Abstract
Drug discrimination procedures use dose-dependent generalization, substitution, and pretreatment with selective agonists and antagonists to evaluate receptor systems mediating interoceptive effects of drugs. Despite the extensive use of these techniques in the nonhuman animal literature, few studies have used human subjects. Specifically, human studies have not routinely used antagonist administration as a pharmacological tool to elucidate the mechanisms mediating the discriminative stimulus effects of drugs. This study evaluated the discriminative-stimulus effects of tramadol, an atypical analgesic with monoamine and mu opioid activity. Three human subjects first learned to discriminate 100 mg tramadol from placebo. A range of tramadol doses (25 to 150 mg) and hydromorphone (4 mg) with and without naltrexone pretreatment (50 mg) were then administered to subjects after acquiring the discrimination. Tramadol produced dose-dependent increases in drug-appropriate responding and hydromorphone partially or fully substituted for tramadol in all subjects. These effects were attenuated by naltrexone. Individual subject records indicated a relationship between mu opioid activity (i.e., miosis) and drug discrimination performance. Our findings indicate that mu opioid activity may mediate the discriminative-stimulus effects of tramadol in humans. The correspondence of generalization, substitution, and pretreatment findings with the animal literature supports the neuropharmacological specificity of the drug discrimination procedure.
Keywords: drug discrimination, opioids, tramadol, naltrexone, miosis, point-distribution, humans
The drug discrimination procedure is an important tool for evaluating the receptor systems mediating interoceptive drug effects. Toward this end, researchers may use a variety of techniques, such as evaluation of dose-related generalization gradients, substitution testing, and pretreatment with selective agonists and antagonists, as evidence of receptor mediation (Colpaert, 1999; Kelly, Stoops, Perry, Prendergast, & Rush, 2003). These methods are common in the nonhuman animal literature, and have been crucial for determining the functional role of many receptor systems in interoceptive drug effects. In contrast, the human drug discrimination literature has not advanced at this same pace. The available human studies have supported the neuropharmacological specificity of drug discrimination when used with stimulant drugs, however, less information is available about other drug classes (see review by Kelly et al., 2003). Such a discrepancy is notable because the predictive validity of animal models rests on the cross-species generality of nonhuman and human outcomes.
Previous research with opioid drugs has demonstrated that drugs with similar neuropharmacological profiles (e.g., mu opioid receptor agonists or kappa opioid receptor agonists) produce similar discriminative-stimulus effects and substitute for one another in humans (e.g., Preston & Bigelow, 1994, 2000). In addition, administration of mu opioid antagonists (e.g., naltrexone, naloxone) attenuates the subject-rated effects of prototypic mu opioid agonists (e.g., hydromorphone; Preston & Bigelow, 1993). To date, few human laboratory studies have used antagonist testing as a pharmacological tool to elucidate the mechanisms mediating the discriminative stimulus effects of drugs and no published studies have determined the discriminative-stimulus effects of an opioid agonist after antagonist pretreatment. Doing so is important because the use of antagonists is a prominent preclinical pharmacological strategy for evaluating interoceptive drug effects. Concordance between the human and animal literature would provide further support for the neuropharmacological specificity of the drug discrimination procedure and the conservation of discriminative-stimulus effects across species.
The interoceptive effects of a drug may vary across multiple dimensions, particularly when a drug acts at multiple receptor sites. Drug discrimination procedures can reveal the relative role these distinct interoceptive effects play in the stimulus control of behavior, and help to disentangle the pharmacological action of atypical drugs. One drug with a mixed mode of action is the atypical analgesic tramadol. Tramadol is a racemic compound that blocks serotonin and norepinephrine reuptake and whose primary metabolite (o-desmethyltramadol; M1) binds to the mu opioid receptor with moderate intrinsic affinity and efficacy (Raffa et al., 1992; Gillen, Haurand, Kobelt, & Wnendt, 2000; Volpe et al., 2011). In several human laboratory studies, oral tramadol produced opioid agonist effects, including miosis (i.e., pupil constriction) and positive subject-rated effects (e.g., “Like Drug” or “Good Effects”) in opioid users (Zacny, 2005; Stoops et al., 2012; Stoops, Glaser, & Rush, 2013). Pretreatment with the mu opioid antagonist naltrexone reversed tramadol-induced miosis and attenuated some of the positive subjective effects, which together support a putative role of mu opioid receptor activation (Stoops et al., 2012). Although subjective effects quantify interoceptive drug states, these data are less sensitive to specific receptor activation and are thus less suited for receptor mediation studies (Kelly et al., 2003). Drug-discrimination studies provide valuable information about the neuropharmacological mechanisms of a tested compound that produce a discrete interoceptive state. As such, drug discrimination findings are needed to understand the specific receptor system(s) mediating the distinct interoceptive state of tramadol.
Drug discrimination studies conducted with tramadol also support the importance of mu opioid activation. For instance, tramadol fully substituted for morphine in rats trained to discriminate 4.0 mg/kg morphine from saline, and this effect was blocked by concurrent naltrexone treatment (Ren & Zheng, 2000). Similarly, morphine substituted for tramadol in rats trained to discriminate 20 mg/kg tramadol from saline, and this substitution was blocked by the mu opioid antagonist naloxone (Filip, Wydra, Inan, Dziedzicka-Wasylewska, & Przegalinski, 2004). In contrast, tramadol failed to substitute for the serotonergic compound anpirtoline, the non-opioid analgesic flupirtine, and the stimulant methamphetamine in rats trained to discriminate these respective drugs from saline (Ren & Zheng, 2000; Swedberg, Shannon, Nickel, & Goldberg, 1988, 1992). In human subjects trained to discriminate placebo, hydromorphone, and methylphenidate, higher but not lower doses of tramadol substituted for hydromorphone (Duke, Bigelow, Lanier, & Strain, 2011).
Despite the importance of antagonist treatment as a pharmacological tool used to determine the underlying pharmacological mechanisms mediating the discriminative stimulus effects of a drug in nonhuman studies, few studies in humans have used these techniques. This study was designed to determine how the mu opioid receptor influences the interoceptive effects of tramadol. We chose tramadol to illustrate the utility of drug discrimination in disentangling the neuropharmacological mechanisms affecting the interoceptive effects of drugs acting at multiple receptor sites. To this end, we investigated the dose-related tramadol generalization gradient, substitution testing with hydromorphone, and naltrexone pretreatment antagonism in occasional opioid users trained to discriminate tramadol versus placebo. The use of intensive within-subject design and analysis further allowed investigation of the relationship between discrimination responding and prototypical mu opioid physiological effects (i.e., miosis).
Methods
Subjects
Adult human subjects who reported recreational opioid use in the year prior to screening were recruited via newspaper ads, flyers, and word-of-mouth. Individuals seeking treatment for substance abuse or successfully sustaining abstinence were excluded. All subjects were in good health as verified by medical history, laboratory tests, and an electrocardiogram. No subject was physically dependent on any drug requiring detoxification. Persons with past or current psychiatric or medical illnesses that, in the opinion of the study physician, would interfere with study participation also were excluded. Subjects gave their sober, written informed consent prior to study participation. The consent document stated that oral placebo, tramadol, hydromorphone, and naltrexone would be administered. The Institutional Review Board of the University of Kentucky approved the informed consent and study procedures. The study was conducted in accordance with the Helsinki guidelines.
Three subjects completed the experiment. S709 was a 24 year old Caucasian male and at the time of screening he reported drinking 4 alcoholic drinks per week. In the past 30 days he also reported using marijuana 5 days, benzodiazepines 1 day, and opioids 1 day. S733 was a 33 year old Caucasian male and at the time of screening he reported smoking 20 cigarettes a day and drinking 5 alcoholic drinks per week. In the past 30 days he also reported using marijuana 30 days, benzodiazepines 7 days, cocaine 1 day, and opioids 1 day. S740 was a 24 year old African American male and at the time of screening he reported smoking 20 cigarettes a day and drinking 6 alcoholic drinks per week. In the past 30 days he also reported using benzodiazepines 12 days and opioids 16 days.
Six additional subjects (5 male, 1 female; 5 Caucasian, 1 African American) were enrolled in the study but failed to complete the experiment. Four subjects were removed from the study due to poor attendance and two subjects failed to acquire the tramadol discrimination as described below. Of the 4 subjects removed due to poor attendance, 3 acquired the tramadol discrimination, with 2 completing only 1 test session and 1 completing 7 test sessions before being dismissed. These 6 subjects ranged in age from 19 to 32 (mean = 25.2 years), 4 smoked 10 to 20 cigarettes per day (mean = 11 cigarettes), and 4 drank 1 to 2 alcoholic drinks per week (mean = 1.1 drinks). In the past 30 days all 6 reported marijuana use (mean = 18.4 days) and 4 reported opiate use (mean = 2.75 days).
General Procedures
Subjects were enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology (LHBP) at the University of Kentucky Medical Center for 31 to 32 experimental sessions conducted on weekdays. Subjects were told that during their participation they would receive various drugs and that the purpose of the study was to see if they could detect the presence of a drug, and how drugs affect mood and behavior. Other than receiving this general information, subjects were given no instructions regarding what outcomes might be expected or what they were “supposed” to do, and were blind to the type of drug administered. All subjects received $40/session plus bonus money for performance on the drug discrimination task as outlined below. Subjects were asked to abstain from illicit drugs for the duration of the study (including marijuana) and from alcohol for the 12 h prior to, and following, each experimental session. Subjects also had to abstain from solid food and caffeine for 4 hours before each experimental session. Subjects were not required to abstain from cigarette smoking outside of sessions, and were given an opportunity to smoke halfway through the experimental session.
Upon arrival at the LHBP (~8:00 AM), an expired air sample (Intoximeters, Inc., St Louis, MO) and urine sample were collected. If the air sample was positive for alcohol or the urine sample positive for cocaine, amphetamines, benzodiazepines, opiates, or barbiturates the subject was sent home for the day. Subjects were also required to pass a standard field sobriety test upon arrival. Three sessions were canceled and rescheduled due to subjects providing alcohol or other drug positive samples. Following confirmation of recent abstinence, subjects were fed a standard, low-fat breakfast. A pretreatment of placebo or 50 mg naltrexone was administered at approximately 9:00 AM and placebo, tramadol, or hydromorphone administered 1 h later. On experimental-session days, subjects completed the self-reported drug-effect questionnaire and performance task approximately 30 min before each drug administration. Subjects completed the drug-discrimination task, self-reported drug-effect questionnaires, and performance measures 1, 2, 3, 4, and 5 h after the second drug administration. Lunch was provided after completion of the 3 h tasks. See Table 1 for details on drug administration and task timing.
Table 1.
Drug Discrimination Session Timeline
| Time | Experimental Activity |
|---|---|
| 8:00 AM | Session begins. Sobriety testing completed. |
| 8:30 AM | Subject served a standard low-fat breakfast. Subject completes subjective, performance, and physiological measures. |
| 9:00 AM | Placebo or naltrexone (50 mg) administered. During the sampling phase and test-of-acquisition sessions, this drug administration is always placebo. |
| 9:30 AM | Subject completes subjective, performance, and physiological measures. |
| 10:00 AM | Placebo, tramadol, or hydromorphone administered. |
| 11:00 AM | Drug discrimination, subjective, performance and physiological measures completed at hourly intervals for the remainder of the session. |
| 1:00 PM | Subject served a standard lunch. |
| 3:00 PM | Session ends. |
Drug-Discrimination Procedures
The drug-discrimination procedure consisted of four phases: (1) practice phase, (2) sampling phase, (3) acquisition phase, and (4) test phase. Each phase (except the practice phase) consisted of two drug administrations, a pretreatment (placebo or 50 mg naltrexone), and a training/test dose (placebo, tramadol, or hydromorphone) administered 1 h later. All references to time are made relative to the training/test dose administration.
Practice phase
Subjects completed a “practice” session prior to the start of drug testing. This “practice” session was used to familiarize participants with the daily laboratory routine including the drug-discrimination task, self-reported drug-effect questionnaires, and performance measures. No drugs were administered during the practice phase.
Sampling phase
Two sampling sessions were conducted to acquaint subjects with the drug effects. During each sampling session, subjects ingested capsules that contained a total of 100 mg tramadol. Tramadol was identified by letter code (e.g., DRUG A; A is used for illustrative purposes and a unique letter code was used for each subject), but the subjects were not explicitly informed of the capsules’ contents. Subjects also ingested a pretreatment that always contained placebo 1 h prior to tramadol administration. Each subject was provided an instruction set during the sampling phase and was asked to carefully read the instructions before each sampling session as a research assistant read them aloud. The instructions explained that subjects were receiving DRUG A, but in the future they would be asked to decide whether they received DRUG A or NOT DRUG A (see Rush, Stoops, Hays, Glaser, & Hays, 2003 for additional details).
Acquisition phase
An acquisition phase was conducted following the sampling phase to determine if subjects could discriminate 100 mg tramadol. Subjects were not explicitly informed they would be attempting to acquire a drug versus placebo discrimination. Subjects first ingested a pretreatment that always contained placebo. One hour later, subjects ingested capsules under double-blind procedures and were not told whether the capsules contained 100 mg tramadol (e.g., DRUG A) or placebo (e.g., NOT DRUG A). After this second drug administration, subjects completed the drug-discrimination task, self-reported drug-effect questionnaires, and performance measures at 1 h intervals for 5 h and were instructed that they could change their responses on the drug-discrimination task between each hour. After completing the 5 h tasks, subjects opened a sealed envelope that informed the research assistant and subject of the drug administered during that session. Subjects acquired the discrimination when ≥ 80% correct responding occurred on the drug-discrimination task on four consecutive sessions. If subjects failed to acquire the discrimination in 12 sessions they were removed from the study. The order of the drug administration was random, with the exception that each subject received each training condition, placebo, and 100 mg tramadol, at least twice.
Test phase
Following successful acquisition of the tramadol discrimination, subjects entered a test phase. In this phase, test sessions were interspersed with acquisition sessions. Prior to all sessions during the test phase, subjects were administered a placebo or 50 mg naltrexone pretreatment 1 h prior to test drug administration. During test sessions, subjects were given no feedback regarding the accuracy of their drug-discrimination performance and were credited with the greater number of points allocated to the DRUG A or NOT DRUG A response option. Thus, these sessions were similar to acquisition sessions except that: (1) bonus money was earned based on the greatest number of points allocated to DRUG A or NOT DRUG A and (2) no feedback was provided based on performance (i.e., the sealed envelope only indicated TEST for test sessions). The schedule of test sessions was double-blind and neither the subject nor research assistant knew until the sealed envelope was opened at the 5 h time point.
Acquisition sessions were included during the test phase to ensure that the 100 mg tramadol discrimination remained intact. At least 4 acquisition sessions (i.e., two placebo and two 100 mg tramadol) were conducted during the test phase and were identical to sessions conducted during the acquisition phase. If a subject responded incorrectly during an acquisition session (i.e., < 80% correct), additional acquisition sessions were conducted until the participant correctly identified at least one of the conditions (i.e., placebo or 100 mg tramadol).
Altogether, 12 test sessions were conducted during the test phase in which subjects received oral placebo, tramadol (25, 50, 100, 150 mg), or hydromorphone (4 mg), following pretreatment with placebo or naltrexone (50 mg) under test conditions (i.e., with a blank card at the 5 h time point). Placebo and the training dose of tramadol (i.e., 100 mg) were included in this phase to evaluate the training drug and placebo under identical conditions as the test doses. Each subject was tested on each dose condition once. The order of test sessions was random during the test phase, with the exception that an active drug dose was never administered on more than 3 consecutive test sessions. At least 24 hours separated drug administrations to allow naltrexone to clear from the system (Verebey, Volavka, Mule, & Resnick, 1976).
Drug-Discrimination Measure
Subjects completed a point-distribution drug-discrimination task (Rush et al., 2003; Stoops, Lile, Glaser, & Rush, 2005) 1, 2, 3, 4, and 5 h after test drug administration. In this task, subjects were asked to distribute 100 points between two options (i.e., DRUG A or NOT DRUG A) depending on how certain he/she was of the identity of the administered drug. Responses on the correct option were converted to money. Points accumulated on the correct option were worth $0.08/point meaning subjects could earn up to $40/session on this task.
Self-Report Questionnaires and Performance Task
Subject-rated measures included a series of items rated on a 100-unit visual analog scale (VAS; see Stoops et al., 2013 for more details). Subjects also performed a computerized digit symbol substitution task (DSST) as a performance task. These measures were recorded 30 minutes before drug administration and 1, 2, 3, 4, and 5 h following drug administration. The focus of this manuscript is the mu related discriminative-stimulus effects of tramadol, so for clarity and brevity, only data from the Any Effect measure (a global, representative subjective effect that correlates with discrimination outcomes; Reynolds, Bolin, Stoops, & Rush, 2013) as well as a representative measure of positive valence (i.e., Good Effect) and negative valence (i.e., Bad Effect) are presented here.
Physiological Indices
Heart rate, blood pressure, and oxygen saturation were recorded using an automated monitor (DINAMAP PRO Series 400 NV2, GE Medical Systems, Milwaukee, WI). Pupil diameter was determined with a pupillometer (PLR-200, NeurOptics, Irvine, CA) in constant room lighting. These measures were recorded 30 minutes before drug administration and 1, 2, 3, 4, and 5 h following drug administration.
Drug Administration
All drugs were administered under double-blind conditions and taken orally with approximately 150 ml of water. To ensure that capsules were swallowed the research assistant: (1) watched the subject to ensure the capsules were swallowed and not removed from the mouth, (2) spoke with the subject to determine if he/she had anything in his/her mouth, and (3) conducted a brief oral examination to ensure that the subject was not hiding the capsules under his/her tongue. Tramadol (25, 50, 100, and 150 mg; UDL Labs, Rockford, IL, USA), hydromorphone (4 mg; Halo Pharmaceuticals, Whippany, NJ, USA), and naltrexone (50 mg; Barr Labs, Pomona, NY, USA) were prepared by over-encapsulating drug with cornstarch filler in opaque gelatin capsules. Placebo capsules were identical and contained only cornstarch. The range of doses were selected based on data from previous human laboratory studies with tramadol, hydromorphone, and naltrexone that indicated these doses were well tolerated (Walsh, Sullivan, Preston, Garner, & Bigelow, 1996; Zacny, 2005; Lile, Kelly, Pinsky, & Hays, 2009; Stoops et al., 2012, 2013). The first dose containing placebo or naltrexone was administered at approximately 9:00 AM. The second dose containing placebo, tramadol, or hydromorphone was administered 1 h later and all drug discrimination measures were taken after this point.
Data Analysis
Acquisition data were first analyzed across individual sessions to determine if subjects met the discrimination criteria of four consecutive sessions with ≥ 80% correct responding. For comparison to test sessions, acquisition data were averaged across the four sessions in which subjects met the discrimination criteria (i.e., the final two placebo and two 100 mg tramadol sessions from the acquisition session). All values during the test phase represent the single test session in which those data were obtained. Test phase drug discrimination data were analyzed as the total percentage of points allocated to the drug-appropriate response over the total session (i.e., points allocated to “Drug A” divided by 500). Drug discrimination data were defined as non (≤ 20%), partial (21%–79%), or full (≥ 80%) substitution based on the percentage of points allocated to the drug-appropriate response (e.g., DRUG A). Pupil diameter data were analyzed as the nadir value during the 5 h post drug administration. The relationship between miosis and drug discrimination data was examined using miosis at the 100 mg tramadol training dose as a relative baseline. Previous studies indicated that miosis greater than placebo is achieved at 100 mg but not 50 mg tramadol (Stoops et al., 2013); therefore, we considered the 100 mg data point as a standard for significant miotic effects. Similarly, peak effects for subject ratings of Any Effect, Good Effect, and Bad Effect as well as other physiological effects were examined using peak effects (nadir for oxygen saturation) at the 100 mg tramadol training dose as a relative baseline. Each subject’s drug discrimination, physiological, and subjective effects data were individually examined for tramadol drug discrimination, hydromorphone substitution, naltrexone antagonism, and the miosis-drug discrimination relationship.
Results
Discrimination Acquisition
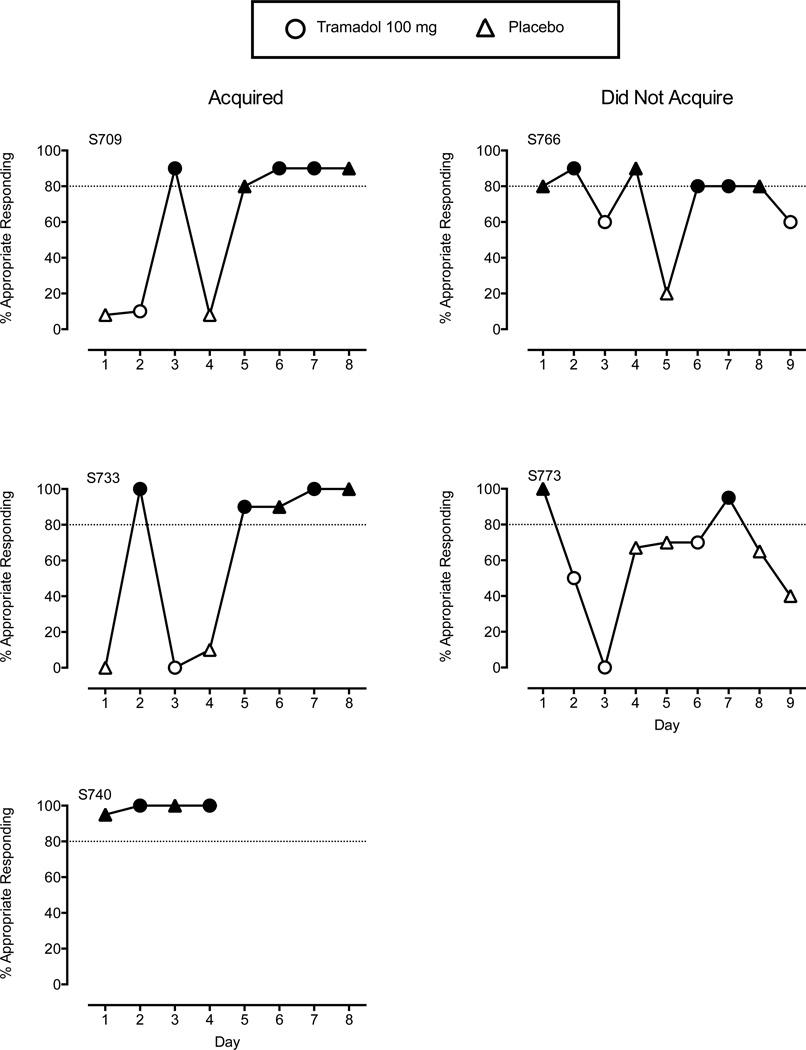
Of the 5 participants who entered the study and were not removed for poor attendance, 3 (60%) successfully acquired the tramadol discrimination. Figure 1 shows the percentage of correct responses during each discrimination training session for subjects who acquired (left panels) and did not acquire (right panels) the tramadol discrimination. S709 and S733 acquired the discrimination in 8 sessions, whereas S740 acquired it in 4 sessions. S766 and S773 were removed from the study because each subject responded incorrectly in the 9th session so could not reach acquisition criteria (i.e., four consecutive sessions) by the 12th session.
Figure 1.
Percent correct responses during tramadol discrimination training. Circles represent sessions with oral 100 mg tramadol. Triangles represent sessions with oral placebo. Filled shapes are sessions with ≥ 80% session-appropriate responding (noted by the dotted line). Subjects acquired the tramadol discrimination when ≥ 80% session-appropriate responding occurred in four consecutive sessions. Left panels are from the 3 subjects who successfully acquired the tramadol discrimination, whereas right panels are from the 2 subjects who failed to acquire the discrimination.
Discrimination Testing
Figure 2 shows the percentage drug-appropriate responses (left panels) and nadir pupil diameter (right panels) across all tramadol, hydromorphone, and naltrexone pretreatment conditions for each subject. Figure 3 and Figure 4 show the peak subject-rated effects and physiological effects (nadir for oxygen saturation) across these doses, respectively. S709 (Figure 2; top panels) generally showed a dose-appropriate increase in responding with full substitution by the 25, 100, and 150 mg tramadol doses. In addition, hydromorphone substituted for tramadol with 90% drug-appropriate responding. Naltrexone reversed these effects with decreases in drug-appropriate responding at all dose conditions with drug-appropriate responding during placebo pretreatment. Of all full substitutions observed for S709, 67% occurred during sessions with miotic effects greater than or equal to the 100 mg tramadol training dose. For only one dose combination (25 mg tramadol + 50 mg naltrexone) did responding fail to show full substitution when miotic effects were greater than or equal to those of the training dose. In contrast, other physiological effects and subject ratings of Any, Good, or Bad Effect were not systematically related to drug discrimination performance (Figures 3 and 4; top panels).
Figure 2.
Percent drug-appropriate responses (left panels) and nadir pupil diameter (right panels). Values over PL (placebo) and 100 on the left side of each panel represent average value observed during the four acquisition sessions (two placebo, two 100 mg tramadol) during which the discrimination was acquired. Values over HY represent data obtained during 4 mg hydromorphone testing. The dotted line in right panel is the nadir pupil diameter observed during the 100 mg tramadol training dose sessions. Circles are data during placebo pretreatment, whereas squares are data during 50 mg naltrexone pretreatment. Filled shapes are sessions where full substitution (≥ 80% drug-appropriate responding) was observed. Half-filled shapes are sessions where partial substitution (21% to 79% drug-appropriate responding) was observed.
Figure 3.
Peak subject-rated effects for Any Effect (left panels), Good Effect (middle panels), and Bad Effect (right panels). Dotted lines represents peak effect observed during the 100 mg tramadol training session. See Figure 2 legend for additional details on axes and legend.
Figure 4.
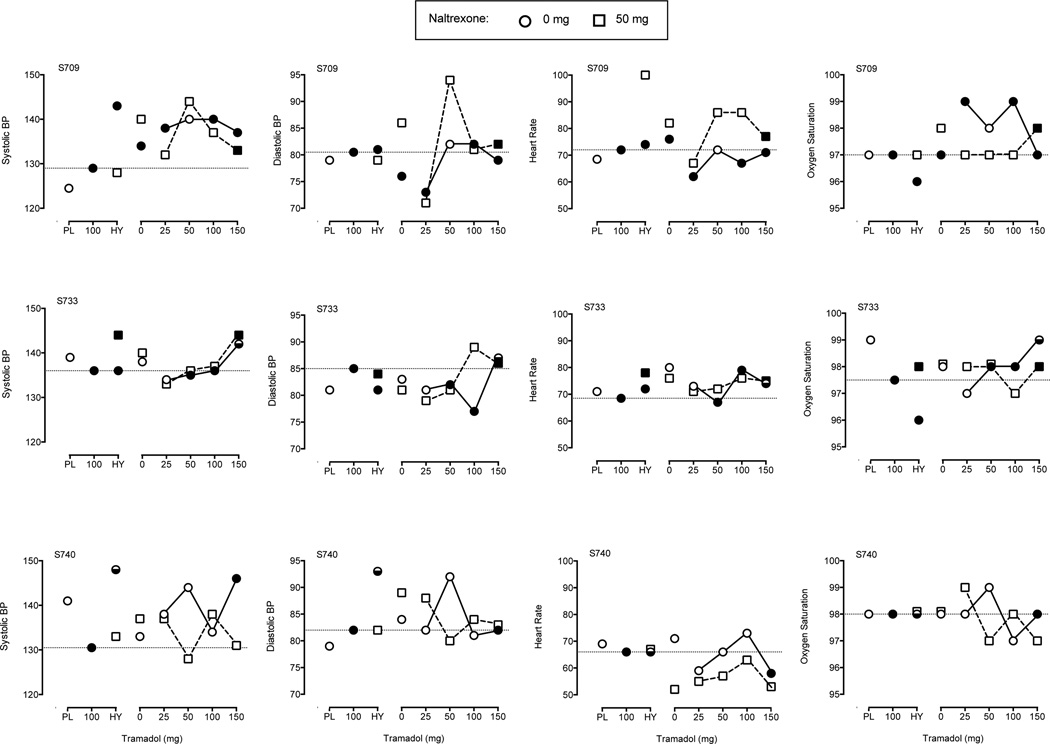
Physiological effects for systolic blood pressure (left panels), diastolic blood pressure (left-middle panels), heart rate (right-middle panels), and oxygen saturation (right panels). Dotted lines represents peak (nadir for oxygen saturation) effect observed during the 100 mg tramadol training session. See Figure 2 legend for additional details on axes and legend.
S733 (Figure 2; middle panels) also generally showed a dose-appropriate increase in responding with full substitution by the 50 and 100 mg tramadol conditions and partial substitution by the 150 mg dose. Hydromorphone fully substituted for tramadol with 100% drug-appropriate responding. Naltrexone attenuated drug-appropriate responding at all dose conditions, except the 150 mg tramadol dose, that had drug-appropriate responding under placebo maintenance. Of all full substitutions observed for S733, only 20% occurred during sessions with miotic effects greater than or equal to the 100 mg tramadol training dose. However, all doses that produced greater than or equal to 100 mg miotic effects also produced full (100 mg tramadol) or partial (150 mg) substitution. Other physiological effects and subject rated effects were not systematically related to drug discrimination performance (Figures 3 and 4; middle panels)
S740 (Figure 2; bottom panels) showed a dose-appropriate increase in responding with full substitution by only the 150 mg dose. Hydromorphone partially substituted for tramadol with 30% drug-appropriate responding. Naltrexone reversed these effects with attenuated responding at all dose conditions with drug-appropriate responding under placebo pretreatment. The only full substitution (i.e., 150 mg tramadol + 0 mg naltrexone) S740 made was during a session with miotic effects and subject ratings greater than or equal to the 100 mg tramadol training dose. Other physiological effects were not systematically related to drug discrimination performance (Figure 4; bottom panels).
Overall, tramadol produced a dose-dependent increase in drug-appropriate responding. Drug-appropriate responding was decreased in 11 out of 12 cases in which an active dose of tramadol was combined with naltrexone (or no change in the case where 0% identification was observed during placebo pretreatment). For all subjects, naltrexone pretreatment decreased drug-appropriate responding for hydromorphone. Notably, in only 2 of 36 test sessions (5.6%) was pupil diameter less than or equal to that observed with the 100 mg training dose and the subject failed to exhibit full substitution. Taken together, other physiological and subject rated measures were generally not related to drug discrimination performance. For example, in only 4 of the 12 sessions where subjects demonstrated full substitution (33%) did subject ratings of Any Effect meet or exceed those observed during the 100 mg training session. Similarly, subjects showed inconsistent drug-appropriate responding relative to changes in systolic blood pressure. Although 9 dose combinations fully substituted, 18 dose combinations failed to substitute for the training stimulus in sessions with changes in systolic blood pressure that were greater than or equal to those observed with the training dose.
Discussion
The present study examined the mu opioid receptor-mediated discriminative-stimulus effects of tramadol in humans using dose-dependent generalization, substitution, and pretreatment with an opioid antagonist. These data support the role of mu opioid receptor activation in the discriminative-stimulus effects of tramadol. First, tramadol produced a dose-dependent generalization gradient with greater doses of tramadol engendering greater percentages of drug-appropriate responding. Second, the mu opioid agonist hydromorphone partially or fully substituted for tramadol in all subjects. Third, pretreatment with the opioid antagonist naltrexone decreased drug-appropriate responding. Finally, responses on the drug-appropriate choice generally corresponded with the mu-mediated effects of tramadol (i.e., miotic effects) but not other, more global physiological effects (e.g., blood pressure, heart rate). Taken together, the dose-related tramadol generalization gradient, substitution testing with hydromorphone, and naltrexone pretreatment correspond with the animal literature, and provide tentative support for the cross-species generality and neuropharmacological specificity of the drug discrimination procedure.
Tramadol functioned as a discriminative stimulus and produced dose-dependent increases in drug-appropriate responding. These findings are concordant with the only nonhuman animal study using tramadol as the training drug (Filip et al., 2004). In that study, administration of tramadol produced a dose-dependent increase in tramadol-appropriate responding in rats trained to discriminate tramadol from saline. Numerous animal and human studies have shown that opioid agonists produce dose-related increases in discriminative responding when compared to the training dose (e.g., Preston, Bigelow, Bickel, & Liebson, 1987; Cleary et al., 1999; Preston & Bigelow, 2000; Stevenson, Canadas, Zhang, Rice, Riley, 2000). Only 60% of subjects acquired the discrimination in the present study, potentially because the training dose of tramadol was too low. The low training dose may also explain the variability observed in discrimination testing performance. For example, S740 acquired the tramadol discrimination rapidly, but only showed full substitution with the highest tramadol dose during testing sessions. It should be noted that we selected this training dose and dose range to enhance the subjects’ safety. Higher doses of tramadol administered to more experienced, but non-dependent, opioid users produced nausea and vomiting in some subjects (Stoops et al., 2012). Although higher doses of tramadol may have resulted in greater discrimination acquisition, these doses would likely have increased the probability of unpleasant and potentially dangerous side effects for the recreational opioid users enrolled in this study.
The substitution of hydromorphone for tramadol provides further evidence that the discriminative-stimulus effects of tramadol are mu opioid receptor mediated. In the only other drug discrimination study using tramadol with humans, tramadol substituted for hydromorphone in subjects trained on a three-choice discrimination with hydromorphone, methylphenidate, and placebo (Duke et al., 2011). A similar symmetric relationship between tramadol and mu opioids is observed in the animal literature. Morphine fully substitutes for tramadol in tramadol-trained rats and tramadol fully substitutes for morphine in morphine-trained rats (Ren & Zheng, 2000; Filip et al., 2004). This cross-substitution is specific because tramadol fails to substitute for the stimulant methamphetamine, the serotonergic compound anpirtoline, and the non-opioid analgesic flupirtine (Swedberg et al., 1988, 1992; Ren & Zheng, 2000). Likewise, the mu opioid antagonist naloxone, the dopamine and serotonergic compound roxindole, and numerous monoamine reuptake inhibitors (e.g., milnacipram, venlafaxine, fluoxetine) fail to fully substitute for tramadol (Filip et al., 2004).
Pretreatment with the opioid antagonist naltrexone attenuated the discriminative-stimulus effects of tramadol as well as hydromorphone substitution. That mu opioid blockade attenuated the discriminative-stimulus effects of the test compounds (i.e., tramadol and hydromorphone) is concordant with the vast animal literature that shows decreased drug-appropriate responding after naltrexone treatment in opioid-trained animals (e.g., White & Holtzman, 1981; Walker, Makhay, House, & Young, 1994). Furthermore, previous studies indicate that naloxone or naltrexone attenuate tramadol-appropriate responding and tramadol substitution for morphine in tramadol- and morphine-trained rats, respectively (Ren & Zheng, 2000; Filip et al., 2004). It should be noted that naltrexone has a complex binding profile with the greatest affinity for mu opioid receptors but some affinity for kappa and delta opioid subtypes (Peng, Knapp, Bidlack, & Neumeyer, 2007). Tramadol itself has limited binding affinity at the mu opioid site (Ki = 2.4 µM) with its metabolite M1 acting with much higher affinity (Ki = 3.4 nM; Gillen et al., 2000). Nevertheless, the above effects support the importance of opioid system activity in tramadol discrimination as well as suggesting the conservation of drug discrimination outcomes across species regarding dose-dependent generalization, substitution testing, and antagonist pretreatment.
We observed additional evidence of mu opioid mediation by comparing individual records of miotic and drug discrimination effects. Specifically, on very few sessions with miotic effects greater than or equal to those of the 100 mg training dose did doses fail to substitute for the training stimulus. These findings indicate that significant mu opioid activation (measured by pupil diameter) corresponded well with drug-appropriate responding. Previous studies evaluating the miotic effects of tramadol have revealed mixed findings. Whereas several reports have reported significant decreases in pupil diameter (Zacny, 2005; Stoops et al., 2012), others have failed to observe miotic effects (Preston, Jasinski, & Testa, 1991; Duke et al., 2011). This discrepancy may be attributed to individual differences in the metabolism of tramadol into M1. Subjects who express a polymorphism associated with poor tramadol metabolism fail to exhibit miosis after tramadol administration (Fliegert, Kurth, & Gohler, 2005). We observed a general dose-dependent effect on pupil diameter, and naltrexone administration attenuated tramadol-induced miosis, as has been shown previously (Stoops et al., 2012). Although we did not examine genotypes in the present study, the observed miotic effects and attenuation by naltrexone indicate that our subjects were sensitive to the mu opioid-related effects of tramadol/M1. However, it is still possible that variations in metabolic phenotypes combined with the low training dose used in this study contributed to differential drug effects and variability in discrimination performance.
Future studies could further explore the discriminative stimulus effects of tramadol using alternative and more complex designs. It is possible that the monoamine reuptake-inhibition activity of tramadol influenced miotic effects because serotonergic drugs also produce miosis (Fanciullacci, Sicuteri, Alessandri, & Geppetti, 1995; Koudas et al., 2009). The use of double-dissociation techniques that also block tramadol’s non-opioid effects (e.g., monoamine reuptake-inhibition activity) would help clarify these mixed-action effects and the relationship with miosis. However, the attenuation of drug discrimination performance by naltrexone and substitution by hydromorphone implicate opioid system over monoamine system activity in the present study. Alternatively, 3-choice models that include monoamine reuptake inhibitors (e.g., Duke et al., 2011) could provide greater specificity of behavioral responding and insight into the mixed-action effects of tramadol.
Worth noting is that in a previous study naltrexone only partially attenuated the positive subjective effects of tramadol, indicating that non-mu opioid action plays a role in the subjective effects of tramadol (Stoops et al., 2012). Naltrexone more fully attenuated subject ratings in this study, perhaps due to the lower doses tested. Moreover, subject ratings were less consistently related to drug discrimination than the miotic effects of tramadol. More global physiological effects, such as blood pressure and heart rate, were also unrelated to drug discrimination performance providing further support for miosis-drug discrimination relationship. Both drug discrimination and subjective effects rely on accessing interoceptive drug states, but these outcomes are not isomorphic and drugs can produce similar discriminative effects but dissimilar subjective effects, or vice versa (e.g., Chait, Uhlenhuth, & Johanson, 1986; Rush, Kollins, & Pazzaglia 1998). These findings highlight the discrepant interoceptive drug states drug discrimination and subjective effects may quantify when determining the neuropharmacological mechanism of a drug.
Drug discrimination is one way to determine the neuropharmacological profile of a drug. Results from such studies help improve pharmacotherapeutic design and contribute to the assessment of abuse potential (Tallman, 1999; Solinas, Panlilio, Justinova, Yasar, & Goldberg, 2006). The current study lends preliminary support for the cross-species generality of the drug discrimination procedure by providing concordant evidence between animal and human studies using generalization, substitution, and selective antagonist pretreatment. In contrast to subject-rated effects measures (e.g., Stoops et al., 2012), drug discrimination data provide information about the specific receptor systems mediating stimulus control of behavior rather than the overt subjective effects a drug might produce. Our use of the atypical analgesic tramadol illustrates the utility of drug discrimination for disentangling the neuropharmacological mechanisms of drug’s affecting multiple receptor sites. Taken together, these findings provide support for the neuropharmacological specificity of the drug discrimination procedure.
References
- Chait LD, Uhlenhuth EH, Johanson CE. The discriminative stimulus and subjective effects of phenylpropanolamine, mazindol and d-amphetamine in humans. Pharmacol Biochem Behav. 1986;24(6):1665–1672. doi: 10.1016/0091-3057(86)90503-4. [DOI] [PubMed] [Google Scholar]
- Cleary JP, O'Hare E, Pomonis JD, Dittel PL, Hofmeister JJ, Fritz MM, Levine AS. Discriminative stimulus effects of morphine: central versus peripheral training. Brain Res. 1999;847(1):26–31. doi: 10.1016/s0006-8993(99)02001-6. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64(2):337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Duke AN, Bigelow GE, Lanier RK, Strain EC. Discriminative stimulus effects of tramadol in humans. J Pharmacol Exp Ther. 2011;338(1):255–262. doi: 10.1124/jpet.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanciullacci M, Sicuteri R, Alessandri M, Geppetti P. Buspirone, but not sumatriptan, induces miosis in humans: Relevance for a serotoninergic pupil control. Clin Pharmacol Ther. 1995;57(3):349–355. doi: 10.1016/0009-9236(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Filip M, Wydra K, Inan SY, Dziedzicka-Wasylewska M, Przegalinski E. Opioid and monoamine systems mediate the discriminative stimulus of tramadol in rats. Eur J Pharmacol. 2004;498(1–3):143–151. doi: 10.1016/j.ejphar.2004.07.090. [DOI] [PubMed] [Google Scholar]
- Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in healthy subjects--the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol. 2005;61(4):257–266. doi: 10.1007/s00228-005-0920-y. [DOI] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(2):116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Stoops WW, Perry AS, Prendergast MA, Rush CR. Clinical neuropharmacology of drugs of abuse: a comparison of drug-discrimination and subject-report measures. Behav Cogn Neurosci Rev. 2003;2(4):227–260. doi: 10.1177/1534582303262095. [DOI] [PubMed] [Google Scholar]
- Koudas V, Kikolaou A, Hourdaki E, Giakoumaki SG, Roussos P, Bitsios P. Comparison of ketanserin, buspirone and propranolol on arousal, pupil size and autonomic function in healthy volunteers. Psychopharmacology (Berl) 2009;205(1):1–9. doi: 10.1007/s00213-009-1508-5. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2009;203(2):241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone and naloxone at μ δ, and κ opioid receptors. J Med Chem. 2007;50(9):2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Differential naltrexone antagonism of hydromorphone and pentazocine effects in human volunteers. J Pharmacol Exp Ther. 1993;264(2):813–823. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Drug discrimination assessment of agonist-antagonist opioids in humans: a three-choice saline-hydromorphone-butorphanol procedure. J Pharmacol Exp Ther. 1994;271(1):48–60. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Effects of agonist-antagonist opioids in humans trained in a hydromorphone/not hydromorphone discrimination. J Pharmacol Exp Ther. 2000;295(1):114–124. [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel W, Liebson IA. Three-choice drug discrimination in opioid-dependent humans: hydromorphone, naloxone and saline. J Pharmacol Exp Ther. 1987;243(3):1002–1009. [PubMed] [Google Scholar]
- Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27(1):7–17. doi: 10.1016/0376-8716(91)90081-9. 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. [PubMed] [Google Scholar]
- Ren YH, Zheng JW. Influence of tramadol on morphine discriminative behavior in rats. Acta Pharmacol Sin. 2000;21(10):924–926. [PubMed] [Google Scholar]
- Reynolds AR, Bolin BL, Stoops WW, Rush CR. Relationship between drug discrimination and ratings of subjective effects: implications for assessing and understanding the abuse potential of D-amphetamine in humans. Behav Pharmacol. 2013;24(5–6):523–532. doi: 10.1097/FBP.0b013e328364505f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol. 1998;6(1):32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306(1):195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Justinova Z, Yasar S, Goldberg SR. Using drug-discrimination techniques to study the abuse-related effects of psychoactive drugs in rats. Nat Protoc. 2006;1(3):1194–1206. doi: 10.1038/nprot.2006.167. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Canadas F, Zhang X, Rice KC, Riley AL. Morphine discriminative control is mediated by the mu opioid receptor: assessment of delta opioid substitution and antagonism. Pharmacol Biochem Behav. 2000;66(4):851–856. doi: 10.1016/s0091-3057(00)00280-x. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Rush CR. Miotic and subject-rated effects of therapeutic doses of tapentadol, tramadol, and hydromorphone in occasional opioid users. Psychopharmacology (Berl) 2013;228(2):255–262. doi: 10.1007/s00213-013-3031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Rush CR. Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine, and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol. 2005;13(1):56–64. doi: 10.1037/1064-1297.13.1.56. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lofwall MR, Nuzzo PA, Craig LB, Siegel AJ, Walsh SL. Pharmacodynamic profile of tramadol in humans: influence of naltrexone pretreatment. Psychopharmacology (Berl) 2012;223(4):427–438. doi: 10.1007/s00213-012-2739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedberg MD, Shannon HE, Nickel B, Goldberg SR. Pharmacological mechanisms of action of flupirtine: a novel, centrally acting, nonopioid analgesic evaluated by its discriminative effects in the rat. J Pharmacol Exp Ther. 1988;246(3):1067–1074. [PubMed] [Google Scholar]
- Swedberg MD, Shannon HE, Nickel B, Goldberg SR. D-16949 (anpirtoline): a novel serotonergic (5-HT1B) psychotherapeutic agent assessed by its discriminative effects in the rat. J Pharmacol Exp Ther. 1992;263(3):1015–1022. [PubMed] [Google Scholar]
- Tallman JF. Neuropsychopharmacology at the new millennium: new industry directions. Neuropsychopharmacology. 1999;20(2):99–105. doi: 10.1016/S0893-133X(98)00104-3. [DOI] [PubMed] [Google Scholar]
- Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20(3):315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Verbois SL. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol. 2011;59(3):385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271(2):959–968. [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. J Pharmacol Exp Ther. 1996;279(2):524–538. [PubMed] [Google Scholar]
- White JM, Holtzman SG. Three-choice drug discrimination in the rat: morphine, cyclazocine and saline. J Pharmacol Exp Ther. 1981;217(2):254–262. [PubMed] [Google Scholar]
- Zacny JP. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80(2):273–278. doi: 10.1016/j.drugalcdep.2005.05.007. [DOI] [PubMed] [Google Scholar]