Abstract
Purpose
Low appendicular skeletal muscle mass (ASMM) is associated with premature mortality, hyperinsulinemia, frailty, disability, and low bone mineral density. We explored the potential efficacy of slowly-progressive weight lifting to attenuate the decline of ASMM among breast cancer survivors by conducting a post hoc analysis of data from the Physical Activity and Lymphedema trial.
Methods
Between October 2005 and August 2008 we conducted a single-blind, randomized controlled trial of twice-weekly slowly-progressive weight lifting or standard care among 295 non-metastatic breast cancer survivors. ASMM was quantified using dual-energy x-ray absorptiometry. Changes in ASMM were evaluated from baseline to 12-months between the weight lifting and control groups using repeated-measures linear mixed-effects regression models.
Results
Over 12-months, participants in the weight lifting group experienced attenuated declines in muscle mass compared to the control group, as reflected by relative ASMM (−0.01±0.02 kg/m2 versus −0.08±0.03 kg/m2; P=0.041) and absolute ASMM (−0.02±0.06 kg versus −0.22±0.07 kg; P=0.038), respectively. Weight lifting did not alter other body composition outcomes including body mass index, total body mass, body fat percentage, and fat mass compared to the control group. Weight lifting significantly increased upper- and lower-body muscle strength compared to the control group. The intervention was well-tolerated with no serious adverse events related to weight lifting.
Conclusions
Slowly-progressive weight lifting attenuated the decline of ASMM among breast cancer survivors compared to standard care over 12-months. These data are hypothesis generating. Future studies should examine the efficacy of weight lifting to improve distal health outcomes among breast cancer survivors.
Keywords: muscle, exercise, physical activity, strength training, aging
INTRODUTION
Each year 232,000 women are diagnosed with breast cancer in the United States [1]. The median age at diagnosis of breast cancer is 61-years [1]. Five-year survival rates for non-metastatic breast cancer exceed 84% [2]. Older age at diagnosis and promising long-term survival rates have created a population of breast cancer survivors that will experience unfavorable alterations in body composition as a result of aging and cancer treatment [3, 4].
An important change in body composition related to aging and cancer treatment is the loss of skeletal muscle mass [3, 4]. Perhaps the most important skeletal muscles are those of the arms and legs, known as appendicular skeletal muscles, which account for ≥75% of total skeletal muscle in the body, and are necessary for ambulatory activity and physical functioning [5]. After the age of 35–45 years, women lose approximately 0.1 kg (0.5%) of appendicular skeletal muscle mass (ASMM) annually [5–8]. This may be relevant to breast cancer survivors because low ASMM is associated with dysregulation of insulin-axis hormones [9–12], which have been implicated in breast cancer recurrence and survival [13]. Low ASMM is associated with all-cause mortality among breast cancer survivors [14]. In addition, low ASMM may be associated with frailty [15], disability [15, 16], and low bone mineral density [12].
These observational data suggest that interventions which attenuate the loss of ASMM may hold clinical importance for breast cancer survivors. It is unknown if lifestyle interventions such as weight lifting preserve ASMM among breast cancer survivors. We tested the hypothesis that slowly-progressive weight lifting attenuates the decline of ASMM among breast cancer survivors by conducting a post hoc analysis of data from the Physical Activity and Lymphedema (PAL) trial [17]. The PAL trial was a randomized controlled trial designed to examine the safety of weight lifting among non-metastatic breast cancer survivors with or at-risk for developing breast cancer-related lymphedema [18, 19].
METHODS
Study Design and Participants
A description of the PAL trial methods are described elsewhere in detail [17]. The primary outcomes of the PAL trial have been published for breast cancer survivors with lymphedema [18], and at-risk for lymphedema [19]. The analysis described herein includes breast cancer survivors with and at-risk for lymphedema. Breast cancer survivors were recruited throughout the metropolitan Philadelphia region [20]. Participants were eligible for the study if they were a female breast cancer survivor, 1–15 years post-diagnosis, free from cancer at study entry, with ≥1 lymph node(s) removed, and no medical conditions or contraindicated medications that would prohibit participation in an exercise program. Additional eligibility criteria included a body mass index (BMI) ≤50 kg/m2, no plans for surgery during the study, no history of bilateral lymph node removal, no weight lifting in the previous year, stable body weight (<10% change in the past year), and not attempting to lose weight.
Participants were stratified on the following baseline variables: lymphedema status (diagnosed or not diagnosed), age (<54 years or ≥54 years), difference in lymphedema arm volume (<10% or 10–20% or >20%), number of lymph nodes removed (<6 or ≥6), obesity (BMI <30 or ≥30 kg/m2), and time since diagnosis (<60 months or ≥60 months) and then randomized to one of the two study groups using a minimization procedure [17].
This trial was approved by the University of Pennsylvania institutional review board and registered as NCT00194363. All participants provided written informed consent and provided written clearance from their physician prior to participating in any study-related activities.
Intervention
Study participants randomized to the weight lifting group were provided with a 12-month membership to a community fitness center. For the first 13-weeks, participants were given instruction on the safe completion of the weight lifting exercises in small groups of two to six participants. Certified exercise professionals employed by the fitness centers led the twice-weekly exercise sessions that each lasted 90-minutes. Each session included stretching of major muscle groups, cardiovascular warm up, abdominal and lower back strengthening exercises, and weight lifting exercises. Weight lifting exercises for the upper-body included the dumbbell press, seated row, lateral or front raise, bicep curls, and triceps extension. Weight lifting exercises for the lower-body included the leg press, back extension, leg extension, and leg curl. One to three new weight lifting exercises were taught at each session. For each exercise session, three-sets of each weight lifting exercise were performed using 10-repetitions per set. For each exercise, weight was increased by the smallest possible increment after two sessions at which three-sets of 10-repetitions could be performed without concurrent changes in arm and hand symptoms. No maximal upper limit was placed on the weight lifted for each exercise.
After 13-weeks of supervised weight lifting, participants were instructed to continue unsupervised weight lifting for 39-weeks adhering to the same exercise prescription used during the supervised portion of the trial. Weight lifting adherence was defined as attendance to the weight lifting sessions at the community fitness centers. Attendance was monitored using logs completed by study participants that were verified for accuracy by the exercise professionals. The exercise professionals contacted study participants if they missed more than one exercise session each week throughout the year. Participants in the control group were asked to maintain their baseline level of physical activity throughout the study. Upon study completion, control group participants were offered a 12-month membership to a community fitness center, with 13-weeks of supervised exercise instruction, similar to that of the weight lifting group.
Safety
All community-based certified exercise professionals who worked with study participants completed a three-day training course that reviewed the exercise protocol and outlined lymphedema management practices. Study participants with lymphedema received custom-fitted compression garments (Jobst, BSN Medical) at baseline and six-months. When participants with lymphedema were asked to do weight lifting they were required to wear a study-provided lymphedema garment during all exercise. The certified exercise professionals systematically asked all participants each week about changes in lymphedema symptoms and measured arm circumference and arm volume each month to detect changes in arm swelling. All study participants were required to complete an educational lecture about lymphedema risk-reduction, management, and exercise safety, based on the National Lymphedema Network clinical practice guidelines (http://www.lymphnet.org/resources).
Measurements
Measurements were obtained from all participants at baseline and 12-months by trained staff that followed a standardized protocol and were blinded to study group. Prior to completing the measurement visit, all study participants were reminded not to disclose their study group to the measurement staff.
Demographic characteristics including age, education, race, and smoking habits were self-reported at baseline. Self-rated general health was assessed using a five-item ordinal scale. Clinical characteristics including time since cancer diagnosis, and cancer stage were collected from the state cancer registry, surgical pathology report, or self-report. The presence and severity of lymphedema was quantified using water displacement volumetry [17]. Cancer treatment including chemotherapy, radiation, and endocrine therapies were self-reported. Caloric consumption was quantified using the Diet History Questionnaire [21]. Physical activity was assessed using the International Physical Activity Questionnaire [22].
Muscular strength was quantified using one-repetition maximum (1-RM) testing with bench press, leg press, and handgrip exercises. 1-RM testing is the maximum amount of weight that can be lifted one time. 1-RM tests are safe for clinical populations with appropriate conduct and supervision, and provide direct quantification of muscular strength [23]. Participants completed a warm-up set of 4–6 repetitions using a weight of 2.25 kg on the bench press and 18.2 kg on the leg press, and then rated exertion using a 1–10 scale. Participant rating established the first weight at which a 1-RM test was attempted. Additional weight was added until exercise biomechanics were compromised or the participant was unwilling or unable to try a heavier weight. Isometric handgrip strength was assessed in the dominate hand in the seated position with the elbow flexed at a right angle and the forearm in a neutral position. The handle was adjusted to the hand size and no extraneous body movement was allowed during testing. Three maximal contractions each lasting four to five seconds with a 60 second rest interval were performed. The maximum of the three readings was taken as the maximum handgrip strength.
Outcome
The outcome of this post hoc analysis was ASMM. ASMM was measured as the sum of lean soft tissue (non-fat and non-bone) mass in the arms and legs using whole-body dual-energy x-ray absorptiometry (DXA; Hologic Discovery, Bedford MA). All DXA scans were reviewed for accuracy by a bio-nutritionist who was blinded to study group. The DXA scanner was calibrated daily using a soft-tissue phantom. ASMM was quantified in two outcomes [24]. The first outcome represents relative ASMM by accounting for participant stature, defined as kg of muscle mass per m2 of height (kg/m2). The second outcome represents absolute ASMM, defined as kg of muscle mass (kg). DXA was also used to quantify body fat percentage (%) and fat mass (kg). Other anthropometric measures included height (m) and body mass (kg), which were used to calculate BMI (kg/m2).
Statistical Analysis
All statistical analyses were completed using Stata MP Version 13.1 (StataCorp, College Station, TX). Descriptive statistics presented for baseline variables include counts and proportions for categorical variables and means ± standard deviations for continuous variables. Categorical variables were compared between the two study groups using Fisher’s exact test, and continuous variables were compared between the two study groups using the Wilcoxon rank-sum test. All inferential analyses were conducted on an intention-to-treat basis. Changes in ASMM were evaluated from baseline to 12-months between the weight lifting and control groups using repeated-measures linear mixed-effects regression models. This statistical approach includes all available data and accounts for the correlation between repeated measures. The baseline value of the dependent variable was included as a covariate in the regression models to reduce variance [25]. A group-by-time interaction term was included as a fixed effect in the regression model. Results from the repeated-measures linear mixed-effects regression models are presented as means ± standard error. Given the exploratory nature of this analysis we made no adjustment for multiplicity. A total of 10 outcomes were examined in this post hoc analysis (three muscle strength outcomes and six body composition outcomes); thus, one statistically significant test (P<0.05) might be expected by chance alone. P values for all analyses should be interpreted conservatively and considered as hypothesis generating.
RESULTS
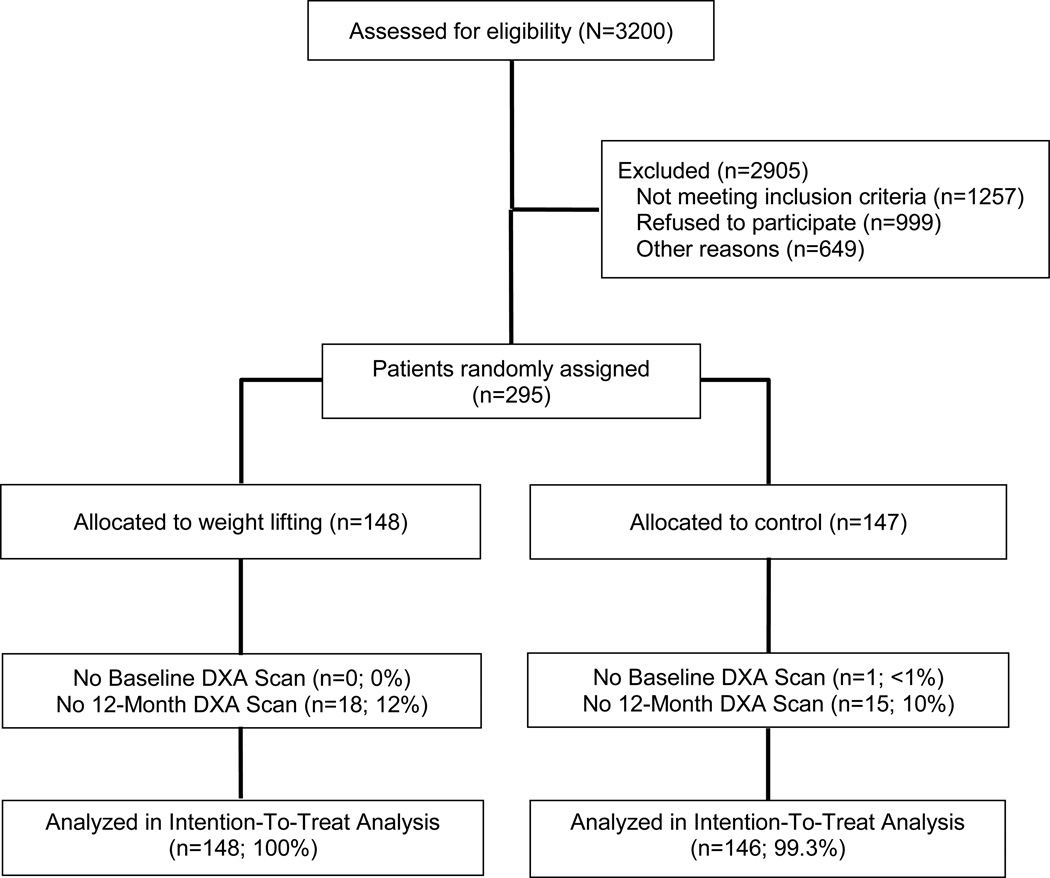
Between October 2005 and February 2007, 295 breast cancer survivors were recruited and randomized with data collection ending for all study participants in August 2008. One randomized participant did not have a baseline DXA scan and was excluded from this analysis. Characteristics of study participants are presented in Table 1. All baseline study characteristics were balanced between groups.
Table 1.
Baseline Characteristics of Study Participants, According to Study Group
| Characteristic | Weight Lifting (N=148) |
Control (N=146) |
P |
|---|---|---|---|
| Age, yr. | 55.3±8.5 | 56.7±9.1 | 0.27 |
| Education, n (%) | |||
| High school or less | 20 (14%) | 27 (18%) | 0.48 |
| Some college | 54 (36%) | 47 (32%) | |
| College degree or more | 74 (50%) | 72 (50%) | |
| Race, n (%) | |||
| White | 47 (32%) | 43 (29%) | 0.07 |
| Black | 90 (61%) | 100 (69%) | |
| Other | 11 (7%) | 3 (2%) | |
| Smoking habits, n (%) | |||
| Never | 74 (50%) | 84 (57%) | 0.50 |
| Former | 66 (44%) | 55 (38%) | |
| Current | 8 (5%) | 7 (5%) | |
| Self-Rated General Health, n (%) | |||
| Excellent | 8 (5%) | 11 (8%) | 0.62 |
| Very Good | 60 (41%) | 54 (37%) | |
| Good | 63 (43%) | 69 (47%) | |
| Fair | 15 (10%) | 9 (6%) | |
| Poor | 0 (0%) | 1 (1%) | |
| Unknown/missing | 2 (1%) | 2 (1%) | |
| Time since cancer diagnosis, months | 57.9±38.1 | 63.7±40.4 | 0.12 |
| Cancer stage, n (%) | |||
| Ductal carcinoma in situ | 1 (<1%) | 0 (0%) | 0.35 |
| I | 69 (47%) | 63 (43%) | |
| II | 3 (2%) | 0 (0%) | |
| III | 44 (30%) | 47 (32%) | |
| Unknown | 31 (21%) | 36 (25%) | |
| Lymphedema, n (%) | 71 (48%) | 69 (47%) | 0.91 |
| Cancer Treatment | |||
| Chemotherapy, n (%) | 115 (78%) | 108 (74%) | 0.50 |
| Radiation, n (%) | 118 (80%) | 110 (75%) | 0.40 |
| Tamoxifen, n (%) | 29 (20%) | 19 (13%) | 0.23 |
| Aromatase Inhibitor, n (%) | 1 (<1%) | 1 (<1%) | 0.99 |
| Caloric Consumption (kcal/d) | 1731±1228 | 1654±1309 | 0.76 |
| Physical Activity (MET-hr/wk) | 64.0±66.9 | 58.4±63.2 | 0.48 |
Data are mean ± standard deviation or number (%). P values are from the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables.
The rates of attrition over 12-months were similar between the two study arms (Figure 1; P=0.712). Participants who did not have a 12-month DXA scan were more likely to be younger (50.3±8.7 versus 56.7±8.6; P<0.001), have greater handgrip strength (28.1±7.5 kg versus 25.2±6.3 kg; P=0.033), have a higher relative ASMM (7.9±1.4 kg/m2 versus 7.4±1.3 kg/m2; P=0.027), and have a higher absolute ASMM (21.2±3.8 kg versus 19.7±3.9; P=0.046). Among the subset of participants who did not have a 12-month DXA scan, there were no between-group differences for any baseline characteristics.
Figure 1.
Enrollment, Randomization, and Follow-Up of Study Participants. DXA, dual-energy x-ray absorptiometry.
Muscular strength outcomes are presented in Table 2. At baseline, maximal bench press strength ranged from 0.0 to 43.1 kg, maximal leg press strength ranged from 11.3 to 186.0 kg, and maximal handgrip strength ranged from 9.0 to 50.0 kg. The median adherence rate to the 12-month weight lifting protocol was 80% [interquartile range: 47–92%]. After 12-months, participants in the weight lifting group significantly improved all outcomes of muscular strength relative to the control group, as reflected by maximal bench press strength (+4.9±0.46 kg versus +0.6±0.35 kg; P<0.001), maximal leg press strength (+21.7±1.54 kg versus +3.8±1.33 kg; P<0.001), and maximal handgrip strength (+2.6±0.33 kg versus +1.3±0.50 kg; P=0.022), respectively.
Table 2.
Muscular Strength Outcomes at Baseline and Change (Δ) During 12-Months, According to Study Group
| Baseline | Δ Baseline to 12-Months | P | |
|---|---|---|---|
| Bench Press, kg | |||
| Weight Lifting Group | 19.1±0.54 | +4.9±0.46 | <0.001 |
| Control Group | 18.4±0.47 | +0.6±0.35 | |
| Leg Press, kg | |||
| Weight Lifting Group | 79.8±2.11 | +21.7±1.54 | <0.001 |
| Control Group | 77.9±2.12 | +3.8±1.33 | |
| Handgrip, kg | |||
| Weight Lifting Group | 25.2±0.55 | +2.6±0.33 | 0.022 |
| Control Group | 25.9±0.54 | +1.3±0.50 |
Data are means ± standard error. Change (Δ) values from baseline to 12-months are from the repeated-measures linear mixed-effects regression models. P values are for the group-by-time interaction term in the regression model.
Body composition outcomes are presented in Table 3. At baseline, relative ASMM ranged from 4.4 to 11.2 kg/m2, and absolute ASMM ranged from 11.2 to 30.5 kg. After 12-months, participants in the weight lifting group experienced attenuated declines in muscle mass compared to the control group, as reflected by relative ASMM (−0.01±0.02 kg/m2 versus −0.08±0.03 kg/m2; P=0.041; Figure 2) and absolute ASMM (−0.02±0.06 kg versus −0.22±0.07 kg; P=0.038), respectively. We explored the hypothesis that breast cancer-related lymphedema may influence the effects of weight lifting on ASMM. We did not find a group-by-time-by-lymphedema interaction for relative (P=0.310) or absolute (P=0.321) ASMM.
Table 3.
Body Composition Outcomes at Baseline and Change (Δ) During 12-Months, According to Study Group
| Baseline | Δ Baseline to 12-Months | P | |
|---|---|---|---|
| Relative Appendicular Skeletal Muscle Mass, kg/m2 | |||
| Weight Lifting Group | 7.5±0.11 | −0.01±0.02 | 0.041 |
| Control Group | 7.4±0.11 | −0.08±0.03 | |
| Absolute Appendicular Skeletal Muscle Mass, kg | |||
| Weight Lifting Group | 20.1±0.32 | −0.02±0.06 | 0.038 |
| Control Group | 19.7±0.32 | −0.22±0.07 | |
| Body Mass Index, kg/m2 | |||
| Weight Lifting Group | 29.6±0.48 | −0.26±0.09 | 0.865 |
| Control Group | 29.7±0.53 | −0.29±0.12 | |
| Total Body Mass, kg | |||
| Weight Lifting Group | 79.1±1.36 | −0.71±0.25 | 0.847 |
| Control Group | 79.3±1.44 | −0.79±0.34 | |
| Body Fat, % | |||
| Weight Lifting Group | 37.8±0.47 | −0.10±0.17 | 0.191 |
| Control Group | 38.4±0.48 | 0.23±0.18 | |
| Fat Mass, kg | |||
| Weight Lifting Group | 30.6±0.82 | −0.35±0.21 | 0.512 |
| Control Group | 31.1±0.88 | −0.13±0.26 | |
Data are means ± standard error. Change (Δ) values from baseline to 12-months are from the repeated-measures linear mixed-effects regression models. P values are for the group-by-time interaction term in the regression model.
Figure 2.
Least-squares mean change in relative appendicular skeletal muscle mass (kg/m2) from the repeated-measures linear mixed-effects regression models. Bars represent standard errors.
We observed no changes in body mass index, total body mass, body fat percentage, and fat mass between groups over 12-months. Self-reported daily caloric consumption (−212±79 kcal/d versus −159±56 kcal/d; P=0.581), and moderate- or vigorous-intensity aerobic activity (+6.9±4.6 MET-hr/wk versus +0.9±5.7 MET-hr/wk; P=0.412) did not differ between the weight lifting and control groups over 12-months, respectively.
No serious weight lifting related adverse events occurred. Non-serious adverse events have been reported elsewhere in detail [26].
DISCUSSION
The principal finding from this post hoc analysis is that breast cancer survivors who participated in slowly-progressive weight lifting for 12-months attenuated the decline of ASMM compared to control group participants. Slowly-progressive weight lifting also increased maximal muscle strength of the upper- and lower-body. These findings expand upon the known benefits of weight lifting for breast cancer survivors.
Women begin to lose approximately 0.1 kg (0.5%) of ASMM each year starting between the age of 35 and 45 years [5–8]. Treatment for breast cancer may accelerate the loss of ASMM [4]. It is noteworthy that the decline in ASMM in the control group of this study over 12-months was 0.22±0.07 kg (1.03%), which is approximately double the observed rate of 0.1 kg (0.5%) lost per year among women without cancer [5–8]. In contrast, the decline in ASMM in the weight lifting group over 12-months was 0.02±0.06 kg (0.05%). Our findings support the hypothesis that treatment for cancer may accelerate the loss of ASMM, and reinforce the importance of identifying interventions that attenuate the decline of ASMM among breast cancer survivors.
ASMM is associated with a variety of health outcomes that may be of importance to breast cancer survivors. Low ASMM is associated with an increased risk of all-cause mortality among breast cancer survivors [14]. ASMM also has endocrine effects, such that low levels of ASMM are associated with higher levels of fasting insulin [9, 10], and other insulin-axis hormones [11, 12]. This is important because high levels of fasting insulin associate with an increased risk of cancer recurrence and death among women diagnosed with early-stage breast cancer [13]. Low levels of ASMM are associated with frailty, disability, and bone mineral density [12, 15, 16]. Frailty occurs at an earlier age in breast cancer survivors compared to that of non-cancer controls [27]. Disability is more prevalent among breast cancer survivors than non-cancer controls and exerts a negative impact on overall self-reported health [28]. Bone mineral density among breast cancer survivors is often lower compared to healthy controls that are of similar age [29]. Collectively, these data suggest that attenuating the decline of ASMM through the use of weight lifting may favorably influence clinically meaningful outcomes among breast cancer survivors. This hypothesis warrants confirmation in larger trials designed to examine the effects of weight lifting on clinical endpoints among breast cancer survivors.
Other randomized studies have examined the effects of weight lifting on body composition outcomes among breast cancer survivors. To our knowledge, our study is the first to demonstrate the efficacy of weight lifting to attenuate the decline of ASMM among breast cancer survivors. Weight lifting may decrease body fat percentage and fat mass, without altering total body mass among breast cancer survivors [30]. We did not observe any changes in body mass index, total body mass, body fat percentage, or fat mass. This was not surprising as self-reported caloric consumption and moderate- to vigorous-intensity aerobic activity did not differ between groups over time.
There are several weaknesses to this analysis. ASMM was not a primary outcome of the PAL trial. Consequently, participants were not enrolled into this trial on the basis of being at-risk of losing ASMM or having low ASMM at baseline. PAL participants were of similar age to women in the cancer registry from which they were recruited [20], but because of our modest enrollment rate (295 randomized of 3200 screened; 9.2%), participants may differ in other ways that are unknown. Therefore our findings may be generalizable only to the population of breast cancer survivors willing to participate in a randomized controlled trial of weight lifting. This analysis was not pre-specified in the PAL trial protocol. Therefore our findings should be interpreted conservatively and considered hypothesis generating. We did not adjust our type-I error rate. Therefore we cannot rule out the possibility of false discovery.
There are several strengths to this analysis. The outcome of this study is important in that ASMM represents ≥75% of skeletal muscle in the body and is essential for ambulatory activity and physical functioning. Outcome data were collected by staff members who were blinded to randomized study group. The sample size of the study was large for a behavioral trial, and participants were diverse with respect to racial background (35% non-white). The intervention was 12-months long, with high rates of follow up that were similar between the two study groups. Adherence to the weight lifting protocol was high. This may be the result of successfully blending supervised and unsupervised exercise to promote long-term sustainability.
In conclusion, the findings from this study demonstrate the feasibility and potential efficacy for slowly-progressive weight lifting to attenuate the decline of ASMM among breast cancer survivors. While these findings are hypothesis generating and require replication, they provide additional evidence that weight lifting may provide important health benefits for breast cancer survivors.
ACKNOWLEDGEMENT
This research was supported by R01-CA106851, F31-CA192560, U54-CA155850, and R21-CA182767 from the National Cancer Institute.
Footnotes
JCB declares no conflicts of interest. KHS declares no conflicts of interest.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians. 2014 doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Siegel R, Jemal A. Breast cancer facts and figures 2013 – 2014. American Cancer Society. 2013:1–38. [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. Journal of clinical oncology. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985) 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 6.Auyeung TW, Lee SWJ, Leung J, et al. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatrics & gerontology international. 2014;14:76–84. doi: 10.1111/ggi.12213. [DOI] [PubMed] [Google Scholar]
- 7.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura I, Koda M, Otsuka R, et al. Six-year longitudinal changes in body composition of middle-aged and elderly Japanese: Age and sex differences in appendicular skeletal muscle mass. Geriatrics & gerontology international. 2014;14:354–361. doi: 10.1111/ggi.12109. [DOI] [PubMed] [Google Scholar]
- 9.Teros MTL, Alemán-Mateo H. Hyperinsulinemia is associated with the loss of appendicular skeletal muscle mass at 4.6 year follow-up in older men and women. Clinical Nutrition. 2014 doi: 10.1016/j.clnu.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Shishikura K, Tanimoto K, Sakai S, et al. Association between skeletal muscle mass and insulin secretion in patients with type 2 diabetes mellitus. Endocr J. 2014;61:281–287. doi: 10.1507/endocrj.ej13-0375. [DOI] [PubMed] [Google Scholar]
- 11.Aleman-Mateo H, Lopez Teros MT, Ramirez FA, et al. Association between insulin resistance and low relative appendicular skeletal muscle mass: evidence from a cohort study in community-dwelling older men and women participants. J Gerontol A Biol Sci Med Sci. 2014;69:871–877. doi: 10.1093/gerona/glt193. [DOI] [PubMed] [Google Scholar]
- 12.LeBrasseur NK, Achenbach SJ, Melton LJ, et al. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. Journal of Bone and Mineral Research. 2012;27:2159–2169. doi: 10.1002/jbmr.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. Journal of Clinical Oncology. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 14.Villaseñor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. Journal of Cancer Survivorship. 2012;6:398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmstrom TK, Miller DK, Herning MM, et al. Low appendicular skeletal muscle mass (ASM) with limited mobility and poor health outcomes in middle-aged African Americans. Journal of cachexia, sarcopenia and muscle. 2013;4:179–186. doi: 10.1007/s13539-013-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanimoto Y, Watanabe M, Sun W, et al. Association between muscle mass and disability in performing instrumental activities of daily living (IADL) in community-dwelling elderly in Japan. Arch Gerontol Geriatr. 2012;54:e230–e233. doi: 10.1016/j.archger.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KH, Troxel AB, Cheville A, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30:233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA. 2010;304:2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 20.Rogerino A, Grant LL, Wilcox H, 3rd, et al. Geographic recruitment of breast cancer survivors into community-based exercise interventions. Med Sci Sports Exerc. 2009;41:1413–1420. doi: 10.1249/MSS.0b013e31819af871. [DOI] [PubMed] [Google Scholar]
- 21.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 22.Booth ML, Ainsworth BE, Pratt M, et al. International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise. 2003;195 doi: 10.1249/01.MSS.0000078924.61453.FB. 3508-1381. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WR, Gordon NF, Pescatello LS, editors. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott, Williams & Wilkins; 2010. [Google Scholar]
- 24.Bosy-Westphal A, Müller M. Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int J Obes. 2014 doi: 10.1038/ijo.2014.161. [DOI] [PubMed] [Google Scholar]
- 25.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. New Jersey: Wiley, Hoboken; 2004. [Google Scholar]
- 26.Brown JC, Troxel AB, Schmitz KH. Safety of weightlifting among women with or at risk for breast cancer-related lymphedema: musculoskeletal injuries and health care use in a weightlifting rehabilitation trial. Oncologist. 2012;17:1120–1128. doi: 10.1634/theoncologist.2012-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett JA, Winters-Stone KM, Dobek J, et al. Frailty in Older Breast Cancer Survivors: Age, Prevalence, and Associated Factors. 2013;40:E126–E134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 29.Body JJ. Increased fracture rate in women with breast cancer: a review of the hidden risk. BMC Cancer. 2011;11 doi: 10.1186/1471-2407-11-384. 384-2407-11-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battaglini CL, Mills RC, Phillips BL, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: A systematic review of the literature. World journal of clinical oncology. 2014;5:177. doi: 10.5306/wjco.v5.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]