Summary
Objective
Temporal lobe epilepsy (TLE) is frequently medically intractable and often progressive. Compromised inhibitory neurotransmission due to altered GABAA receptor α4 (GABARα4) subunit expression has been emphasized as a potential contributor to the initial development of epilepsy following a brain insult (primary epileptogenesis), but the regulation of GABARα4 during chronic epilepsy, specifically, how expression is altered following spontaneous seizures, is less well understood.
Methods
Continuous video-EEG recordings from rats with pilocarpine-induced TLE were used to capture epileptic animals within 3 hours of a spontaneous seizure (SS) or greater than 24 hours after the last SS to determine whether recent occurrence of a seizure was associated with altered levels of GABARα4 subunit expression. We further evaluated whether this GABARα4 subunit plasticity is regulated by signaling mechanisms active in primary epileptogenesis, specifically, increases in brain-derived neurotrophic factor (BDNF) and early growth response factor 3 (Egr3).
Results
Elevated levels of GABARα4 subunit mRNA and protein were observed following spontaneous seizures, and were associated with higher levels of BDNF and Egr3 mRNA
Significance
These data suggest that spontaneous, recurrent seizures that define chronic epilepsy may influence changes in GABARα4 subunit expression, and that signaling pathways known to regulate GABARα4 expression after SE may also be activated after spontaneous seizures in chronically epileptic animals.
Keywords: spontaneous seizure, GABA(A) receptor, pilocarpine
Introduction
Fast synaptic inhibition is primarily mediated by post-synaptic GABAA receptors (GABARs). The most abundant receptor subtype, α1β2γ2, is localized at the synapse, and mediates phasic inhibition1,2. Alterations in subunit expression and composition that effect the localization, function, and pharmacology of GABARs have been demonstrated during primary epileptogenesis and following the onset of spontaneous seizures in animal models of epilepsy3-9, as well as in tissue resected from patients with intractable TLE10,11. Status epilepticus (SE) results in changes in the expression and membrane localization of several GABAR subunits (e.g., α1, α4, γ2, and δ) in hippocampal dentate granule cell neurons; specifically, α1 and δ subunits decrease and α4 and γ2 subunit expression increases, resulting in a change in receptor subunit composition and localization in these neurons7,12,13 that is predicted to impair inhibitory function14. Preventing these changes using viral gene transfer can inhibit development of epilepsy in the pilocarpine model of TLE15.
The signaling mechanisms that mediate GABARα4 subunit plasticity after SE (in primary epileptogenesis) have been defined. Increases in GABARα4 subunits are transcriptionally regulated by BDNF activation of the TrkB receptor, its downstream signaling cascades (protein kinase C (PKC) and mitogen activated protein kinase (MAPK)), as well as upregulation of its target (early growth response factor 3 (Egr3))16. Moreover, levels of both BDNF and Egr3 increase12,16,17, and binding of Egr3 to the Egr response element (ERE) in the endogenous GABARα4 subunit gene increases (Gabra4)17 following SE. Whether similar mechanisms mediate changes in GABAR α4 during chronic epilepsy, and the role, if any, spontaneous seizures may play in this regulation remains unknown.
The current studies investigate whether GABARα4 protein expression in the dentate gyrus of chronically epileptic rats is altered following recent spontaneous seizure (SS), and if this is regulated by activation of signal transduction pathways similar to those that are activated during primary epileptogenesis. We now report for the first time increases in levels of BDNF and GABARα4 within 3h after SS that parallel those seen following SE, and that increases in GABARα4 following SS are transient, but associated with overall higher mean seizure frequencies.
Materials and Methods
Pilocarpine injections
SE was induced in adult Sprague-Dawley rats (Charles-River Labs, Kingston, PA) as previously described12 using 385 mg/kg i.p. pilocarpine after pre-treatment with 1mg/kg i.p. scopolamine and followed by diazepam (6 mg/kg, i.p.) 1h after onset of SE, with additional 3 mg/kg every 2h as needed to ablate persistent motor seizures. Age-matched control rats received subconvulsive doses of pilocarpine (38.5 mg/kg i.p.), did not develop SE nor spontaneous seizures, and were handled and housed identically to the epileptic animals. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus approved and conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
Surgical implantation of electrodes
Chronically epileptic rats (4 weeks post-SE) and controls were implanted with two stainless steel screws placed 4mm caudal to bregma and 2.5mm lateral from midline bilaterally with reference and ground electrodes placed bilaterally behind lambda, as previously described18. Animals were allowed to recover from surgery for 1 week before proceeding with any further experimentation.
EEG Acquisition and Analysis
Rats subjected to pilocarpine-induced SE and control rats were video-EEG recorded 24h/day using a Pinnacle digital video-EEG system with flexible cables to monitor seizure frequency as previously described18. EEG signals were sampled at 1kHz, amplified by 100x, and band-pass filtered between 0.3 Hz and 600 Hz. Prior to inclusion in the study, all pilocarpine treated SE animals were confirmed to have at least 2 EEG-documented spontaneous seizures (SS) and thus meet criteria for epilepsy. EEG records of these chronically epileptic animals were then reviewed daily, and animals were sacrificed if they had (1) experienced a seizure within the past 3 h (recent SS or <3h group) or (2) had been seizure-free for > 24 h (no recent SS or >24h group). After sacrifice, and tissue collection, electrographic recordings spanning the 7 days prior to sacrifice were visually examined by a trained technician blinded to all experimental parameters to determine the number of electrographic seizures that had occurred for each animal. Electroencephalographic seizures were differentiated from background noise by the appearance of large-amplitude (> 3 times baseline), high-frequency (> 5 Hz) activity, with progression of the spike frequency lasting > 10 sec. Seizure quantification was conducted based on seizure number and type (convulsive or non-convulsive). Motor seizures were scored by standard behavioral classes19, with class 3 or greater seizures categorized as convulsive seizures and class 2 or below considered non-convulsive.
RT-PCR and Western Blots
Epileptic animals (56.21 ± 13.99 days after pilocarpine-induced SE) confirmed to have had at least 2 SS were sacrificed <3h from the time of a SS or >24h after the last SS. Tissue from age-matched controls was also analyzed to assess changes in protein and mRNA potentially induced by spontaneous seizure activity. RNA was extracted from microdissected hippocampal dentate gyrus tissue using RNeasy Mini RNA extraction kit (Qiagen). Primers and probe for BDNF (Rn02531967), Gabra4 (Rn00589846_m1), and cyclophilin (Rn00690933_m1) were purchased from Applied Biosystems. Primers and probe for Egr3 were designed using primer express software (PE Biosystems). Primer and probe sequences for Egr3 were: Egr3 forward 5′-GAGATCCCCAGCGCGC-3′, Egr3 reverse 5′-CATCTGAGTGTAATGGGCTACCG-3′, Egr3 Taqman 5′-CAACCTCTTCTCCGGCAGCAGTGAC-3′. Samples were repeated in duplicate with each reaction split into two wells in a total volume of 20 μl containing 16ng of RNA using an ABI Prism 7900HT machine. PCR cycling parameters were 50°C for 30 minutes, 95°C for 10 minutes, 50 cycles of 95°C for 15 sec, and 60°C for 1 minute. All values were normalized to cyclophilin expression to control for loading variability and expressed as fold change with respect to the mean control values (defined as 1).
Western blot was performed on protein (25 μg for GABARα4, 30 μg for Egr3) extracted from microdissected DG as previously published15. Membranes were incubated with rabbit polyclonal antibodies raised against GABARα4 (anti-GABARα4, Millipore AB5457; RRID: AB_177479; 1:2,000) in 1% milk/TBS-T or Egr3 (anti-Egr-3 (H-180) N –terminus, Santa Cruz, sc22801; RRID: AB_2097199; 1:400) in 2% milk/TBST overnight in 4°C. Membranes were then washed and incubated with anti-rabbit secondary antibody (GE health, 1:10,000 in 1% milk/TBST for GABAR) or (Veryblot, Abcam AB131366; 1:500 in 2% milk/TBST for Egr3) for 1h at room temperature. Bands were detected using chemiluminescent solution (Pierce), membranes were stripped and reprobed with rabbit polyclonal antibody raised against β-actin (1:40,000, Sigma) in 1% milk/TBS-T overnight. GABARα4 and Egr3 values were normalized to β-actin expression in the same samples and expressed as percent change relative to mean control values in the same run (defined as 1). Densitometry was performed with NIH Image J version 1.42q (RRID:nif-0000-30467). Statistical significance was was calculated with Prism software (RRID:rid_000081) using a One-way ANOVA with a Tukey's test for multiple comparisons or an unpaired student's t-test as indicated.
Chromatin Immunoprecipitation
Rats were perfused with cold 1X PBS (with 1:1000 phosphatase inhibitors) then 4% formaldehyde. Whole brains were immersed overnight in 4% formaldehyde and sectioned at 600 μm. Microdissected DG was sonicated to produce 300-500 bp fragments of crosslinked protein-DNA complexes and precipitated using an Egr3 specific antibody (10μgs; Santa Cruz Biotechnology) or normal rabbit IgG (2μgs; Santa Cruz Biotechnology) to account for non-specific DNA pulldown. Immunoprecipitated protein-DNA complexes were reverse-crosslinked and purified for PCR analysis using the Protein A-conjugated magnetic bead ChIP kit (Millipore). Quantitative real-time RT-PCR was performed using primers that flank the Egr3 binding site in the GABRA4 promoter and a Taqman probe: forward, 5′- GAACAAACTTGCCTAGCTTCGCGT-3′; reverse, 5′- TCCTCCAGCCTAGCCGC-3′; Taqman probe, 5′- AAGTTCACCGGCGAGCAGCGCTTTCA-3′. Data were normalized to input signal [Egr3 / Input] and expressed as fold change with respect to control (defined as 1). Input represents the DNA signal from sample preparation prior to immunoprecipitation.
Results
Regulation of GABARα4 protein by spontaneous seizure activity
Previous experiments have demonstrated that increased transcription of Gabra4 following prolonged pilocarpine-induced seizures (SE) is mediated by binding of Egr3 to the Gabra4 ERE site in the core promoter region 24h following SE17. Here for the first time, we evaluated whether increased GABARα4 expression may also occur during secondary epileptogenesis in response to acute SS. Animals sacrificed within 3h of a recent SS had significantly higher GABARα4 protein levels than control rats (Fig 1A and 1C), yet epileptic rats that had not had a seizure for >24h have GABARα4 protein levels similar to control rats (Fig 1B and 1C). Most importantly, epileptic rats having a SS within the last 3h also had significantly higher GABARα4 protein levels when compared to epileptic rats without a seizure for >24h (Fig 1C) suggesting recent SS activity induces increased GABARα4 protein expression in chronically epileptic animals. Continuous video-EEG collected from the same animals for 7-days prior to sacrifice was analyzed to determine whether there was a difference in overall seizure frequency between the two groups. Video-EEG data demonstrated that rats sacrificed <3h after SS which had higher levels of GABARα4 protein expression, also had significantly higher daily seizure frequency than the rats sacrificed >24h after the last seizure (Fig 1D). These results suggest that spontaneous seizures may induce a cascade of events that increase GABARα4 on a relatively short timescale, and that animals with frequent SS (ie., more severe epilepsy) may have higher overall GABARα4 levels.
Fig. 1. Spontaneous seizures increase GABARα4 protein expression in DG.
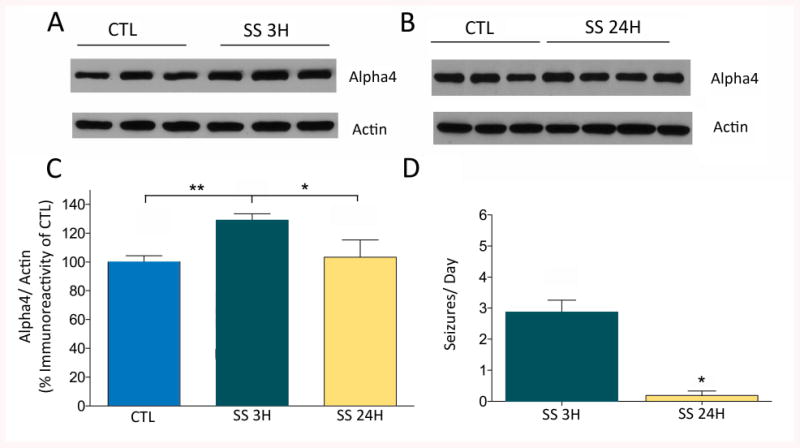
Western blots of DG protein homogenates of control rats or epileptic rats sacrificed within (A) 3h of a spontaneous seizure (n=6 controls, n=6 SS) or (B) > 24h after last spontaneous seizure (n=3 controls, n=4 No SS) reacted with anti-Alpha4 and anti-Actin antibodies. (C) Densitometry analysis demonstrates that rats with recent seizure activity show ∼23% increase in GABARα4 expression relative to controls (p<0.01) and ∼20% increase relative to epileptic rats without recent spontaneous seizures (p<0.05) using a one-way ANOVA with Tukey's test for multiple comparisons. Normalized data are presented as mean ± SEM and expressed as percent change. (D) The same rats that express increased GABARα4 protein levels in DG following a recent SS (i.e., <3h SS (SS 3H,n=6)) demonstrate a significantly higher seizure frequency (i.e., seizures/day) during the 7-day monitoring period prior to sacrifice relative to the >24h SS group (SS 24H, n=4; *p=0.0139). Significant differences in seizure frequency were detected using a Mann-Whitney test. For all graphs, Error bars, mean±S.E.M.
Spontaneous seizures increase GABARα4, BDNF and Egr3 mRNA levels
Evaluation of the mechanisms leading to increased GABARα4 protein levels demonstrated that rats with a recent SS had significantly higher levels of GABARα4 mRNAs in the DG than controls (Fig 2A), while epileptic rats with no seizures for >24h had GABARα4 mRNA levels similar to controls. Levels of BDNF mRNAs in the DG were increased 5-fold within 3h of SS in chronically epileptic rats relative to control rats (Fig 2B). BDNF mRNA levels in epileptic rats with no SS within 24h of sacrifice were similar to controls, suggesting changes in BDNF expression may be driven by recent seizure activity rather than being a residual effect of SE, or inherent to the epileptic state. As BDNF has previously been shown to regulate GABARα4 expression via its control over Egr3 levels, and the binding of Egr3 to the Gabra4 core promoter after SE16, we examined whether Egr3 mRNA levels also change following recent SS. Levels of Egr3 mRNAs increase by greater than eight-fold following a recent (<3h) spontaneous seizure and were significantly higher than epileptic rats with no seizures for >24h (Fig 2C). However, the 14.37% increase in mean Egr3 protein expression following recent spontaneous seizures (i.e., in the <3h SS group) in the dentate gyrus was not significantly different from controls. Chromatin immunoprecipitation was used to examine whether increased binding of Egr3 protein to the promoter of the Gabra4 subunit gene occurred within 3h of a spontaneous seizure, and might account for the increased GABARα4 mRNA levels. Although Egr3 protein binding to the ERE promoter of the Gabra4 subunit gene was observed in the dentate gyrus following spontaneous seizures, the levels were not significantly different than that seen in controls (Fig 2D).
Fig 2. Spontaneous seizures increase GABARα4, BDNF and Egr3 mRNA expression in DG.
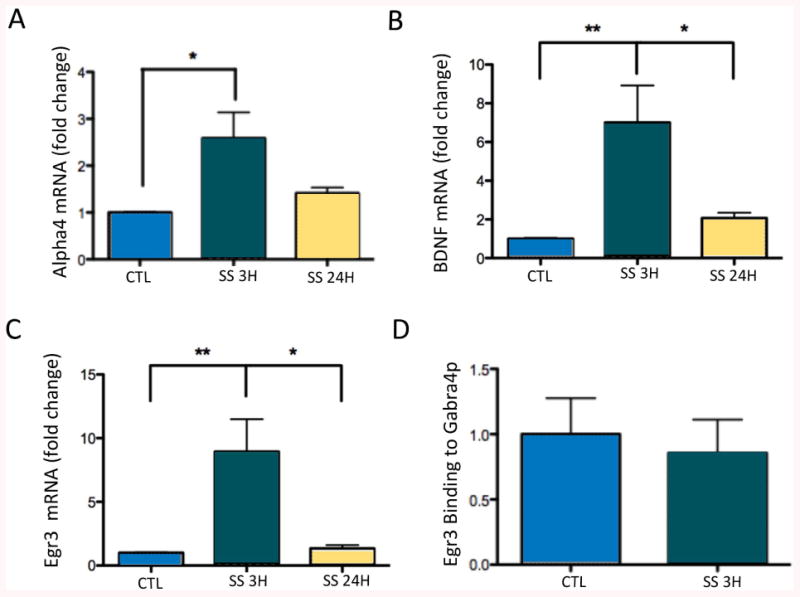
(A) Quantification of RT-PCR analysis of GABARα4 mRNA expression in DG <3 and >24h after SS (controls, n=4; SS 3H, n=5; SS 24H, n=4) demonstrates a significant (p<0.05) increase in GABARα4 mRNA levels following recent spontaneous seizure activity (within 3h) relative to control rats. Epileptic rats that had not had a seizure for >24h have GABARα4 mRNA levels similar to controls. (B) DG from epileptic rats with recent SS (within 3h, n=8) showed significant (p<0.05) increases in BDNF mRNA expression compared to control (n=7), and compared to rats without SS for >24h (n=6). (C) DG from epileptic rats with recent SS (within 3h) showed significant (p<0.05) increases in Egr3 mRNA expression compared to rats that had not had a SS for >24h and controls (controls, n=4; SS 3H, n=8; SS 24H, n=4). Expression of all mRNAs was normalized to cyclophilin and expressed as fold change compared to controls (defined as 1). (D) Quantification of protein-DNA binding in DG <3h and >24h after SS (controls, n=3; SS 24H, n=4) demonstrates no difference in Egr3 protein binding to the ERE promoter of Gabra4 following recent spontaneous seizure activity (within 3h) relative to control rats. Data were normalized to input signal [Egr3/Input] and expressed as fold change with respect to control (defined as 1). Input represents the DNA signal from sample preparation prior to immunoprecipitation. Statistically significant differences in mRNA levels and in protein-DNA binding were determined using a One-way ANOVA with Tukey's test for multiple comparisons. For all graphs, Error bars, mean±S.E.M.
Changes in seizure semiology related to GABAR differences
Continuous video-EEG monitoring was used to characterize seizure frequency, seizure type, and temporal distribution for 7 days prior to sacrifice (Fig 3). Rats sacrificed within 3h of a recent seizure had significantly higher seizure frequencies overall than rats sacrificed >24h after the last spontaneous seizure (Fig 3A), suggesting they had more severe epilepsy in general. However, despite the difference in seizure frequency between the <3h SS and >24h SS groups, each group had similar seizure durations and severity (i.e. convulsive vs. nonconvulsive [3B and 3C]). Interestingly, rats sacrificed >24h after SS, had significantly more prolonged interictal intervals (defined as >300 min or ∼6h) than animals sacrificed <3h after SS (3D). Increases in GABARα4 mRNA and protein levels occurred following high frequency seizure clusters with or without prolonged inter-cluster intervals (fig 3A, Fig 3E-F) suggesting that spontaneous seizure clusters can induce GABARα4 subunit plasticity even when they occur infrequently. High frequency seizure clusters were more common in <3h SS animals, but were present in some of the >24h SS group, however, the group consistently had GABARα4 mRNA and protein levels similar to control rats when sacrificed following long periods of seizure freedom (i.e., long interictal intervals (G-H)). This suggests that GABARα4 levels are quite plastic, increasing transiently following a spontaneous seizure and then falling again after a long period of seizure freedom.
Fig. 3. Characterization of seizures in epileptic rats.
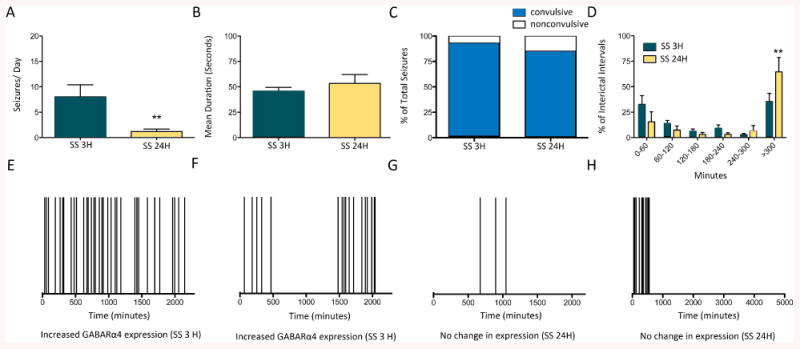
(A) EEG data across all rats used for above experiments for <3h SS group (n=16) demonstrate a significantly higher seizure frequency (i.e., seizures/day) during the 7-day monitoring period prior to sacrifice relative to the >24h SS group (n=6; **p=0.0019). Statistically significant differences were determined using a Mann-Whitney test. During the same 7-day period, continuous EEG monitoring demonstrated no significant difference in seizure duration (B) or in the proportion of convulsive seizures to non-convulsive seizures present in the <3h SS group or the >24h SS group (C). Statistically significant differences were determined using an unpaired t-test and a Fischer's exact test, respectively. (D) Frequency histograms of the interseizure intervals for the group of epileptic rats sacrificed within 3h of last spontaneous seizure and >24h after last spontaneous seizure. The first bin (<60 min or >1 seizure/h) had a high number of interseizure intervals for both groups suggesting the presence of seizure clusters. A significant difference between the two groups was found in <300 (or ∼6 h) bin demonstrating the SS 24 H group had more long interseizure intervals (or inter-cluster intervals). Statistically significant differences were determined using a One-way ANOVA with a Newman-Keul's test for multiple comparisons. Representative raster plots depicting the time of previous seizure occurrence under the conditions of increased GABARα4 mRNA and protein expression within 3h of a spontaneous seizure (E and F) or unchanged GABARα4 mRNA and protein expression (G and H) in DG of rats without seizures for >24h.
Discussion
The results of these studies are the first demonstration that spontaneous seizures may acutely regulate GABARα4 RNA and protein expression, suggesting that spontaneous seizures (regardless of whether convulsive or non-convulsive in nature) result in acute transcriptional up-regulation of the Gabra4 gene in the DG of epileptic rats.
Spontaneous seizures, GABARα4 increases, and chronic epilepsy severity
Increased levels of GABARα4 RNAs and protein were associated with higher overall seizure frequencies in the epileptic rats sacrificed within 3h of a SS. These data could support two different, but not mutually exclusive, interpretations. One interpretation is that in chronic epilepsy the spontaneous, recurrent seizures drive GABARα4 increases via acute activation of cellular signaling pathways after each epileptic event (i.e., reactive plasticity of GABAR subunits). Long periods of seizure freedom allow the system to “reset” and GABAR subunit levels return to control levels until a SS again induces GABARα4 increases. In addition, it is possible that the increased seizure frequency observed in these animals may, in part, be a consequence of long-term changes in GABAA-receptor subunit expression. The most parsimonious explanation of our data is that these scenarios exist together, with spontaneous seizures themselves stimulating the change in GABARα4 subunit expression, and that these alterations, in turn, may contribute to higher overall seizure frequencies that then perpetuate and/or exacerbate the GABAR alterations.
Changes in GABAR function in chronically epileptic rats with spontaneous seizures
Increases in GABAA receptor α4 subunit expression have been demonstrated in epileptic animals in several TLE models3,5,7,20. The functional consequences of these increases in GABARα4 subunit levels depends in large part on what other subunits it pairs with to compose a receptor. Immuno-precipitation and immunogold labeling studies suggest that α4 subunits can assemble with either δ or γ2 subunits12,21,22, with α4δ-containing receptors predominating in normal dentate gyrus and α4γ-containing receptors becoming more abundant in epileptic tissue5,7,12. Delta-containing GABAA receptors are located primarily at extrasynaptic sites and have traditionally been thought to mediate tonic inhibition, while phasic inhibition is governed by γ-containing-GABAA receptors located at synaptic or perisynaptic regions23-26. Despite a diminished expression of δ subunit mRNA7,20 and a reduction in functional α4δ-containing GABARs in the hippocampus of epileptic animals13, tonic GABAergic inhibition is maintained or increased in epileptic animals27. Some studies in chronically epileptic animals suggest that the novel α4γ2-containing receptors are responsible for maintaining tonic inhibition13, while others find that an increase in such receptors in synaptic and/or perisynaptic locations are responsible for altered phasic GABA currents in epileptic hippocampus7. Thus, alterations in GABARα4 subunit composition may be associated with changes in both tonic and phasic GABAR-mediated inhibition.
Alterations in α-subunit subtype can result in differences in GABAAR modulation by benzodiazepines, neurosteroids, and zinc28-31. Pharmacological experiments suggest that increased levels of α4γ-containing receptors in epileptic brain may contribute to an altered response of GABARs to GABA and GABAergic drugs. Lagrange et al., 2007 reported that α4γ-containing receptors are more rapidly desensitized and recover more slowly from desensitization. They further found that exposure to prolonged low levels of GABA greatly suppressed the response of α4γ-containing receptor currents to higher concentrations of GABA. Overall, these receptors appeared less efficacious when exposed to prolonged low levels of GABA or during repetitive stimulation, as may occur during seizures. These results are consistent with the observaton that, in epileptic animals, GABARs become largely insensitive to higher GABA concentrations32. Thus, drug treatments that increase the levels of extrasynaptic GABA may negatively modulate the ability of extrasynaptic α4γ-containing receptors to respond to variations in ambient GABA that may occur following SSs, as well as during status epilepticus33. Additionally, as α4-containing GABAARs are more sensitive to zinc blockade, a shift to more α4-containing GABAARs may be associated with the enhanced blockade of the GABAA response by zinc in the dentate gyrus seen in both acutely after SE and in chronic epilepsy in both animals28-30,34 and in humans with TLE35. Zinc is distributed by aberrant mossy fiber axons of dentate granule cells that innervate the inner molecular layer of the DG in late stages of epileptogenesis (i.e., after the onset of SSs). The α4-containing GABAA receptors present on dendrites of epileptic DG neurons are exquisitely sensitive to blockade by zinc, and thus may contribute to loss of inhibitory function and increased excitability29,35. The transcriptional upregulation of GABAARα4 subunit gene expression seen in association with spontaneous seizures may thus result in changes in both tonic and phasic GABAR-mediated inhibition that could impact seizure susceptibility in chronically epileptic rats.
Caveats related to assessing Egr3 regulation of Gabra4 changes
The increases in BDNF levels following recent spontaneous seizure activity (i.e., within 3h) suggest that SSs may recurrently “reactivate” signaling pathways that could contribute to the perpetuation and progression of epilepsy. However, although there was a marked and signficant increase in levels of Egr3 mRNA, as well as BDNF, following recent SS, we did not detect an associated increase in Egr3 binding to the Gabra4 promoter. Without this evidence we cannot definitively conclude that BDNF-induced increases in Egr3 are driving the induction of GABARα4 transcription following SS. Our evidence does allow us to conclude, however, that Egr3 is present at the promoter region and is part of the transcriptional complex that most likely underlies increased rates of transcription in our studies. Little is known about the mechanism of Egr3 directed gene regulation in neurons and research in this area has been hampered by the lack of antibodies with which to probe the expression of its many variants, an active area of investigation in our laboratories
Conclusions
These studies provide the first evidence of acute molecular regulation of GABAR subunit expression in association with spontaneous seizures during chronic epilepsy. The current study specifically examines changes in GABARα4, and as it is known that expression of multiple GABAR subunits may be alerted in chronic epilepsy, the role of spontaneous seizures in regulating other subunits, and the subsequent effects on receptor composition and function, merits additional study. Better understanding of the molecular changes induced by spontaneous seizures and their functional consequences could contribute to the development of disease modifying therapies that do not just symptomatically treat seizures but potentially inhibit their progression and long-term adverse consequences.
Acknowledgments
The authors would like to thank the University of Colorado Neurophysiology Core for assistance related to EEG monitoring. Funding was provided by the NIH, National Institute of Neurological Disorders and Stroke R01NS051710 (to ABK and SJR) and the Epilepsy Foundation (to HG).
Footnotes
Disclosure of Conflicts of Interests: None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Brooks-Kayal AR, Shumate MD, Jin H, et al. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 4.Lauren HB, Pitkanen A, Nissinen J, et al. Selective changes in gamma-aminobutyric acid type A receptor subunits in the hippocampus in spontaneously seizing rats with chronic temporal lobe epilepsy. Neurosci Lett. 2003;349:58–62. doi: 10.1016/s0304-3940(03)00735-3. [DOI] [PubMed] [Google Scholar]
- 5.Peng Z, Huang CS, Stell BM, et al. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperk G, Furtinger S, Schwarzer C, et al. GABA and its receptors in epilepsy. Adv Exp Med Biol. 2004;548:92–103. doi: 10.1007/978-1-4757-6376-8_7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Wei W, Mody I, et al. Altered localization of GABA(A) receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamphuis W, De Rijk TC, Lopes da Silva FH. Expression of GABAA receptor subunit mRNAs in hippocampal pyramidal and granular neurons in the kindling model of epileptogenesis: an in situ hybridization study. Brain Res Mol Brain Res. 1995;31:33–47. doi: 10.1016/0169-328x(95)00022-k. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzer C, Tsunashima K, Wanzenbock C, et al. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–1017. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 10.Brooks-Kayal AR, Shumate MD, Jin H, et al. Human neuronal gamma-aminobutyric acid(A) receptors: coordinated subunit mRNA expression and functional correlates in individual dentate granule cells. J Neurosci. 1999;19:8312–8318. doi: 10.1523/JNEUROSCI.19-19-08312.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund IV, Hu Y, Raol YH, et al. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci Signal. 2008;1:ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajasekaran K, Joshi S, Sun C, et al. Receptors with low affinity for neurosteroids and GABA contribute to tonic inhibition of granule cells in epileptic animals. Neurobiol Dis. 2010;40:490–501. doi: 10.1016/j.nbd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of alpha4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raol YH, Lund IV, Bandyopadhyay S, et al. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts DS, Hu Y, Lund IV, et al. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DS, Raol YH, Bandyopadhyay S, et al. Egr3 stimulation of GABRA4 promoter activity as a mechanism for seizure-induced up-regulation of GABA(A) receptor alpha4 subunit expression. Proc Natl Acad Sci U S A. 2005;102:11894–11899. doi: 10.1073/pnas.0501434102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabenstatter HL, Cruz Del Angel Y, Carlsen J, et al. The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis. 2013;62:73–85. doi: 10.1016/j.nbd.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura T, Schwarzer C, Gasser E, et al. Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Sur C, Farrar SJ, Kerby J, et al. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 22.Jia F, Pignataro L, Schofield CM, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 23.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 24.Wisden W, Cope D, Klausberger T, et al. Ectopic expression of the GABA(A) receptor alpha6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- 25.Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- 26.Chandra D, Jia F, Liang J, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs JW, 3rd, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 29.Cohen AS, Lin DD, Quirk GL, et al. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, Mtchedlishvili Z, Erisir A, et al. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the alpha4 subunit of GABA(A) receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overstreet LS, Jones MV, Westbrook GL. Slow desensitization regulates the availability of synaptic GABA(A) receptors. J Neurosci. 2000;20:7914–7921. doi: 10.1523/JNEUROSCI.20-21-07914.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naylor DE, Wasterlain CG. GABA synapses and the rapid loss of inhibition to dentate gyrus granule cells after brief perforant-path stimulation. Epilepsia. 2005;46(Suppl 5):142–147. doi: 10.1111/j.1528-1167.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 34.Leroy C, Poisbeau P, Keller AF, et al. Pharmacological plasticity of GABA(A) receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol. 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shumate MD, Lin DD, Gibbs JW, 3rd, et al. GABA(A) receptor function in epileptic human dentate granule cells: comparison to epileptic and control rat. Epilepsy Res. 1998;32:114–128. doi: 10.1016/s0920-1211(98)00045-x. [DOI] [PubMed] [Google Scholar]


