 2A. The JHH DIPG1 cell line was derived from the former. In summary, 10/12 (83%) of the DIPG tumors examined express NOTCH pathway receptors, ligands, and downstream effectors.
γ-Secretase Inhibition Decreases NOTCH Activity, Growth, and Proliferation in DIPG Cell Lines and Induces Apoptosis
We found that DIPG cell lines also express high levels of NOTCH effectors (Fig. 2C). The canonical NOTCH pathway can be disrupted by inhibition of γ-secretase, one of the enzymes that mediates the critical cleavage of NOTCH receptors, thereby preventing nuclear translocation of the NOTCH intracellular domain (NICD) (21). Treatment of DIPG cell lines with the γ-secretase inhibitor (GSI) MRK003 led to a greater than 75% reduction of the NOTCH effectors HES1, HES4, and HES5 as measured by quantitative PCR (Fig. 2D).
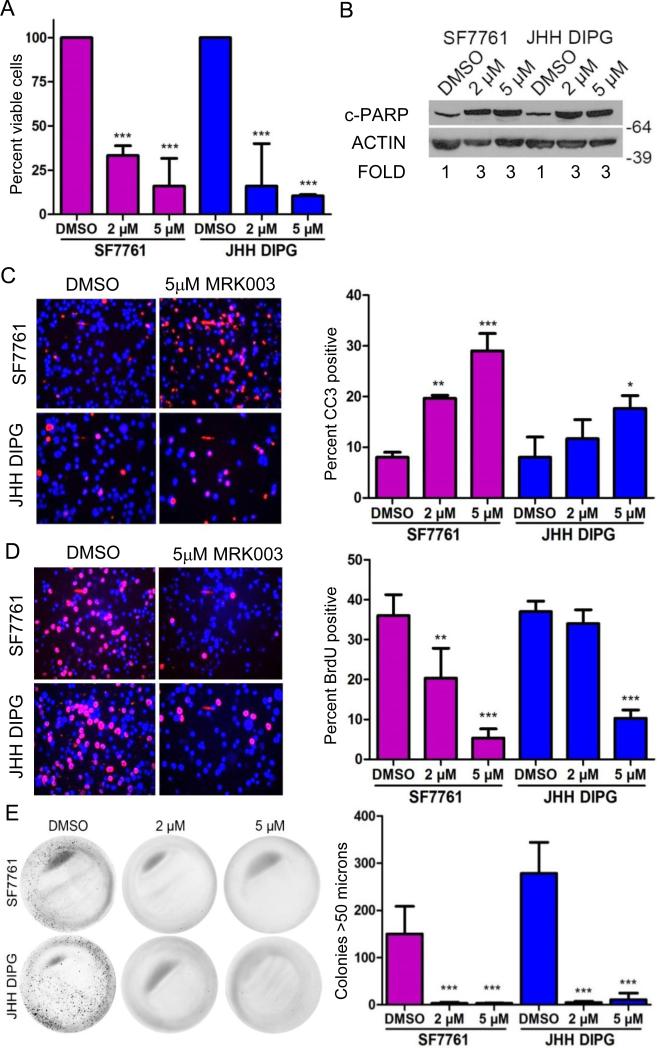
Incubation of DIPG cell lines with 5 μM MRK003 for 5 days caused a modest decrease in viability as measured by MTS assay which only reached statistical significance for the JHH DIPG1 cell line (Supplemental Fig. 1). However, incubation of DIPG cell lines for seven days led to a greater than 75% decrease in DIPG viability as measured by MTS assay (Fig. 3A; p < 0.001 for JHH-DIPG1 compared to DMSO control and p < 0.001 for SF7761 compared to DMSO control). Investigating the mechanism of the loss of viability, we found increased apoptosis, as measured by cleaved PARP Western blot (Fig. 3B). MRK003 treatment of JHH-DIPG1 and SF7761 led to a 3-fold increase in cleaved PARP expression compared to DMSO control as measured by IMAGE J quantification normalized to actin. We confirmed the induction of apoptosis by MRK003 by performing anti-CC3 immunofluorescence staining and found that GSI treatment led to a 2- and 3-fold induction of apoptosis in JHH-DIPG1 and SF7761, respectively (p < 0.05 for JHH-DIPG1 compared to DMSO control; p < 0.001 for SF7761 treated with 5 μM MRK003 compared to DMSO control) (Fig. 3C).
Figure 3.
The γ-secretase inhibitor MRK003 decreases cell proliferation, growth and increases apoptosis in diffuse intrinsic pontine glioma (DIPG) cell lines. (A) Incubation of DIPG cell lines with 5 μM MRK003 for seven days led to a greater than 75% decrease in DIPG viability as measured by MTS assay. (B) Western blot showing increased cleaved PARP expression in SF7761 and JHH DIPG1 after treatment with MRK003 for 7 days. Numbers under the blot indicate fold increase compared to DMSO. (C) Representative 400X photomicrographs showing increased apoptosis after treatment of DIPG cells with 5 μM MRK003, as measured by Cleaved caspase 3 (CC3) immunofluorescence (red color) (left). DAPI counterstains nuclei blue. Quantification of percent CC3 positivity in SF7761 and JHH DIPG1 treated with MRK003 for 7 days (right). (D) Representative 400X photomicrographs showing decreased proliferation of SF7761 and JHH DIPG1 cells as measured by anti-bromodeoxyuridine (BrdU) immunofluorescence (red) after treatment with 5 μM MRK003 for 7 days (left). DAPI counterstains nuclei blue. Quantification of percent BrdU positivity of DIPG cells after treatment with MRK003 for 7 days (right). (E) Low-power images show suppression of colony formation in methylcellulose after pretreatment with MRK003 (left). Quantification of methylcellulose assay colonies (right). Cells were treated with MRK003 for 5 days, allowed to recover in normal media for two days, and then triturated to single cells and plated in methylcellulose. Wells were photographed after 4 weeks. *p < 0.05; **p < 0.01; ***p < 0.001 by ANOVA compared to DMSO-treated cells.
In addition to inducing apoptosis, MRK003 treatment for 7 days also suppressed DIPG proliferation. We found a 75% decrease in the number of proliferating DIPG cells as measured by BrdU incorporation (p < 0.001 for JHH-DIPG1 treated with 5 μM MRK003 compared to DMSO control and p < 0.001 for SF7761 treated with 5 μM MRK003 compared to DMSO control) (Fig. 3D).
MRK003 Decreases DIPG Clonogenicity
Next we determined the effect of GSI treatment on the clonogenicity of DIPG cell lines. We treated cells for 5 days with MRK003 or DMSO control and then allowed them to recover for 2 days in normal growth media before plating equal numbers of viable cells in methylcellulose in normal growth media. After 4 weeks, we stained colonies with NBT and quantified the number of colonies larger than 50 μm. We found that pre-treatment with MRK003 suppressed colony formation by more than 95% (p < 0.001 by ANOVA compared to DMSO-treated cells for both JHH-DIPG1 and SF7761, Fig. 3E).
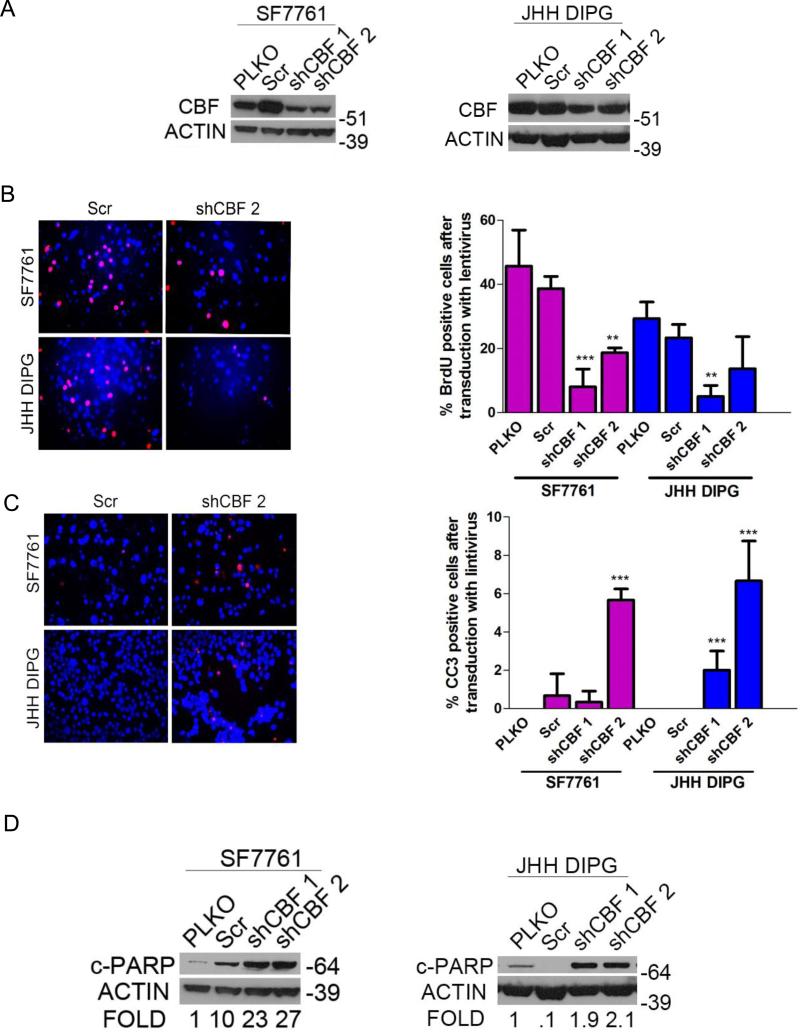
Targeting Canonical NOTCH Signaling with Lentiviral shRNA Mimics the Effects of GSI in DIPG
Because GSI can act on multiple pathways within the cell, we used lentivirally delivered shRNA against the core binding factor (RBPJ or CBF1), an essential component of canonical NOTCH signaling (21) to verify the importance of NOTCH signaling in DIPG (28). Multiple CBF1-targeting shRNAs led to knockdown of CBF1 in DIPG cell lines (Fig. 4A). Inhibition of NOTCH canonical signaling through CBF1 knockdown led to decreased proliferation as measured by BrdU incorporation for SF7761 and JHH-DIPG1 (greater than 50% reduction in BrdU positivity compared to empty vector control) (Fig. 4B). Knockdown of CBF1 also led to a modest, but statistically significant, induction of apoptosis as measured by CC3 (Fig. 4C). The percent CC3 positivity increased from 1 percent to more than 6 percent for both JHH-DIPG1 and SF7761 (p < 0.0001 by ANOVA, comparing shCBF2 to empty vector control). We confirmed the induction of apoptosis after CBF1 knockdown by cleaved PARP Western blot (Fig. 4D). We found that CBF1 knockdown led to decreased HES1 protein expression by Western blot (Supplemental Fig. 2).
Figure 4.
Core binding factor (CBF) shRNA phenocopies γ-secretase inhibitor (GSI) in diffuse intrinsic pontine glioma (DIPG). (A) Western blot showing decreased CBF1 expression with SF7761 (left) and JHH-DIPG1 (right) cells after infection with lentiviral vectors for shCBF. (B) Left, representative anti-bromodeoxyuridine (BrdU) immunofluorescence (red) showing decreased proliferation in cells transduced with lentiviral vectors for shCBF compared to scramble (Scr). DAPI (blue) counterstains nuclei. Magnification: 400X. Right, quantification of percent BrdU positivity in SF7761 and JHH DIPG1 transduced with lentiviral vectors for shCBF (right) **p < 0.01; ***p < 0.001 by ANOVA. (C) Left, representative anti-cleaved caspase 3 (CC3) immunofluorescence (red) showing increased apoptosis in cells transduced with lentiviral vectors for shCBF compared to Scr control. DAPI (blue) counterstains nuclei. Magnification: 400X. Right, quantification of percent CC3 positivity in SF7761 and JHH DIPG1 transduced with lentiviral vectors for shCBF. **p < 0.01; ***p < 0.001 by ANOVA. (D) Western blot showing increased cleaved PARP expression with SF7761 (left) and JHH-DIPG1 (right) cells transduced with lentiviral vectors for shCBF. Numbers below the blot represent fold change compared to pLKO control.
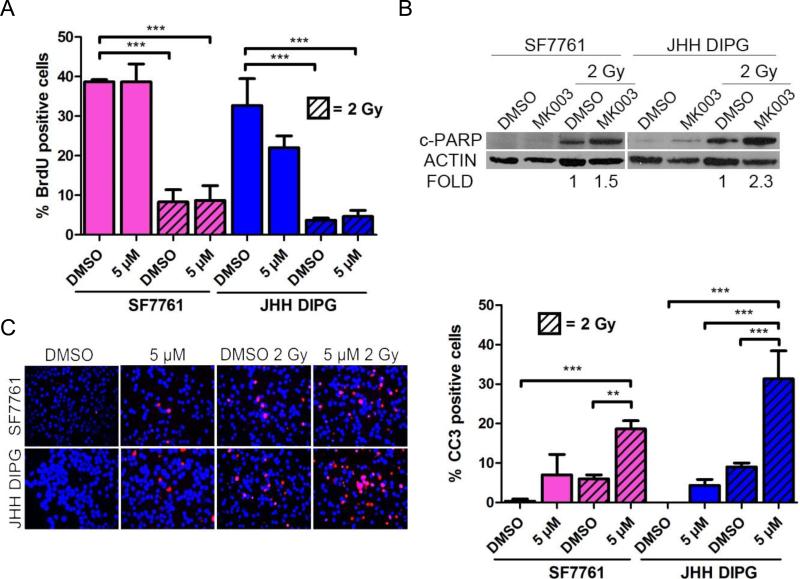
Combination Therapy of MRK003 and Radiation Increases Cell Death
Because cancer stem cells are often resistant to radiation therapy, we hypothesized that treatment of DIPG with GSI would sensitize them to radiation therapy, which is a mainstay of DIPG treatment. SF7761 and JHH-DIPG1 were treated with 5 μM MRK003 for 48 hours, then radiated with 2 Gy. We measured proliferation and apoptosis 48 hours after irradiation. We chose this time point because it entailed a total of 4 days of incubation with GSI, which was earlier than the timeframe at which we observed a cytotoxic effect of single-agent MRK003. After irradiation both DIPG cell lines arrested their proliferation, as measured by BrdU incorporation; pretreatment with MRK003 did not cause a further decrease in proliferation (Fig. 5A). However, we did detect an increase in apoptosis as measured by cleaved PARP Western blotting when cells were pretreated with MRK003 followed by 2 Gy radiation (Fig. 5B). MRK003 increased cleaved PARP by 1.5-fold in SF7761 and 2.3-fold in JHH DIPG1 compared to radiation alone. Similar to our results with cleaved PARP, radiation treatment caused a modest increase in CC3 positivity in the vehicle-only treated cells; however, pre-treatment with MRK003 followed by 2 Gy radiation led to a significant increase in apoptosis in both cell lines (Fig. 5C). SF7761 cells treated with vehicle and radiation showed 6% apoptosis while 16.6% of those treated with MRK003 and radiation were apoptotic (p < 0.001 by ANOVA). JHH-DIPG1 cells treated with vehicle and radiation showed 9% CC3 immunoreactivity, while JHH-DIPG1 cells treated with 5 μM MRK003 and radiation were 31.3% CC3-positive (p < 0.001 by ANOVA).
Figure 5.
γ-Secretase inhibitor (GSI) restores radiation sensitivity to heavily pre-treated diffuse intrinsic pontine glioma (DIPG) cell lines. (A) Quantification of percent BrdU positivity in SF7761 and JHH DIPG1 treated with MRK003 for 48 hours and/or then radiated at 2 Gy. ***p < 0.001 by ANOVA. MRK003 treatment did not augment the arrest in proliferation caused by radiation. (B) Western blot showing increased cleaved PARP expression in DIPG cells after treatment with 5 μM MRK003 for 48 hours followed by 2 Gy radiation. Numbers below the blot represent fold change compared to DMSO control. (C) Representative cleaved caspase 3 (CC3) immunofluorescence (red) in SF7761 and JHH-DIPG after treatment with 5 μM MRK003 for 48 hours followed by 2 Gy radiation (left). DAPI (blue) counterstains nuclei. Magnification = 400X. Quantification of percent CC3 positivity (right); ***p < 0.001; **p < 0.01 by ANOVA.
Combination Therapy of MRK003 and JQ1 Decreases Proliferation and Increases Apoptosis in DIPG
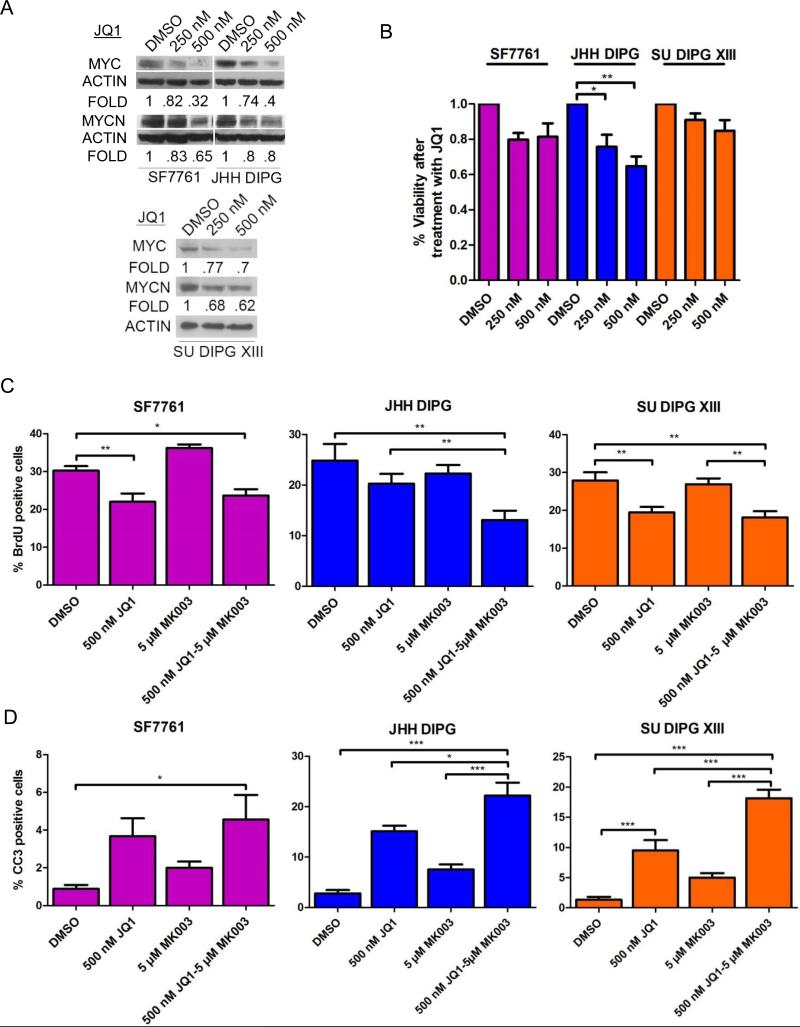
The high level of MYCN detected in primary DIPG tumors led us to hypothesize that bromodomain inhibitors may be effective in DIPG (20). We used the BET bromodomain inhibitor JQ1 at well-established concentrations (35) and assessed the ability of JQ1 to suppress MYCN and MYC in DIPG. We added a third DIPG cell line to our studies of bromodomain inhibition and NOTCH targeting due to a heterogeneous response of 7761 and JHH DIPG1 to combination therapy. After treatment of SF7761, JHH-DIPG1, and SU DIPG XIII cells with JQ1 at 250 nM and 500 nM for 72 hours, knockdown of MYCN and MYC was most significant at 500 nM (Fig. 6A). When we performed a dose-response curve for JQ1 in DIPG, we found that the viability of SF7761, JHH-DIPG1, and SU DIPG XIII cells after 5 days of treatment with JQ1 decreased by 10% to 30% (p < 0.05 for JHH-DIPG1 250 μM JQ1 compared to DMSO control and p < 0.001 for 500 μM JQ1 compared to DMSO control; SF7761 and SU DIPG XIII showed a modest 10% to 20% reduction at both concentrations that was not significant) (Fig. 6B).
Figure 6.
Combination therapy of the bromodomain inhibitor JQ1 and the γ-secretase inhibitor MRK003 leads to increased cell death. (A) Western blot of MYC and MYCN expression in SF7761 and JHH DIPG1 cells after 4 days of treatment with JQ1 showing that expression decreases in a dose-dependent manner. (B) Percent viability of SF7761 and JHH DIPG1 cells after 5 days of treatment with JQ1, normalized to DMSO control. *p < 0.05; **p < 0.01 by ANOVA. (C) Quantification of percent bromodeoxyuridine (BrdU) positivity in SF7761, JHH DIPG1, and SU DIPG XIII treated with JQ1 for 48 hours followed by MRK003 treatment for 48 hours, or JQ1 alone for 96 hours, or MRK003 alone for 48 hours. *p < 0.05; **p < 0.01 by ANOVA. (D) Quantification of percent cleaved caspase 3 (CC3) positivity in SF7761, JHH-DIPG1, and SU DIPG XIII treated with JQ1 for 48 hours followed by MRK003 for 48 hours, or JQ1 alone for 96 hours, or MRK003 alone for 48 hours). *p < 0.05; **p < 0.01; ***p < 0.001 by ANOVA.
Based on results reported recently in T-cell ALL (36), we hypothesized that dual treatment targeting both NOTCH and MYC might be more efficacious in DIPG in a shorter time course than either monotherapy. We treated SF7761, JHH-DIPG1, and SU DIPG XIII with 500 nM JQ1 for 2 days followed by addition of 5 μM MRK003 for 2 more days, continuing the JQ1 treatment during that time. We found that combination therapy did not affect the cell proliferation of SF7761 and SU DIPG XIII more than JQ1 treatment alone (Fig. 6C).
When we investigated apoptosis by CC3 immunofluorescence, we found that in SF7761 there was not a significant increase in apoptosis when MRK003 was added to JQ1, compared to monotherapy with each agent (Fig. 6D). In contrast, in JHH-DIPG1, JQ1 followed by MRK003 led to increased apoptosis compared to monotherapy with each agent (22% CC3-positive for MRK003 and JQ1 combination compared to 15% in JQ1-only treated cells, 8% in MRK003-only treated, and 3% in DMSO control; p < 0.001, by ANOVA) (Fig. 6D). Similarly, in SU DIPG XIII, JQ1 followed by MRK003 led to increased apoptosis compared to monotherapy with each agent (18% CC3 positive for MRK003 and JQ1 combination compared to 9.5% in JQ1-treated cells, 5% in MRK003-treated, and 1% in DMSO control; p < 0.001,by ANOVA) (Fig. 6D).
DISCUSSION
Because of the dismal clinical outcome of DIPG, there is a need to identify new therapeutic targets (3). Here, we show that the NOTCH pathway is highly expressed in DIPG primary tumors and can be successfully disrupted using either a GSI or shRNAs targeting canonical NOTCH signaling in vitro. We identified increased NOTCH pathway expression in 5 of 7 tumors that harbored mutations in H3F3A and HIST1H3B as well as the 1 wild-type tumor. These data suggest that NOTCH pathway activity is not strictly associated with H3K27M-mutated DIPG. In DIPG cell lines, inhibition of the NOTCH pathway led to reduced cell growth, proliferation, clonogenicity, and increased apoptosis, suggesting that NOTCH is a potential therapeutic target in patients with this tumor.
γ-Secretase inhibitors are known to lead to downregulation of the STAT and AKT pathways in addition to the NOTCH pathway (24). The doses for MRK003 we chose are those that are standard in our laboratory for treatment of GBM (22). The increasing effect of GSI on growth, proliferation and apoptosis at 5 μM compared to 2 μM may be due to targeting these other pathways. However, we found that lentiviral shRNA against the key canonical NOTCH pathway effector CBF1/RBPJ mimics MRK003 treatment, suggesting that the majority of the phenotype we observe after GSI treatment is due to NOTCH blockade.
We found that phenotypic changes with MRK003 (i.e. suppression of growth and increased apoptosis) were nonexistent or modest at early time points and became most prominent after 7 days of treatment. These data are consistent with what we have observed in slow-growing adult GBM cell lines after GSI treatment (24). Therefore, we believe that the slow time course is likely due to the indolent growth of DIPG cell lines in vitro.
In radiation therapy experiments, we pre-treated the cells for 2 days with MRK003, irradiated the cells, and then evaluated the cells for viability and apoptosis 48 hours after irradiation. The total treatment time in MRK003 was 96 hours, which is earlier than we identified any significant cytotoxicity with MRK003 alone, indicating that deprivation of NOTCH signaling was able to augment radiation therapy independent of the cytotoxic effects of NOTCH blockade per se.
Because DIPGs are known to express MYCN (17, 20), we hypothesized that drug therapy targeting the BET bromodomain, which is essential for MYC and MYCN transcription, would be effective in DIPG. However, we found that the bromodomain inhibitor JQ1 had modest effects on these DIPG cell lines, suppressing their viability by 10% to 30% after 5 days of treatment. We then hypothesized that combination therapy with JQ1 and MRK003 would lead to decreased DIPG proliferation and increased cell death in a shorter time frame. When we co-treated DIPG cells with the bromodomain inhibitor JQ1 and NOTCH, we chose to pre-treat cells with JQ1 for 48 hours and then add MRK003 for 48 hours. Thus, the total treatment time in JQ1 was 96 hours. Cells were exposed to MRK003 for 48 hours only, which is earlier than we identified significant cytotoxicity with MRK003 alone. Dual treatment of JHH-DIPG1 and SU DIPG XIII with JQ1 and MRK003 induced more apoptosis in a shorter time frame than we observed for MRK003 or JQ1 alone. These findings are similar to those recently reported in T-ALL, in which JQ1 treatment primed cells to be more sensitive to NOTCH inhibition (36). Dual therapy with JQ1 and NOTCH showed combinatorial/additive efficacy in JHH DIPG1 and SU DIPG XIII. However, SF7761 cells were more resistant than JHH-DIPG1 or SUDIPGXIII to JQ1 and MRK003 combination therapy, even though the 3 cell lines showed equivalent downregulation of MYCN and MYC after JQ1 treatment. These findings emphasize the molecular heterogeneity of DIPG and suggest that combination therapy would not be effective for all tumors. Our group previously showed that disruption of the NOTCH pathway in adult GBM decreases cell viability, clonogenicity and tumorigenicity (22-24). NOTCH is known to be important in the development of the mammalian brain (21, 37). While the effect of blocking NOTCH in young children is unknown, radiation and chemotherapy are known to cause severe neuro-cognitive side effects in pediatric patients with other types of brain tumors who survive (38). The current 100% mortality of patients with DIPG even with intensive therapy suggests that potential new treatments that offer improvement in survival with manageable short-term side effects should be considered.
Effective targeting of NOTCH in human subjects with GSI is limited by gastrointestinal toxicity (39). This toxicity in laboratory animals can be mitigated by co-administration of glucocorticoids (40, 41). Radiation therapy is the initial treatment for DIPG, and reirradiation is increasingly being used in patients with relapses (42). Most of these patients receive dexamethasone to mitigate the cerebral edema associated with their tumor and radiation. Therefore, these patients may tolerate GSI inhibitors better than patients who are not routinely receiving dexamethasone. A phase I trial of a GSI in children showed that it could safely be administered; however, the drug did not reproducibly inhibit NICD1 in peripheral blood lymphocytes, indicating it may not have sufficient potency to disrupt NOTCH signaling in vivo in brain tumors (43).
The GSI PF-03084014 had minimal gastrointestinal toxicity in a phase I clinical trial and demonstrated activity against some solid tumors (44). Clinical trials of PF-03084014 for non-CNS tumors (NCT01876251, NCT01981551) are ongoing. PF-03084014 does have good brain penetration (45), raising the possibility of its usefulness for treatment of patients with DIPG. Although the Pediatric Pre-clinical Testing Program did not find high-level single-agent activity of PF-03084014, DIPG are not included in the panel of xenografts, due to the long latency of tumor formation and lack of availability of DIPG cell lines (46). Based on our present data, we believe that testing PF-03084014 or other GSI in vivo against DIPG xenografts is warranted. Possible combination therapy with radiation and bromodomain targeting agents may demonstrate improved efficacy of GSI in DIPG and other pediatric brain tumors.
Supplementary Material
Acknowledgments
Financial Support: Matthew Larson Foundation (E.H.R); NCI Core Grant to the Johns Hopkins SKCCC P30 CA006973; E.H.R. is a St. Baldrick's Scholar.
REFERENCES
- 1.Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol. 2012;2:205. doi: 10.3389/fonc.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballester LY, Wang Z, Shandilya S, et al. Morphologic characteristics and immunohistochemical profile of diffuse intrinsic pontine gliomas. Am J Surg Pathol. 2013;37:1357–64. doi: 10.1097/PAS.0b013e318294e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen MH, van Vuurden DG, Vandertop WP, et al. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38:27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13:410–6. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warren K, Bent R, Wolters PL, et al. A phase 2 study of pegylated interferon alpha-2b (PEG-Intron((R))) in children with diffuse intrinsic pontine glioma. Cancer. 2012;118:3607–13. doi: 10.1002/cncr.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels U, Hawkins C, Vezina G, et al. Proceedings of the diffuse intrinsic pontine glioma (DIPG) Toronto Think Tank: advancing basic and translational research and cooperation in DIPG. J Neurooncol. 2011;105:119–25. doi: 10.1007/s11060-011-0704-4. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Rubio MP, Correa KM, et al. Molecular genetics of pediatric brain stem gliomas. Application of PCR techniques to small and archival brain tumor specimens. J Neuropathol Exp Neurol. 1993;52:507–15. doi: 10.1097/00005072-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Angelini P, Hawkins C, Laperriere N, et al. Post mortem examinations in diffuse intrinsic pontine glioma: challenges and chances. J Neurooncol. 2011;101:75–81. doi: 10.1007/s11060-010-0224-7. [DOI] [PubMed] [Google Scholar]
- 9.Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108:4453–8. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kambhampati M, Perez JP, Yadavilli S, et al. A standardized autopsy procurement allows for the comprehensive study of DIPG biology. Oncotarget. 2015 Jan 24; doi: 10.18632/oncotarget.3374. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puget S, Philippe C, Bax DA, et al. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7:e30313. doi: 10.1371/journal.pone.0030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107:1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume R, Smirnov I, Liu S, et al. Characterization of a diffuse intrinsic pontine glioma cell line: implications for future investigations and treatment. J Neurooncol. 2012;110:305–13. doi: 10.1007/s11060-012-0973-6. [DOI] [PubMed] [Google Scholar]
- 14.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28:3061–8. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22:425–37. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–31. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 17.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–47. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahsan S, Raabe EH, Haffner MC, et al. Increased 5-hydroxymethylcytosine and decreased 5-methylcytosine are indicators of global epigenetic dysregulation in diffuse intrinsic pontine glioma. Acta Neuropathol Commun. 2014;2:59. doi: 10.1186/2051-5960-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saratsis AM, Kambhampati M, Snyder K, et al. Comparative multidimensional molecular analyses of pediatric diffuse intrinsic pontine glioma reveals distinct molecular subtypes. Acta neuropathologica. 2014;127:881–95. doi: 10.1007/s00401-013-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierfelice TJ, Schreck KC, Eberhart CG, et al. Notch, neural stem cells, and brain tumors. Cold Spring Harb Symp Quant Biol. 2008;73:367–75. doi: 10.1101/sqb.2008.73.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu Q, Orr BA, Semenkow S, et al. Prolonged inhibition of glioblastoma xenograft initiation and clonogenic growth following in vivo Notch blockade. Clin Cancer Res. 2013;19:3224–33. doi: 10.1158/1078-0432.CCR-12-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–52. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao XG, Hutt-Cabezas M, Orr BA, et al. LIN28A facilitates the transformation of human neural stem cells and promotes glioblastoma tumorigenesis through a pro-invasive genetic program. Oncotarget. 2013;4:1050–64. doi: 10.18632/oncotarget.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasso CS, Tang Y, Truffaux N, et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015 doi: 10.1038/nm0715-827a. 2015 May 4. doi: 10.1038/nm.3855. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asnaghi L, Ebrahimi KB, Schreck KC, et al. Notch signaling promotes growth and invasion in uveal melanoma. Clin Cancer Res. 2012;18:654–65. doi: 10.1158/1078-0432.CCR-11-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 30.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Kaur H, Hutt-Cabezas M, Weingart MF, et al. The chromatin-modifying protein HMGA2 promotes atypical teratoid/rhabdoid cell tumorigenicity. J Neuropathol Exp Neurol. 2015;74:177–85. doi: 10.1097/NEN.0000000000000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raabe EH, Lim KS, Kim JM, et al. BRAF activation induces transformation and then senescence in human neural stem cells: a pilocytic astrocytoma model. Clin Cancer Res. 2011;17:3590–9. doi: 10.1158/1078-0432.CCR-10-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weingart MF, Roth JJ, Hutt-Cabezas M, et al. Disrupting LIN28 in atypical teratoid rhabdoid tumors reveals the importance of the mitogen activated protein kinase pathway as a therapeutic target. Oncotarget. 2015;6:3165–77. doi: 10.18632/oncotarget.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutt-Cabezas M, Karajannis MA, Zagzag D, et al. Activation of mTORC1/mTORC2 signaling in pediatric low-grade glioma and pilocytic astrocytoma reveals mTOR as a therapeutic target. Neuro-oncology. 2013;15:1604–14. doi: 10.1093/neuonc/not132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandopadhayay P, Bergthold G, Nguyen B, et al. BET-bromodomain inhibition of MYC-amplified medulloblastoma. Clin Cancer Res. 2014;20:912–25. doi: 10.1158/1078-0432.CCR-13-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knoechel B, Roderick JE, Williamson KE, et al. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nature genetics. 2014;46:364–70. doi: 10.1038/ng.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizutani K, Yoon K, Dang L, et al. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–5. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 38.Glauser TA, Packer RJ. Cognitive deficits in long-term survivors of childhood brain tumors. Childs Nerv Syst. 1991;7:2–12. doi: 10.1007/BF00263824. [DOI] [PubMed] [Google Scholar]
- 39.Takebe N, Nguyen D, Yang SX. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol Ther. 2014;141:140–9. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguirre SA, Liu L, Hosea NA, et al. Intermittent oral coadministration of a gamma secretase inhibitor with dexamethasone mitigates intestinal goblet cell hyperplasia in rats. Toxicol Pathol. 2014;42:422–34. doi: 10.1177/0192623313486315. [DOI] [PubMed] [Google Scholar]
- 41.Samon JB, Castillo-Martin M, Hadler M, et al. Preclinical analysis of the gamma-secretase inhibitor PF-03084014 in combination with glucocorticoids in T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2012;11:1565–75. doi: 10.1158/1535-7163.MCT-11-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. 2014;118:305–12. doi: 10.1007/s11060-014-1428-z. [DOI] [PubMed] [Google Scholar]
- 43.Fouladi M, Stewart CF, Olson J, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol. 2011;29:3529–34. doi: 10.1200/JCO.2011.35.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Messersmith WA, Shapiro GI, Cleary JM, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral gamma-secretase inhibitor PF-03084014. Clin Cancer Res. 2015;21:60–7. doi: 10.1158/1078-0432.CCR-14-0607. [DOI] [PubMed] [Google Scholar]
- 45.Lanz TA, Wood KM, Richter KE, et al. Pharmacodynamics and pharmacokinetics of the gamma-secretase inhibitor PF-3084014. J Pharm Exp Ther. 2010;334:269–77. doi: 10.1124/jpet.110.167379. [DOI] [PubMed] [Google Scholar]
- 46.Carol H, Maris JM, Kang MH, et al. Initial testing (stage 1) of the notch inhibitor PF-03084014, by the pediatric preclinical testing program. Pediat Blood Cancer. 2014;61:1493–6. doi: 10.1002/pbc.25026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.